Submitted:
05 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Methods
2.2. Experimental Conditions for HS
2.3. Experimental Conditions for SPME:
2.4. Experimental Condition for All Three Analyses
2.5. Identification
3. Results
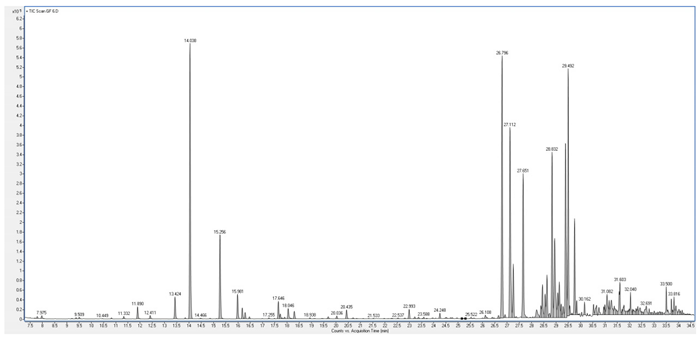
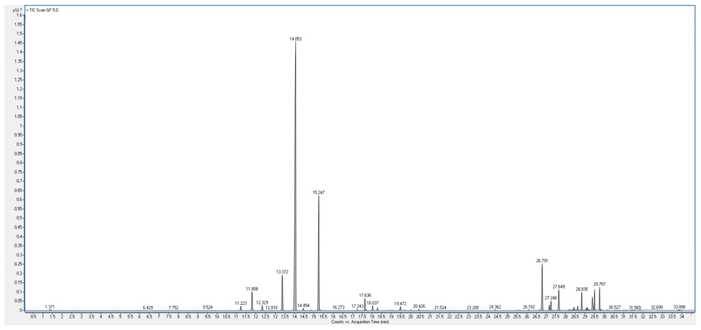
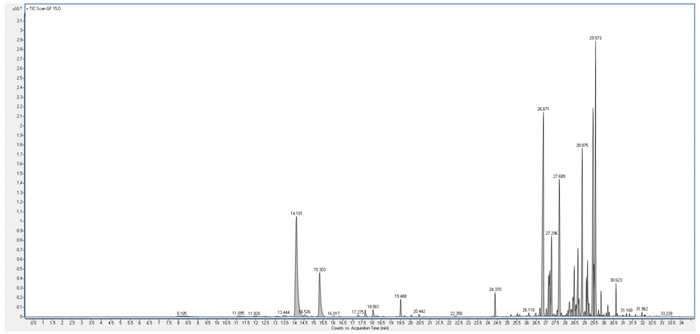
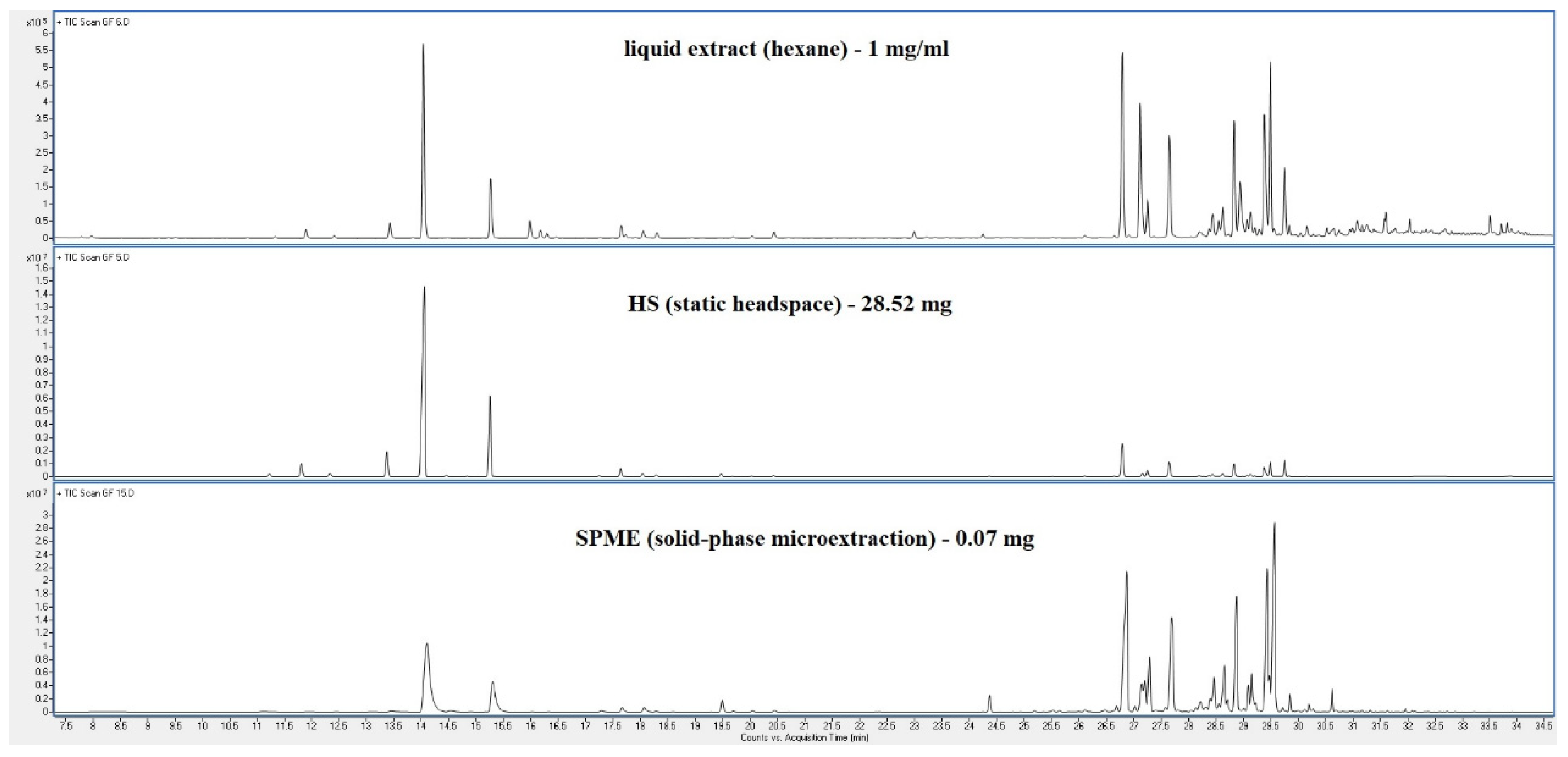
4. Discussion
5. Conclusions
Acknowledgments
Statement of Ethics
Conflicts of Interest Statement
Funding Sources
Abbreviations
References
- Booth, J.K., Page, J.E., Bohlmann, J. Terpene synthases from Cannabis sativa, PLoS ONE. 2017; 12(3): e0173911. [CrossRef]
- Booth, J. Terpene and isoprenoid biosynthesis in Cannabis sativa. PhD thesis, The University of British Columbia, Vancouver, Canada, 2020; 223 pages.
- Zhao, L., Chang, W., Xiao, Y., Liu, H., Liu, P. Methylerythritol Phosphate Pathway of Isoprenoid Biosynthesis. Ann Rev Biochem 2013; 82: 497–530. [CrossRef]
- Lichtenthaler, H.K. The plants’ 1-deoxy-D-xylulose-5-phosphate pathway for biosynthesis of isoprenoids, Fett/Lipid 1998; 100 (4-5): 128–138. [CrossRef]
- Lichtenthaler, H.K. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants, Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999; 50: 47–65. [CrossRef] [PubMed]
- Mechoulam, R., Gaoni, Y. The isolation and structure of cannabinolic, cannabidiolic and cannabigerolic acids, Tetrahedron 1965; 21: 1223-1229. [PubMed]
- Fellermeier, M., Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol, FEBS Letters 1998; 427: 283-285. [CrossRef]
- Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants, Nat Prod Rep. 1999; 16: 565–574.
