Submitted:
03 March 2024
Posted:
05 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. Replicability of Methods and Research Findings in Studies of Nest-Box Breeders
3.1. Replicability of Methodologies
3.1.1. Nest-Size Components
3.1.2. Animal-Derived Nest Material
3.1.3. Greenery
2.2. Replicability of Research Findings
3.2.1. Nest-Size Components
3.2.2. Animal-Derived Nest Material
3.2.3. Greenery
4. Concluding Remarks and Perspectives
Future Research Directions
Author Contributions
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hansell, M. Bird nests and construction behaviour. Cambridge University Press, 2000.
- Healy, S.; Walsh, P.; Hansell, M. Nest building by birds. Current Biology 2008, 18, R271–R273. [Google Scholar] [CrossRef]
- Schaedelin, F.C.; Taborsky, M. Extended phenotypes as signals. Biological Reviews 2009, 84, 293–313. [Google Scholar] [CrossRef]
- Møller, A.P.; Adriaensen, F.; Artemyev, A.V.; Bańbura, J.; Barba, E.; Biard, C.; Blondel, J.; Bouslama, Z.; Bouvier, J.-C.; Camprodon, J.; et al. Variation in nest size in relation to clutch size in birds. Ecol. Evol. 2014, 4, 3583–3595. [Google Scholar] [CrossRef] [PubMed]
- Deeming, D.C. A review of the roles materials play in determining functional properties of bird nests. Acta Ornithol. 2023, 58, 1–28. [Google Scholar] [CrossRef]
- Deeming, D. C. Nest construction in mammals: a review of the patterns of construction and functional roles. Phil. Trans. R. Soc. B 2023, 378, 20220138. [Google Scholar] [CrossRef] [PubMed]
- Svensson, O.; Kvarnemo, C. How sexual and natural selection interact and shape the evolution of nests and nesting behaviour in fishes. Phil. Trans R. Soc. B 2023, 378, 20220139. [Google Scholar] [CrossRef]
- Fischer, E.K. Form, function, foam: evolutionary ecology of anuran nests and nesting behaviour. Phil. Trans R. Soc. B 2023, 378, 20220141. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, S.; Drager, K.; Zhao, A.; Suarez, A.; Pinter-Wollman, N. Foraging behaviour affects nest architecture in a cross-species comparison of ant nests. Phil. Trans R. Soc. B 2023, 378, 20220146. [Google Scholar] [CrossRef]
- Bodensteiner, B.L.; Iverson, J.B.; Lea, C.A.; Milne-Zelman, C.L.; Michell, T.S.; Refsnider, J.H.; Voves, K.; Warner, D.A.; Janzen, F.J. Mother knows best: nest-site choice homogenises embryo thermal environments among populations in a widespread ectotherm. Phil. Trans R. Soc. B 2023, 378, 20220155. [Google Scholar]
- Dawkins, R. The Extended Phenotype. The Gene as the Unit of Selection. Oxford University Press, 1982.
- Laland, K.N.; Sterelny, K. Perspective: seven reasons (not) to neglect niche construction. Evolution 2006, 60, 1751–1762. [Google Scholar]
- Biddle, L.E.; Broughton, R.E.; Deeming, D.C.; Goodman, A.M. Composition of bird nests is a species-specific characteristic. Avian Biol. Res. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Dickinson, A.M.; Locke, E.; Gray, L.A.; Bennett, S.L.; Biddle, L.E.; Goodman, A.M.; Deeming, D.C. Composition of nests constructed by species in the Motacillidae, Sylviidae and Prunellidae. Avian Biol. Res. 2022, 15, 21–33. [Google Scholar] [CrossRef]
- Deeming, D.C.; Dickinson, A.M.; Broughton, R.E.; Locke, E.; Gray, L.A.; Bennett, S.L.; Gilchrist, R.; Muniz, S.; Goodman, A.M.; Biddle, L.E. Factors affecting thermal insulation of songbird nests as measured using temperature loggers. Physiol. Biochem. Zool. 2020, 93, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Deeming, D.C.; Gilchrist, R.; Szafraniec, M.; Pollins, J.M. Water vapour conductance of passerine nest walls. Acta Ornithol. 2020, 55, 13–21. [Google Scholar] [CrossRef]
