Submitted:
12 August 2025
Posted:
14 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
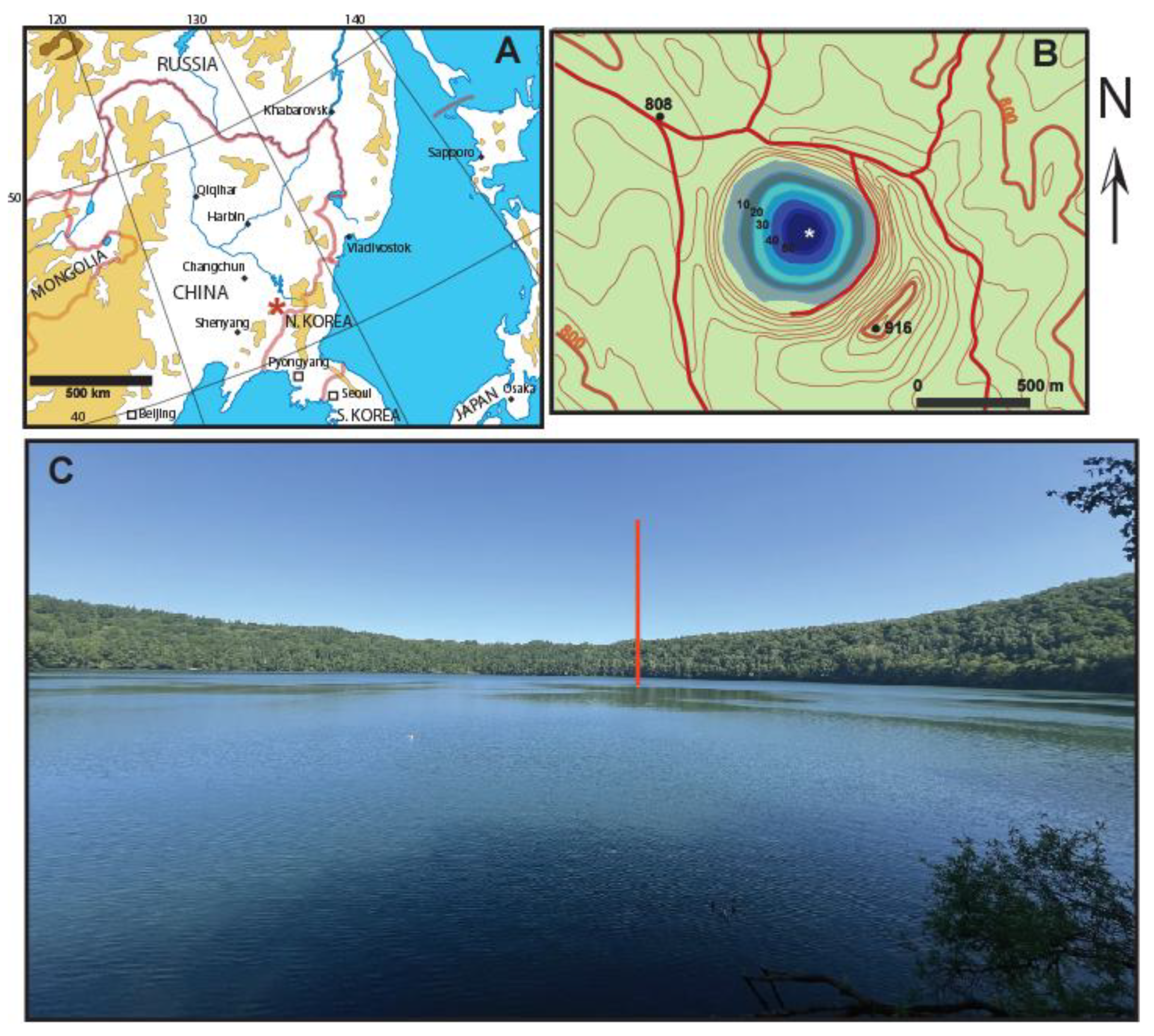
2.1. Study Site
2.2. Limnology of Lake Sihailongwan
2.3. Diatom Sampling and Sample Preparation
3. Results
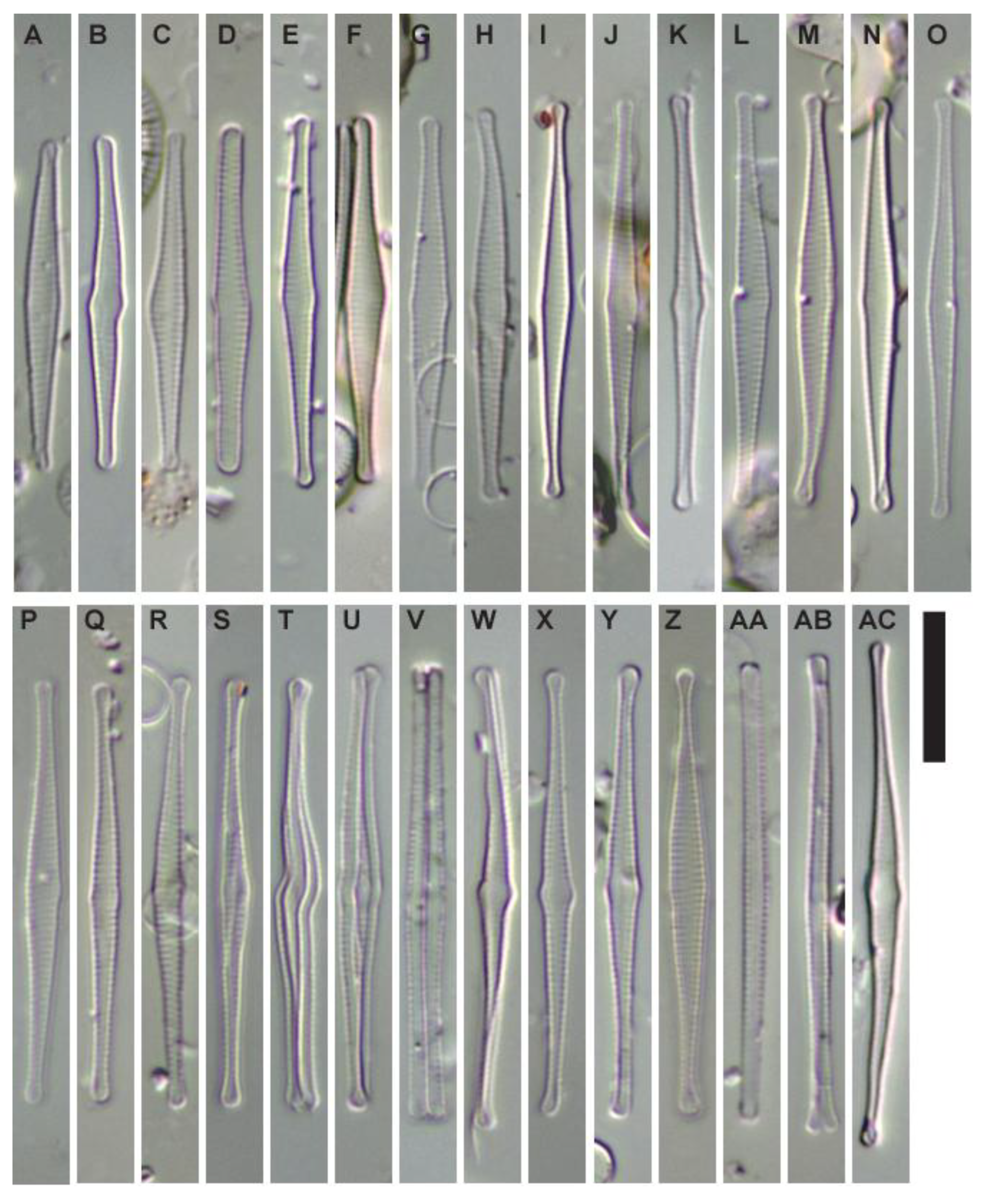
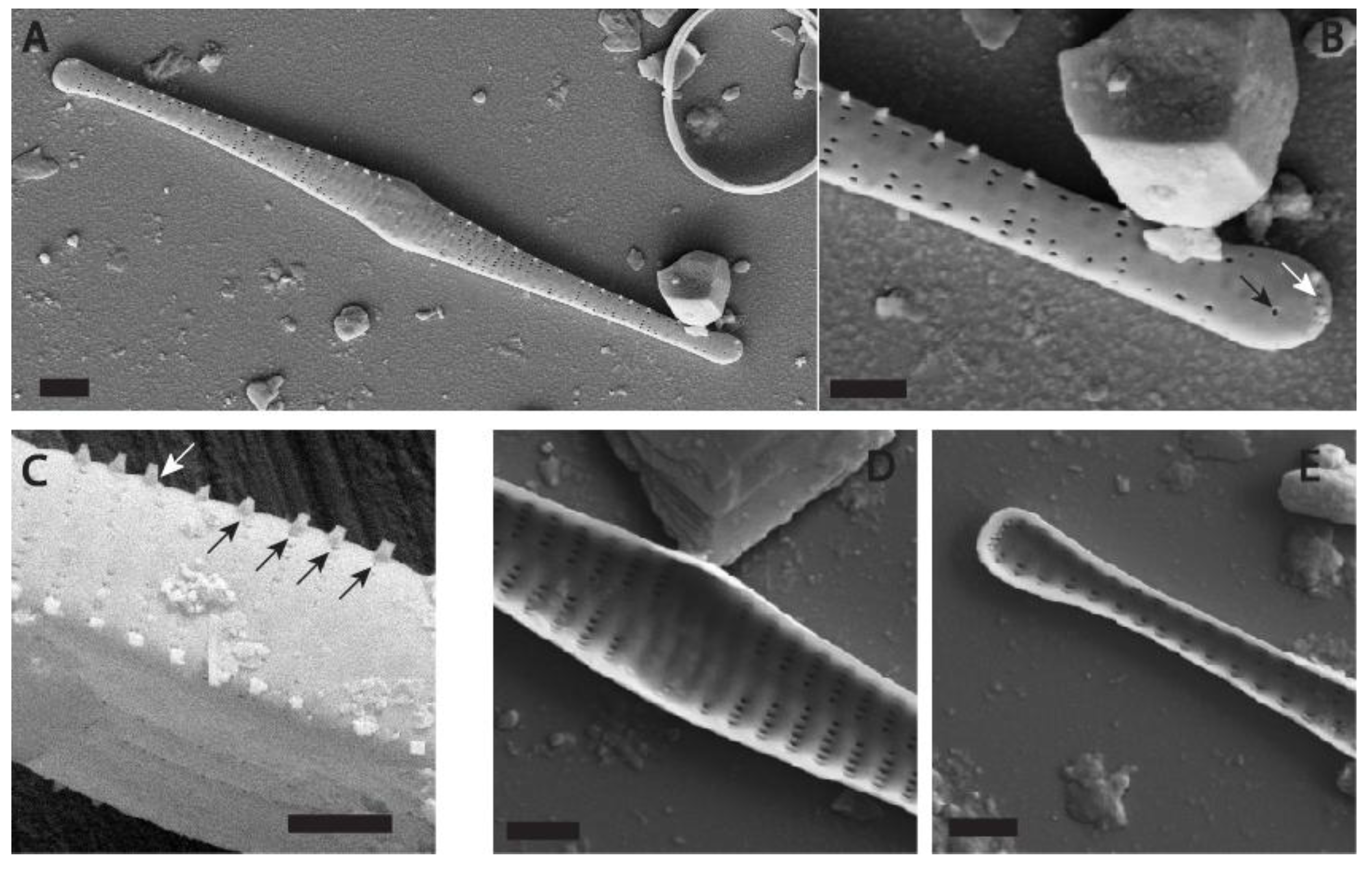
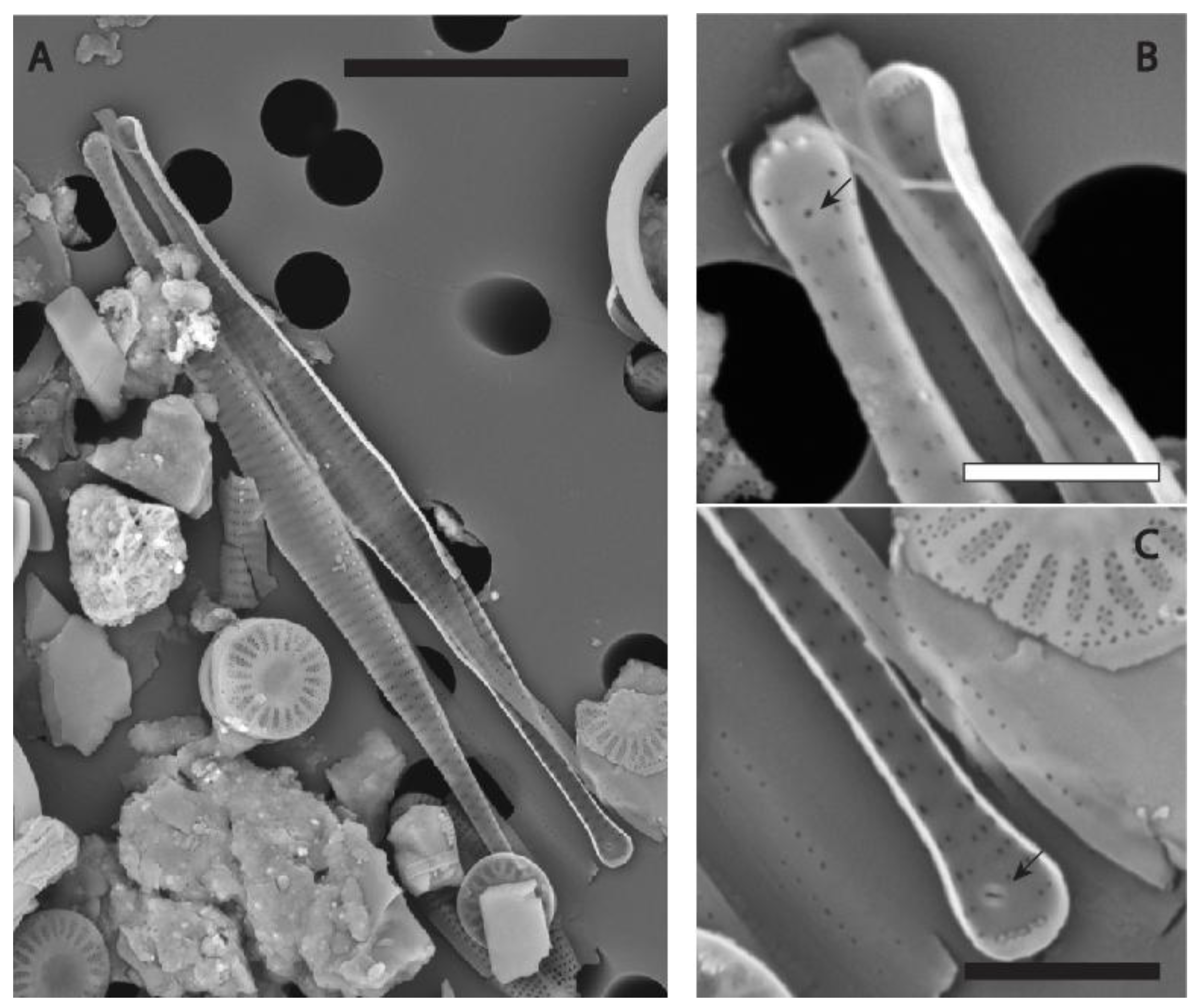
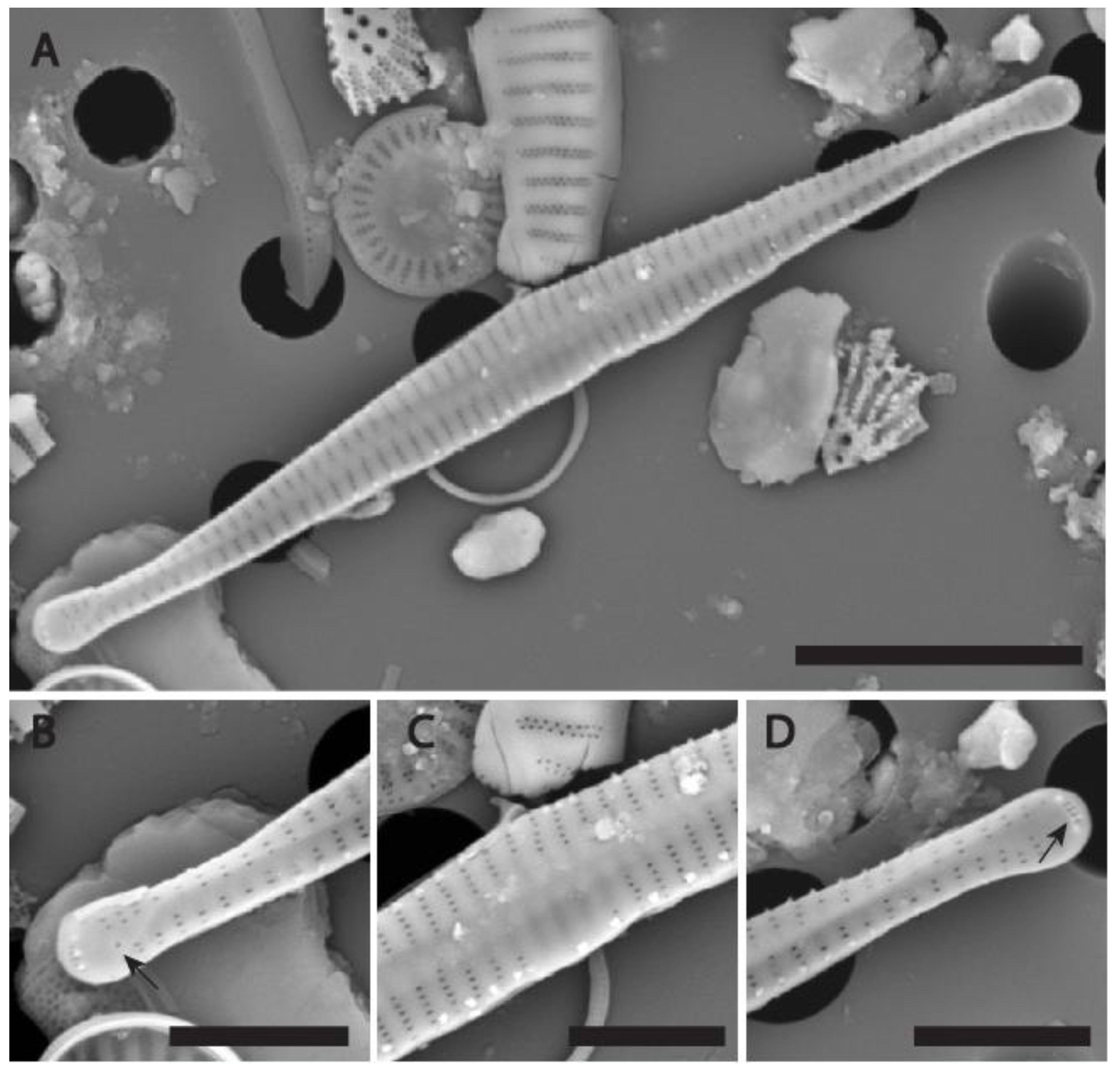
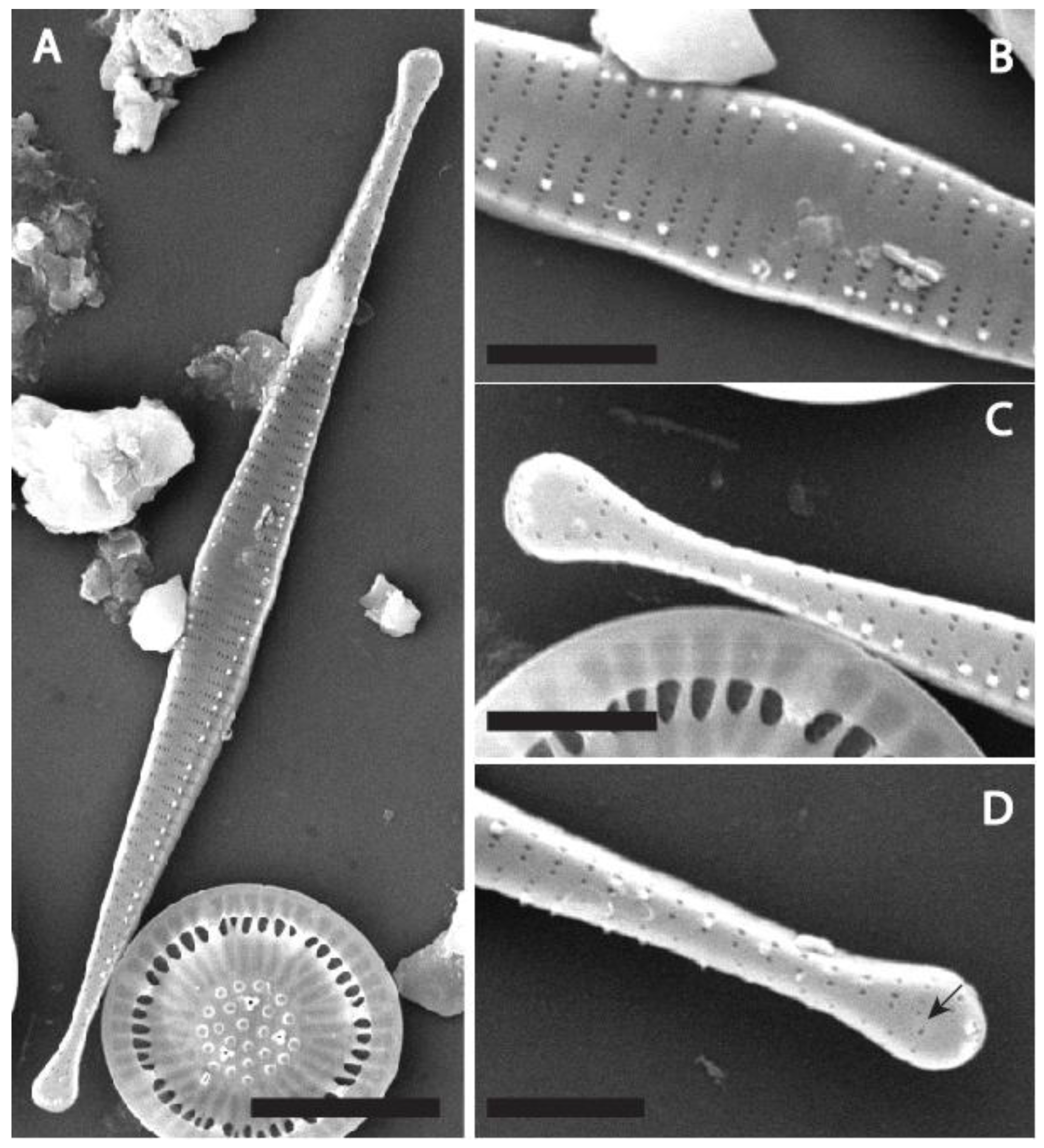
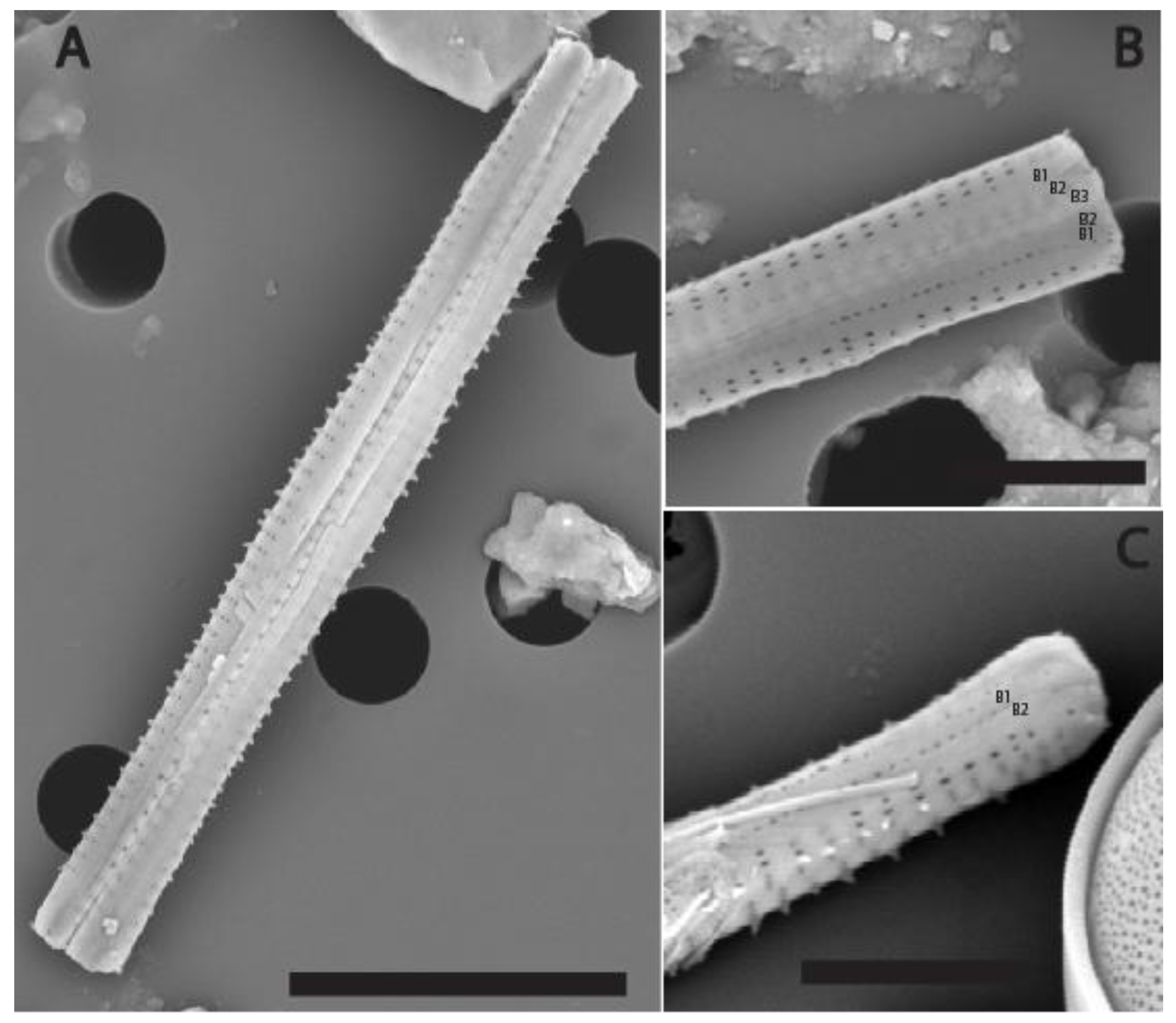
3.1. New Species Description
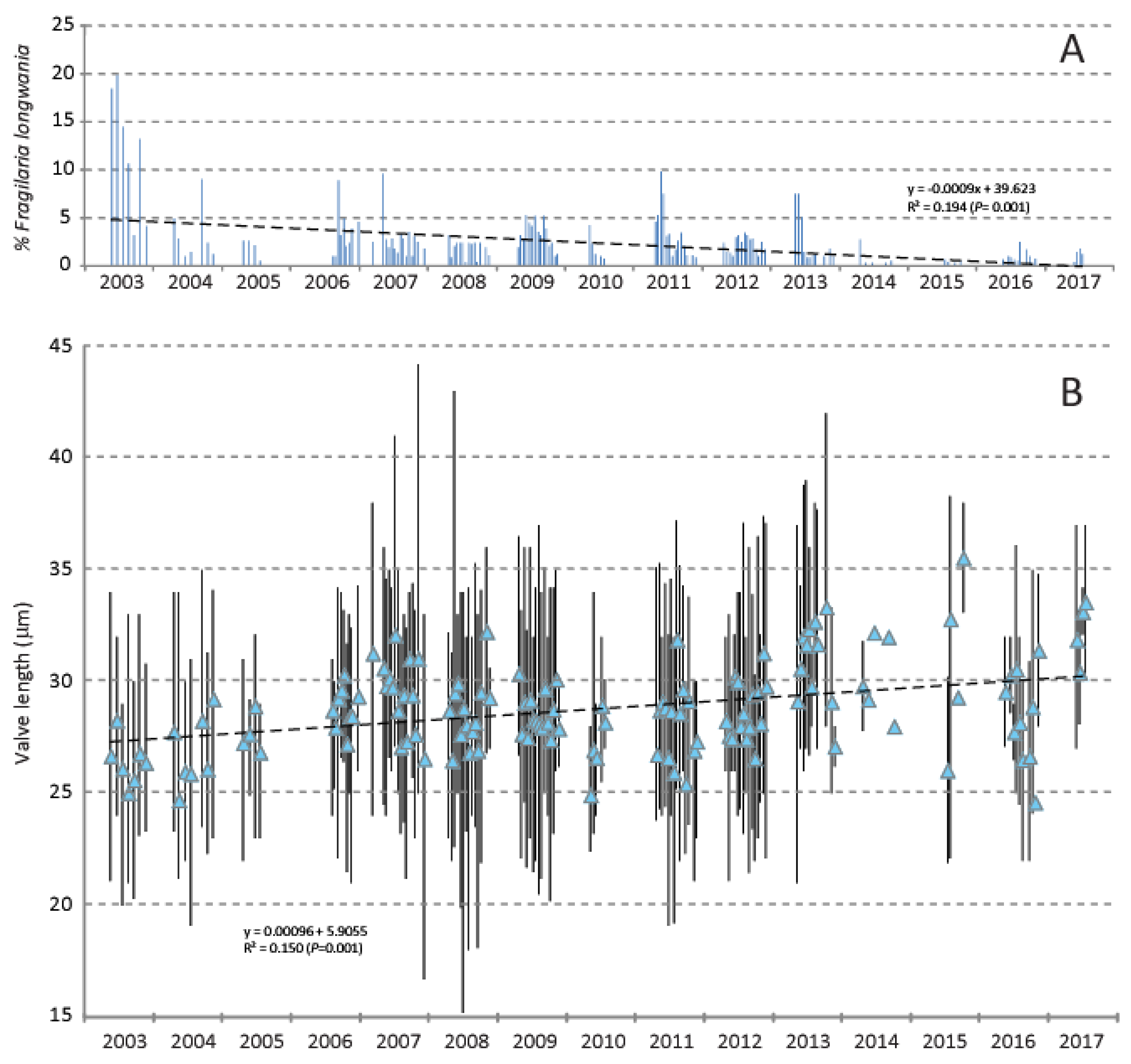
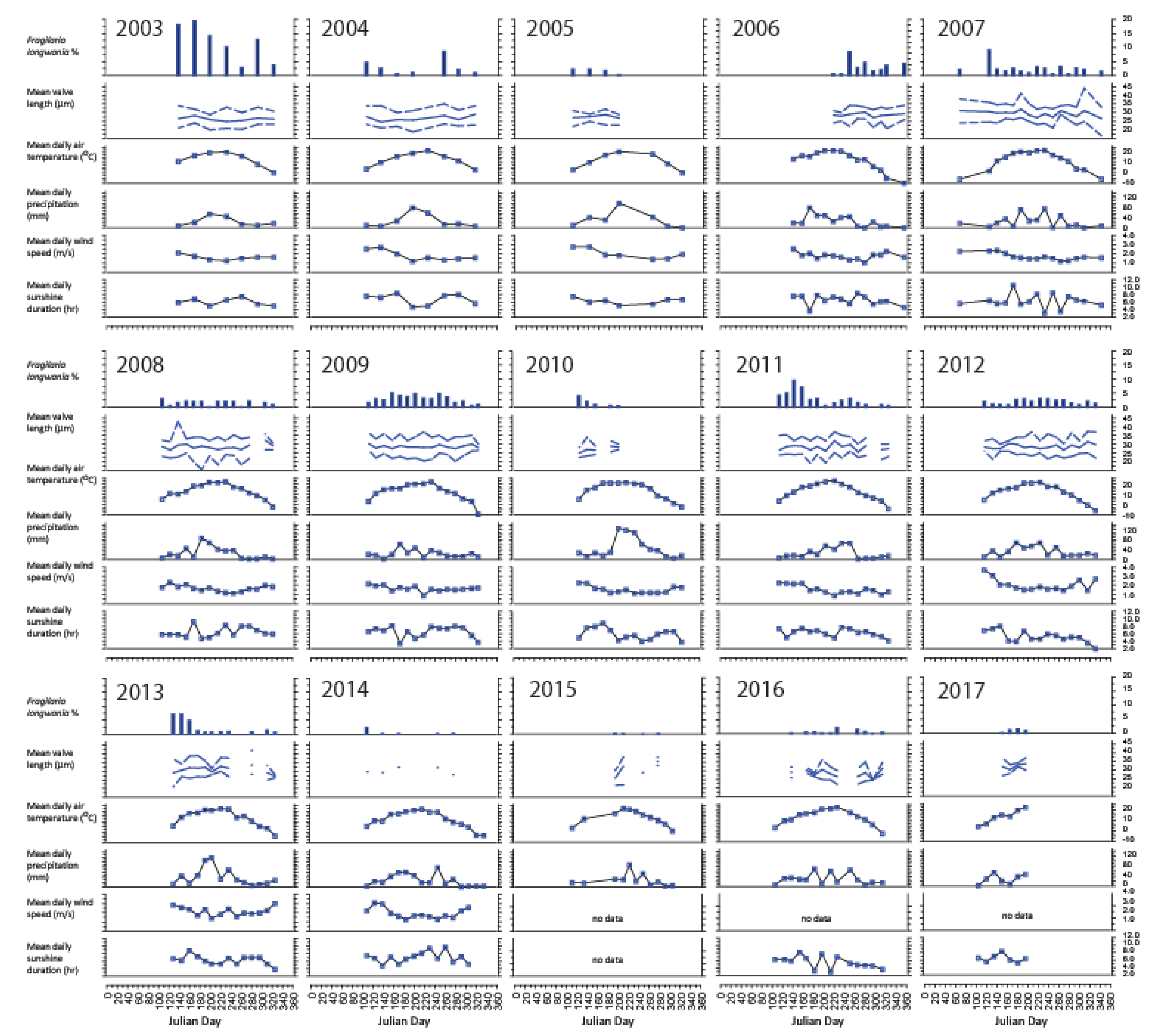
3.2. Analysis of the Trap Samples
3.3. Analysis of Additional Samples
4. Discussion
4.1. Generic Placement of the New Species
4.2. Comparison with Other Species of the Genus Fragilaria
| Taxon | Length (µm) | Width (µm) | Striae in 10 µm | Valve outline | Central area | Pore fields | Distribution | Reference |
| F.longwania | 15—44 | 2.0—3.5 | 21—25 | spindle shaped | clearly swollen | small, only one or two rows of small poroids | Lake Sihailongwan, NE China | This study |
| F.campyla | 35—45 | 2.5—3.0 | 19—20 | linear to linear-lanceolate | not swollen | well-developed, several rows of small poroids | Europe | [3] |
| F. parva | 20—40 | 3.0—3.5 | 19—21 | linear to linear-lanceolate | swollen | well-developed, 4 rows of large poroids | Europe | [3] |
