Submitted:
31 October 2024
Posted:
01 November 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
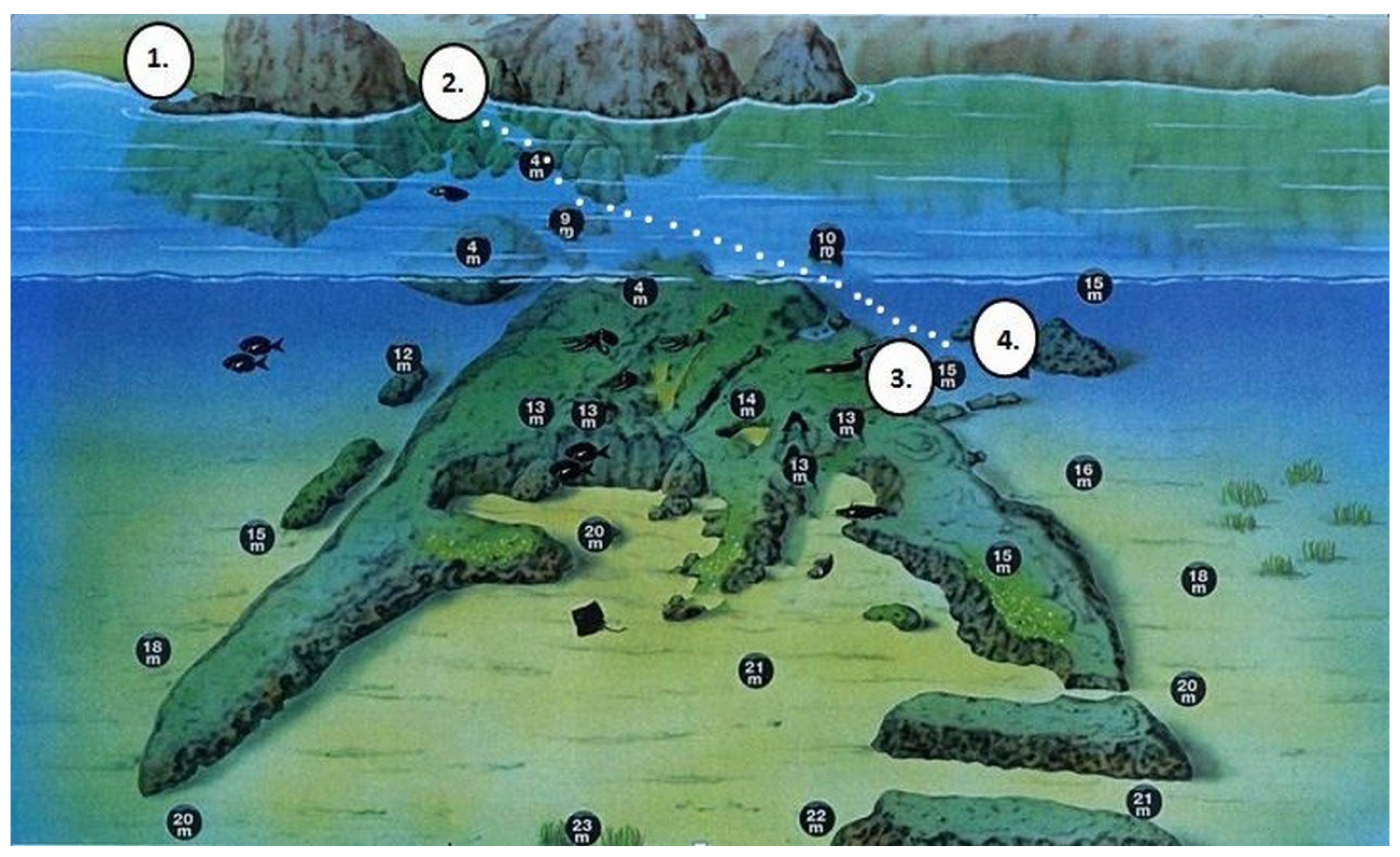
2.1. Study Area
2.2. The Studied Communities
2.3. Sampling and Processing of Samples
2.4. Work in the Laboratory
2.5. Data Analysis and Statistical Methods
3. Results
3.1. General Abundance and Specific Richness
| Species | Trophic categories |
Total abundances |
Sc | Ph |
|---|---|---|---|---|
| Bittium latreillii | HE | 2106 | 55 | 2,051 |
| Musculus costulatus | F | 1282 | 486 | 796 |
| Pusillina philippi | C | 817 | 272 | 545 |
| Pusillina radiata | F | 546 | 179 | 367 |
| Hiatella arctica | HE | 404 | 162 | 242 |
| Rissoella diaphana | HE | 228 | 0 | 228 |
| Odostomia striolata | HE | 217 | 50 | 167 |
| Alvania lineata | HE | 176 | 49 | 127 |
| Chauvetia mamillata | C | 130 | 22 | 108 |
| Parvicardium scriptum | F | 130 | 29 | 101 |
| Triphoridae indet. | C | 130 | 16 | 114 |
| Bittium reticulatum | HE | 96 | 10 | 86 |
| Clanculus cruciatus | HE | 88 | 8 | 80 |
| Striarca lactea | F | 88 | 13 | 75 |
| Tritia incrassata | SC | 80 | 20 | 60 |
| Ammonicera fischeriana | HE | 79 | 13 | 66 |
| Modiolula phaseolina | F | 67 | 11 | 56 |
| Vitreolina perminima | C | 65 | 6 | 59 |
| Jujubinus ruscurianus | HE | 62 | 13 | 49 |
| Mytilus galloprovincialis juv. | F | 57 | 11 | 46 |
| Crisilla semistriata | HE | 53 | 29 | 24 |
| Cerithium vulgatum | SDF | 41 | 2 | 39 |
| Parvamussium fenestratum | F | 41 | 22 | 19 |
| Aplysia punctata juv. | HE | 40 | 10 | 30 |
| Arca noae | F | 40 | 1 | 39 |
| Runcina sp. | HE | 39 | 4 | 35 |
| Aequipecten opercularis | F | 36 | 23 | 13 |
| Parthenina emaciata | C | 36 | 0 | 36 |
| Alvania cancellata | HE | 35 | 3 | 31 |
| Runcina adriatica | C | 30 | 3 | 27 |
| Cyrillia linearis | C | 29 | 1 | 28 |
| Cerithiopsis tubercularis | C | 25 | 2 | 23 |
| Limaria tuberculata | F | 24 | 13 | 11 |
| Irus irus | F | 22 | 5 | 17 |
| Mimachlamys varia | F | 22 | 13 | 8 |
| Scissurella costata | HE | 22 | 1 | 21 |
| Gibberula turgidula | C | 20 | 0 | 20 |
| Odostomia plicata | EP | 20 | 11 | 9 |
| Tricolia sp. | HE | 20 | 3 | 17 |
| Chauvetia brunnea | C | 17 | 9 | 8 |
| Cerithiopsis jeffreysi | C | 16 | 0 | 16 |
| Gregariella semigranata | F | 16 | 8 | 8 |
| Mitromorpha columbellaria | C | 16 | 5 | 11 |
| Ocinebrina aciculata | C | 16 | 2 | 14 |
| Odostomella doliolum | EC | 16 | 1 | 15 |
| Bulla striata | C | 15 | 3 | 12 |
| Setia pulcherrima | HE | 15 | 5 | 10 |
| Haminoea navicula | HE | 13 | 0 | 13 |
| Petricola lithophaga | F | 13 | 0 | 13 |
| Chama gryphoides | F | 12 | 8 | 4 |
| Rissoa lia | HE | 11 | 1 | 10 |
| Monophorus erythrosoma | C | 10 | 2 | 8 |
| Alvania nestaresi | HE | 9 | 0 | 9 |
| Bittium sp. | HE | 9 | 0 | 9 |
| Cerithiopsis sp. | C | 9 | 0 | 9 |
| Modiolus barbatus | F | 9 | 1 | 8 |
| Rissoa variabilis | HE | 9 | 1 | 8 |
| Bivalvia morph. 1 | F | 8 | 0 | 8 |
| Doridina nudibranch | C | 8 | 3 | 5 |
| Hexaplex trunculus | C | 8 | 4 | 4 |
| Pusia tricolor | C | 8 | 2 | 6 |
| Raphitoma echinata | C | 8 | 2 | 6 |
| Anomia ephippium | F | 6 | 4 | 2 |
| Diodora graeca | HE | 6 | 2 | 4 |
| Marshallora adversa | C | 6 | 2 | 4 |
| Muricopsis cristata | C | 6 | 4 | 2 |
| Philine sp. | C | 6 | 0 | 6 |
| Pseudochama gryphina | F | 6 | 3 | 3 |
| Rhyssoplax corallina | HE | 6 | 3 | 3 |
| Alvania aspera | HE | 5 | 0 | 5 |
| Alvania sp. | HE | 5 | 0 | 5 |
| Gastropoda morph. 1 | UN | 5 | 0 | 5 |
| Gibberula miliaria | C | 5 | 0 | 5 |
| Mitromorpha olivoidea | C | 5 | 1 | 4 |
| Tricolia tingitana | HE | 5 | 0 | 5 |
| Trinchesia genovae | C | 5 | 0 | 5 |
| Acanthochitona sp. | HE | 4 | 0 | 4 |
| Chauvetia recondita | C | 4 | 0 | 4 |
| Nodulus contortus | HE | 4 | 0 | 4 |
| Smithiela costulata | C | 4 | 1 | 3 |
| Spiralina alpinoligustica | EC | 4 | 2 | 2 |
| Acanthochitona fascicularis | HE | 3 | 2 | 1 |
| Aegires leuckartii | C | 3 | 1 | 2 |
| Bivalvia morph. 2 | F | 3 | 0 | 3 |
| Gibberula morph. 1 | UN | 3 | 0 | 3 |
| Haliotis tuberculata | HE | 3 | 2 | 1 |
| Lima lima | F | 3 | 0 | 3 |
| Limaria hians | F | 3 | 2 | 1 |
| Pusillina inconspicua | HE | 3 | 0 | 3 |
| Veneridae indet. | F | 3 | 1 | 2 |
| Bivalvia morph. 4 | F | 2 | 1 | 1 |
| Brachystomia scalaris | EC | 2 | 0 | 2 |
| Calliostoma zizyphinum | C | 2 | 0 | 2 |
| Crisilla simulans | HE | 2 | 0 | 2 |
| Diodora italica | HE | 2 | 2 | 0 |
| Doris ocelligera | C | 2 | 2 | 0 |
| Emarginula pustula | C | 2 | 1 | 1 |
| Episcomitra cornicula | C | 2 | 2 | 0 |
| Folinella excavata | EC | 2 | 0 | 2 |
| Fusinus rudis | C | 2 | 0 | 2 |
| Gastropoda morph. 2 | UN | 2 | 0 | 2 |
| Jujubinus exasperatus | HE | 2 | 1 | 1 |
| Polyplacophoran indet. | HE | 2 | 0 | 2 |
| Raphitoma laviae | C | 2 | 0 | 2 |
| Raphitoma morph. 1 | C | 2 | 0 | 2 |
| Rissoa guerinii | HE | 2 | 0 | 2 |
| Weinkauffia turgidula | C | 2 | 0 | 2 |
| Alvania cimex | HE | 1 | 1 | 0 |
| Alvania discors | HE | 1 | 0 | 1 |
| Aplus dorbignyi | SC | 1 | 0 | 1 |
| Babelomurex cariniferus | C | 1 | 1 | 0 |
| Bivalvia morph. 3 | F | 1 | 0 | 1 |
| Caloria elegans | C | 1 | 1 | 0 |
| Cardiidae morph. 1 | F | 1 | 0 | 1 |
| Cardiidae morph. 2 | F | 1 | 0 | 1 |
| Chauvetia morph. 1 | UN | 1 | 0 | 1 |
| Chauvetia morph. 2 | UN | 1 | 0 | 1 |
| Columbella rustica | HE | 1 | 1 | 0 |
| Doto sp. | C | 1 | 1 | 0 |
| Felimare sp. | C | 1 | 0 | 1 |
| Gari tellinella | F | 1 | 0 | 1 |
| Gastropoda morph 3 | UN | 1 | 1 | 0 |
| Gibberula morph. 2 | UN | 1 | 0 | 1 |
| Idas sp. | F | 1 | 1 | 0 |
| Ischnochitonidae indet. | HE | 1 | 0 | 1 |
| Lithophaga lithophaga | F | 1 | 1 | 0 |
| Mangelia multilineolata | C | 1 | 0 | 1 |
| Mangelia morph. 1 | UN | 1 | 0 | 1 |
| Mangelia morph. 2 | UN | 1 | 0 | 1 |
| Mitrella broderipii | C | 1 | 0 | 1 |
| Muricidae indet. | C | 1 | 0 | 1 |
| Nodulus spiralis | HE | 1 | 0 | 1 |
| Otina ovata | UN | 1 | 0 | 1 |
| Paradoris indecora | C | 1 | 0 | 1 |
| Patella sp. | HE | 1 | 0 | 1 |
| Philine intricata | C | 1 | 0 | 1 |
| Phorcus richardi | HE | 1 | 0 | 1 |
| Pseudomangelia vauquelini | C | 1 | 0 | 1 |
| Pusia ebenus | C | 1 | 0 | 1 |
| Raphitoma morph. 2 | C | 1 | 0 | 1 |
| Retilaskeya horrida | C | 1 | 1 | 0 |
| Retusa mammillata | C | 1 | 0 | 1 |
| Runcina coronata | HE | 1 | 0 | 1 |
| Spondylus gaederopus | F | 1 | 1 | 0 |
| Talochlamys multistriata | F | 1 | 0 | 1 |
| Tarantinaea lignaria | C | 1 | 0 | 1 |
| Tectura virginea | HE | 1 | 1 | 0 |
| Williamia gussoni | HE | 1 | 1 | 0 |
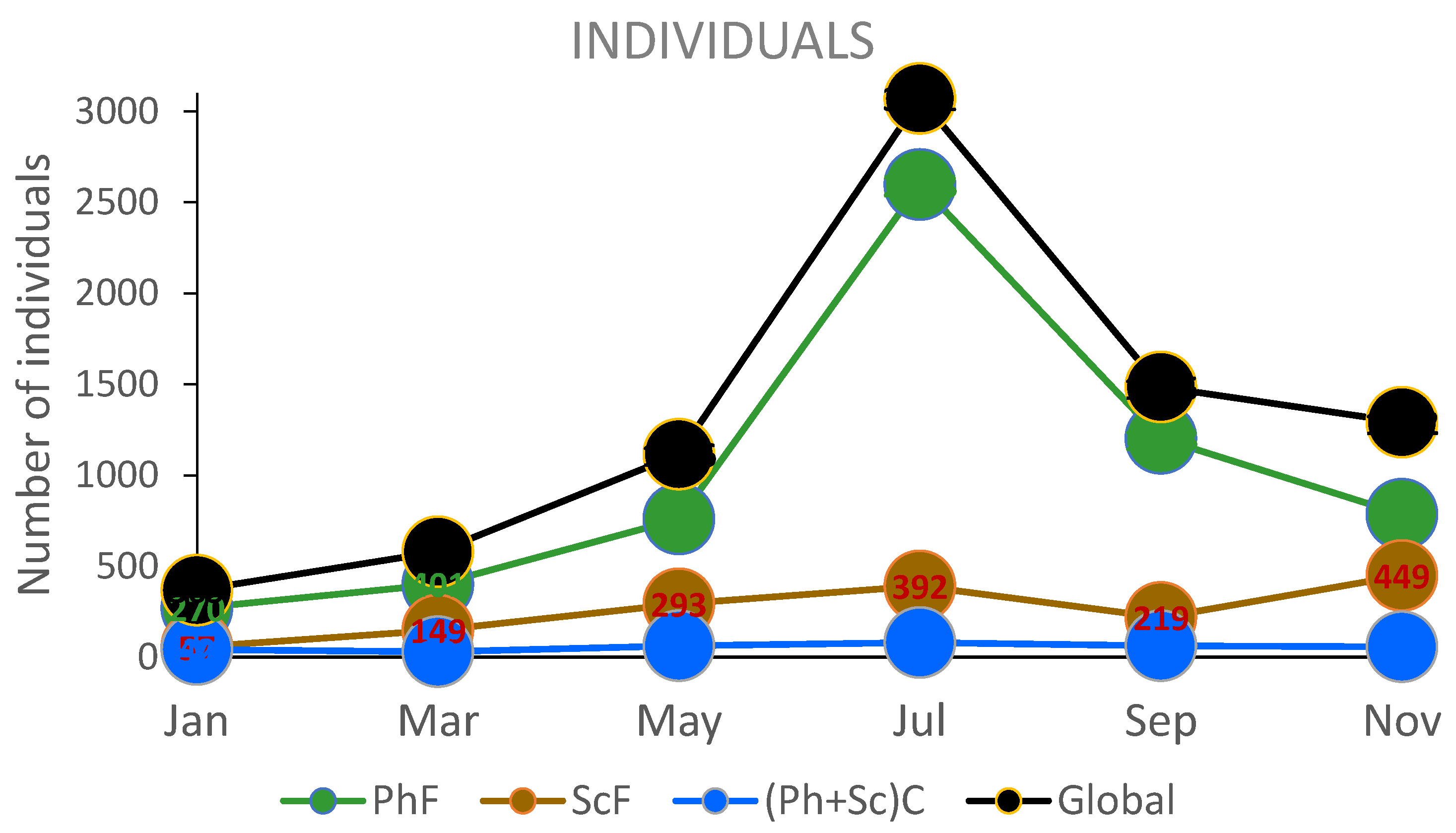
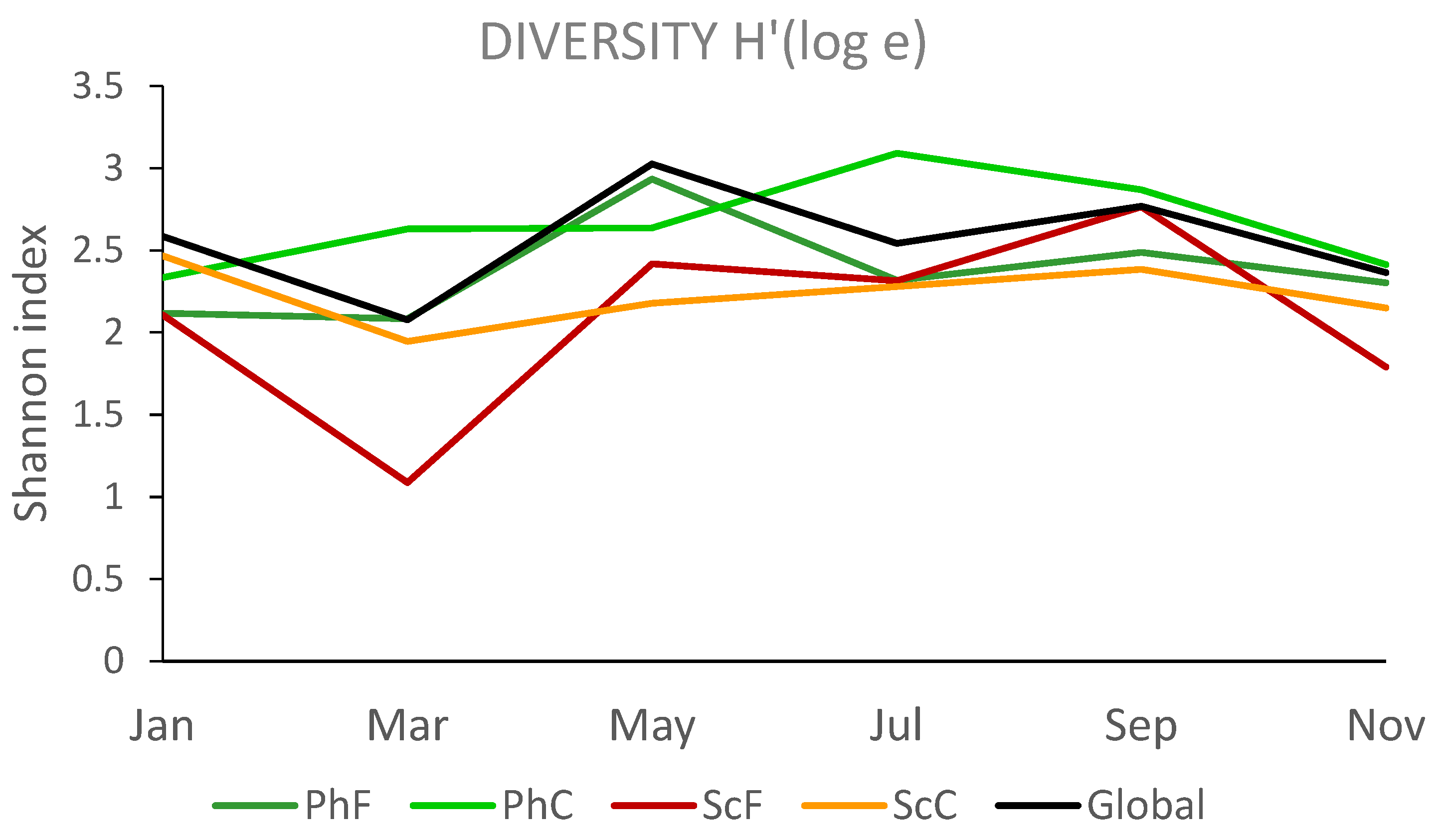
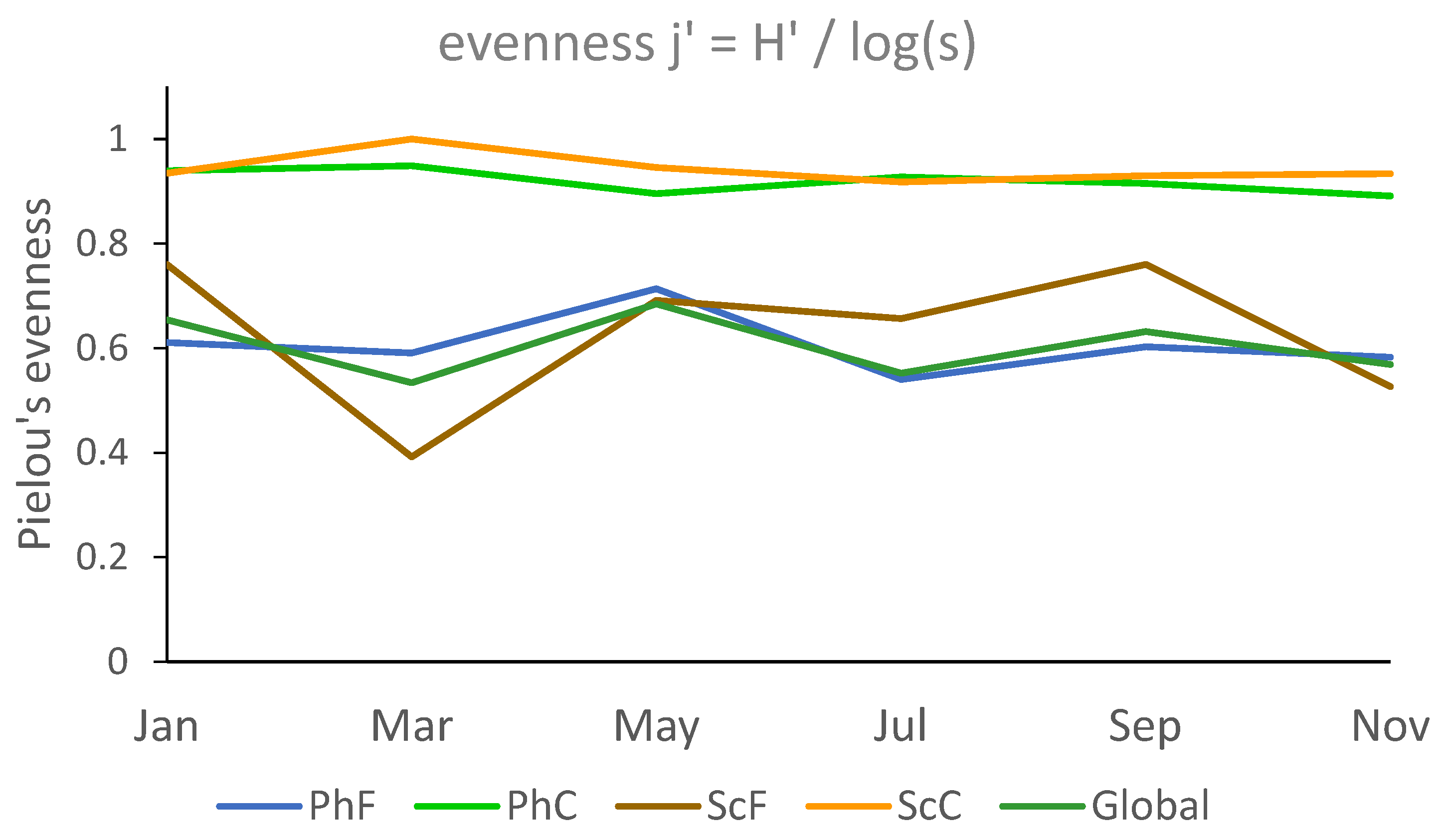
3.2. Species Richness, Abundance, Diversity and Evenness Throughout the Year
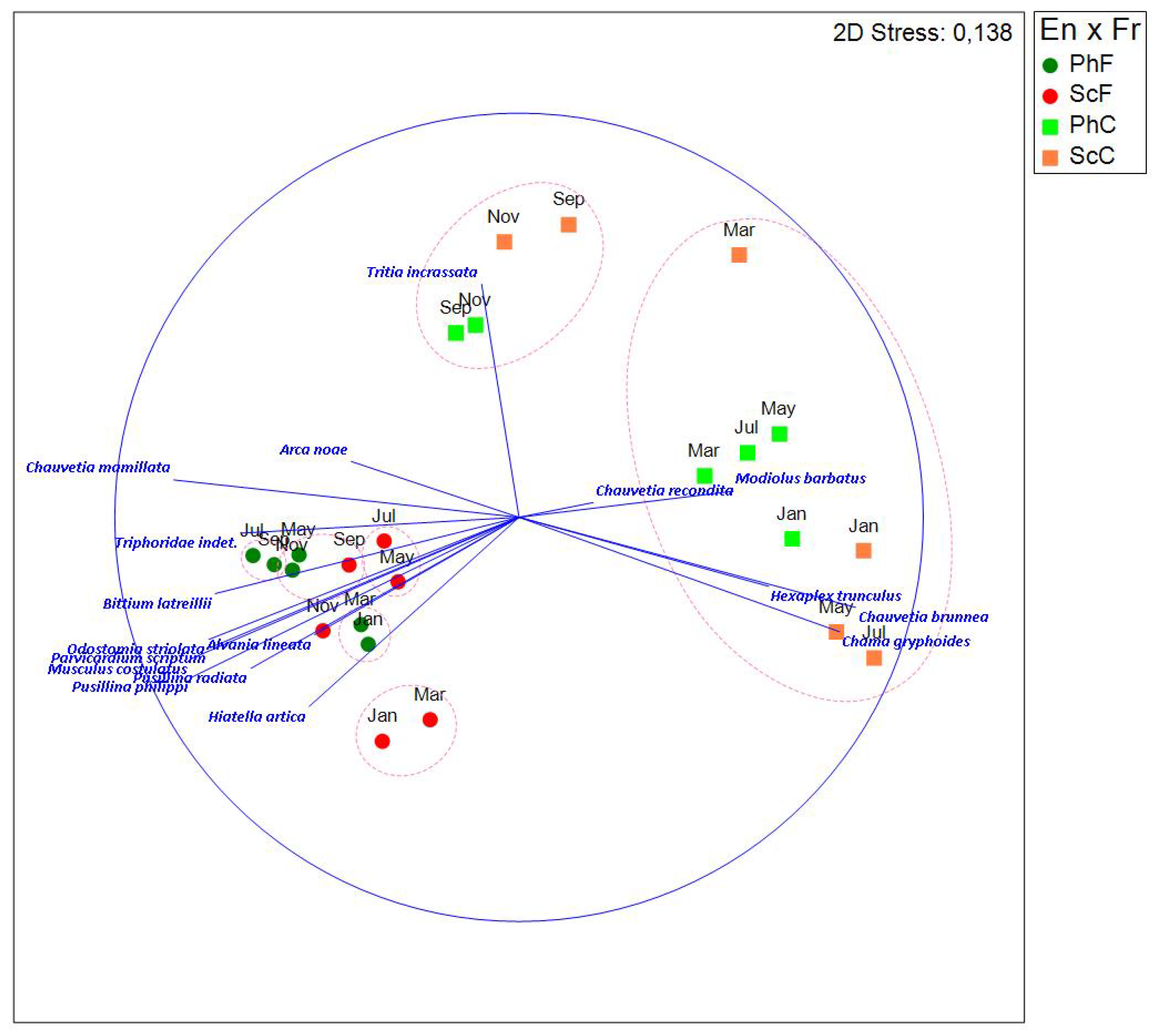
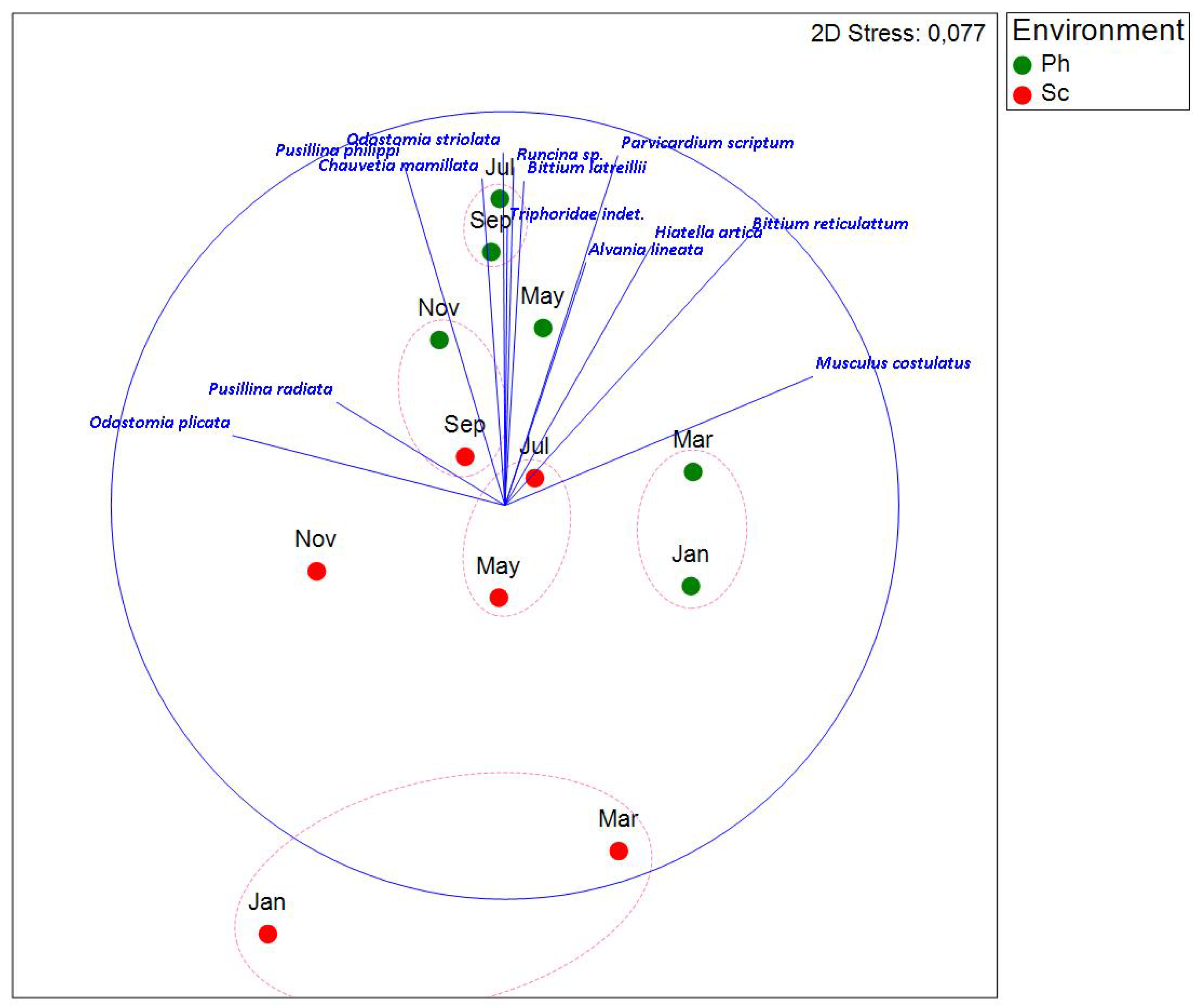
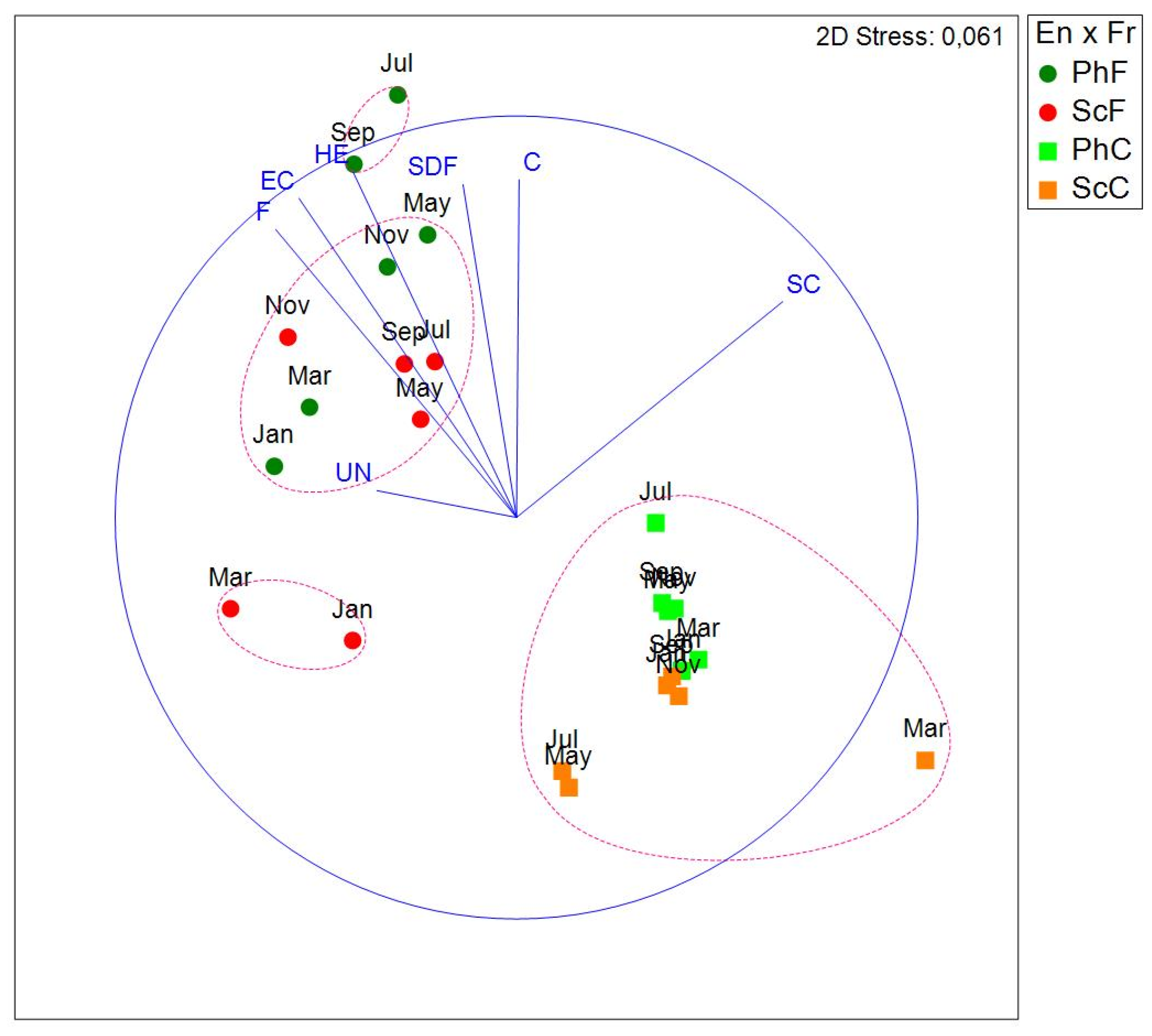
3.3. Community Composition According to Environment, Fraction and Changes over the Year
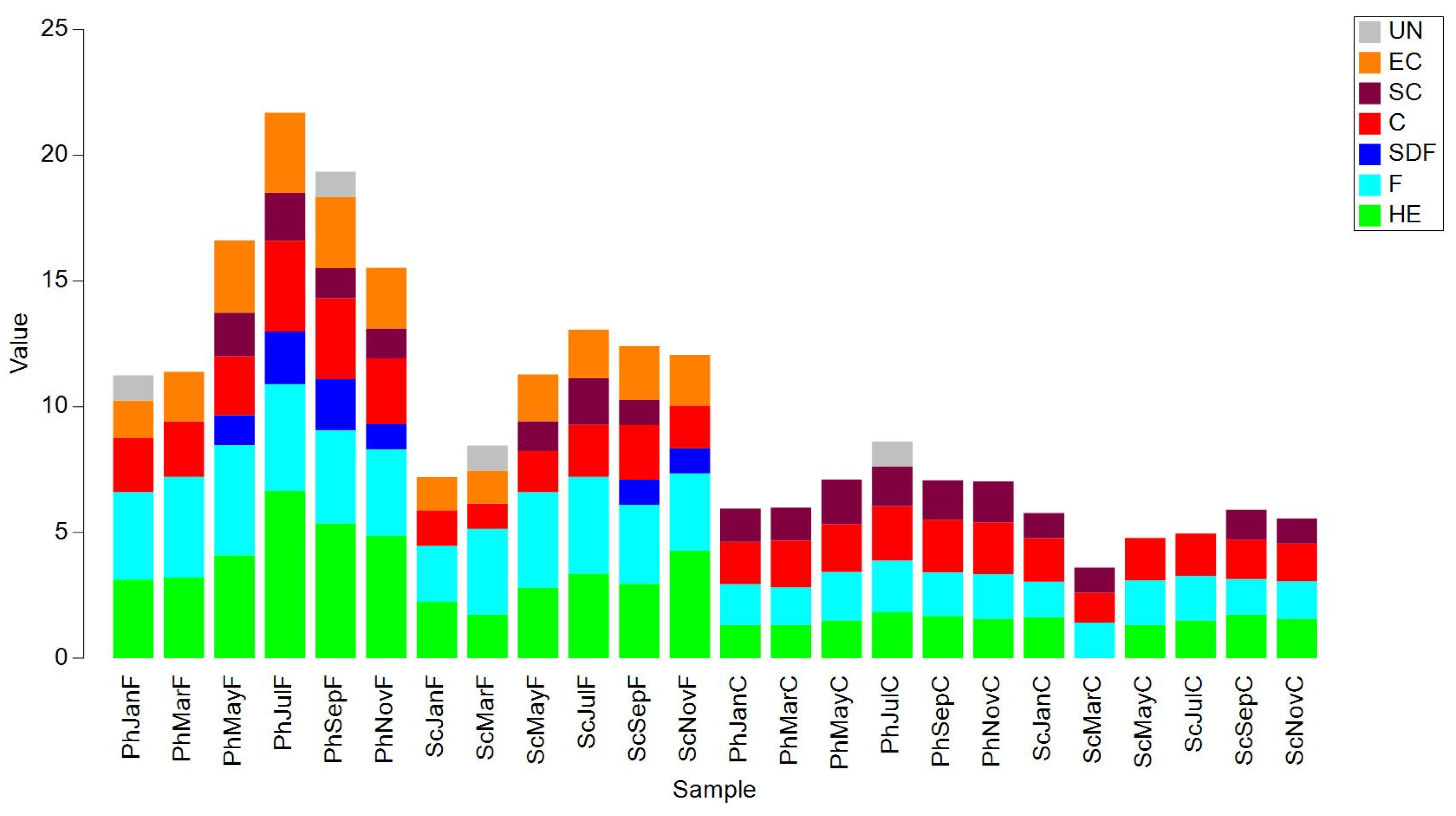
3.4. Trophic Level According to Environment, Fraction and Evolution over the Year
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Sciophilous environment (Sc) |
Photophilous environment (Ph) |
Total Sc + Ph |
||||
|---|---|---|---|---|---|---|---|
| Sc Coarse |
Sc Fine |
Total Sc |
Ph Coarse |
Ph Fine |
Total Ph |
||
| Acanthochitona fascicularis | 2 | 2 | 1 | 1 | 3 | ||
| Acanthochitona sp. | 4 | 4 | 4 | ||||
| Aegires leuckartii | 1 | 1 | 2 | 2 | 3 | ||
| Aequipecten opercularis | 23 | 23 | 13 | 13 | 36 | ||
| Alvania aspera | 5 | 5 | 5 | ||||
| Alvania cancellata | 4 | 3 | 31 | 31 | 35 | ||
| Alvania cimex | 1 | 1 | 1 | ||||
| Alvania discors | 1 | 1 | 1 | ||||