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science, Headache 2018; 58(7): 1139-1186. [CrossRef]
- Tomko, A.M., Whynot, E.G/, Ellis, L.D., Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis, Cancers 2020; 12(7): 1985. [CrossRef]
- Kumar P, Mahato DK, Kamle M, Borah R, Sharma B, Pandhi S, Tripathi V, Yadav HS, Devi S, Patil U , Xiao J, Mishra AK. Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: An overview. Phytother. Res. 2021; 35: 6010-29. [CrossRef]
- Liktor-Busa E, Keresztes A, LaVigne J, Streicher JM, Largent-Milnes TM. Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacol. Rev. 2021; 73: 1269–97. [CrossRef]
- Laws III JS, Shrestha S, Smid SD. Cannabis terpenes display variable protective and anti-aggregatory actions against neurotoxic β amyloid in vitro: highlighting the protective bioactivity of α-bisabolol in motorneuronal-like NSC-34 cells. Neurotoxicology 2022; 90: 81–87. [CrossRef]
- Rodriguez CEB, Ouyang L, Kandasamy R. Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis. Behav. Pharmacol. 2022; 33: 130-157. [CrossRef]
- Di Sotto A|, Gullì M, Acquaviva A, Tacchini M, Di Simone SC, Chiavaroli A, Recinella L, Leone S, Brunetti L, Orlando G, Flores GA, Venanzoni R, Angelini P, Menghini L, Ferrante C. Phytochemical and pharmacological profiles of the essential oil from the inflorescences of the Cannabis sativa L. Industrial Crops & Products 2022; 183: 114980.
- Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011; 163(7): 1344-64. [CrossRef] [PubMed]
- Blasco-Benito S, Seijo-Vila M, Caro-Villalobos M, Tundidor I, Andradas C, Garcia-Taboada E, Wade J, Smith S, Guzmán M, Pérez-Gómez E, Gordon M, Sanchez C. Appraising the "entourage effect": Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem Pharmacol. 2018; 157: 285-93. [CrossRef]
- Worth T.Unpicking the entourage effect. Nature 2019; 572(7771): S12-S13. [CrossRef] [PubMed]
- Santiago M, Sachdev S, Arnold JC, McGregor IS, Connor M. Absence of entourage: terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis and Cannabinoid Research 2019; 4(3): 165-176. [CrossRef]
- Finlay DB, Sircombe KJ, Nimick M, Jones C, Glass M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front Pharmacol. 2020; 11: 359. [CrossRef]
- Ferber SG, Namdar D, Hen-Shoval D, Eger G, Koltai H, Shoval G, Shbiro L, Weller A. The "Entourage Effect": Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr Neuropharmacol. 2020; 18(2): 87-96.
- LaVigne JE, Hecksel R, Keresztes A, Streicher JM. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci Rep 2021; 11(1): 8232. [CrossRef]
- D’Amour FE, Smith Donn L. A method for determining loss of pain sensation. Journal of Pharmacology and Experimental Therapeutics 1941; 72(1): 74-9.
- Dewey WL, Harris LS, Howes JF, Nuite JA. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. Journal of pharmacology and experimental therapeutics 1970; 175(2): 435-42.
- Pertwee RG. The ring test: a quantitative method for assessing the 'cataleptic' effect of cannabis in mice. Br J Pharmacol. 1972; 46(4): 753-63. [CrossRef]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988; 247(3): 1046-51.
- Turek C, Stintzing FC. Stability of essential oils: a review. Comprehensive Reviews in Food Science and Food Safety 2013; 12(1): 40-53. [CrossRef]
- Bueno J, Leuer E, Kearney M Jr, Green EH, Greenbaum EA. The preservation and augmentation of volatile terpenes in cannabis inflorescence. J Cannabis Res. 2020; 2(1): 27. [CrossRef]
- Prakash Chanda Gupta. Method Validation of Analytical Procedures. PharmaTutor Magazine 3(1), 32-39 (2015).
- Stahl Egon: Chemical varieties of plants containing terpenoids. Essenze e Derivati Agrumari 1957; 27: 188-220.
- Martin L, Smith DM, Farmilo CG. Essential oil from fresh Cannabis sativa and its use in identification. Nature 1961; 191: 774-76. [CrossRef]
- Nigam MC, Handa KL, Nigam IC, Levi L. Essential oils and their constituents XXIX. The essential oil of marihuana: composition of genuine Indian Cannabis sativa L. Can J Chem. 1965; 43(12): 3372-76. [CrossRef]
- Hood LVS, Dames ME, Barry GT. Headspace volatiles of marijuana. Nature 1973; 242(5397): 402-3. [CrossRef]
- Hood LVS, Barry GT. Headspace volatiles of marihuana and hashish: gas chromatographic analysis of samples of different geographic origin. J Chromatogr. 1978; 166(2): 499-506. [CrossRef]
- Shapira A, Berman P, Futoran K, Guberman O, Meiri D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal Chem. 2019; 91(17): 11425-32. [CrossRef]
- Hanuš LO, Hod Y. Terpenes/terpenoids in Cannabis – are they important? Medical Cannabis and Cannabinoids 2020; 3: 25-60.