| F. metcalfeana | 60—120 | 2.5—3.5 | ca. 18 | linear but strongly elongated | distinctly inflated | large, 2-3 rows of large poroids | Fossil, Neogene, Dubravica, Croatia | [3] |
| F. pseudofamiliaris | 30—50 | 2.0—3.0 | 18—19 | elongated, narrowly lanceolate | not swollen | well-developed, at least 4 rows of small poroids | Artesian well, Dresden, Germany | [3] |
| F. scotica | 65 | 4 | 15 | linear-lanceolate | clearly inflated | not observed | Loch Leven, Scotland | [3] |
| F. nanoides | 40—90 | 1.8—2.4 | 22.5—24 | linear-lanceolate | weakly inflated | not observed | Lake Julma Ölkky, Finland | [26] |
| F.billingsii | 54—76 | 2.0—2.5 | 17—20 | narrow lanceolate | bilaterally gibbous | well-developed | Reservoir, Sao Paulo State, Brazil | [27] |
| F.huebeneri | 99—121 | 2.0—4.0 | 17—21 | spindle to needle-shaped, narrowly lanceolate | indistinct | well-developed, 3-5 rows of poroids | Lake NamCo, Tibet,China | [8] |
| F.stoermeriana | 60—110 | 1.5—3.0 | 20—24 | long, lanceolate | distinctly swollen | small ocellulimbi | Lake Superior, Ontario, Canada | [28] |
| F. tenera var. nanana | 29—85 | 2.0—2.3 | 18.5—20 | linear to linear-lanceolate | barely inflated | well-developed, 3 rows of poroids | cosmopolitan | [29] |
4.3. Ecology, Seasonality, Cell-Size and Distribution
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | Scanning Electron Microscopy |