| Alvania lineata | 17 | 32 | 49 | 16 | 111 | 127 | 176 |
| Alvania nestaresi | 2 | 7 | 9 | 9 | |||
| Alvania sp. | 5 | 5 | 5 | ||||
| Ammonicera fischeriana | 13 | 13 | 66 | 66 | 79 | ||
| Anomia ephippium | 1 | 3 | 4 | 1 | 1 | 2 | 6 |
| Aplus dorbignyi | 1 | 1 | 1 | ||||
| Aplysia punctata juv. | 10 | 10 | 2 | 28 | 30 | 40 | |
| Arca noae | 1 | 1 | 11 | 28 | 39 | 40 | |
| Babelomurex cariniferus | 1 | 1 | 1 | ||||
| Bittium latreillii | 1 | 54 | 55 | 1 | 2.050 | 2.051 | 2.106 |
| Bittium reticulatum | 10 | 10 | 4 | 82 | 86 | 96 | |
| Bittium sp. | 2 | 7 | 9 | 9 | |||
| Bivalvia morph. 1 | 2 | 6 | 8 | 8 | |||
| Bivalvia morph. 2 | 2 | 1 | 3 | 3 | |||
| Bivalvia morph. 3 | 1 | 1 | 1 | ||||
| Bivalvia morph. 4 | 1 | 1 | 1 | 1 | 2 | ||
| Brachystomia scalaris | 2 | 2 | 2 | ||||
| Bulla striata | 3 | 3 | 12 | 12 | 15 | ||
| Calliostoma zizyphinum | 2 | 2 | 2 | ||||
| Caloria elegans | 1 | 1 | 1 | ||||
| Cardiidae morph. 1 | 1 | 1 | 1 | ||||
| Cardiidae morph. 2 | 1 | 1 | 1 | ||||
| Cerithiopsis jeffreysi | 16 | 16 | 16 | ||||
| Cerithiopsis tubercularis | 2 | 2 | 23 | 23 | 25 | ||
| Cerithiopsis sp. | 9 | 9 | 9 | ||||
| Cerithium vulgatum | 2 | 2 | 39 | 39 | 41 | ||
| Chama gryphoides | 8 | 8 | 4 | 4 | 12 | ||
| Chauvetia brunnea | 9 | 9 | 8 | 8 | 17 | ||
| Chauvetia mamillata | 5 | 17 | 22 | 16 | 92 | 108 | 130 |
| Chauvetia recondita | 4 | 4 | 4 | ||||
| Chauvetia morph. 1 | 1 | 1 | 1 | ||||
| Chauvetia morph. 2 | 1 | 1 | 1 | ||||
| Clanculus cruciatus | 1 | 7 | 8 | 1 | 79 | 80 | 88 |
| Columbella rustica | 1 | 1 | 1 | ||||
| Crisilla semistriata | 29 | 29 | 24 | 24 | 53 | ||
| Crisilla simulans | 2 | 2 | 2 | ||||
| Cyrillia linearis | 1 | 1 | 10 | 18 | 28 | 29 | |
| Diodora graeca | 2 | 2 | 2 | 2 | 4 | 6 | |
| Diodora italica | 2 | 2 | 2 | ||||
| Doridina nudibranch | 3 | 3 | 5 | 5 | 8 | ||
| Doris ocelligera | 2 | 2 | 2 | ||||
| Doto sp. | 1 | 1 | 1 | ||||
| Emarginula pustula | 1 | 1 | 1 | 1 | 2 | ||
| Episcomitra cornicula | 2 | 2 | 2 | ||||
| Felimare sp. | 1 | 1 | 1 | ||||
| Folinella excavata | 2 | 2 | 2 | ||||
| Fusinus rudis | 2 | 2 | 2 | ||||
| Gari tellinella | 1 | 1 | 1 | ||||
| Gastropoda morph. 1 | 2 | 3 | 5 | 5 | |||
| Gastropoda morph. 2 | 1 | 1 | 2 | 2 | |||
| Gastropoda morph. 3 | 1 | 1 | 1 | ||||
| Gibberula miliaria | 5 | 5 | 5 | ||||
| Gibberula turgidula | 2 | 18 | 20 | 20 | |||
| Gibberula morph. 1 | 3 | 3 | 3 | ||||
| Gibberula morph. 2 | 1 | 1 | 1 | ||||
| Gregariella semigranata | 8 | 8 | 8 | 8 | 16 | ||
| Haliotis tuberculata | 2 | 2 | 1 | 1 | 3 | ||
| Haminoea navicula | 13 | 13 | 13 | ||||
| Hexaplex trunculus | 4 | 4 | 4 | 4 | 8 | ||
| Hiatella arctica | 9 | 153 | 162 | 3 | 239 | 242 | 404 |
| Idas sp. | 1 | 1 | 1 | ||||
| Irus irus | 5 | 5 | 17 | 17 | 22 | ||
| Ischnochitonidae sp. | 1 | 1 | 1 | ||||
| Jujubinus exasperatus | 1 | 1 | 1 | 1 | 2 | ||
| Jujubinus ruscurianus | 7 | 6 | 13 | 3 | 46 | 49 | 62 |
| Lima lima | 3 | 3 | 3 | ||||
| Limaria hians | 2 | 2 | 1 | 1 | 3 | ||
| Limaria tuberculata | 13 | 13 | 1 | 10 | 11 | 24 | |
| Lithophaga lithophaga | 1 | 1 | 1 | ||||
| Mangelia multilineolata | 1 | 1 | 1 | ||||
| Mangelia morph. 1 | 1 | 1 | 1 | ||||
| Mangelia morph. 2 | 1 | 1 | 1 | ||||
| Marshallora adversa | 2 | 2 | 4 | 4 | 6 | ||
| Mimachlamys varia | 4 | 9 | 13 | 7 | 2 | 8 | 22 |
| Mitrella broderipii | 1 | 1 | 1 | ||||
| Mitromorpha columbellaria | 1 | 4 | 5 | 2 | 9 | 11 | 16 |
| Mitromorpha olivoidea | 1 | 1 | 4 | 4 | 5 | ||
| Modiolula phaseolina | 11 | 11 | 3 | 53 | 56 | 67 | |
| Modiolus barbatus | 1 | 1 | 8 | 8 | 9 | ||
| Monophorus erythrosoma | 1 | 1 | 2 | 8 | 8 | 10 | |
| Muricidae indet. | 1 | 1 | 1 | ||||
| Muricopsis cristata | 4 | 4 | 2 | 2 | 6 | ||
| Musculus costulatus | 2 | 484 | 486 | 8 | 788 | 796 | 1.282 |
| Mytilus galloprovincialis juv. | 1 | 10 | 11 | 46 | 46 | 57 | |
| Nodulus contortus | 4 | 4 | 4 | ||||
| Nodulus spiralis | 1 | 1 | 1 | ||||
| Ocinebrina aciculata | 1 | 1 | 2 | 3 | 11 | 14 | 16 |
| Odostomella doliolum | 1 | 1 | 15 | 15 | 16 | ||
| Odostomia plicata | 11 | 11 | 9 | 8 | 20 | ||
| Odostomia striolata | 50 | 50 | 167 | 167 | 217 | ||
| Otina ovata | 1 | 1 | 1 | ||||
| Paradoris indecora | 1 | 1 | 1 | ||||
| Parthenina emaciata | 36 | 36 | 36 | ||||
| Parvamussium fenestratum | 22 | 22 | 19 | 19 | 41 | ||
| Parvicardium scriptum | 2 | 27 | 29 | 1 | 100 | 101 | 130 |
| Patella sp. | 1 | 1 | 1 | ||||
| Petricola lithophaga | 13 | 13 | 13 | ||||
| Philine intricata | 1 | 1 | 1 | ||||
| Philine sp. | 6 | 6 | 6 | ||||
| Phorcus richardi | 1 | 1 | 1 | ||||
| Polyplacophora indet. | 2 | 2 | 2 | ||||
| Pseudochama gryphina | 3 | 3 | 3 | 3 | 6 | ||
| Pseudomangelia vauquelini | 1 | 1 | 1 | ||||
| Pusia ebenus | 1 | 1 | 1 | ||||
| Pusia tricolor | 2 | 2 | 4 | 2 | 6 | 8 | |
| Pusillina inconspicua | 3 | 3 | 3 | ||||
| Pusillina philippi | 272 | 272 | 545 | 545 | 817 | ||
| Pusillina radiata | 179 | 179 | 367 | 367 | 546 | ||
| Raphitoma echinata | 2 | 2 | 1 | 5 | 6 | 8 | |
| Raphitoma laviae | 2 | 2 | 2 | ||||
| Raphitoma morph. 1 | 2 | 2 | 2 | ||||
| Raphitoma morph. 2 | 1 | 1 | 1 | ||||
| Retilaskeya horrida | 1 | 1 | 1 | ||||
| Retusa mammillata | 1 | 1 | 1 | ||||
| Rhyssoplax corallina | 2 | 1 | 3 | 3 | 3 | 6 | |
| Rissoa guerinii | 2 | 2 | 2 | ||||
| Rissoa lia | 1 | 1 | 10 | 10 | 11 | ||
| Rissoa variabilis | 1 | 1 | 1 | 7 | 8 | 9 | |
| Rissoella diaphana | 228 | 228 | 228 | ||||
| Runcina adriatica | 3 | 3 | 27 | 27 | 30 | ||
| Runcina coronata | 1 | 1 | 1 | ||||
| Runcina sp. | 4 | 4 | 35 | 35 | 39 | ||
| Scissurella costata | 1 | 1 | 21 | 21 | 22 | ||
| Setia pulcherrima | 5 | 5 | 10 | 10 | 15 | ||
| Smithiella costulata | 1 | 1 | 1 | 2 | 3 | 4 | |
| Spiralina alpinoligustica | 2 | 2 | 2 | 2 | 4 | ||
| Spondylus gaederopus | 1 | 1 | 1 | ||||
| Striarca lactea | 4 | 9 | 13 | 9 | 66 | 75 | 88 |
| Talochlamys multistriata | 1 | 1 | 1 | ||||
| Tarantinaea lignaria | 1 | 1 | 1 | ||||
| Tectura virginea | 1 | 1 | 1 | ||||
| Tricolia tingitana | 5 | 5 | 5 | ||||
| Tricolia sp. | 3 | 3 | 17 | 17 | 20 | ||
| Trinchesia genovae | 5 | 5 | 5 | ||||
| Triphoridae indet. | 16 | 16 | 5 | 109 | 114 | 130 | |
| Tritia incrassata | 5 | 15 | 20 | 35 | 25 | 60 | 80 |
| Veneridae indet. | 1 | 1 | 2 | 2 | 3 | ||
| Vitreolina perminima | 6 | 6 | 59 | 59 | 65 | ||
| Weinkauffia turgidula | 2 | 2 | 2 | ||||
| Williamia gussoni | 1 | 1 | 1 | ||||
| Total specimens | 110 | 1.559 | 1.669 | 227 | 6.017 | 6.244 | 7.913 |
| Total species | 38 | 62 | 84 | 58 | 105 | 133 | 148 |
|
