Submitted:
27 May 2024
Posted:
27 May 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. S. cerevisiae Strains Origin
2.2. Genotyping Characterization of S. cerevisiae Strains
2.3. Analyses of Biotypes for H2S Production and Killer Activity
2.4. Fermentation Trials
2.5. Main Analytical Characters and Volatile Compounds of Microfermentation Trials
2.6. Data Analyses
3. Results
3.1. Genotyping, Occurrence, H2S Production and Killer Activity of S. cerevisiae Strains
3.2. Oenologically characterization
3.2.1. Fermentation rate, volatile acidity and ethanol production of biotypes
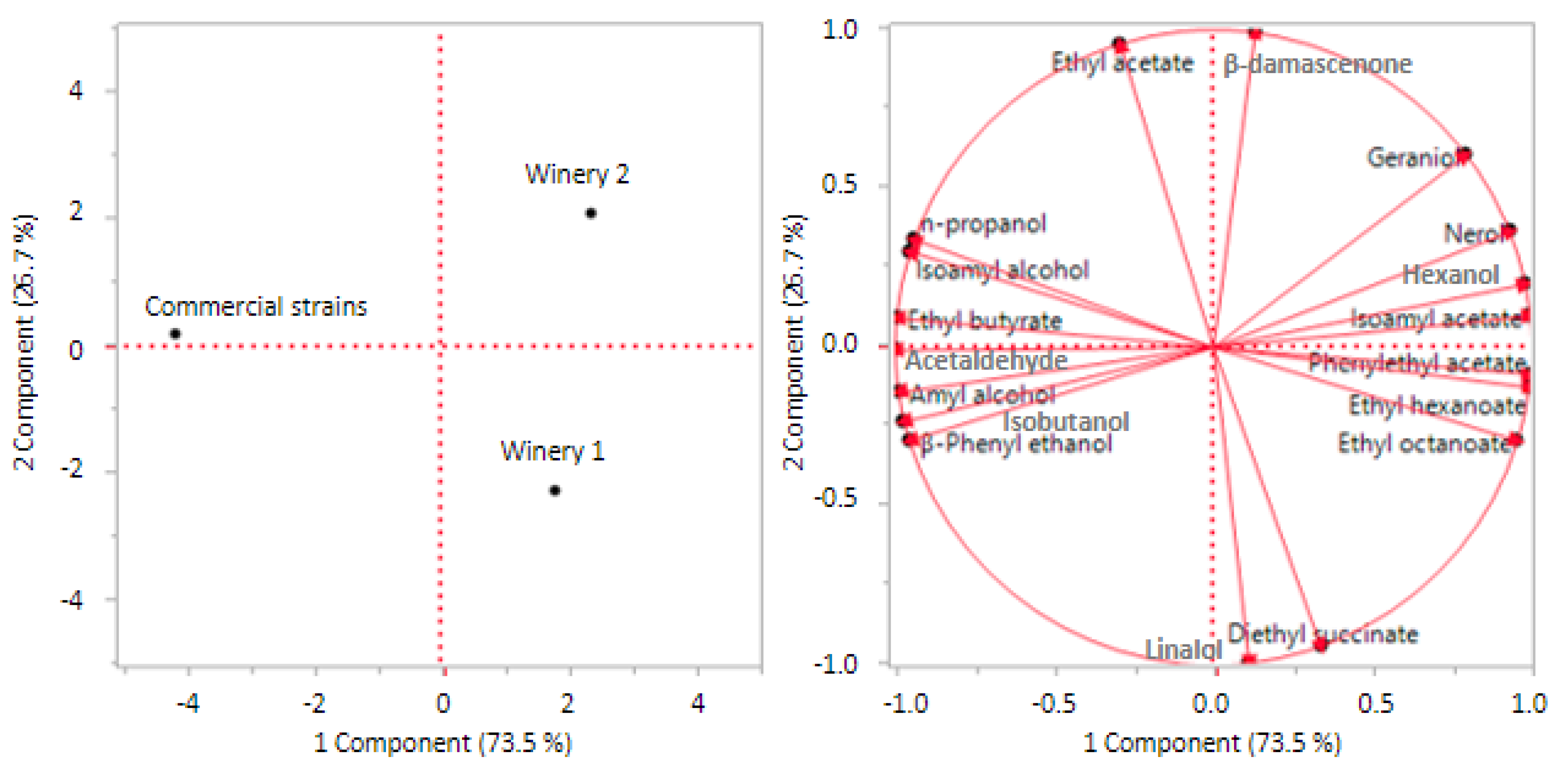
3.2.2. Main by-products of fermentation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Longo, E.; Cansado, J.; Agrelo, D.; Villa, T.G. Effect of Climatic Conditions on Yeast Diversity in Grape Musts from Northwest Spain. Am J Enol Vitic. 1991, 42, 141–144. [Google Scholar] [CrossRef]
- Capece, A.; Pietrafesa, R.; Siesto, G.; Romaniello, R.; Condelli, N.; Romano, P. Selected Indigenous Saccharomyces Cerevisiae Strains as Profitable Strategy to Preserve Typical Traits of Primitivo Wine. Fermentation 2019, 5, 87. [Google Scholar] [CrossRef]
- Santamaría, P.; López, R.; del Patrocinio Garijo, M.; Escribano, R.; González-Arenzana, L.; López-Alfaro, I., Rosa Gutiérrez, A. Biodiversity of Saccharomyces cerevisiae yeasts in spontaneous alcoholic fermentations: typical cellar or zone strains?. In Morata, A.; Loira, I. Advances in Grape and Wine Biotechnology; 2019 1-15.