| LM | Light Microscopy |
References
- Lyngbye, H.C. Tentamen hydrophytologiae danicae continens omnia hydrophyta cryptogama Daniae, Holsatiae, Faeroae, Islandiae, Groenlandiae hucusque cognita, systematice disposita, descripta et iconibus illustrata, adjectis simul speciebus norvegicis. Hafniae [Copenhagen]: typis Schultzianis, in commissis Librariae Gyldendaliae. 1819, pp. [i]–xxxii, [1]–248, 70 pls. [CrossRef]
- Van de Vijver, B.; Williams, D.M.; Kelly, M.; Jarlman, A.; Wetzel, C.E.; Ector, L. Analysis of some species resembling Fragilaria capucina (Fragilariaceae, Bacillariophyta). Fottea 2021, 21, 128–151. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Williams, D.M.; Schuster, T.M.; Kusber, W.-H.; Cantonati, M.; Wetzel, C.E.; Ector, L. Analysis of the Fragilaria rumpens complex (Fragilariaceae, Bacillariophyta) with the description of two new species. Fottea 2022, 22, 93–121. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Williams, D.M.; Kusber, W.-H.; Cantonati, M.; Hamilton, P.B.; Wetzel, C.E.; Ector, L. Fragilaria radians (Kützing) D. M. Williams et Round, the correct name for F. gracilis (Fragilariaceae, Bacillariophyta): a critical analysis of this species complex in Europe. Fottea 2022, 22, 256–291. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Schuster, T.M.; Jónsson, G.S.; Hansen, I.; Williams, D.M.; Kusber, W.-H.; Wetzel, C.E.; Ector, L. A critical analysis of the Fragilaria vaucheriae complex (Bacillariophyta) in Europe. Fottea 2023, 23, 62–96. [Google Scholar] [CrossRef]