Class GASTROPODA 257 species Sub Class Caenogastropoda Order Littorinimorpha Littorinidae Melarhaphe neritoides Skeneopsidae Skeneopsis planorbis Naticidae Notocochlis dillwynii Rissoidae *Alvania aspera *Alvania cancellata *Alvania cimex *Alvania discors *Alvania lineata *Alvania nestaresi Alvania subcrenulata Alvania tenera *Crisilla semistriata *Crisilla simulans *Pusillina incospicua Pusillina lineolata *Pusillina philippi *Pusillina radiata Rissoa decorata *Rissoa guerini *Rissoa lia Rissoa splendida *Rissoa variabilis *Setia pulcherrima Anabathridae *Nodulus contortus *Nodulus spìralis Cypraeidae Luria lurida Eulimidae Vitreolina antiflexa *Vitreolina perminima Ovulidae Simnia spelta Triviidae Trivia monacha Vermetidae Vermetus triquetrus Order Neogastropoda Chauvetiidae *Chauvetia brunnea *Chauvetia mamillata *Chauvetia recondita Columbellidae *Columbella rustica Mitrella broderipii Mitrella scripta Fasciolariidae *Fusinus rudis Pseudofusus rostratus *Tarantinaea lignaria Nassariidae Tritia corrugata *Tritia incrassata Tritia reticulata Tritia varicosa Pisaniidae *Aplus dorbignyi Pisania striata Conidae Conus ventricosus Mangeliidae *Mangelia multilineolata *Pseudomangelia vauquelini *Smithiela costulata Mitromorphidae *Mitromorphacolumbellaria *Mitromorpha olivoidea Raphitomidae *Cyrillia linearis *Raphitoma echinata *Raphitoma laviae Raphitoma leufroyi Mitridae *Episcomitra cornicula Isara cornea Muricidae *Babelomurex cariniferus Coralliophila meyendorffii *Hexaplex trunculus Hirtomurex squamosus *Muricopsis cristata Pseudofusus rostratus Ocenebra edwardsii *Ocinebrina aciculata Stramonita haemastoma Cystiscidae *Gibberula miliaria *Gibberula turgidula Costellariidae *Pusia ebenus *Pusia tricolor Order Caenogastropoda incertae sedis Cerithiidae *Bittium latreillii *Bittium reticulatum Cerithium africanum *Cerithium vulgatum Cerithiopsidae *Cerithiopsis jeffreysi *Cerithiopsis tubercularis Newtoniellidae *Retilaskeya horrida Triphoridae Marshallora adversa *Monophorus erythrosoma Turritellidae Turritellinella tricarinata Sub Class Heterobranchia SuperOrder Acochlidiimorpha Hedylopsidae Hedylopsis spiculifera Order Aplysiida Aplysiidae Aplysia depilans Aplysia fasciata *Aplysia punctata Petalifera petalifera Phyllaplysia lafonti Order Cephalaspidea Aglajidae Aglaja tricolorata Camachoaglaja africana Melanochlamys miqueli Spinoaglaja wildpretii Bullidae *Bulla striata Haminoeidae Haminoea exigua Haminoea hydatis *Haminoea navicula *Weinkauffia turgidula Philinidae *Philine intricata Philine catena Retusidae *Retusa mammillata Retusa truncatula Order Ellobiida Otinidae *Otina ovata Order Nudibranchia Aegiridae *Aegires leuckartii Aegires punctilucens Aeolidiidae Aeolidiella alderi Berghia coerulescens Spurilla neapolitana Arminidae Armina tigrina Cadlinidae Aldisa banyulensis Calycidorididae Diaphorodoris alba Diaphorodoris luteocincta Diaphorodoris papillata Chromodorididae Felimare bilineata Felimare fontandraui Felimare orsinii Felimare picta Felimare tricolor Felimare villafranca Felimida binza Felimida krohni Felimida luteorosea Felimida purpurea Coryphellidae Coryphella lineata Dendrodorididae Dendrodoris grandiflora Dendrodoris limbata Discodorididae Discodoris rosi Discodoris stellifera Geitodoris planata Jorunna tomentosa *Paradoris indecora Peltodoris atromaculata Platydoris argo Rostanga rubra Tayuva lilacina Dorididae *Doris ocelligera Dotidae Doto coronata Doto dunnei Doto eireana Doto floridicola Doto fragaria Doto koenneckeri Doto paulinae Doto rosea Embletoniidae Embletonia pulchra Eubranchidae Amphorina farrani Capellinia doriae Eubranchus capellinii Eubranchus exiguus Eubranchus vittatus Facelinidae *Caloria elegans Caloria quatrefagesi Cratena peregrina Facelina annulicornis Facelina auriculata Facelina rubrovittata Facelinopsis marioni Favorinus branchialis Flabellinidae Calmella cavolini Calmella gaditana Edmundsella pedata Flabellina affinis Paraflabellina gabinierei Paraflabellina ischitana Goniodorididae Okenia mediterranea Trapania lineata Trapania maculata Hancockiidae Hancockia uncinata Janolidae Antiopella cristata Myrrhinidae Nemesignis banyulensis Onchidorididae Atalodoris pictoni Atalodoris sparsa Idaliadoris neapolitana Phyllidiidae Phyllidia flava Piseinotecidae Piseinotecus soussi Polyceridae Crimora papillata Kaloplocamus ramosus Limacia inesae Polycera elegans Polycera quadrilineata Samlidae Luisella babai Trinchesiidae Rubramoena amoena Trinchesia caerulea Trinchesia cuanensis Trinchesia foliata *Trinchesia genovae Trinchesia miniostriata Trinchesia morrowae Trinchesia ocellata Tritoniidae Candiella manicata Candiella odhneri Candiella striata Marionia blainvillea Order Pleurobranchida Pleurobranchidae Berthella ocellata Pleurehdera stellata Pleurobranchus testudinarius Order Runcinida Runcinidae *Runcina adriatica Runcina africana Runcina bahiensis *Runcina coronata Runcina hansbechi Super Order Sacoglossa Hermaeidae Cyerce cristallina Hermaea bifida Hermaea variopicta Limapontiidae Placida cremoniana Placida dendritica Placida verticilata Plakobranchidae Bosellia mimètica Elysia margaritae Elysia timida Elysia viridis Thuridilla hopei Order Umbraculida Tylodinidae Tylodina perversa Umbraculidae Umbraculum umbraculum Order Siphonariida Siphonariidae *Williamia gussoni Super family Omalogyroidea Omalogyridae Ammonicera rota Ammonicera fischeriana Superfamily Pyramidelloidea Pyramidellidae *Brachystomia scalaris *Folinella excavata *Odostomella doliolum *Odostomia plicata *Odostomia striolata *Parthenina emaciata Parthenina monozona *Spiralina alpinoligustica Super family Rissoelloidea Rissoellidae *Rissoella diaphana Sub Class Patellogastropoda Super Family Lottioidea Lotiidae *Tectura virginea Superfamily Patelloidea Patellidae Patella aspera Patella caerulea Patella rustica Patella ulyssiponensis Sub Class Vetigastropoda Order Lepetellida Fissurellidae Diodora gibberula *Diodora graeca *Diodora italica Emarginula huzardii Emarginula octaviana *Emarginula pustula Emarginula sicula Fissurella nubecula Puncturella noachina Haliotidae *Haliotis tuberculata Scissurellidae *Scissurella costata Order Trochida Phasianellidae *Tricolia tingitana Calliostomatidae Calliostoma laugieri *Calliostoma zizyphinum Calliostoma conulus Trochidae Clanculus corallinus *Clanculus cruciatus Gibbula ardents Gibbula divaricata Gibbula drepanensis *Jujubinus exasperatus *Jujubinus ruscurianus Jujubinus striatus *Phorcus richardi Phorcus turbinatus Steromphala divaricata Steromphala umbilicalis Turbinidae Bolma rugosa Class BIVALVIA 32 species Sub Class Autobranchia Order Cardiida Cardiidae *Parvicardium scriptum Psammobiidae *Gari tellinella Order Venerida Chamidae *Chama gryphoides *Pseudochama gryphina Veneridae Gouldia minima *Irus irus *Petricola lithophaga Venus casina Venus verrucosa Order Adapedonta Hiatellidae *Hiatella arctica Super order Anomalodesmata Thraciidae Thracia phaseolina Order Arcoida Arcidae *Arca noae Noetiidae *Striarca lactea Order Galeommatida Galiommatidae Galeomma turtoni Lasaeidae Kellia suborbicularis Order Gastrochaenida Gastrochaenidae Rocellaria dubia Order Limida Limidae *Lima lima *Limaria hians *Limaria tuberculata Order Mytilida Mytilidae *Gregariella semigranata *Lithophaga lithophaga *Modiolula phaseolina *Modiolus barbatus *Musculus costulatus *Mytilus galloprovincialis Order Pectinida Anomiidae *Anomia ephippium Pododesmus patelliformis Pectinidae *Aequipecten opercularis *Mimachlamys varia *Talochlamys multistriata Propeamussiidae *Parvamussium fenestratum Spondylidae *Spondylus gaederopus Class POLYPLACOPHORA 7 species Sub Classe Neoloricata Order Chitonida Acanthochitonidae Acanthochitona crinita *Acanthochitona fascicularis Chitonidae *Rhyssoplax corallina Rhyssoplax olivacea Ischnochiton rissoi Lepidochitona caprearum Order Lepidopleurida Leptochitonidae Leptochiton sarsi |
| PhF & ScF - Average dissimilarity = 57,05 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species |
Group PhF Av.Abund |
Group ScF Av.Abund |