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Diversity of Saccharomyces Cerevisiae Yeasts Associated to Spontaneously Fermenting Grapes from an Italian “Heroic Vine-Growing Area”. Food Microbiology 2012, 31, 159–166. [Google Scholar] [CrossRef]
- Pérez-Torrado, R.; Rantsiou, K.; Perrone, B.; Navarro-Tapia, E.; Querol, A.; Cocolin, L. Ecological Interactions among Saccharomyces cerevisiae Strains: Insight into the Dominance Phenomenon. Sci Rep 2017, 7, 43603. [Google Scholar] [CrossRef]
- Granchi, L.; Ganucci, D.; Buscioni, G.; Mangani, S.; Guerrini, S. The Biodiversity of Saccharomyces cerevisiae in Spontaneous Wine Fermentation: The Occurrence and Persistence of Winery-Strains. Fermentation 2019, 5, 86. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Comitini, F.; Ciani, M. Ecological Distribution and Oenological Characterization of Native Saccharomyces cerevisiae in an Organic Winery. Fermentation 2022, 8, 224. [Google Scholar] [CrossRef]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in Alcoholic Fermentation Processes: Role of Physiological Fitness and Microbial Interactions. Appl Microbiol Biotechnol 2016, 100, 2035–2046. [Google Scholar] [CrossRef]
- Ganucci, D.; Guerrini, S.; Mangani, S.; Vincenzini, M.; Granchi, L. Quantifying the Effects of Ethanol and Temperature on the Fitness Advantage of Predominant Saccharomyces cerevisiae Strains Occurring in Spontaneous Wine Fermentations. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Börlin, M.; Venet, P.; Claisse, O.; Salin, F.; Legras, J.-L.; Masneuf-Pomarede, I. Cellar-Associated Saccharomyces Cerevisiae Population Structure Revealed High-Level Diversity and Perennial Persistence at Sauternes Wine Estates. Applied and Environmental Microbiology 2016, 82, 2909–2918. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous Yeasts: Emerging Trends and Challenges in Winemaking. Current Opinion in Food Science 2020, 32, 133–143. [Google Scholar] [CrossRef]
- Legras, J.-L.; Karst, F. Optimisation of Interdelta Analysis for Saccharomyces cerevisiae Strain Characterisation. FEMS Microbiology Letters 2003, 221, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, A.; Canonico, L.; Ciani, M.; Comitini, F. The Impact of Fungicide Treatments on Yeast Biota of Verdicchio and Montepulciano Grape Varieties. PLOS ONE 2019, 14, e0217385. [Google Scholar] [CrossRef] [PubMed]
- Agarbati, A.; Canonico, L.; Comitini, F.; Ciani, M. Reduction of Sulfur Compounds through Genetic Improvement of Native Saccharomyces cerevisiae Useful for Organic and Sulfite-Free Wine. Foods 2020, 9, 658. [Google Scholar] [CrossRef]
- Comitini, F.; Agarbati, A.; Canonico, L.; Galli, E.; Ciani, M. Purification and Characterization of WA18, a New Mycocin Produced by Wickerhamomyces anomalus Active in Wine Against Brettanomyces bruxellensis Spoilage Yeasts. Microorganisms 2021, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- OIV-Compendium of International Methods of Wine and Must Analysis MA-AS313-02. Available online: https://www.oiv.int/standards/annex-a-methods-of-analysis-of-wines-and-musts/section-3-chemical-analysis/section-3-1-organic-compounds/section-3-1-3-acids/volatile-acidity-%28type-i%29 (accessed on 15 May 2024).