- Hall BJ, Satterfield-Doerr M, Parikh A., Brodbelt JS. Determination of Cannabinoids in Water and Human Saliva by Solid-Phase Microextraction and Quadrupole Ion Trap Gas Chromatography/Mass Spectrometry. Anal Chem. 1998; 70(9): 1788-96. [CrossRef]
- Ilias Y, Rudaz S, Mathieu P, Christen P, Veuthey JL. Extraction and analysis of different Cannabis samples by headspace solid-phase microextraction combined with gas chromatography-mass spectrometry. J Sep Sci. 2005; 28: 2293–2300. [CrossRef]
- Yang Zaibo, Long Chengmei, Zhong Caining, Sun Chengbin, Mao Haili: Analysis of chemical constituents of the volatile oil from Cannabis sativa by SPME-GC-MS. Zhongguo Yao Fang (Journal of China Pharmacy) 2008; 19(33): 2613-14.
- Krill C, Rochfort S, Spangenberg G. A High-Throughput Method for the Comprehensive Analysis of Terpenes and Terpenoids in Medicinal Cannabis Biomass. Metabolites 2020; 10: 276. [CrossRef]
- Myers C, Herrington JS, Hamrah P, Anderson K. Accelerated Solvent Extraction of Terpenes in Cannabis Coupled With Various Injection Techniques for GC-MS Analysis. Front Chem. 2021; 9: 619770. [CrossRef]
- Russo, EB. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No "Strain," No Gain. Frontiers in plant science 2018; 9: 1969. [CrossRef]
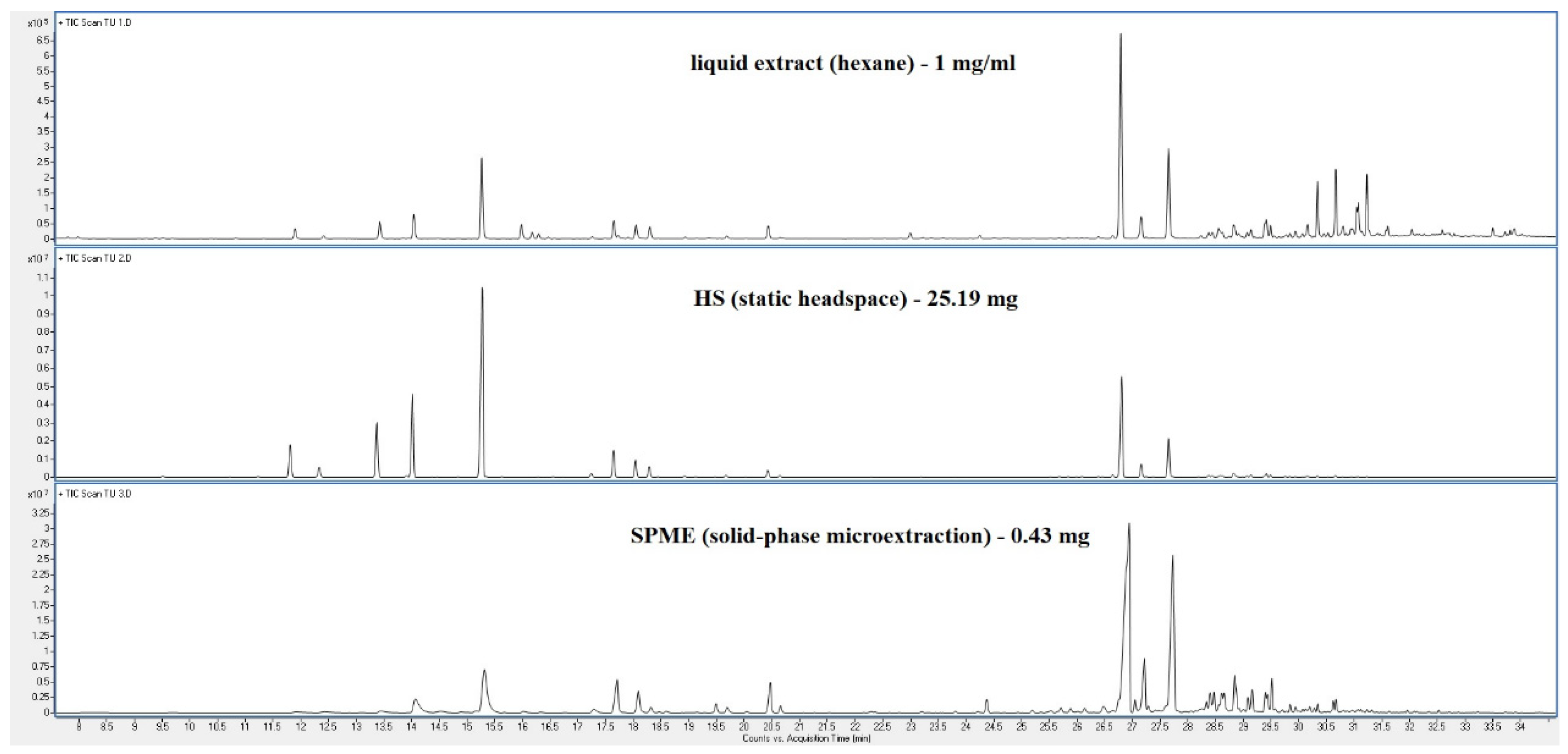
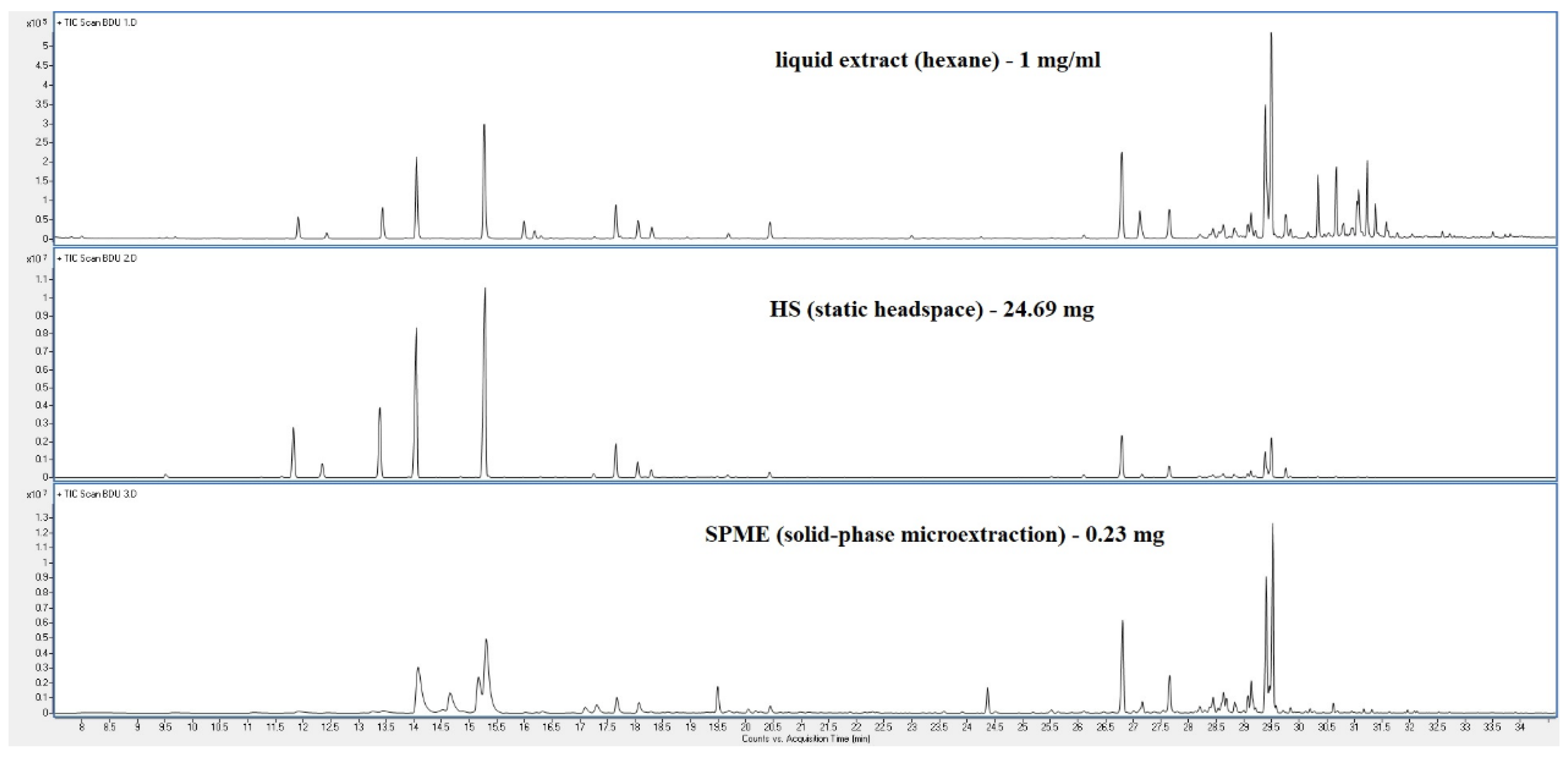
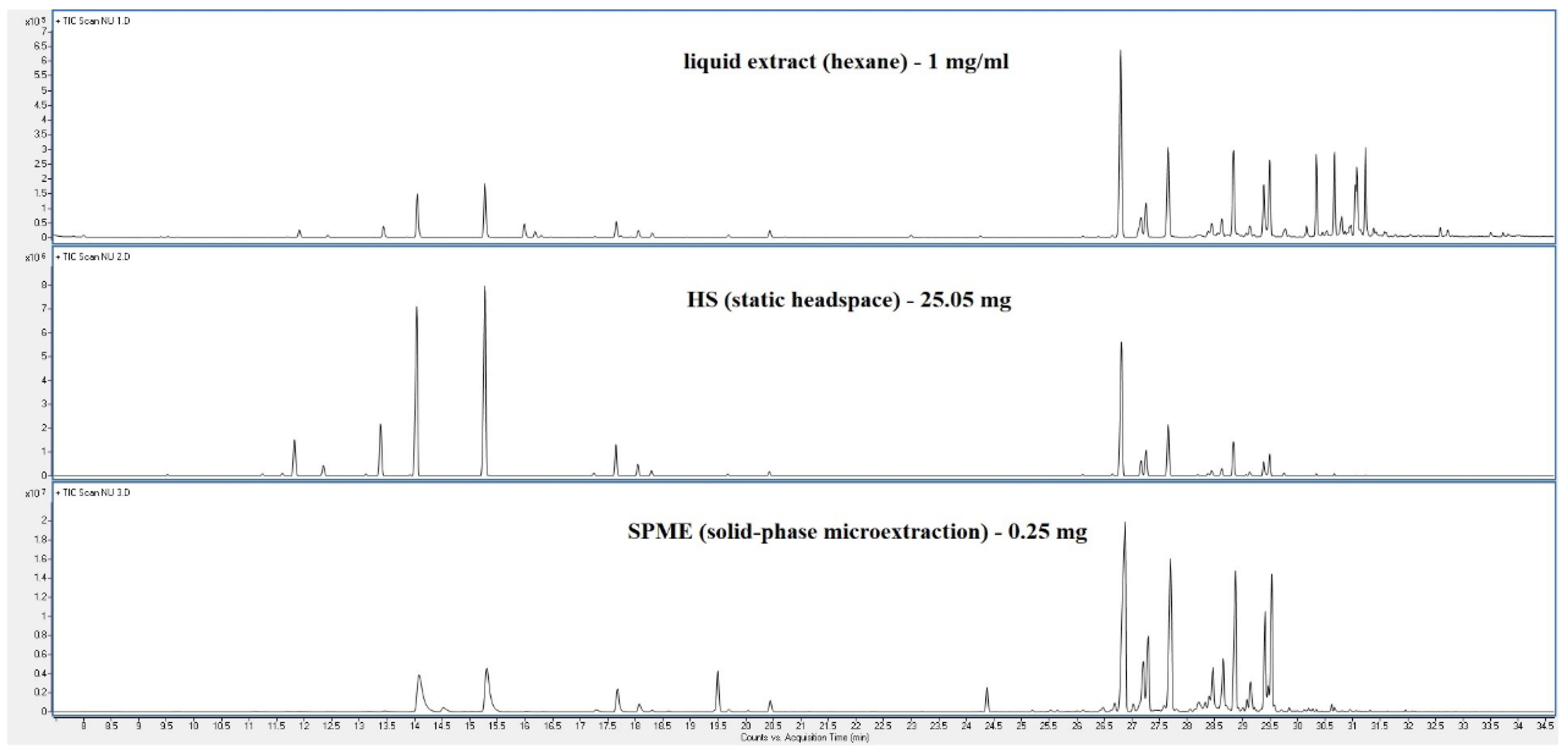
| Peak | RT | % norm (Liq) | % norm (HS) |
% norm (SPME) |
Compounds | Type | RI |
| 1 | 6.429 | 0.02 | 2,3-butanediol | glycol | 788 | ||
| 2 | 7.789 | 0.72 | 2,4-dimethylheptane | hydrocarbon | 821 | ||
| 3 | 9.212 | 0.20 | ethylbenzene | aromatic hydrocarbon | 855 | ||
| 4 | 9.379 | 0.48 | 4-methyloctane | hydrocarbon | 863 | ||
| 5 | 9.509 | 0.75 | p-xylene | aromatic hydrocarbon | 865 | ||
| 6 | 9.524 | 0.08 | 1-hexanol | organic alcohol | 868 | ||
| 7 | 10.718 | 0.37 | 0.05 | heptanal | alkyl aldehyde | 901 | |
| 8 | 11.223 | 0.92 | 1.29 |
5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 |
|
| 9 | 11.600 | 0.04 | α-thujene | bicyclic monoterpene | 929 | ||
| 10 | 11.808 | 4.56 | 5.55 | 0.54 | α-pinene | bicyclic monoterpene | 937 |
| 11 | 12.329 | 1.35 | 1.52 | 0.26 | camphene | bicyclic monoterpene | 952 |
| 12 | 12.810 | 0.02 | benzaldehyde | aromatic aldehyde | 962 | ||
| 13 | 13.372 | 8.38 | 10.07 | 1.50 | β-pinene | bicyclic monoterpene | 979 |
| 14 | 13.893 | 0.03 |
6-methyl-5-hepten-2-one |
unsaturated methylated ketone | 986 |
||
| 15 | 14.053 | 95.78 | 100.00 | 86.64 | β-myrcene | acyclic monoterpene | 991 |
| 16 | 14.887 | 0.23 | α-terpinene | monocyclic monoterpene | 1017 | ||
| 17 | 15.111 | 0.02 | 0.08 | p-cymene | monocyclic monoterpene | 1025 | |
| 18 | 15.247 | 32.07 | 30.90 | 30.66 | limonene | monocyclic monoterpene | 1030 |
| 19 | 15.328 | 0.13 | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 20 | 15.624 | 0.06 | cis-β-ocimene | acyclic monoterpene | 1038 | ||
| 21 | 15.969 | 0.01 | 0.19 | trans-β-ocimene | acyclic monoterpene | 1049 | |
| 22 | 16.273 | 0.06 | 0.15 | γ-terpinene | monocyclic monoterpene | 1060 | |
| 23 | 16.554 | 0.04 | sabinene hydrate | bicyclic monoterpenoid | 1068 | ||
| 24 | 17.243 | 0.34 | 1.13 | terpinolene | monocyclic monoterpene | 1088 | |
| 25 | 17.636 | 6.38 | 2.90 | 2.86 | linalool | acyclic monoterpenoid | 1099 |
| 26 | 18.037 | 4.64 | 1.29 | 3.46 | fenchyl alcohol | bicyclic monoterpenoid | 1113 |
| 27 | 18.286 | 3.07 | 0.71 | cis-pinene hydrate | bicyclic monoterpenoid | 1121 | |
| 28 | 18.590 | 0.25 | neo-allo-ocimene | acyclic monoterpene | 1131 | ||
| 29 | 19.079 | 0.03 | ipsdienol | acyclic monoterpenoid | 1147 | ||
| 30 | 19.672 | 0.96 | 0.15 | 0.52 | borneol | bicyclic monoterpenoid | 1166 |
| 31 | 20.025 | 1.12 | 0.20 | 0.70 | terpinen-4-ol | monocyclic monoterpenoid | 1177 |
| 32 | 20.426 | 3.40 | 0.39 | 0.91 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 33 | 20.706 | 0.10 | dodecane | alkane hydrocarbon | 1200 | ||
| 34 | 24.931 | 0.06 | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 35 | 25.516 | 0.56 | 0.09 | 1.18 | ylangene | tricyclic sesquiterpene | 1372 |
| 36 | 25.637 | 0.23 | 0.07 | 0.63 | α-copaene | tricyclic sesquiterpene | 1376 |
| 37 | 25.813 | 0.18 | β-patchoulene | tricyclic sesquiterpene | 1381 | ||
| 38 | 25.997 | 0.03 | 0.31 | 7-epi-sesquithujene | bicyclic sesquiterpene | 1391 | |
| 39 | 26.206 | 0.31 | tetradecane | alkane hydrocarbon | 1400 | ||
| 40 | 26.462 | 0.03 | 1.56 | cis-β-caryophyllene | bicyclic sesquiterpene | 1406 | |
| 41 | 26.639 | 1.11 | 0.19 | 3.33 | cis-α-bergamotene | bicyclic sesquiterpene | 1415 |