Av.Diss | Diss/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 0,71 | 2,92 | 1,79 | 5,12 | 5,12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 1,17 | 0,35 | 1,98 | 1,10 | 3,46 | 8,58 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 1,48 | 1,96 | 1,02 | 3,44 | 12,02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 0,61 | 1,65 | 1,19 | 2,89 | 14,91 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 0,56 | 1,54 | 1,14 | 2,69 | 17,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 0,86 | 1,45 | 0,91 | 2,54 | 20,14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 0,18 | 1,36 | 1,13 | 2,38 | 22,51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 0,81 | 1,35 | 1,00 | 2,37 | 24,89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 0,29 | 1,33 | 1,07 | 2,34 | 27,22 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 2,08 | 1,33 | 1,00 | 2,33 | 29,55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 0,00 | 1,30 | 0,92 | 2,28 | 31,84 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 0,38 | 1,17 | 0,91 | 2,06 | 33,89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 0,37 | 1,15 | 1,05 | 2,01 | 35,90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 0,25 | 1,13 | 1,17 | 1,99 | 37,89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,77 | 0,06 | 1,13 | 1,11 | 1,98 | 39,87 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Limaria tuberculata | 0,47 | 0,49 | 1,02 | 0,87 | 1,80 | 41,67 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 0,22 | 1,01 | 1,01 | 1,77 | 43,44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cerithiopsis tubercularis | 0,57 | 0,07 | 0,95 | 0,78 | 1,67 | 45,10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parthenina emaciata | 0,68 | 0,00 | 0,95 | 0,85 | 1,66 | 46,76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crisilla semistriata | 0,45 | 0,53 | 0,93 | 0,66 | 1,64 | 48,40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoa lia | 0,42 | 0,06 | 0,91 | 0,57 | 1,59 | 49,99 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 1,43 | 0,86 | 0,69 | 1,51 | 51,50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| PhF & PhC - Average dissimilarity = 88,50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species |
Group PhF Av.Abund |
Group PhC Av.Abund |
Av.Diss | Diss/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 0,30 | 5,26 | 1,45 | 5,95 | 5,95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 0,06 | 4,28 | 2,22 | 4,84 | 10,79 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 0,00 | 4,27 | 2,30 | 4,83 | 15,62 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 0,12 | 3,21 | 2,08 | 3,63 | 19,25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 0,00 | 2,78 | 2,36 | 3,15 | 22,39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 0,31 | 2,62 | 1,47 | 2,96 | 25,36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 0,00 | 2,54 | 0,96 | 2,87 | 28,22 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,43 | 0,00 | 2,53 | 1,83 | 2,86 | 31,08 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 1,17 | 0,11 | 2,43 | 1,19 | 2,75 | 33,83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 0,11 | 1,98 | 1,44 | 2,24 | 36,07 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 0,57 | 1,91 | 1,12 | 2,16 | 38,24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 0,00 | 1,70 | 1,19 | 1,92 | 40,16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 0,12 | 1,63 | 1,13 | 1,85 | 42,00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 0,06 | 1,62 | 1,40 | 1,83 | 43,83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 0,00 | 1,56 | 0,92 | 1,77 | 45,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 0,00 | 1,47 | 1,09 | 1,67 | 47,26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 0,00 | 1,47 | 1,18 | 1,66 | 48,92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,63 | 1,05 | 1,46 | 0,85 | 1,65 | 50,57 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ScF & PhC - Average dissimilarity = 89,04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species |
Group ScF Av.Abund |
Group PhC Av.Abund |
Av.Diss | Diss/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,08 | 0,30 | 7,51 | 1,61 | 8,43 | 8,43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,48 | 0,00 | 5,13 | 1,57 | 5,76 | 14,19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,43 | 0,12 | 5,07 | 2,05 | 5,69 | 19,88 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,86 | 0,00 | 3,62 | 0,87 | 4,06 | 23,94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,04 | 0,00 | 3,53 | 1,48 | 3,96 | 27,90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,27 | 1,05 | 3,43 | 1,33 | 3,85 | 31,75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,81 | 0,57 | 2,82 | 0,91 | 3,16 | 34,92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,61 | 0,31 | 2,71 | 0,93 | 3,04 | 37,96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,71 | 0,06 | 2,13 | 0,93 | 2,39 | 40,35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 0,37 | 0,11 | 1,93 | 0,68 | 2,17 | 42,52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,06 | 0,52 | 1,91 | 0,84 | 2,14 | 44,66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,56 | 0,00 | 1,84 | 0,81 | 2,07 | 46,73 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Limaria tuberculata | 0,49 | 0,06 | 1,79 | 0,84 | 2,01 | 48,75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crisilla semistriata | 0,53 | 0,00 | 1,70 | 0,66 | 1,91 | 50,66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| PhF & ScC - Average dissimilarity = 93,59 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species |
Group PhF Av.Abund |
Group ScC Av.Abund |
Av.Diss | Diss/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 0,12 | 5,99 | 1,78 | 6,40 | 6,40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 0,00 | 4,54 | 2,44 | 4,85 | 11,25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 0,06 | 4,53 | 2,12 | 4,84 | 16,09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 0,39 | 3,11 | 1,62 | 3,32 | 19,41 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 0,00 | 2,98 | 2,34 | 3,18 | 22,59 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 0,20 | 2,89 | 1,80 | 3,09 | 25,68 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 0,00 | 2,77 | 0,96 | 2,96 | 28,63 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,43 | 0,00 | 2,72 | 1,75 | 2,90 | 31,54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulattum | 1,17 | 0,00 | 2,72 | 1,33 | 2,90 | 34,44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 0,63 | 2,16 | 1,27 | 2,31 | 36,75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 0,00 | 2,16 | 1,67 | 2,31 | 39,06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 0,06 | 1,92 | 1,44 | 2,05 | 41,10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 0,00 | 1,78 | 1,18 | 1,91 | 43,01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 0,00 | 1,78 | 1,13 | 1,90 | 44,91 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 0,00 | 1,61 | 1,15 | 1,72 | 46,63 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 0,00 | 1,58 | 0,88 | 1,69 | 48,32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 0,00 | 1,54 | 1,06 | 1,64 | 49,96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,77 | 0,00 | 1,47 | 1,13 | 1,57 | 51,54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ScF & ScC - Average dissimilarity = 89,73 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species |
Group ScF Av.Abund |
Group ScC Av.Abund |