- Kociolek, J.P.; You, Q.; Liu, Q.; Liu, Y.; Wang, Q. Continental diatom biodiversity discovery and description in China: 1848 through 2019. PhytoKeys 2020, 160, 45–97. [Google Scholar] [CrossRef]
- Rioual, P.; Flower, R.J.; Chu, G.; Lu, Y.; Zhang, Z.; Zhu, B.; Yang, X. Observations on a fragilarioid diatom found in inter-dune lakes of the Badain Jaran Desert (Inner Mongolia, China), with a discussion on the newly erected genus Williamsella Graeff, Kociolek & Rushforth. Phytotaxa 2017, 329, 28–50. [Google Scholar] [CrossRef]
- Krahn, K.J.; Schwarz, A.; Wetzel, C.E.; Cohuo-Durán, S.; Daut, G.; Macario-González, L.; Pérez, L.; Wang, J.; Schwalb, A. Three new needle-shaped Fragilaria species from Central America and the Tibetan Plateau. Phytotaxa 2021, 479, 1–22. [Google Scholar] [CrossRef]
- Liu, B. The diatom genus Ulnaria (Bacillariophyceae) in China. PhytoKeys 2023, 228, 1–118. [Google Scholar] [CrossRef]
- Stebich, M.; Rehfeld, K.; Schlütz, F.; Tarasov, P.; Liu, J.; Mingram, J. Holocene vegetation and climate dynamics of NE China based on the pollen record from Sihailongwan Maar Lake. Quaternary Science Reviews 2015, 124, 275–289. [Google Scholar] [CrossRef]