- OIV-MA-AS313-24. Available online: https://www.oiv.int/standards/annex-a-methods-of-analysis-of-wines-and-musts/section-3-chemical-analysis/section-3-1-organic-compounds/section-3-1-3-acids/determination-of-total-ethanol-in-wine-by-high-performance-liquid-chromatography-%28type-iv%29 (accessed on 15 May 2024).
- Canonico, L.; Comitini, F.; Ciani, M. Influence of Vintage and Selected Starter on Torulaspora delbrueckii/Saccharomyces cerevisiae Sequential Fermentation. Eur Food Res Technol 2015, 241, 827–833. [Google Scholar] [CrossRef]
- Canonico, L.; Ciani, E.; Galli, E.; Comitini, F.; Ciani, M. Evolution of Aromatic Profile of Torulaspora delbrueckii Mixed Fermentation at Microbrewery Plant. Fermentation 2020, 6, 7. [Google Scholar] [CrossRef]
- Alexandre, H. Wine Yeast Terroir: Separating the Wheat from the Chaff—for an Open Debate. Microorganisms 2020, 8, 787. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.S. Tasting the Terroir of Wine Yeast Innovation. FEMS yeast research 2020, 20, foz084. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial Biogeography of Wine Grapes Is Conditioned by Cultivar, Vintage, and Climate. Proceedings of the National Academy of Sciences 2014, 111, E139–E148. [Google Scholar] [CrossRef]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional Microbial Signatures Positively Correlate with Differential Wine Phenotypes: Evidence for a Microbial Aspect to Terroir. Sci Rep 2015, 5, 14233. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. mBio 2016, 7. [Google Scholar] [CrossRef]
- Vigentini, I.; De Lorenzis, G.; Fabrizio, V.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Imazio, S.; Failla, O.; Foschino, R. The Vintage Effect Overcomes the Terroir Effect: A Three Year Survey on the Wine Yeast Biodiversity in Franciacorta and Oltrepò Pavese, Two Northern Italian Vine-Growing Areas. Microbiology 2015, 161, 362–373. [Google Scholar] [CrossRef]
- Torija, M.J.; Rozès, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Yeast Population Dynamics in Spontaneous Fermentations: Comparison between Two Different Wine-Producing Areas over a Period of Three Years. Antonie Van Leeuwenhoek 2001, 79, 345–352. [Google Scholar] [CrossRef]