| 42 | 26.791 | 100.00 | 12.73 | 100.00 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 43 | 26.879 | 0.02 | γ-maaliene | tricyclic sesquiterpene | 1430 | ||
| 44 | 27.024 | 0.02 | β-copaene | tricyclic sesquiterpene | 1433 | ||
| 45 | 27.144 | 71.31 | 14.69 | γ-elemene | monocyclic sesquiterpene | 1434 | |
| 46 | 27.160 | 5.60 | 1.35 | 15.36 | α-bergamotene | bicyclic sesquiterpene | 1436 |
| 47 | 27.248 | 19.06 | 2.44 | 25.89 | α-guaiene | bicyclic sesquiterpene | 1439 |
| 48 | 27.376 | 0.43 | 0.06 | 1.39 | guaia-6,9-diene | bicyclic sesquiterpene | 1443 |
| 49 | 27.585 | 2.32 | humulen-(v1) | bicyclic sesquiterpene | 1455 | ||
| 50 | 27.649 | 55.13 | 5.56 | 61.27 | α-humulene | monocyclic sesquiterpene | 1454 |
| 51 | 28.042 | 0.27 | 0.02 | 0.86 | 4,5-di-epi-aristolochene | bicyclic sesquiterpene | 1467 |
| 52 | 28.194 | 6.30 | 0.39 | 6.68 | γ-muurolene | bicyclic sesquiterpene | 1477 |
| 53 | 28.282 | 0.07 | α-amorphene | bicyclic sesquiterpene | 1485 | ||
| 54 | 28.370 | 4.35 | 0.36 |
5.68 |
4a,8-Dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 |
| 55 | 28.434 | 13.06 | 0.92 | 16.48 | β-selinene | bicyclic sesquiterpene | 1486 |
| 56 | 28.555 | 3.73 | δ-selinene | bicyclic sesquiterpene | 1488 | ||
| 57 | 28.627 | 16.16 | 1.21 | 23.47 | α-selinene | bicyclic sesquiterpene | 1494 |
| 58 | 28.835 | 56.69 | 4.39 | 56.51 | α-bulnesene | bicyclic sesquiterpene | 1505 |
| 59 | 28.988 | 0.08 | 1.86 | γ-cadinene | bicyclic sesquiterpene | 1513 | |
| 60 | 29.124 | 13.65 | 0.87 | 16.32 | δ-amorphene | bicyclic sesquiterpene | 1519 |
| 61 | 29.380 | 69.01 | 3.66 | 67.76 | γ-selinene | bicyclic sesquiterpene | 1544 |
| 62 | 29.493 | 73.42 | 4.35 | 89.15 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 63 | 29.757 | 31.07 | 4.40 | 0.37 | germacrene B | monocyclic sesquiterpene | 1557 |
| 64 | 30.158 | 3.53 | 0.08 | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 65 | 31.312 | 0.57 |
cadalene |
bicyclic aromatic hydrocarbon | 1674 | ||
| 66 | 31.575 | 4.56 | juniper camphor | bicyclic sesquiterpenoid | 1691 | ||
| 67 | 32.378 | 0.17 | guaiazulene | bicyclic sesquiterpene | 1775 |
| Peak | RT | % norm (Liq) | % norm (HS) | % norm (SPME) | Compound | Type | RI |
| 1 | 2.966 | 0.12 | 2-methylbutanal | saturated fatty aldehyde | 662 | ||
| 2 | 4.578 | 0.21 | 2-methyl-1-butanol | alcohol | 739 | ||
| 3 | 6.373 | 0.67 | DL-2,3-butanediol | glycol | 773 | ||
| 4 | 6.510 | 0.11 | 2,3-butanediol | glycol | 788 | ||
| 5 | 7.799 | 0.12 | 2,4-dimethyl-heptane | hydrocarbon | 821 | ||
| 6 | 9.508 | 0.53 | 1-hexanol | alcohol | 868 | ||
| 7 | 10.718 | 0.16 | heptanal | saturated fatty aldehyde | 901 | ||
| 8 | 11.231 | 0.32 |
5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 |
||
| 9 | 11.608 | 0.09 | α-thujene | bicyclic monoterpene | 929 | ||
| 10 | 11.808 | 4.55 | 15.82 | 0.93 | α-pinene | bicyclic monoterpene | 937 |
| 11 | 12.33 | 1.39 | 4.88 | 0.98 | camphene | bicyclic monoterpene | 953 |
| 12 | 12.811 | 0.05 | benzaldehyde | aromatic aldehyde | 962 | ||
| 13 | 13.372 | 7.74 | 25.48 | 1.24 | β-pinene | bicyclic monoterpene | 979 |
| 14 | 13.901 | 0.75 | 2,2,4,6,6-pentamethylheptane | hydrocarbon | 991 | ||
| 15 | 14.013 | 10.24 | 37.32 | 8.73 | β-myrcene | acyclic monoterpene | 991 |
| 16 | 14.358 | 0.12 | ethyl hexanoate | fatty acid ester | 1000 | ||
| 17 | 14.839 | 0.16 | 0.52 | α-terpinene | monocyclic monoterpene | 1017 | |
| 18 | 14.983 | 0.05 | p-menth-1-ene | monocyclic monoterpene | 1025 | ||
| 19 | 15.111 | 0.07 | 0.74 | p-cymene | monocyclic monoterpene | 1025 | |
| 20 | 15.272 | 36.55 | 100.00 | 23.48 | limonene | monocyclic monoterpene | 1030 |
| 21 | 15.336 | 0.31 | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 22 | 15.624 | 0.20 | 0.13 | cis-β-ocimene | acyclic monoterpene | 1038 | |
| 23 | 16.017 | 0.65 | trans-β-ocimene | acyclic monoterpene | 1049 | ||
| 24 | 16.282 | 0.11 | 0.29 | γ-terpinene | monocyclic monoterpene | 1060 | |
| 25 | 16.546 | 0.21 | cis-sabinene hydrate | bicyclic monoterpenoid | 1070 | ||
| 26 | 16.739 | 0.13 | linalool oxide | monocyclic monoterpenoid | 1086 | ||
| 27 | 17.283 | 1.69 | terpinolene | monocyclic monoterpene | 1088 | ||
| 28 | 17.644 | 7.96 | 11.36 | 11.42 | linalool | acyclic monoterpenoid | 1099 |
| 29 | 17.730 | 1.53 | undecane | hydrocarbon | 1100 | ||
| 30 | 17.789 | 0.06 | nonanal | saturated fatty aldehyde | 1104 | ||
| 31 | 18.037 | 7.21 | 7.27 | 6.74 | fenchol | bicyclic monoterpenoid | 1113 |
| 32 | 18.286 | 5.65 | 4.61 | 1.64 | cis-pinene hydrate | bicyclic monoterpenoid | 1121 |
| 33 | 18.438 | 0.19 | 0.25 | methyl octanoate | fatty acid ester | 1126 | |