- Biddle, L.E.; Dickinson, A.M.; Broughton, R.E.; Gray, L.A.; Bennett, S.L.; Goodman, A.M.; Deeming, D.C. Nest materials affect the hydrological properties of bird nests. J. Zool. 2019, 309, 161–171. [Google Scholar] [CrossRef]
- Mertens, J.A.L. Thermal conditions for successful breeding in Great Tits (Parus major L.). Oecologia 1977, 28, 1–56. [Google Scholar] [CrossRef]
- Moreno, J. Avian nests and nest-building as signals. Avian Biol. Res. 2012, 5, 238–251. [Google Scholar] [CrossRef]
- Mainwaring, M.C.; Hartley, I.R.; Lambrechts, M.M.; Deeming, D.C. The design and function of birds’ nests. Ecol. Evol. 2014, 4, 3909–3928. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Nest predation selects for small nest size in the blackbird. Oikos 1990, 57, 237–240. [Google Scholar] [CrossRef]
- Grégoire, A.; Garnier, S.; Dréano, N.; Faivre, B. Nest predation in blackbirds and the influence of nest characteristics. Ornis Fenn. 2003, 80, 1–10. [Google Scholar]
- Heeb, P.; Werner, I.; Richner, H.; Kölliker, M. Horizontal Transmission and Reproductive Rates of Hen Fleas in Great 432 Tit Nests. J. Anim. Ecol. 1996, 65, 474–484. [Google Scholar] [CrossRef]
- Mennerat, A.; Charmantier, A.; Perret, P.; Hurtrez-Boussès, S.; Lambrechts, M.M. Parasite intensity is driven by temperature in a wild bird. Peer Community Journal 2021, 1, e60. [Google Scholar] [CrossRef]
- Deeming, D.C.; Reynolds, S.J. Nests, Eggs, and Incubation: New Ideas about Avian Reproduction. Oxford University Press, Oxford, 2015.
- Perez, D.M.; Gardner, J.L.; Medina, I. Climate as an evolutionary driver of nest morphology in birds: A Review. Front Ecol. Evol. 2020, 8, 566018. [Google Scholar] [CrossRef]
- McCabe, R.A. Nest construction by house wrens. Condor 1965, 67, 229–234. [Google Scholar] [CrossRef]
- Petit, C.; Hossaert-McKey, M.; Perret, P.; Blondel, J.; Lambrechts, M.M. Blue tits use selected plants and olfaction to maintain an aromatic environment for nestlings. Ecol. Lett. 2002, 5, 585–589. [Google Scholar] [CrossRef]
- Mennerat, A.; Perret, P.; Lambrechts, M.M. Local individual preferences for nest materials in a passerine bird. PLoS ONE 2009, 4, e5104. [Google Scholar] [CrossRef]
- Harničárová, K.; Adamík, P. Mammal hair in nests of four cavity-nesting songbirds: occurrence, diversity and seasonality. Bird Study 2016, 63, 181–186. [Google Scholar] [CrossRef]
- Glądalski, M.; Wolski, G.J.; Bańbura, M.; Kaliński, A.; Markowski, M.; Skwarska, J.; Wawrzyniak, J.; Bańbura, J. Differences in use of bryophyte species in tit nests between two contrasting habitats: an urban park and a forest. Eur. Zoo. J. 2021, 88, 807–815. [Google Scholar] [CrossRef]
- Hauber, M.E.; Nagy, J.; Sheard, C.; Antonson, N.D.; Street, S.E.; Healy, S.D.; Lala, K.N.; Mainwaring, M.C. Nest architecture influences host use by avian brood parasites and is shaped by coevolutionary dynamics. Proc. R. Soc. Lond. Biol. Sci. 2024, 291, 20231734. [Google Scholar] [CrossRef]
- Mainwaring, M.C. Causes and consequences of intraspecific variation in nesting behaviors: insights from blue tits and great tits. Front Ecol. Evol. 2017, 5, 39. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H. (Ed.) Reproductive success. Studies of individual variation in contrasting breeding systems. The University of Chicago Press, 1988.
- Otter, K.A. (Ed.) Ecology and behavior of chickadees and titmice. An integrated approach. Oxford University Press, 2007.