- Rosini, G. Assessment of Dominance of Added Yeast in Wine Fermentation and Origin of Saccharomyces cerevisiae in Wine-Making. The Journal of General and Applied Microbiology 1984, 30, 249–256. [Google Scholar] [CrossRef]
- Cocolin, L.; Pepe, V.; Comitini, F.; Comi, G.; Ciani, M. Enological and Genetic Traits of Saccharomyces Cerevisiae Isolated from Former and Modern Wineries. FEMS Yeast Research 2004, 5, 237–245. [Google Scholar] [CrossRef]
- Abdo, H.; Catacchio, C.R.; Ventura, M.; D’Addabbo, P.; Calabrese, F.M.; Laurent, J.; David-Vaizant, V.; Alexandre, H.; Guilloux-Bénatier, M.; Rousseaux, S. Colonization of Wild Saccharomyces Cerevisiae Strains in a New Winery. Beverages 2020, 6, 9. [Google Scholar] [CrossRef]
- Vezinhet, F.; Hallet, J.-N.; Valade, M.; Poulard, A. Ecological Survey of Wine Yeast Strains by Molecular Methods of Identification. Am J Enol Vitic. 1992, 43, 83–86. [Google Scholar] [CrossRef]
- Gutiérrez, A.R.; Santamaría, P.; Epifanio, S.; Garijo, P.; Ópez, R.L. Ecology of Spontaneous Fermentation in One Winery during 5 Consecutive Years. Letters in Applied Microbiology 1999, 29, 411–415. [Google Scholar] [CrossRef]
- Stefanini, I.; Albanese, D.; Cavazza, A.; Franciosi, E.; De Filippo, C.; Donati, C.; Cavalieri, D. Dynamic Changes in Microbiota and Mycobiota during Spontaneous ‘Vino Santo Trentino’ Fermentation. Microbial Biotechnology 2016, 9, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Jouhten, P.; Ponomarova, O.; Gonzalez, R.; Patil, K.R. Saccharomyces Cerevisiae Metabolism in Ecological Context. FEMS Yeast Research 2016, 16, fow080. [Google Scholar] [CrossRef] [PubMed]
- Conacher, C.; Luyt, N.; Naidoo-Blassoples, R.; Rossouw, D.; Setati, M.; Bauer, F. The Ecology of Wine Fermentation: A Model for the Study of Complex Microbial Ecosystems. Appl Microbiol Biotechnol 2021, 105, 3027–3043. [Google Scholar] [CrossRef] [PubMed]
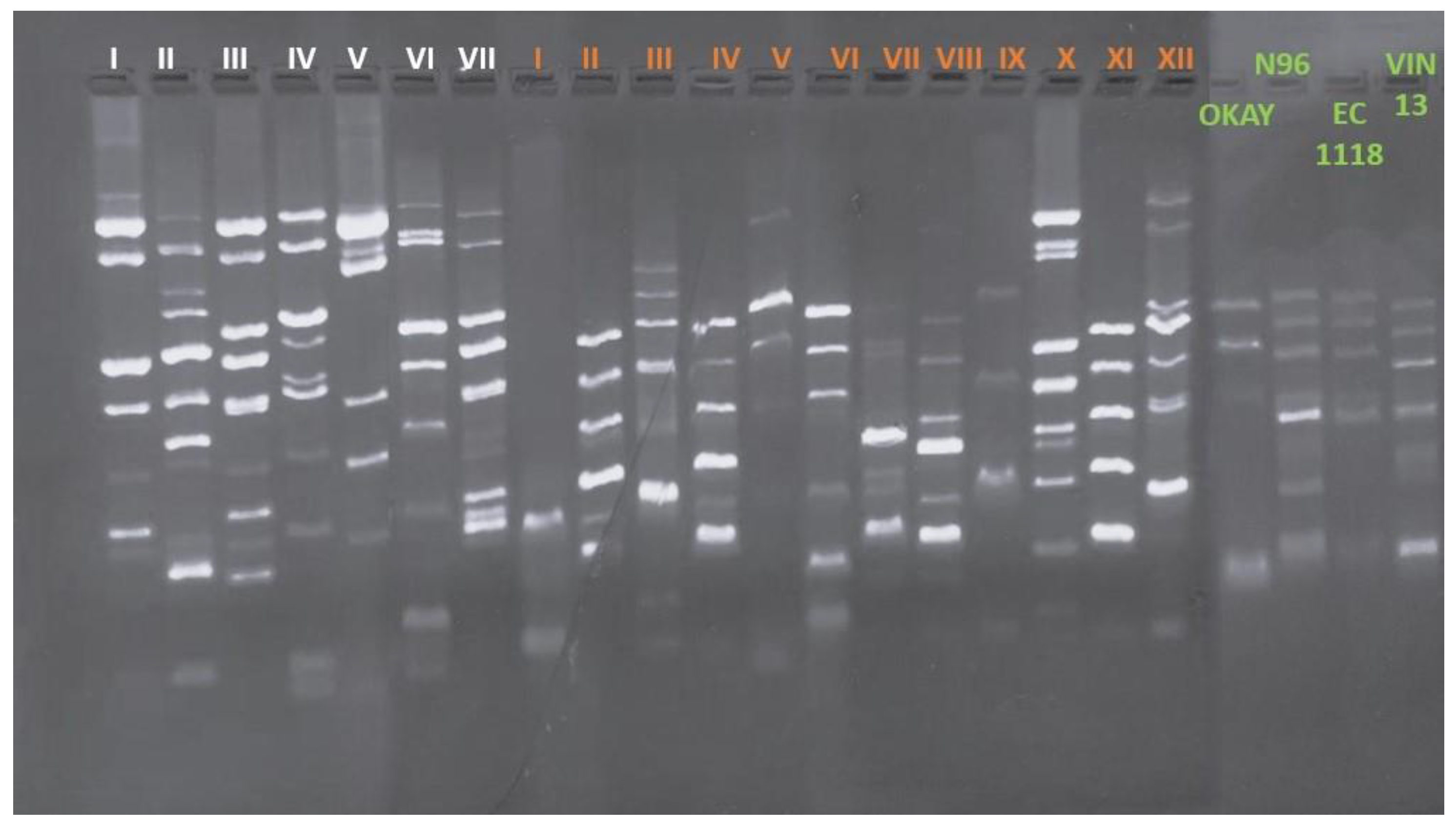
| Winery | Biotype | Frequency (%) | H2S production | Killer activity |
|---|---|---|---|---|
| 1 | I* | 49.5 | 2.0 | +/− |
| II* | 43.4 | 0.0 | + | |
| III | 1.0 | 1.5 | + | |
| IV* | 2.0 | 0.0 | + | |
| V | 1.0 | 0.5 | + | |
| VI | 1.0 | 0.5 | +/− | |
| VII | 2.0 | 0.8 | + | |
| 2 | I | 23.4 | 0.5 | + |