| 34 | 18.590 | 0.49 | neo-allo-ocimene | acyclic monoterpene | 1131 | ||
| 35 | 18.919 | 0.67 | 0.53 | trans-pinene hydrate | bicyclic monoterpenoid | 1140 | |
| 36 | 19.015 | 0.07 | camphor | bicyclic monoterpenoid | 1145 | ||
| 37 | 19.119 | 0.22 | 0.20 | camphene hydrate | bicyclic monoterpenoid | 1148 | |
| 38 | 19.408 | 0.03 | isoborneol | bicyclic monoterpenoid | 1157 | ||
| 39 | 19.665 | 1.42 | 0.94 | 1.69 | borneol | bicyclic monoterpenoid | 1166 |
| 40 | 20.025 | 0.11 | 0.32 | terpinen-4-ol | monocyclic monoterpenoid | 1177 | |
| 41 | 20.418 | 5.93 | 3.01 | 7.99 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 42 | 20.635 | 0.89 | 0.79 | 1.63 | ethyl octanoate | fatty acid ester | 1196 |
| 43 | 23.192 | 0.12 | 0.32 | bornyl acetate | bicyclic monoterpenoid | 1286 | |
| 44 | 23.801 | 0.07 | 0.33 | (E)-4-decenoic acid methyl ester | fatty acid ester | 1299 | |
| 45 | 24.202 | 0.04 | 0.32 | methyl decanoate | fatty acid ester | 1325 | |
| 46 | 24.939 | 0.10 | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 47 | 25.525 | 0.15 | 0.79 | ylangene | tricyclic sesquiterpene | 1372 | |
| 48 | 25.685 | 0.33 | 1.60 | ethyl trans-4-decenoate | fatty acid ester | 1375 | |
| 49 | 25.837 | 0.28 | 1.20 | hexyl hexanoate | fatty acid ester | 1384 | |
| 50 | 25.990 | 0.17 | 0.43 | 7-epi-sesquithujene | bicyclic sesquiterpene | 1391 | |
| 51 | 26.086 | 0.30 | 1.27 | ethyl decanoate | fatty acid ester | 1396 | |
| 52 | 26.246 | 0.07 | tetradecane | hydrocarbon | 1400 | ||
| 53 | 26.302 | 0.15 | cyperene | tricyclic sesquiterpene | 1399 | ||
| 54 | 26.390 | 0.79 | 0.26 | sesquithujene | bicyclic sesquiterpene | 1402 | |
| 55 | 26.463 | 0.17 | 2.37 | cis-caryophyllene | bicyclic sesquiterpene | 1406 | |
| 56 | 26.647 | 1.33 | 0.97 | cis-α-bergamotene | bicyclic sesquiterpene | 1415 | |
| 57 | 26.807 | 100.00 | 48.85 | 100.00 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 58 | 27.047 | 2.19 | 10,10-dimethyl-2,6-dimethylenebicyclo[7.2.0]undecane | bicyclic sesquiterpene | 1440 | ||
| 59 | 27.16 | 10.57 | 5.28 | 14.84 | α-bergamotene | bicyclic sesquiterpene | 1435 |
| 60 | 27.248 | 0.62 | 0.26 | 1.15 | α-guaiene | bicyclic sesquiterpene | 1439 |
| 61 | 27.376 | 0.18 | guaia-6,9-diene | bicyclic sesquiterpene | 1444 | ||
| 62 | 27.465 | 0.06 | epi-β-santalene | bicyclic sesquiterpene | 1448 | ||
| 63 | 27.480 | 0.30 | α-himachalene | bicyclic sesquiterpene | |||
| 64 | 27.649 | 42.09 | 17.38 | 55.63 | α-humulene | monocyclic sesquiterpene | 1454 |
| 65 | 27.809 | 0.24 | β-santalene | bicyclic sesquiterpene | 1462 | ||
| 66 | 28.338 | 2.08 | α-curcumene | aromatic sesquiterpene | 1483 | ||
| 67 | 28.370 | 2.17 | 0.85 | selina-4,11-diene | bicyclic sesquiterpene | 1474 | |
| 68 | 28.402 | 4.05 | 4a,8-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 |
||
| 69 | 28.443 | 2.29 | 0.67 | 4.04 | β-selinene | bicyclic sesquiterpene | 1486 |
| 70 | 28.587 | 0.84 | 4.19 | valencene | bicyclic sesquiterpene | 1492 | |
| 71 | 28.627 | 1.93 | 0.67 | 3.90 | α-selinene | bicyclic sesquiterpene | 1494 |
| 72 | 28.747 | 0.09 | 0.36 | β-dihydroagarofuran | tricyclic sesquiterpenoid | 1496 | |
| 73 | 28.819 | 7.14 | 2.28 | 9.18 | α-farnesene | acyclic sesquiterpene | 1508 |
| 74 | 28.931 | 0.62 | β-curcumene | monocyclic sesquiterpene | 1514 | ||
| 75 | 28.972 | 0.11 | sesquicineole | bicyclic sesquiterpenoid | 1516 | ||
| 76 | 29.140 | 3.00 | 0.88 | 4.59 | β-sesquiphellandrene | monocyclic sesquiterpene | 1524 |
| 77 | 29.389 | 4.11 | 0.36 | 3.48 | γ-selinene | bicyclic sesquiterpene | 1538 |
| 78 | 29.493 | 3.88 | 0.83 | 5.12 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 79 | 29.757 | 0.29 | germacrene B | monocyclic sesquiterpene | 1557 | ||
| 80 | 29.781 | 0.94 | nerolidol | acyclic sesquiterpenoid | 1544 | ||
| 81 | 30.158 | 4.86 | 0.51 | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 82 | 30.334 | 16.49 | 0.46 | 1.11 | guaiol | bicyclic sesquiterpenoid | 1596 |
| 83 | 30.455 | 0.84 | 0.02 | 5-epi-7-epi-α-eudesmol | bicyclic sesquiterpenoid | 1616 | |
| 84 | 30.527 | 0.06 | humulene epoxide II | bicyclic sesquiterpenoid | 1606 | ||
| 85 | 30.663 | 22.81 | 0.55 | 1.91 | 10-epi-γ-eudesmol | bicyclic sesquiterpenoid | 1619 |
| 86 | 30.799 | 5.38 | 0.04 | γ-eudesmol | bicyclic sesquiterpenoid | 1631 | |
| 87 | 30.859 | 1.07 | agarospirol | bicyclic sesquiterpenoid | 1645 | ||
| 88 | 31.040 | 7.59 | 0.07 | β-eudesmol | bicyclic sesquiterpenoid | 1649 | |
| 89 | 31.072 | 13.73 | 0.11 | α-eudesmol | bicyclic sesquiterpenoid | 1653 | |
| 90 | 31.224 | 19.51 | 0.09 | 0.35 | bulnesol | bicyclic sesquiterpenoid | 1667 |