Av.Diss | Diss/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,08 | 0,12 | 9,43 | 1,87 | 10,51 | 10,51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,48 | 0,00 | 5,83 | 1,54 | 6,50 | 17,01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,43 | 0,39 | 4,71 | 1,13 | 5,25 | 22,26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,86 | 0,00 | 4,31 | 0,96 | 4,81 | 27,06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,04 | 0,00 | 4,24 | 1,33 | 4,72 | 31,79 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,61 | 0,20 | 3,17 | 1,03 | 3,54 | 35,32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,71 | 0,06 | 2,55 | 0,95 | 2,85 | 38,17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,00 | 0,47 | 2,18 | 0,80 | 2,43 | 40,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,56 | 0,00 | 2,13 | 0,76 | 2,37 | 42,97 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,27 | 0,29 | 1,97 | 0,73 | 2,20 | 45,17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,81 | 0,63 | 1,95 | 0,72 | 2,18 | 47,35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aequipecten opercularis | 0,48 | 0,00 | 1,90 | 0,73 | 2,12 | 49,47 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crisilla semistriata | 0,53 | 0,00 | 1,86 | 0,70 | 2,08 | 51,54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| PhC & ScC - Average dissimilarity = 78,87 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species |
Group PhC Av.Abund |
Group ScC Av.Abund |
Av.Diss | Diss/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 1,05 | 0,29 | 6,45 | 1,20 | 8,17 | 8,17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,57 | 0,63 | 4,64 | 0,98 | 5,89 | 14,06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,52 | 0,00 | 4,15 | 0,80 | 5,26 | 19,32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 0,12 | 0,39 | 3,57 | 0,73 | 4,52 | 23,85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Striarca lactea | 0,36 | 0,14 | 2,92 | 0,77 | 3,70 | 27,55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolus barbatus | 0,35 | 0,06 | 2,60 | 0,79 | 3,29 | 30,84 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,18 | 0,47 | 2,48 | 0,60 | 3,14 | 33,98 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 0,30 | 0,12 | 2,40 | 0,61 | 3,05 | 37,03 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Muricopsis cristata | 0,11 | 0,24 | 2,21 | 0,63 | 2,80 | 39,83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusia tricolor | 0,22 | 0,12 | 2,19 | 0,54 | 2,78 | 42,61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marshallora adversa | 0,22 | 0,12 | 2,12 | 0,53 | 2,69 | 45,29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mimachlamys varia | 0,25 | 0,19 | 2,09 | 0,51 | 2,65 | 47,95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cyrillia linearis | 0,33 | 0,06 | 2,08 | 0,59 | 2,64 | 50,58 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| January - Average similarity: 34,80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,05 | 7,45 | 0,91 | 21,40 | 21,40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,83 | 7,32 | 0,97 | 21,02 | 42,42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,43 | 5,19 | 0,65 | 14,92 | 57,35 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 0,62 | 2,60 | 0,65 | 7,49 | 64,83 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,48 | 2,60 | 0,58 | 7,47 | 72,30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hexaplex trunculus | 0,35 | 2,20 | 0,43 | 6,33 | 78,64 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,46 | 1,69 | 0,39 | 4,86 | 83,50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| March - Average similarity: 29,72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,45 | 7,26 | 0,86 | 24,42 | 24,42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,01 | 5,33 | 0,84 | 17,93 | 42,34 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,47 | 1,75 | 0,59 | 5,89 | 48,23 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,54 | 1,64 | 0,55 | 5,53 | 53,76 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia recondita | 0,19 | 1,62 | 0,29 | 5,44 | 59,20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 0,52 | 1,44 | 0,55 | 4,86 | 64,06 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,27 | 1,25 | 0,29 | 4,19 | 68,25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,41 | 1,21 | 0,55 | 4,06 | 72,31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,35 | 1,20 | 0,38 | 4,03 | 76,34 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,51 | 1,13 | 0,55 | 3,81 | 80,15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| May - Average similarity: 46,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,33 | 4,73 | 0,55 | 10,14 | 10,14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,30 | 4,55 | 0,83 | 9,77 | 19,92 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,82 | 4,49 | 0,70 | 9,64 | 29,56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,14 | 4,21 | 0,84 | 9,04 | 38,60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,38 | 3,23 | 0,90 | 6,92 | 45,52 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 0,92 | 2,31 | 0,94 | 4,95 | 50,47 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,84 | 2,18 | 0,93 | 4,68 | 55,14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Muricopsis cristata | 0,17 | 1,74 | 0,29 | 3,74 | 58,88 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aequipecten opercularis | 0,48 | 1,25 | 0,55 | 2,69 | 61,57 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,66 | 1,19 | 0,67 | 2,54 | 64,11 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Junjubinus ruscurianus | 0,63 | 1,15 | 0,67 | 2,46 | 66,58 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mimachlamys varia | 0,49 | 1,05 | 0,55 | 2,25 | 68,83 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gregariella semigranata | 0,33 | 0,99 | 0,55 | 2,13 | 70,96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 0,66 | 0,96 | 0,65 | 2,06 | 73,01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,55 | 0,96 | 0,67 | 2,05 | 75,07 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crisilla semistriata | 0,57 | 0,94 | 0,61 | 2,02 | 77,09 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,25 | 0,87 | 0,29 | 1,86 | 78,95 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mitromorpha olivoidea | 0,18 | 0,87 | 0,29 | 1,86 | 80,80 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| July - Average similarity: 54,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,25 | 6,39 | 0,98 | 11,71 | 11,71 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia brunnea | 0,46 | 4,47 | 0,64 | 8,19 | 19,90 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,36 | 2,76 | 0,82 | 5,06 | 24,96 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,24 | 2,72 | 0,84 | 4,98 | 29,94 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,10 | 2,63 | 0,67 | 4,82 | 34,75 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,80 | 2,17 | 0,71 | 3,98 | 38,74 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triphoridae indet. | 0,90 | 1,92 | 0,87 | 3,51 | 42,24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,88 | 1,87 | 0,86 | 3,43 | 45,68 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolus barbatus | 0,28 | 1,87 | 0,55 | 3,42 | 49,10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mimachlamys varia | 0,49 | 1,86 | 0,42 | 3,41 | 52,50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania aspera | 0,27 | 1,76 | 0,55 | 3,22 | 55,72 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 1,42 | 1,65 | 0,66 | 3,02 | 58,74 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ammonicera fischeriana | 0,79 | 1,63 | 0,88 | 2,98 | 61,73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,68 | 1,60 | 0,82 | 2,93 | 64,65 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,17 | 1,44 | 0,29 | 2,64 | 67,29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aequipecten opercularis | 0,57 | 1,30 | 0,79 | 2,38 | 69,67 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Striarca lactea | 0,64 | 1,03 | 0,49 | 1,89 | 71,56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cyrillia linearis | 0,52 | 0,95 | 0,46 | 1,74 | 73,31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,66 | 0,89 | 0,63 | 1,62 | 74,93 