- Mingram, J.; Stebich, M.; Schettler, G.; Hu, Y.; Rioual, P.; Nowaczyk, N.; Dulski, P. , You, H.; Opitz, S.; Liu, Q.; Liu, J. Millennial-scale East Asian monsoon variability of the last glacial deduced from annually laminated sediments from Lake Sihailongwan, N. E. China. Quaternary Science Reviews 2018, 201, 57–76. [Google Scholar] [CrossRef]
- Li, P.; Chu, G.; Rioual, P.; Zhan, N.; Zhang, G.; Zhu, Z.; Qi, L.; Xie, M.; Sun, Q. Independent temperature records since the last deglaciation from the varved sediments of Sihailongwan maar lake, northeastern China. Quaternary Science Reviews 2025, 349, 109139. [Google Scholar] [CrossRef]
- Chu, G.; Liu, J.; Schettler, G.; Li, J.; Sun, Q.; Gu, Z.; Lu, H.; Liu, Q.; Liu, T. Sediment fluxes and varve formation in Sihailongwan, a maar lake from northeastern China. Journal of Paleolimnology 2005, 34, 311–324. [Google Scholar] [CrossRef]
- Schettler, G.; Liu, Q.; Mingram, J. , Negendank, J.F.W. Palaeovariations in the East-Asian monsoon regime geochemically recorded in varved sediments of Lake Sihailongwan (Northeast China, Jilin Province). Part 1: Hydrological conditions and dust flux. Journal of Paleolimnology 2006, 35, 239–270. [Google Scholar] [CrossRef]