| 91 | 31.320 | 0.34 | cadalene | bicyclic aromatic hydrocarbon | 1674 | ||
| 92 | 31.575 | 1.62 | juniper camphor | bicyclic sesquiterpenoid | 1691 | ||
| 93 | 32.523 | 0.31 | α-phellandrene dimer | tricyclic terpene | 1801 | ||
| 94 | 33.733 | 0.13 | hexadecanoic acid | saturated fatty acid | 1968 |
| Peak | RT | % norm (Liq) | % norm HS |
% norm (SPME) |
Compound | Type | RI |
| 1 | 8.987 | 0.10 | 3-hexen-1-ol | 856 | |||
| 2 | 9.398 | 0.51 | 4-methyl-octane | hydrocarbon | 863 | ||
| 3 | 9.528 | 0.74 | p-xylene | aromatic hydrocarbon | 865 | ||
| 4 | 9.508 | 1.02 | 1.70 | 1.58 | 1-hexanol | organic alcohol | 868 |
| 5 | 11.239 | 0.21 |
5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 |
||
| 6 | 11.608 | 0.50 | 0.45 | α-thujene | bicyclic monoterpene | 929 | |
| 7 | 11.824 | 11.86 | 24.64 | 4.11 | α-pinene | bicyclic monoterpene | 937 |
| 8 | 12.345 | 3.43 | 7.13 | 0.93 | camphene | bicyclic monoterpene | 952 |
| 9 | 13.388 | 17.21 | 33.51 | 4.92 | β-pinene | bicyclic monoterpene | 979 |
| 10 | 14.045 | 41.18 | 76.91 | 70.38 | β-myrcene | acyclic monoterpene | 991 |
| 11 | 14.406 | 0.11 | α-phellandrene | monocyclic monoterpene | 1005 | ||
| 12 | 14.646 | 29.77 | Δ3-carene | bicyclic monoterpene | 1011 | ||
| 13 | 14.879 | 2.48 | α-terpinene | monocyclic monoterpene | 1017 | ||
| 14 | 14.999 | 0.10 | p-menth-1-ene | monocyclic monoterpene | 1025 | ||
| 15 | 15.119 | 0.13 | 38.91 | p-cymene | monocyclic monoterpene | 1025 | |
| 16 | 15.288 | 63.52 | 100.00 | 100.00 | limonene | monocyclic monoterpene | 1030 |
| 17 | 15.344 | 0.75 | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 18 | 15.632 | 0.20 | cis-β-ocimene | acyclic monoterpene | 1038 | ||
| 19 | 15.977 | 0.10 | 0.79 | trans-β-ocimene | acyclic monoterpene | 1049 | |
| 20 | 16.290 | 0.25 | 1.92 | γ-terpinene | monocyclic monoterpene | 1060 | |
| 21 | 16.554 | 0.21 | cis-sabinene hydrate | bicyclic monoterpenoid | 1070 | ||
| 22 | 16.322 | 0.42 | p-cresol | phenol derivative | 1077 | ||
| 23 | 16.875 | 5.67 | m-cymenene | aromatic compound | 1082 | ||
| 24 | 17.252 | 1.68 | terpinolene | monocyclic monoterpene | 1088 | ||
| 25 | 17.308 | 8.92 | p-cymenene | aromatic compound | 1090 | ||
| 26 | 17.652 | 18.06 | 14.53 | 11.77 | linalool | acyclic monoterpenoid | 1099 |
| 27 | 17.730 | 1.46 | undecane | hydrocarbon | 1100 | ||
| 28 | 18.045 | 11.22 | 7.01 | 8.98 | fenchol | bicyclic monoterpenoid | 1113 |
| 29 | 18.286 | 6.74 | 3.40 | trans-pinene hydrate | bicyclic monoterpenoid | 1132 | |
| 30 | 19.127 | 0.40 | 0.22 | camphene hydrate | bicyclic monoterpenoid | 1148 | |
| 31 | 18.871 | 0.34 | 5-methyl-undecane | hydrocarbon | 1156 | ||
| 32 | 19.296 | 0.17 | 2,3-dimethyldecane | hydrocarbon | 1157 | ||
| 33 | 19.673 | 3.14 | 1.34 | 2.52 | borneol | bicyclic monoterpenoid | 1166 |
| 34 | 19.817 | 0.25 | 0.70 | 3-methyl-undecane | hydrocarbon | 1170 | |
| 35 | 20.025 | 0.15 | 2.48 | terpinen-4-ol | monocyclic monoterpenoid | 1177 | |
| 36 | 20.178 | 1.34 | p-cymene-8-ol | monocyclic monoterpenoid | 1183 | ||
| 37 | 20.426 | 8.93 | 2.37 | 4.41 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 38 | 20.627 | 0.34 | myrtenal | bicyclic monoterpenoid | 1193 | ||
| 39 | 20.705 | 0.56 | dodecane | hydrocarbon | 1200 | ||
| 40 | 23.192 | 0.10 | bornyl acetate | bicyclic monoterpenoid | 1286 | ||
| 41 | 23.585 | 1.08 | carvacrol | monoterpenoid phenol | 1299 | ||
| 42 | 24.931 | 0.10 | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 43 | 25.525 | 0.57 | 0.36 | 2.34 | ylangene | tricyclic sesquiterpene | 1372 |
| 44 | 25.637 | 0.15 | 0.71 | copaene | tricyclic sesquiterpene | 1376 | |
| 45 | 25.845 | 0.08 | hexyl hexanoate | fatty acid ester | 1384 | ||
| 46 | 25.893 | 0.22 | α-bourbonene | tricyclic sesquiterpene | 1384 | ||
| 47 | 26.182 | 0.42 | tetradecane | hydrocarbon | 1400 | ||
| 48 | 26.471 | 0.69 | cis-β-caryophyllene | bicyclic sesquiterpene | 1406 | ||
| 49 | 26.791 | 50.61 | 19.34 | 54.32 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 50 | 27.121 | 14.06 | 1.55 | γ-elemene | monocyclic sesquiterpene | 1434 | |
| 51 | 27.160 | 3.24 | 1.49 | 6.27 | α-bergamotene | bicyclic sesquiterpene | 1435 |
| 52 | 27.248 | 0.10 | 0.49 | α-guaiene | bicyclic sesquiterpene | 1439 | |
| 53 | 27.376 | 0.13 | 0.88 | guaia-6,9-diene | bicyclic sesquiterpene | 1444 | |
| 54 | 27.545 | 1.70 | humulen-(v1) | bicyclic sesquiterpene | 1455 | ||
| 55 | 27.649 | 17.43 | 5.37 | 22.06 | α-humulene | monocyclic sesquiterpene | 1454 |
| 56 | 28.202 | 2.60 | 0.79 | 4.01 | γ-muurolene | bicyclic sesquiterpene | 1477 |
| 57 | 28.290 | 0.17 | 1.77 | α-amorphene | bicyclic sesquiterpene | 1482 | |