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,63 | 0,88 | 0,61 | 1,61 | 76,54 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,61 | 0,87 | 0,61 | 1,60 | 78,14 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 0,68 | 0,83 | 0,60 | 1,53 | 79,66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 0,68 | 0,81 | 0,55 | 1,49 | 81,15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| September - Average similarity: 44,42 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,77 | 4,62 | 0,78 | 10,40 | 10,40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 1,44 | 3,10 | 1,15 | 6,98 | 17,38 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,26 | 2,75 | 1,08 | 6,18 | 23,56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,12 | 2,73 | 1,12 | 6,14 | 29,70 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 0,89 | 2,63 | 0,97 | 5,91 | 35,61 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triphoridae indet. | 0,90 | 2,51 | 1,12 | 5,65 | 41,26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,88 | 2,38 | 1,08 | 5,35 | 46,61 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,90 | 1,95 | 1,10 | 4,40 | 51,01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,67 | 1,86 | 1,05 | 4,18 | 55,19 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,84 | 1,72 | 0,48 | 3,88 | 59,06 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,81 | 1,49 | 0,73 | 3,36 | 62,42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jujubinus ruscurianus | 0,51 | 1,34 | 0,32 | 3,03 | 65,45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,53 | 1,26 | 0,58 | 2,84 | 68,28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusia tricolor | 0,27 | 1,13 | 0,32 | 2,54 | 70,83 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,62 | 1,13 | 0,74 | 2,53 | 73,36 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 0,51 | 0,97 | 0,61 | 2,18 | 75,54 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 0,53 | 0,97 | 0,67 | 2,18 | 77,72 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,55 | 0,87 | 0,39 | 1,95 | 79,67 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mitromorpha columbellaria | 0,36 | 0,69 | 0,32 | 1,56 | 81,23 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| November - Average similarity: 41,53 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,98 | 6,89 | 0,72 | 16,60 | 16,60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,37 | 3,83 | 0,92 | 9,22 | 25,82 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,48 | 3,09 | 0,54 | 7,43 | 33,25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,43 | 3,08 | 0,59 | 7,42 | 40,67 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,08 | 2,97 | 0,91 | 7,15 | 47,81 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,03 | 2,69 | 0,64 | 6,48 | 54,29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,75 | 2,55 | 0,87 | 6,15 | 60,44 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 0,75 | 2,28 | 0,90 | 5,48 | 65,92 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pseudochama gryphina | 0,33 | 2,20 | 0,40 | 5,29 | 71,21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia plicata | 0,56 | 1,91 | 0,87 | 4,60 | 75,81 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,77 | 1,54 | 0,54 | 3,71 | 79,52 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Striarca lactea | 0,78 | 1,17 | 0,66 | 2,83 | 82,34 | |||||||||||||||||||||||||||||||||||||||||||||||||||||
References
- Hayward, P.J. Invertebrate epiphytes of coastal marine algae. In: The shore environment, Ecosystems, vol 2; Price, J.H., Irvine, D.E.G. & Farnham, W.F. (eds). Systematics Association, Academic Press, London, 1980; pp. 761–787.
- Hayward, P.J. Animals on seaweed. Naturalist’s handbooks, vol. 9. Richmond Publishing, Richmond, 1988; pp. 1-108.
- Ballesteros, M.; Castelló, J.; Gallés, M.; Sardà, R. Invertebrados Alguícolas Marinos de las Islas Pitiusas. Consell Insular d’Eivissa i Formentera, Conselleria d’Ecologia i Medi Ambient, Ibiza: 1987; 96 pp.
- Bégin, C.; Johnson, L.E.; Himmelman, J.H. Macroalgal canopies: Distribution and diversity of associated invertebrates and effects on the recruitment and growth of mussels. Mar. Ecol. Prog. Ser. 2004, 271, 121–132. [Google Scholar] [CrossRef]
- Guerra-García, J.M.; Sánchez, J.A.; Ros, M.; Baeza-Rojano, E.; Cabezas, M.P.; Izquierdo, D.; Corzo, J. Macrofauna asociada al alga Stycopaulon scoparium en el Estrecho de Gibraltar y comparación con el resto de la Península Ibérica. Almoraima 2010, 40, 123–132. [Google Scholar]
- Bracken, M.E.S.; González-Dorantes, C.A.; Stachowicz, J.J. Whole-community mutualism: associated invertebrates facilitate a dominant habitat-forming seaweed. Ecology 2007, 88, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Chemello, R.; Dipiazza, F.; Dieli, T.; Riggio, S. . Struttura della Malacocenosi Associata at Alghe Fotofile: Effetti della Profondità e della Complessità d’Habitat. Biol. Mar. Mediterr 1997, 4, 167–168. [Google Scholar]
- García, R.C.; Soto, F.S.; Colón, R.R.; Medina, H.J. Gasterópodos asociados al alga calcárea Halimeda tuna (Udoteaceae) en Puerto Rico. Rev. Biol. Trop. 2008, 56, 1665–1675. [Google Scholar]
- Ávila, S.P.; Santos, A.C.; Penteado, A.M.; Rodriguez, A.M.; Quitino, I.; Machado, M. The molluscs of the intertidal algal turf in the Azores. Iberus 2005, 23, 67–76. [Google Scholar]
- Chemello, R.; Milazzo, M. Effect of algal architecture on associated fauna: some evidence from phytal molluscs. Mar. Biol. 2002, 140, 981–990. [Google Scholar]
- Gallardo, D.; Oliva, F.; Ballesteros, M. Marine invertebrates epibiont on photophilic seaweeds: importance of algal architecture. Mar. Biodivers. 2021, 51, 16. [Google Scholar] [CrossRef]
- Terlizzi, A.; Scuderi, D.; Fraschetti, S.; Guidetti, P.; Boero, F. Molluscs on subtidal cliffs: patterns of spatial distribution. J. Mar. Biol. Assoc. U. K., 2003, 83, 165–172. [Google Scholar] [CrossRef]
- Salas, C.; Hergueta, E. La fauna de moluscos de las concreciones calcáreas de Mesophyllum lichenoides (Ellis) Lemoine. Estudio de la Diversidad de un Ciclo Anual. Iberus 1986, 6, 57–65. [Google Scholar]
- Martin, D.; Dantart, L.; Ballesteros, M. Moluscos de las concreciones de algas calcáreas del litoral catalán (NE España). Lavori SIM 1990, 23, 445–456. [Google Scholar]
- Milazzo, M.; Chemello, R.; Badalamendi, F.; Riggio, S. Molluscan assemblages associated with photophilic alae in the Marine Reserve of Ustica Island (Lower Tyrrhenian Sea, Italy). Ital. J. Zool. 2000, 67, 287–295. [Google Scholar] [CrossRef]
- Urra, J.; Rueda, J.L.; Mateo Ramírez, Á.; Marina, P.; Tirado, C.; Salas, C.; Gofas, S. Seasonal variation of molluscan assemblages in diferent strata of photophilous algae in the Alboran Sea (Western Mediterranean). J. Sea Res. 2013, 83, 83–93. [Google Scholar] [CrossRef]
- Sánchez-Moyano, J.E.; Estacio, F.J.; García-Adiego, E.M.; García-Gómez, J.C. The molluscan epifauna of the alga Halopteris scoparia in Southern Spain as a bioindicator of coastal environmental conditions. J. Mollus. Stud. 2000, 66, 431–448. [Google Scholar] [CrossRef]
- Pitacco,V. ; Orlando-Bonaca, M.; Mavrič, B.; Popović, A.; Lipej, L. Mollusc fauna associated with the Cystoseira algal associations in the Gulf of Trieste (Northern Adriatic Sea). Mediterr. Mar. Sci. 2014, 15, 225–238. [Google Scholar] [CrossRef]
- Peñas, A.; Almera, J. Malacofauna Asociada a una pradera de Posidonia oceanica (L.) en Mataró (NE de la Península Ibérica). Spira 2001, 1, 25–31. [Google Scholar]
- Llamas, A. Costa Brava. Las 200 Mejores Inmersiones, A Fondo. Barcelona. Geoplaneta,2001. 280 pp.