- Han, Y.; An, Z.; Lei, D.; Zhou, W.; Zhang, L.; Zhao, X.; Yan, D.; Arimoto, R.; Rose, N.L.; Roberts, S.L.; Li, L.; Tang, Y.; Liu, X.; Fu, X.; Schneider, T.; Hou, X.; Lan, J.; Tan, L.; Liu, X.; Hu, J.; Cao, Y.; Liu, W.; Wu, F.; Wang, T.; Qiang, X.; Chen, N.; Cheng, P.; Hao, Y.; Wang, Q.; Chu, G.; Guo, M.; Han, M.; Tan, Z.; Wei, C.; Dusek, U. The Sihailongwan Maar Lake, northeastern China as a candidate Global Boundary Stratotype Section and point for the Anthropocene Series. The Anthropocene Review 2023, 10, 177–200. [Google Scholar] [CrossRef]
- Rioual, P.; Lu, Y.; Yang, H.; Scuderi, L.; Chu, G.; Holmes, J.; Zhu, B.; Yang, X. Diatom-environment relationships and a transfer function for conductivity in lakes of the Badain Jaran Desert, Inner Mongolia, China. Journal of Paleolimnology 2014, 50, 207–229. [Google Scholar] [CrossRef]
- Battarbee, R.W.; Jones, V.J.; Flower, R.J.; Cameron, N.G.; Bennion, H.; Carvalho, L.R.; Juggins, S. Diatoms. In Smol, J.P.; Birks, H.J.B., Ed.; Last, W.M. (Eds), Tracking environmental change using lake sediments, Volume 3: terrestrial, algal, and siliceous indicators. Kluwer Academic Publishers, Dordrecht, The Netherlands, 2001; pp. 155–202. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Silva, P.C. Classification of algae. In Lewin, R.A. (ed.): Physiology and Biochemistry of Algae. Academic Press, New York & London, 1962; pp. 827–837.