| 58 | 28.386 | 2.92 |
4a,8-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 |
||
| 59 | 28.378 | 1.49 | 0.52 | selina-4,11-diene | bicyclic sesquiterpene | 1474 | |
| 60 | 28.443 | 5.84 | 1.34 | 9.13 | β-selinene | bicyclic sesquiterpene | 1486 |
| 61 | 28.531 | 0.22 | δ-selinene | bicyclic sesquiterpene | 1495 | ||
| 62 | 28.627 | 8.87 | 2.26 | 13.55 | α-selinene | bicyclic sesquiterpene | 1494 |
| 63 | 28.749 | 0.87 | β-dihydroagarofuran | tricyclic sesquiterpenoid | 1496 | ||
| 64 | 28.819 | 7.49 | 1.74 | 6.98 | α-farnesene | acyclic sesquiterpene | 1508 |
| 65 | 28.996 | 0.73 | 0.19 | 1.31 | γ-cadinene | bicyclic sesquiterpene | 1513 |
| 66 | 29.132 | 18.11 | δ-amorphene | bicyclic sesquiterpene | 1497 | ||
| 67 | 29.388 | 73.83 | 11.61 | 63.98 | γ-selinene | bicyclic sesquiterpene | 1544 |
| 68 | 29.469 | 9.56 | α-bisabolene | monocyclic sesquiterpene | 1540 | ||
| 69 | 29.501 | 100.00 | 15.20 | 84.02 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 70 | 29.757 | 12.81 | 3.04 | germacrene B | monocyclic sesquiterpene | 1557 | |
| 71 | 30.158 | 2.54 | 0.12 | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 72 | 30.334 | 22.79 | 0.36 | guaiol | bicyclic sesquiterpenoid | 1596 | |
| 73 | 30.450 | 1.30 | 5-epi-7-epi-α-eudesmol | bicyclic sesquiterpenoid | 1616 | ||
| 74 | 30.663 | 27.80 | 0.38 | 10-epi-γ-eudesmol | bicyclic sesquiterpenoid | 1619 | |
| 75 | 30.799 | 9.26 | 0.06 | γ-eudesmol | bicyclic sesquiterpenoid | 1631 | |
| 76 | 30.859 | 1.46 | agarospirol | bicyclic sesquiterpenoid | 1645 | ||
| 77 | 31.045 | 10.68 | 0.10 | β-eudesmol | bicyclic sesquiterpenoid | 1649 | |
| 78 | 31.072 | 23.23 | 0.14 | α-eudesmol | bicyclic sesquiterpenoid | 1653 | |
| 79 | 31.224 | 26.57 | 0.17 | bulnesol | bicyclic sesquiterpenoid | 1667 | |
| 80 | 31.312 | 1.17 |
cadalene |
bicyclic aromatic hydrocarbon | 1674 |
||
| 81 | 31.377 | 14.80 | 0.08 | α-bisabolol | monocyclic sesquiterpenoid | 1684 | |
| 82 | 31.575 | 7.52 | juniper camphor | bicyclic sesquiterpenoid | 1692 | ||
| 83 | 32.523 | 0.36 | α-phellandrene dimer | tricyclic terpene | 1801 | ||
| 84 | 32.719 | 1.67 | selina-4,7-diol | bicyclic sesquiterpenoid | 1826 |
| Peak | RT | % norm (Liq) | % norm (HS) |
% norm (SPME) |
Compound | Type | RI |
| 1 | 9.398 | 0.36 | 4-methyl-octane | hydrocarbon | 863 | ||
| 2 | 9.515 | 0.98 | 1-hexanol | organic alcohol | 868 | ||
| 3 | 0.51 | o-xylene | aromatic hydrocarbon | 887 | |||
| 4 | 10.341 | 0.10 | 2-heptanone | ketone | 891 | ||
| 5 | 10.734 | 0.20 | heptanal | alkyl aldehyde | 901 | ||
| 6 | 11.239 | 1.29 |
5,5-Dimethyl-1-vinylbicyclo[2.1.1]hexane | bicyclic monoterpene | 921 |
||
| 7 | 11.600 | 1.58 |
0.52 | 3-methyl-2-butenoic acid ethyl ester | fatty acid ester | 924 |
|
| 8 | 11.824 | 3.89 | 19.50 | 0.81 | α-pinene | bicyclic monoterpene | 937 |
| 9 | 12.345 | 1.19 | 5.88 | 0.99 | camphene | bicyclic monoterpene | 952 |
| 10 | 12.818 | 0.10 | benzaldehyde | aromatic aldehyde | 962 | ||
| 11 | 13.379 | 5.53 | 26.49 | β-pinene | bicyclic monoterpene | 979 | |
| 12 | 13.941 | 0.23 | 6-methyl-5-heptene-2-one | unsaturated methylated ketone | 986 | ||
| 13 | 13.917 | 0.73 | 2,2,4,6,6-pentamethylheptane | hydrocarbon | 991 | ||
| 14 | 14.037 | 20.17 | 87.07 | 32.79 | β-myrcene | acyclic monoterpene | 991 |
| 15 | 14.398 | 0.10 | α-phellandrene | monocyclic monoterpene | 1005 | ||
| 16 | 14.846 | 0.22 | 0.29 | α-terpinene | monocyclic monoterpene | 1017 | |
| 17 | 14.999 | 0.07 | p-menth-1-ene | monocyclic monoterpene | 1025 | ||
| 18 | 15.039 | 0.39 | isomyrcenol | acyclic monoterpenoid | 1022 | ||
| 19 | 15.119 | 0.16 | 0.32 | p-cymene | monocyclic monoterpene | 1025 | |
| 20 | 15.271 | 26.80 | 100.00 | 32.54 | limonene | monocyclic monoterpene | 1030 |
| 21 | 15.343 | 0.53 | 1,8-cineole | bicyclic monoterpenoid | 1032 | ||
| 22 | 15.632 | 0.22 | cis-β-ocimene | acyclic monoterpene | 1038 | ||
| 23 | 16.025 | 0.58 | trans-β-ocimene | acyclic monoterpene | 1049 | ||
| 24 | 16.289 | 0.22 | 0.26 | γ-terpinene | monocyclic monoterpene | 1060 | |
| 25 | 16.562 | 0.16 | cis-sabinene hydrate | bicyclic monoterpenoid | 1070 | ||
| 26 | 17.291 | 1.41 | terpinolene | monocyclic monoterpene | 1088 | ||
| 27 | 17.644 | 7.85 | 14.47 | 10.37 | linalool | acyclic monoterpenoid | 1099 |
| 28 | 17.813 | 0.19 | nonanal | aldehyde | 1104 | ||
| 29 | 18.045 | 3.93 | 5.52 | 3.88 | fenchol | bicyclic monoterpenoid | 1113 |
| 30 | 18.294 | 2.38 | 2.79 | 0.82 | trans-pinene hydrate | bicyclic monoterpenoid | 1140 |
| 31 | 18.598 | 0.53 | allo-ocimene | acyclic monoterpene | 1144 | ||