- Parenzan, P. Carta d’Identità delle Conchiglie del Mediterraneo. Vol. I Gasteropodi. Bios Taras, Taranto, Italy, 1970, 283 pp.
- Parenzan, P. Carta d’Identità delle Conchiglie del Mediterraneo. Vol. II Bivalvi prima parte. Bios Taras, Taranto, Italy, 1974, 282 pp.
- Parenzan, P. Carta d’Identità delle Conchiglie del Mediterraneo. Vol. II Bivalvi seconda parte. Bios Taras, Taranto, Italy, 1976, pp. 283 - 546.
- D’Angelo, G.; Gargiullo, S. Guida alle Conchiglie Mediterranee. Fabbri Editori, Milano, Italy, 1987, 224 pp.
- Poppe, G.T.; Goto, Y. European seashells. Verlag Christa Hemmen, Wiesbaden, Germany, 1991, 352 pp.
- Doneddu, M.; Trainito, E. Conchiglie del Mediterraneo. Il Castello Editore, Trezzano Sul naviglio, Italy, 2005, 256 pp.
- Gofas, S.; Moreno, D.; Salas, C. Moluscos Marinos de Andalucía. Universidad de Málaga, Servicio de Publicaciones e Intercambio Científico, vols. I & II, Málaga, Spain, 2011, 798 pp.
- Scaperrotta, M.; Bartolini, S.; Bogi, C. Accrescimenti. Stadi di accrescimento dei molluschi marini del Mediterraneo. Vols. I-VI, L´Informatore Piceno, Ancona, Italia, 2009-2014.
- WoRMS, World Register of Marine species. 2024. Published on: http://www.marinespecies.org/ Accessed: 03/10/2024.
- Dantart, L.; Frechilla, M.; Ballesteros, M. Fauna Malacologica del Estany des Peix (Formentera). Iberus 1990, 9, 111–125. [Google Scholar]
- Antit, M.; Daoulatli, A.; Rueda, J.L.; Salas, C. Temporal variation of the algae associated molluscan assemblage of artificial substrata in the Bay of Tunis (Tunisia). Mediterr. Mar. Sci. 2013, 14, 390–402. [Google Scholar] [CrossRef]
- Chemello, R.; Russo, G.F. The molluscan Taxocoene of photophilic algae from the Island of Lampedusa (strait of Sicily, Southern Mediterranean). Boll. Malacol. 1997, 33(5–8), 95–104.
- Chintiroglou, C.; Antoniadou, C.; Vafidis, D.; Koutsoubas, D. VI. 5. Zoobenthos –hard Substrata communities’. In: State the Hellenic Marine Environment, E. Papathanassiou & A. Zenetos (Eds), Athens, Greece, 2005, pp. 247-253.
- Pereira, F.; Ballesteros, M. Gasterópodos del litoral mediterráneo español. II. Tossa de Mar. Actas I Simp.o Ibér. Bent. Mar. 1982, 1, 223–235. [Google Scholar]
- BIOCAT, Banc de Dades de Biodiversitat de Catalunya. 2024. Published on: http://biodiver.bio.ub.es/biocat/ Accessed: 03/10/2024.
- Ballesteros, M.; Madrenas, E.; Pontes, M. Actualización del catálogo de los moluscos opistobranquios (Gastropoda: Heterobranchia) de las costas catalanas. Spira 2016, 6, 1–28. [Google Scholar]
- OPK Opistobranquis. 2024. Published on: https://opistobranquis.info/en/. Accessed: 02/10/2024.
- GROC, Grup de Recerca d´Opistobranquis de Catalunya. 2024. Published on: https://opistobranquis.org/ca/home. Accessed: 03/10/2024. Accessed, 2024.
- Milazzo, M.; Chemello, R.; Badalamendi, F.; Riggio, S. Molluscan assemblages associated with photophilic algae in the Marine Reserve of Ustica Island (Lower Tyrrhenian Sea, Italy). Ital. J. Zool. 2000, 67, 287–295. [Google Scholar] [CrossRef]
- Waller, C.L. Variability in intertidal communities along a latitudinal gradient in the Southern Ocean. Polar Biol. 2008, 31, 809–816. [Google Scholar] [CrossRef]
- Bick, A.; Arlt, G. Description of intertidal macro- and meiobenthic assemblages in Maxwell Bay, King George Island, South Shetland Islands, Southern Ocean. Polar Biol. 2013, 36, 673–689. [Google Scholar] [CrossRef]
- Aghmich, A.; Taboada, S.; Toll, S.; Ballesteros, M. First assessment of the rocky intertidal communities of Fildes Bay, King George Island (South Shetland Islands, Antarctica). Polar Biol. 2015, 39, 198–198. [Google Scholar] [CrossRef]
- AnimalBase. 2024. Published on: http://www.animalbase.uni-goettingen.de/zooweb/servlet/AnimalBase/AnimalBase/search Accessed: 03/10/2024.
- Forli, M. : Dell´Angelo, B.; Sosso, M.; Bonfitto, A. Note su alcune specie fossili di Williamia (Gastropoda: Siphonariidae) con descrizione di una nuova specie. Boll. Malacol. 2009, 45, 5–8. [Google Scholar]
- GBIF, Global Biodiversity Information Facility. 2024. Published on: https://www.gbif.org/es/ Accessed: 03/10/2024.
- Hodgson, A.N. The Biology of Siphonariid limpets (Gastropoda: Pulmonata). Oceanography and Marine Biology, CRC Press, 1999, 70 pp.
- Ruthensteiner, B. Redescription and 3D morphology of Williamia gussonii (Gastropoda: Siphonariidae). J. Mollus. Stud. 2006, 72, 327–336. [Google Scholar] [CrossRef]
- Gofas, S.; Luque, A.A.; Templado, J.; Salas, C. A national checklist of marine Mollusca in Spanish waters. Sci. Mar. 2017, 81, 241–254. [Google Scholar] [CrossRef]
- Ballesteros, E. Species composition and structure of a photophilic algal community dominated by Halopteris scoparia (L.) Sauvageau from the North-Western Mediterranean. Collect. Bot. 1993, 22, 5–24. [Google Scholar] [CrossRef]


| PhF (photophilous, fine fraction) Average similarity: 60,28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 6,53 | 1,65 | 10,83 | 10,83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 4,51 | 1,91 | 7,49 | 18,31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 4,30 | 2,03 | 7,13 | 25,45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 3,51 | 2,42 | 5,83 | 31,27 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 3,51 | 1,79 | 5,82 | 37,09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 2,76 | 2,14 | 4,58 | 41,67 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,43 | 2,38 | 1,48 | 3,95 | 45,62 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 2,12 | 1,21 | 3,52 | 49,14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 2,02 | 1,52 | 3,36 | 52,50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 1,17 | 1,96 | 0,85 | 3,26 | 55,76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 1,87 | 0,71 | 3,09 | 58,85 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 1,35 | 0,94 | 2,23 | 61,08 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 1,31 | 1,05 | 2,17 | 63,25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Striarca lactea | 0,87 | 1,29 | 0,98 | 2,14 | 65,39 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 1,25 | 0,77 | 2,07 | 67,46 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 1,13 | 0,81 | 1,87 | 69,33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 1,09 | 0,79 | 1,82 | 71,15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ammonicera fischeriana | 0,84 | 1,08 | 0,86 | 1,79 | 72,93 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 1,06 | 0,82 | 1,77 | 74,70 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triphoridae indet. | 0,88 | 0,93 | 0,76 | 1,55 | 76,25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,77 | 0,91 | 0,79 | 1,51 | 77,76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jujubinus ruscurianus | 0,60 | 0,82 | 0,63 | 1,37 | 79,13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mytilus galloprovincialis | 0,74 | 0,78 | 0,74 | 1,30 | 80,43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ScF (sciophilous, fine fraction) Average similarity: 49,55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,08 | 10,77 | 1,92 | 21,74 | 21,74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,43 | 6,83 | 1,52 | 13,78 | 35,52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,48 | 5,06 | 1,37 | 10,21 | 45,73 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,04 | 4,14 | 1,36 | 8,36 | 54,08 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,81 | 3,18 | 0,72 | 6,42 | 60,50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,86 | 2,73 | 0,61 | 5,51 | 66,01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,61 | 1,88 | 0,53 | 3,78 | 69,80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triphoridae indet. | 0,51 | 1,49 | 0,67 | 3,00 | 72,80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aequipecten opercularis | 0,48 | 1,47 | 0,67 | 2,97 | 75,77 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia plicata | 0,47 | 1,27 | 0,59 | 2,57 | 78,33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,71 | 1,22 | 0,59 | 2,47 | 80,80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| PhC (photophilous, coarse fraction) Average similarity: 27,62 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 1,05 | 9,04 | 1,50 | 32,72 | 32,72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,52 | 2,88 | 0,58 | 10,43 | 43,16 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,31 | 2,37 | 0,43 | 8,60 | 51,75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolus barbatus | 0,35 | 1,73 | 0,51 | 6,25 | 58,01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,57 | 1,48 | 0,43 | 5,35 | 63,36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania aspera | 0,23 | 1,17 | 0,43 | 4,24 | 67,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cyrillia linearis | 0,33 | 1,15 | 0,33 | 4,17 | 71,76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia recondita | 0,13 | 1,08 | 0,24 | 3,90 | 75,66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triphoridae indet. | 0,23 | 0,88 | 0,34 | 3,20 | 78,86 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,18 | 0,75 | 0,24 | 2,70 | 81,56 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ScC (sciophilous, coarse fraction) Average similarity: 28,56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,47 | 7,68 | 0,83 | 26,89 | 26,89 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,63 | 5,61 | 0,72 | 19,66 | 46,55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 0,39 | 4,23 | 0,56 | 14,81 | 61,36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia brunnea | 0,33 | 2,90 | 0,46 | 10,15 | 71,51 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,20 | 1,88 | 0,25 | 6,60 | 78,11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Muricopsis cristata | 0,24 | 1,31 | 0,25 | 4,57 | 82,68 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| PhF & ScF Average dissimilarity = 57,05 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Group PhF #break#Av.Abund | Group ScF #break#Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 0,71 | 2,92 | 1,79 | 5,12 | 5,12 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 1,17 | 0,35 | 1,98 | 1,10 | 3,46 | 8,58 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 1,48 | 1,96 | 1,02 | 3,44 | 12,02 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 0,61 | 1,65 | 1,19 | 2,89 | 14,91 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 0,56 | 1,54 | 1,14 | 2,69 | 17,60 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 0,86 | 1,45 | 0,91 | 2,54 | 20,14 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 0,18 | 1,36 | 1,13 | 2,38 | 22,51 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 0,81 | 1,35 | 1,00 | 2,37 | 24,89 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 0,29 | 1,33 | 1,07 | 2,34 | 27,22 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 2,08 | 1,33 | 1,00 | 2,33 | 29,55 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 0,00 | 1,30 | 0,92 | 2,28 | 31,84 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 0,38 | 1,17 | 0,91 | 2,06 | 33,89 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 0,37 | 1,15 | 1,05 | 2,01 | 35,90 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 0,25 | 1,13 | 1,17 | 1,99 | 37,89 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,77 | 0,06 | 1,13 | 1,11 | 1,98 | 39,87 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Limaria tuberculata | 0,47 | 0,49 | 1,02 | 0,87 | 1,80 | 41,67 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 0,22 | 1,01 | 1,01 | 1,77 | 43,44 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Cerithiopsis tubercularis | 0,57 | 0,07 | 0,95 | 0,78 | 1,67 | 45,10 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Parthenina emaciata | 0,68 | 0,00 | 0,95 | 0,85 | 1,66 | 46,76 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Crisilla semistriata | 0,45 | 0,53 | 0,93 | 0,66 | 1,64 | 48,40 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoa lia | 0,42 | 0,06 | 0,91 | 0,57 | 1,59 | 49,99 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 1,43 | 0,86 | 0,69 | 1,51 | 51,50 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| PhF & PhC Average dissimilarity = 88,50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Group Ph#break#Av.Abund | Group PhC#break#Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 0,30 | 5,26 | 1,45 | 5,95 | 5,95 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 0,06 | 4,28 | 2,22 | 4,84 | 10,79 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 0,00 | 4,27 | 2,30 | 4,83 | 15,62 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 0,12 | 3,21 | 2,08 | 3,63 | 19,25 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 0,00 | 2,78 | 2,36 | 3,15 | 22,39 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 0,31 | 2,62 | 1,47 | 2,96 | 25,36 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 0,00 | 2,54 | 0,96 | 2,87 | 28,22 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,43 | 0,00 | 2,53 | 1,83 | 2,86 | 31,08 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 1,17 | 0,11 | 2,43 | 1,19 | 2,75 | 33,83 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 0,11 | 1,98 | 1,44 | 2,24 | 36,07 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 0,57 | 1,91 | 1,12 | 2,16 | 38,24 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 0,00 | 1,70 | 1,19 | 1,92 | 40,16 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 0,12 | 1,63 | 1,13 | 1,85 | 42,00 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 0,06 | 1,62 | 1,40 | 1,83 | 43,83 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 0,00 | 1,56 | 0,92 | 1,77 | 45,60 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 0,00 | 1,47 | 1,09 | 1,67 | 47,26 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 0,00 | 1,47 | 1,18 | 1,66 | 48,92 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,63 | 1,05 | 1,46 | 0,85 | 1,65 | 50,57 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ScF & PhC Average dissimilarity = 89,04 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Group ScF#break#Av.Abund | Group PhC#break#Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,08 | 0,30 | 7,51 | 1,61 | 8,43 | 8,43 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,48 | 0,00 | 5,13 | 1,57 | 5,76 | 14,19 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,43 | 0,12 | 5,07 | 2,05 | 5,69 | 19,88 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,86 | 0,00 | 3,62 | 0,87 | 4,06 | 23,94 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,04 | 0,00 | 3,53 | 1,48 | 3,96 | 27,90 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,27 | 1,05 | 3,43 | 1,33 | 3,85 | 31,75 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,81 | 0,57 | 2,82 | 0,91 | 3,16 | 34,92 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,61 | 0,31 | 2,71 | 0,93 | 3,04 | 37,96 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,71 | 0,06 | 2,13 | 0,93 | 2,39 | 40,35 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 0,37 | 0,11 | 1,93 | 0,68 | 2,17 | 42,52 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,06 | 0,52 | 1,91 | 0,84 | 2,14 | 44,66 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,56 | 0,00 | 1,84 | 0,81 | 2,07 | 46,73 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Limaria tuberculata | 0,49 | 0,06 | 1,79 | 0,84 | 2,01 | 48,75 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Crisilla semistriata | 0,53 | 0,00 | 1,70 | 0,66 | 1,91 | 50,66 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| PhF & ScC Average dissimilarity = 93,59 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Group PhF#break#Av.Abund | Group ScC#break#Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 0,12 | 5,99 | 1,78 | 6,40 | 6,40 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 0,00 | 4,54 | 2,44 | 4,85 | 11,25 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 0,06 | 4,53 | 2,12 | 4,84 | 16,09 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 0,39 | 3,11 | 1,62 | 3,32 | 19,41 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 0,00 | 2,98 | 2,34 | 3,18 | 22,59 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 0,20 | 2,89 | 1,80 | 3,09 | 25,68 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 0,00 | 2,77 | 0,96 | 2,96 | 28,63 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,43 | 0,00 | 2,72 | 1,75 | 2,90 | 31,54 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 1,17 | 0,00 | 2,72 | 1,33 | 2,90 | 34,44 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 0,63 | 2,16 | 1,27 | 2,31 | 36,75 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 0,00 | 2,16 | 1,67 | 2,31 | 39,06 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 0,06 | 1,92 | 1,44 | 2,05 | 41,10 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 0,00 | 1,78 | 1,18 | 1,91 | 43,01 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 0,00 | 1,78 | 1,13 | 1,90 | 44,91 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 0,00 | 1,61 | 1,15 | 1,72 | 46,63 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 0,00 | 1,58 | 0,88 | 1,69 | 48,32 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 0,00 | 1,54 | 1,06 | 1,64 | 49,96 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,77 | 0,00 | 1,47 | 1,13 | 1,57 | 51,54 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ScF & ScC Average dissimilarity = 89,73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Group PhF#break#Av.Abund | Group ScC#break#Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 2,50 | 0,12 | 5,99 | 1,78 | 6,40 | 6,40 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 2,09 | 0,00 | 4,54 | 2,44 | 4,85 | 11,25 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 2,49 | 0,06 | 4,53 | 2,12 | 4,84 | 16,09 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,71 | 0,39 | 3,11 | 1,62 | 3,32 | 19,41 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 1,40 | 0,00 | 2,98 | 2,34 | 3,18 | 22,59 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 1,46 | 0,20 | 2,89 | 1,80 | 3,09 | 25,68 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,25 | 0,00 | 2,77 | 0,96 | 2,96 | 28,63 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 1,43 | 0,00 | 2,72 | 1,75 | 2,90 | 31,54 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 1,17 | 0,00 | 2,72 | 1,33 | 2,90 | 34,44 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,28 | 0,63 | 2,16 | 1,27 | 2,31 | 36,75 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 1,00 | 0,00 | 2,16 | 1,67 | 2,31 | 39,06 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 1,05 | 0,06 | 1,92 | 1,44 | 2,05 | 41,10 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,93 | 0,00 | 1,78 | 1,18 | 1,91 | 43,01 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,95 | 0,00 | 1,78 | 1,13 | 1,90 | 44,91 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania cancellata | 0,82 | 0,00 | 1,61 | 1,15 | 1,72 | 46,63 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 1,04 | 0,00 | 1,58 | 0,88 | 1,69 | 48,32 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,77 | 0,00 | 1,54 | 1,06 | 1,64 | 49,96 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,77 | 0,00 | 1,47 | 1,13 | 1,57 | 51,54 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| PhC & ScC Average dissimilarity = 78,87 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Group PhC#break#Av.Abund | Group ScC#break#Av.Abund | Av.Diss | Diss/SD | Contrib% | Cum.% | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 1,05 | 0,29 | 6,45 | 1,20 | 8,17 | 8,17 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,57 | 0,63 | 4,64 | 0,98 | 5,89 | 14,06 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,52 | 0,00 | 4,15 | 0,80 | 5,26 | 19,32 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 0,12 | 0,39 | 3,57 | 0,73 | 4,52 | 23,85 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Striarca lactea | 0,36 | 0,14 | 2,92 | 0,77 | 3,70 | 27,55 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolus barbatus | 0,35 | 0,06 | 2,60 | 0,79 | 3,29 | 30,84 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,18 | 0,47 | 2,48 | 0,60 | 3,14 | 33,98 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 0,30 | 0,12 | 2,40 | 0,61 | 3,05 | 37,03 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Muricopsis cristata | 0,11 | 0,24 | 2,21 | 0,63 | 2,80 | 39,83 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusia tricolor | 0,22 | 0,12 | 2,19 | 0,54 | 2,78 | 42,61 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Marshallora adversa | 0,22 | 0,12 | 2,12 | 0,53 | 2,69 | 45,29 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mimachlamys varia | 0,25 | 0,19 | 2,09 | 0,51 | 2,65 | 47,95 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Cyrillia linearis | 0,33 | 0,06 | 2,08 | 0,59 | 2,64 | 50,58 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| January Average similarity: 34,80 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,05 | 7,45 | 0,91 | 21,40 | 21,40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,83 | 7,32 | 0,97 | 21,02 | 42,42 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,43 | 5,19 | 0,65 | 14,92 | 57,35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 0,62 | 2,60 | 0,65 | 7,49 | 64,83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,48 | 2,60 | 0,58 | 7,47 | 72,30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hexaplex trunculus | 0,35 | 2,20 | 0,43 | 6,33 | 78,64 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,46 | 1,69 | 0,39 | 4,86 | 83,50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| March Average similarity: 29,72 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,45 | 7,26 | 0,86 | 24,42 | 24,42 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,01 | 5,33 | 0,84 | 17,93 | 42,34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,47 | 1,75 | 0,59 | 5,89 | 48,23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,54 | 1,64 | 0,55 | 5,53 | 53,76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia recondita | 0,19 | 1,62 | 0,29 | 5,44 | 59,20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 0,52 | 1,44 | 0,55 | 4,86 | 64,06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,27 | 1,25 | 0,29 | 4,19 | 68,25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,41 | 1,21 | 0,55 | 4,06 | 72,31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,35 | 1,20 | 0,38 | 4,03 | 76,34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,51 | 1,13 | 0,55 | 3,81 | 80,15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| May Average similarity: 46,60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,33 | 4,73 | 0,55 | 10,14 | 10,14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,30 | 4,55 | 0,83 | 9,77 | 19,92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,82 | 4,49 | 0,70 | 9,64 | 29,56 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,14 | 4,21 | 0,84 | 9,04 | 38,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,38 | 3,23 | 0,90 | 6,92 | 45,52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 0,92 | 2,31 | 0,94 | 4,95 | 50,47 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,84 | 2,18 | 0,93 | 4,68 | 55,14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Muricopsis cristata | 0,17 | 1,74 | 0,29 | 3,74 | 58,88 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aequipecten opercularis | 0,48 | 1,25 | 0,55 | 2,69 | 61,57 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,66 | 1,19 | 0,67 | 2,54 | 64,11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jujubinus ruscurianus | 0,63 | 1,15 | 0,67 | 2,46 | 66,58 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mimachlamys varia | 0,49 | 1,05 | 0,55 | 2,25 | 68,83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gregariella semigranata | 0,33 | 0,99 | 0,55 | 2,13 | 70,96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Clanculus cruciatus | 0,66 | 0,96 | 0,65 | 2,06 | 73,01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,55 | 0,96 | 0,67 | 2,05 | 75,07 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crisilla semistriata | 0,57 | 0,94 | 0,61 | 2,02 | 77,09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,25 | 0,87 | 0,29 | 1,86 | 78,95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mitromorpha olivoidea | 0,18 | 0,87 | 0,29 | 1,86 | 80,80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| July Average similarity: 54,60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 1,25 | 6,39 | 0,98 | 11,71 | 11,71 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia brunnea | 0,46 | 4,47 | 0,64 | 8,19 | 19,90 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,36 | 2,76 | 0,82 | 5,06 | 24,96 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,24 | 2,72 | 0,84 | 4,98 | 29,94 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 1,10 | 2,63 | 0,67 | 4,82 | 34,75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,80 | 2,17 | 0,71 | 3,98 | 38,74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triphoridae indet. | 0,90 | 1,92 | 0,87 | 3,51 | 42,24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,88 | 1,87 | 0,86 | 3,43 | 45,68 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolus barbatus | 0,28 | 1,87 | 0,55 | 3,42 | 49,10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mimachlamys varia | 0,49 | 1,86 | 0,42 | 3,41 | 52,50 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania aspera | 0,27 | 1,76 | 0,55 | 3,22 | 55,72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 1,42 | 1,65 | 0,66 | 3,02 | 58,74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ammonicera fischeriana | 0,79 | 1,63 | 0,88 | 2,98 | 61,73 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,68 | 1,60 | 0,82 | 2,93 | 64,65 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chama gryphoides | 0,17 | 1,44 | 0,29 | 2,64 | 67,29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aequipecten opercularis | 0,57 | 1,30 | 0,79 | 2,38 | 69,67 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Striarca lactea | 0,64 | 1,03 | 0,49 | 1,89 | 71,56 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cyrillia linearis | 0,52 | 0,95 | 0,46 | 1,74 | 73,31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,66 | 0,89 | 0,63 | 1,62 | 74,93 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 0,63 | 0,88 | 0,61 | 1,61 | 76,54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,61 | 0,87 | 0,61 | 1,60 | 78,14 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium reticulatum | 0,68 | 0,83 | 0,60 | 1,53 | 79,66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 0,68 | 0,81 | 0,55 | 1,49 | 81,15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| September Average similarity: 44,42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,77 | 4,62 | 0,78 | 10,40 | 10,40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 1,44 | 3,10 | 1,15 | 6,98 | 17,38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,26 | 2,75 | 1,08 | 6,18 | 23,56 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,12 | 2,73 | 1,12 | 6,14 | 29,70 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 0,89 | 2,63 | 0,97 | 5,91 | 35,61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triphoridae indet. | 0,90 | 2,51 | 1,12 | 5,65 | 41,26 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,88 | 2,38 | 1,08 | 5,35 | 46,61 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,90 | 1,95 | 1,10 | 4,40 | 51,01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Runcina sp. | 0,67 | 1,86 | 1,05 | 4,18 | 55,19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alvania lineata | 0,84 | 1,72 | 0,48 | 3,88 | 59,06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Parvicardium scriptum | 0,81 | 1,49 | 0,73 | 3,36 | 62,42 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jujubinus ruscurianus | 0,51 | 1,34 | 0,32 | 3,03 | 65,45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,53 | 1,26 | 0,58 | 2,84 | 68,28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusia tricolor | 0,27 | 1,13 | 0,32 | 2,54 | 70,83 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vitreolina perminima | 0,62 | 1,13 | 0,74 | 2,53 | 73,36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rissoella diaphana | 0,51 | 0,97 | 0,61 | 2,18 | 75,54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aplysia punctata | 0,53 | 0,97 | 0,67 | 2,18 | 77,72 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modiolula phaseolina | 0,55 | 0,87 | 0,39 | 1,95 | 79,67 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mitromorpha columbellaria | 0,36 | 0,69 | 0,32 | 1,56 | 81,23 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| November Average similarity: 41,53 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Av.Abund | Av.Sim | Sim/SD | Contrib% | Cum.% | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chauvetia mamillata | 0,98 | 6,89 | 0,72 | 16,60 | 16,60 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina radiata | 1,37 | 3,83 | 0,92 | 9,22 | 25,82 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tritia incrassata | 0,48 | 3,09 | 0,54 | 7,43 | 33,25 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arca noae | 0,43 | 3,08 | 0,59 | 7,42 | 40,67 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Musculus costulatus | 1,08 | 2,97 | 0,91 | 7,15 | 47,81 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pusillina philippi | 1,03 | 2,69 | 0,64 | 6,48 | 54,29 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia striolata | 0,75 | 2,55 | 0,87 | 6,15 | 60,44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hiatella arctica | 0,75 | 2,28 | 0,90 | 5,48 | 65,92 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pseudochama gryphina | 0,33 | 2,20 | 0,40 | 5,29 | 71,21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Odostomia plicata | 0,56 | 1,91 | 0,87 | 4,60 | 75,81 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bittium latreillii | 0,77 | 1,54 | 0,54 | 3,71 | 79,52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Striarca lactea | 0,78 | 1,17 | 0,66 | 2,83 | 82,34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