- Greville, R.K. Div. IV. Diatomaceae. In: The English Flora of Sir James Edward Smith. Class XXIV. Cryptogamia. Vol. V. (or Vol. II of Dr. Hooker’s British flora). Part I. Comprising the Mosses, Hepaticae, Lichens, Characeae and Algae. (Hooker, W.J. Eds), London: Longman, Brown, Green & Longmans Paternoster-Row. 1833; pp. 401–415.
- Rioual, P.; Morales, E.A.; Chu, G.; Han, J.; Li, D.; Liu, J.; Liu, Q.; Mingram, J.; Ector, L. Staurosira longwanensis sp. nov., a new araphid diatom (Bacillariophyta) from Northeast China. Fottea 2014, 14, 91–100. [Google Scholar] [CrossRef]
- Dubois, A. Describing new species. Taprobanica 2010, 2, 6–24. [Google Scholar] [CrossRef]
- Williams, D.M. Spines and homologues in `araphid’ diatoms. Plant Ecology and Evolution 152, 150–162. [CrossRef]
- Graeff, C.L.; Kociolek, J.P.; Rushforth,S. R. New and interesting diatoms (Bacillariophyta) from Blue Lake Warm Springs, Tooele County, Utah. Phytotaxa 2013, 153, 1–38. [Google Scholar] [CrossRef]
- Al-Handal, A.Y.; Kociolek, J.P.; Abdullah, D.S. Williamsella iraqiensis sp. nov., a new diatom (Bacillariophyta, Fragilariophyceae) from Sawa lake, South Iraq. Phytotaxa 2016, 244, 289–297. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Metzeltin, D. Indicators of oligotrophy. 800 taxa representative of three ecologically distinct lake types. Carbonate buffered - oligodystrophic - weakly buffered soft water. In: Lange-Bertalot, H. (ed.). Iconographia Diatomologica vol. 2. Koeltz Scientific Books, Königstein, Germany, 390 pp.