| II | 15.0 | 0.0 | − | |
| III | 16.8 | 0.0 | + | |
| IV* | 19.6 | 1.0 | + | |
| V | 2.8 | 0.5 | +/− | |
| VI | 7.5 | 0.5 | + | |
| VII | 1.9 | 1.5 | +/− | |
| VIII | 1.9 | 0.8 | +/− | |
| IX | 3.7 | 0.0 | +/− | |
| X | 0.9 | 1.0 | + | |
| XI | 5.6 | 1.0 | + | |
| XII | 0.9 | 1.0 | +/− |
| Strains population | Fermentation rate (gCO2/day)* | Volatile acidity (g/L) | Ethanol (% v/v) |
|---|---|---|---|
| Winery 1 | 1.4±0.2a | 0.4±0.1a | 12.8±0.3a |
| Winery 2 | 1.2±0.1ab | 0.5±0.1a | 12.4±0.3a |
| OKAY® | 0.9±0.0c | 0.4±0.0a | 12.4±0.0a |
| N96 | 1.1±0.0bc | 0.5±0.0a | 12.4±0.1a |
| Fermentation by-products | Winery 1 | Winery 2 | OKAY® | N96 |
|---|---|---|---|---|
| Alcohols | ||||
| Hexanol (mg/L) | 11.84±0.99a | 12.66±1.77a | 10.38±0.61ab | 9.13±0.74b |
| β-Phenyl ethanol (mg/L) | 7.48±1.12a | 7.39±1.11a | 7.96±0.03b | 7.32±0.00b |
| n-propanol (mg/L) | 12.60±8.26b | 16.88±2.96b | 42.70±0.09a | 13.51±0.42b |
| Amyl alcohol (mg/L) | 7.28±4.44b | 4.85±2.39b | 15.42±0.39a | 15.55±0.38a |
| Isoamyl alcohol (mg/L) | 36.96±10.7b | 41.60±12.07b | 52.01±0.17ab | 60.78±0.37a |
| Isobutanol (mg/L) | 5.22±3.94a | 8.24±1.78a | 5.29±0.03a | 7.89±0.04a |
| Carbonyl compounds | ||||
| Acetaldehyde (mg/L) | 15.59±9.85b | 12.21±2.06b | 15.05±0.47b | 80.97±0.23a |
| Esters | ||||
| Isoamyl acetate (mg/L) | 0.80±0.39ab | 0.90±0.20a | 0.39±0.00b | 0.35±0.01b |
| Phenyl ethyl acetate (mg/L) | 0.47±0.16a | 0.47±0.09a | 0.21±0.01b | 0.38±0.02ab |
| Ethyl hexanoate (mg/L) | 0.65±0.19a | 0.63±0.22a | 0.39±0.00ab | 0.16±0.01b |
| Ethyl butyrate (mg/L) | 0.03±0.03a | 0.03±0.05a | 0.06±0.00a | 0.05±0.01a |
| Ethyl octanoate (µg/L) | 3.75±1.04a | 3.60±1.13a | 3.45±0.34a | 2.78±0.05a |
| Diethyl succinate (mg/L) | 0.04±0.01b | 0.03±0.01b | 0.01±0.00c | 0.06±0.00a |
| Ethyl acetate (mg/L) | 10.16±3.34b | 11.61±4.61ab | 17.62±0.20a | 5.13±0.03b |
| Terpenes | ||||
| Linalol (µg/L) | 17.85±8.96a | 7.12±5.04a | 8.89±0.53a | 12.51±1.07a |
| Nerol (µg/L) | 3.41±1.48ab | 4.56±1.50a | 1.95±0.50b | 1.88±0.39b |
| Geraniol (µg/L) | 4.71±1.58a | 5.69±1.53a | 4.02±0.57a | 4.49±0.47a |
| Enones | ||||
| β-Damascenone (µg/L) | 4.56±2.05a | 4.15±1.41a | 5.89±0.34a | 4.98±0.28a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