| 32 | 18.927 | 0.26 | cis-pinene hydrate | bicyclic monoterpenoid | 1121 | ||
| 33 | 19.135 | 0.22 | camphene hydrate | bicyclic monoterpenoid | 1148 | ||
| 34 | 19.672 | 1.34 | 1.07 | 1.08 | borneol | bicyclic monoterpenoid | 1166 |
| 35 | 20.033 | 0.10 | 0.56 | terpinen-4-ol | monocyclic monoterpenoid | 1177 | |
| 36 | 20.426 | 3.63 | 2.13 | 4.27 | α-terpineol | monocyclic monoterpenoid | 1189 |
| 37 | 21.147 | 0.21 | 2,4-dimethyl-benzaldehyde | aromatic aldehyde | 1181 | ||
| 38 | 24.931 | 0.10 | α-cubebene | tricyclic sesquiterpene | 1351 | ||
| 39 | 15.196 | 0.54 | clovene | tricyclic sesquiterpene | 1440 | ||
| 40 | 25.524 | 0.17 | 0.81 | ylangene | tricyclic sesquiterpene | 1372 | |
| 41 | 25.645 | 0.18 | 0.45 | copaene | tricyclic sesquiterpene | 1376 | |
| 42 | 25.989 | 0.22 | 0.36 | 7-epi-sesquithujene | bicyclic sesquiterpene | 1391 | |
| 43 | 26.390 | 0.35 | sesquithujene | bicyclic sesquiterpene | 1402 | ||
| 44 | 26.462 | 0.25 | 2.04 | cis-caryophyllene | bicyclic sesquiterpene | 1406 | |
| 45 | 26.647 | 1.01 | 1.05 | cis-α-bergamotene | bicyclic sesquiterpene | 1415 | |
| 46 | 26.807 | 100.00 | 68.80 | 100.00 | β-caryophyllene | bicyclic sesquiterpene | 1419 |
| 47 | 27.023 | 0.08 | β-copaene | tricyclic sesquiterpene | 1432 | ||
| 48 | 27.168 | 13.59 | 7.26 | 20.70 | trans-α-bergamotene | bicyclic sesquiterpene | 1435 |
| 49 | 27.256 | 17.05 | 12.19 | 25.39 | α-guaiene | bicyclic sesquiterpene | 1439 |
| 50 | 27.464 | 0.08 | epi-β-santalene | bicyclic sesquiterpene | 1448 | ||
| 51 | 27.585 | 2.17 | humulene-(v1) | bicyclic sesquiterpene | 1455 | ||
| 52 | 27.657 | 45.78 | 24.62 | 63.07 | α-humulene | monocyclic sesquiterpene | 1454 |
| 53 | 28.042 | 0.11 | aristolochene | bicyclic sesquiterpene | 1476 | ||
| 54 | 28.050 | 0.62 | drima-7,9-diene | bicyclic sesquiterpene | 1461 | ||
| 55 | 28.330 | 3.10 | α-curcumene | monocyclic sesquiterpene | 1483 | ||
| 56 | 28.378 | 1.15 | 4.70 | 4a,8-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | bicyclic sesquiterpene | 1492 | |
| 57 | 28.442 | 7.71 | 2.95 | 16.02 | β-selinene | bicyclic sesquiterpene | 1486 |
| 58 | 28.627 | 9.44 | 3.48 | 20.62 | α-selinene | bicyclic sesquiterpene | 1494 |
| 59 | 28.835 | 42.10 | 14.65 | 45.64 | α-bulnesene | bicyclic sesquiterpene | 1505 |
| 60 | 29.012 | 1.09 | γ-cadinene | bicyclic sesquiterpene | 1513 | ||
| 61 | 29.380 | 25.36 | 6.27 | 27.14 | γ-selinene | bicyclic sesquiterpene | 1538 |
| 62 | 29.468 | 5.77 | α-bisabolene | monocyclic sesquiterpene | 1540 | ||
| 63 | 29.493 | 33.77 | 8.89 | 37.76 | selina-3,7(11)-diene | bicyclic sesquiterpene | 1542 |
| 64 | 29.757 | 5.04 | 1.32 | germacrene B | monocyclic sesquiterpene | 1557 | |
| 65 | 30.158 | 4.70 | 0.31 | caryophyllene oxide | bicyclic sesquiterpenoid | 1581 | |
| 66 | 30.342 | 28.03 | 0.82 | 0.41 | guaiol | bicyclic sesquiterpenoid | 1596 |
| 67 | 30.438 | 0.09 | β-atlantol |
monocyclic sesquiterpenoid | 1607 |
||
| 68 | 30.454 | 2.11 | 0.04 | 5-epi-7-epi-α-eudesmol | bicyclic sesquiterpenoid | 1616 | |
| 69 | 30.663 | 30.65 | 0.83 | 1.04 | 10-epi-γ-eudesmol | bicyclic sesquiterpenoid | 1619 |
| 70 | 30.799 | 11.05 | 0.22 | γ-eudesmol | bicyclic sesquiterpenoid | 1631 | |
| 71 | 30.859 | 2.03 | agarospirol | bicyclic sesquiterpenoid | 1645 | ||
| 72 | 31.040 | 15.65 | 0.24 | β-eudesmol | bicyclic sesquiterpenoid | 1649 | |
| 73 | 31.072 | 29.06 | 0.45 | α-eudesmol | bicyclic sesquiterpenoid | 1653 | |
| 74 | 31.232 | 29.44 | 0.42 | 0.14 | bulnesol | bicyclic sesquiterpenoid | 1667 |
| 75 | 31.312 | 0.30 | cadalene | bicyclic aromatic hydrocarbon | 1674 | ||
| 76 | 31.380 | 3.67 | α-bisabolol | monocyclic sesquiterpenoid | 1684 | ||
| 77 | 31.575 | 3.29 | juniper camphor | bicyclic sesquiterpenoid | 1691 | ||
| 88.10% | 97.17% | 88.85% | |||||
| 34 cpd | 57 cpd | 47 cpd |
| GC/MS | LOH LL1 | LOH LL2 | LOH LL3 | LOH LL4 |
| Liquid | 37 | 38 | 45 | 34 |
| HS | 51 | 74 | 55 | 57 |
| SPME | 46 | 57 | 49 | 47 |
| all identified different compounds | 67 | 94 | 84 | 77 |
| Compound |
LOH LL1 µg/g |
LOH LL2 µg/g |
LOH LL3 µg/g |
LOH LL4 µg/g |
| α-pinene | 45.6 | 60.0 | 101.6 | 47.0 |
| camphene | 13.5 | 18.33 | 29.38 | 14.4 |
| β-pinene | 83.7 | 102.0 | 147.5 | 62.0 |
| β-myrcene | 2136.2 | 294.3 | 744.1 | 544.2 |
| limonene | 473.3 | 705.9 | 803.7 | 457.1 |
| linalool | 151.5 | 236.1 | 367.6 | 231.3 |
| β-caryophyllene | 1322.1 | 1704.0 | 542.1 | 1599.4 |
| α-humulene | 535.0 | 538.4 | 152.1 | 605.2 |
| caryophyllene oxide | 0.1 | 0.2 | 0.2 | 0.2 |
| guaiol | - | 313.7 | 288.8 | 522.3 |
| α-bisabolol | - | - | 170.0 | 64.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