- Wengrat, S.; Morales, E.A.; Wetzel, C.E.; Almeida, P.D.; Ector, L.; Bicudo, D.C. Taxonomy and ecology of Fragilaria billingsii sp. nov. and analysis of type material of Synedra rumpens var. fusa (Fragilariaceae, Bacillariophyta) from Brazil. Phytotaxa 2016, 270, 191–202. [Google Scholar] [CrossRef]
- Alexson, E.E.; Reavie, E.D.; Van de Vijver, B.; Wetzel, C.E.; Ector, L.; Wellard Kelly, H.A.; Aliff, M.N.; Estepp, L.R. Revision of the needle-shaped Fragilaria species (Fragilariaceae, Bacillariophyta) in the Laurentian Great Lakes (United States of America, Canada). Journal of Great Lakes Research 2022, 48, 999–1020. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Ulrich, S. Contributions to the taxonomy of needle-shaped Fragilaria and Ulnaria species. Lauterbornia 2014, 78, 1–73. [Google Scholar]
- Soumya, D.; Cai, J.; Hodoki, Y.; Goda, Y.; Akatsuka, T.; Nakano, S. Seasonal changes in the cell size and density of the diatom Fragilaria crotonensis Kitton in Lake Biwa. [CrossRef]

| Variable | unit | nb | Min. | Max. | Median | St. dev. |
| pH | 99 | 5.53 | 7.21 | 6.55 | 0.24 | |
| Elec. Conductivity | µS/L | 99 | 51 | 105 | 62 | 7 |
| Alkalinity | µeq/L | 99 | 338 | 660 | 478 | 52 |
| Tot. Phosphorus | µg/L | 99 | 5.0 | 88.6 | 14.9 | 14.4 |
| Tot. Nitrogen | µg/L | 97 | 7 | 2443 | 360 | 294 |
| Diss. Organic Carbon | mg/L | 92 | 0.2 | 10.0 | 1.7 | 1.2 |
| Cl | mg/L | 78 | 0.6 | 3.8 | 1.2 | 0.6 |
| NO3 | mg/L | 73 | 0 | 2.4 | 0.6 | 0.5 |
| SO4 | mg/L | 78 | 3.4 | 7.8 | 5.7 | 1.0 |
| Ca | mg/L | 78 | 2.7 | 28.8 | 5.3 | 5.3 |
| K | mg/L | 78 | 0.6 | 3.3 | 1.3 | 0.6 |
| Mg | mg/L | 78 | 1.2 | 3.6 | 2.3 | 0.6 |
| Na | mg/L | 78 | 1.2 | 4.4 | 2.2 | 0.7 |
| Si | mg/L | 99 | 0.1 | 0.8 | 0.4 | 0.2 |
| Secchi Disk depth | m | 22 | 2.4 | 7.6 | 3.8 | 1.3 |
| Date | Sample | Dominant species | % Fragilaria longwania |
| From 19-May-03 to 15-Jul-17 | Sediment trap samples (centre of the lake, -45m water depth), (n =187) | Discostella stelligeroides (43.3, 0.4-92.0%), Lindavia balatonis (26.2, 1.7-76.3%)1 | 2.1 (0—19.8%)1 |
| 26-Apr-05 | Epilithon (small stones, near shore, <0.5m depth) | Lindavia balatonis (19.3%), Achnanthidium sieminskae (16.0%), Encyonopsis perborealis (7.6%), Discostella stelligeroides (5.9%), Encyonema silesiacum (5.0%) | Not observed |
| 21-Oct-05 | Surface-sediment (-50m water depth, lake centre) | Discostella stelligeroides (57.2%), Lindavia balatonis (17.6%), Pseudostaurosira parasitoides (3.2%), Nanofrustulum trainorii (2.2%), Fragilaria pectinalis (1.9%) | 1.8% |
| 18-Nov-06 | Periphyton on floating buoy (lake centre) | Achnanthidium sieminskae (50.6%), Brachysira neoexilis (15.2%), Encyonopsis subminuta (7.2%), Staurosirella ovata (5.9%), Discostella stelligeroides (4.6%) | 3.0% |
| 19-Nov-06 | Epilithon (small stones, near shore, <0.5m depth) | Nitzschia cf sociabilis (12.6%), Achnanthidium sieminskae (9.6%), Lindavia balatonis (9.1%), Nitzschia archibaldii (7.8%), Tabellaria hercynica (7.4%) | 2.6% |
| 19-Nov-06 | Epiphyton (-10m water depth, filamentous green) | Discostella stelligeroides (18.7%), Lindavia balatonis (9.6%), Pseudostaurosira pseudoconstruens (8.7%), Staurosirella ovata (6.5%), Sellaphora chistiakovae (3.0%) | 1.3% |
| 19-Nov-06 | Epipelon (-17m water depth) | Discostella stelligeroides (32.0%), Staurosirella ovata (15.4%), Lindavia balatonis (10.6%), Pseudostaurosira parasitoides (5.9%), Staurosira longwanensis (5.9%) | 1.2% |
| 14-Aug-07 | Surface-sediment (-51m water depth, lake centre) | Discostella stelligeroides (80.4%), Lindavia balatonis (6.3%), Fragilaria longwania (1.2%) | 1.2% |
| 22-Apr-08 | Periphyton on floating buoy (lake centre) | Achnanthidium sieminskae (38.5%), Discostella stelligeroides (16.3%) Brachysira neoexilis (14.4%), Encyonopsis perborealis (4.4%), Lindavia balatonis (3.0%), | 0.4% |
| 22-Apr-08 | Epilithon (small stones, near shore, <0.5m depth) | Encyonopsis perborealis (23.6%), Encyonema silesiacum (14.6%), Discostella stelligeroides (12.2%), Lindavia balatonis (10.6%), Achnanthidium sieminskae (8.9) | Present (<0.1%) |
| 3-Aug-16 | Periphyton on floating buoy (lake centre) | Achnanthidium sieminskae (27.5%), Encyonopsis perborealis (19.0%), Lindavia balatonis (14.2%), Eucocconeis laevis (13.3%), Rossithidium pusillum (7.3%) | Not observed |
| 3-Aug-16 | Epilithon (small stones, near shore, <0.5m depth) | Encyonopsis perborealis (29.3%), Achnanthidium sieminskae (9.30%), Lindavia balatonis (8.7%), Achnanthidium sp cf duriense (8.0%), Nitzschia lacuum (6.0%) | 1.3% |
| 3-Aug-16 | Epixylon (dead tree, near shore, <0.5m depth) | Encyonopsis perborealis (32.8%), Achnanthidium sieminskae (18.0%), Lindavia balatonis (14.8%), Nitzschia gessneri (6.9%), Brachysira neoexilis (4.8%) | Not observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