- Clutton-Brock, T.; Sheldon, B.C. Individuals and populations: the role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 2010, 25, 562–573. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Adriaensen, F.; Ardia, D.R.; Artemyev, A.V.; Atienzar, F.; Banbura, J.; Barba, E.; Bouvier, J.-C.; Camprodon, J.; Cooper, C.B.; et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 2010, 45, 1–26. [Google Scholar] [CrossRef]
- Wesołowski, T. Nest-sites of hole-nesters in a primaeval temperate forest (Białowieża National Park, Poland). Acta Ornithol. 1989, 25, 321–351. [Google Scholar]
- Czeszczewik, D.; Walankiewicz, W.; Mitrus, C.; Nowakowski, W.K. Nest-box data of pied flycatcher Ficedula hypoleuca may lead to erroneous generalizations. Vogelwelt 1999, 120, 361–365. [Google Scholar]
- Maziarz, M.; Broughton, R.K.; Wesołowski, T. Microclimate in tree cavities and nest-boxes: Implications for hole-nesting birds. Forest Ecol. Manage. 2017, 389, 306–313. [Google Scholar] [CrossRef]
- Sudyka, J.; Di Lecce, I.; Szulkin, M. Microclimate shifts in nest-boxes and natural cavities throughout reproduction. J. Avian Biol. 2023, e03000. [Google Scholar] [CrossRef]
- Thompson, E.K.; Keenan, R.J.; Kelly, L.T. The use of nest boxes to support bird conservation in commercially managed forests: a systematic review. For. Ecol. Manage. 2023, 550, 121504. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Chen, Z. et al. Negative effects of artificial nest boxes on birds: a review. Avian Research 2023, 14, 100101. [CrossRef]
- Markus, J.M.; Hart, A.G.; Goodenough, A.E. Predator-proofing avian nest boxes: a review of interventions, opportunities, and challenges. Birds 2024, 5, 1–23. [Google Scholar] [CrossRef]
- Kelly, C.D. Rate and success of study replication in ecology and evolution. PeerJ 2019, 7, e7654. [Google Scholar] [CrossRef]
- Fraser, H.; Barnett, A.; Parker, T.H.; Fidler, F. The role of replication studies in ecology. Ecol. Evol. 2020, 10, 5197–5207. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Perret, P.; Maistre, M.; Blondel, J. Do experiments with captive non-domesticated animals make sense without population field studies? A case study with Blue Tits’ breeding time. Proc. R. Soc. Lond. B Biol. Sci. 1999, 266, 1311–1315. [Google Scholar] [CrossRef]
- Stroebe, W.; Strack, F. The alleged crisis and the illusion of exact replication. Pers. Psychol. Sci. 2014, 9, 59–71. [Google Scholar] [CrossRef]
- Alambiaga, I.; Álvarez, E.; Diez-Méndez, D.; Verdejo, J.; Barba, E. “The tale of the three little tits”: different nest building solutions under the same environmental pressures. Avian Biol. Res. 2020, 13, 49–56. [Google Scholar] [CrossRef]
- Møller, A.P.; Adriaensen, F.; Artemyev, A.V.; Bańbura, J.; Barba, E.; Biard, C.; Blondel, J.; Bouslama, Z.; Bouvier, J.-C.; Camprodon, J.; et al. Clutch-size variation in Western Palaearctic secondary hole-nesting passerine birds in relation to nest box design. Methods Ecol. Evol. 2014, 5, 353–362. [Google Scholar] [CrossRef]
- Loukola, O.J.; Adamik, P.; Adriaensen, F.; Barba, E.; Doligez, B.; Flensted-Jensen, E.; Eeva, T.; Kivelä, S.M.; Laaksonen, T.; Morosinotto, C.; et al. The roles of temperature, nest predators and information parasites for geographical variation in egg covering behaviour of tits (Paridae). J. Biogeogr. 2020, 47, 1482–1493. [Google Scholar] [CrossRef]
- Alabrudzińska, J.; Kaliński, A.; Słomczyński, R.; Wawrzyniak, J.; Zieliński, P.; Bańbura, J. Effects of nest characteristics on breeding success of Great Tits Parus major. Acta. Ornithol. 2003, 38, 151–154. [Google Scholar] [CrossRef]
- Álvarez, E.; Belda, E.J.; Verdejo, J.; Barba, E. Variation in great tit nest mass and composition and its breeding consequences: a comparative study in four Mediterranean habitats. Avian Biol. Res. 2013, 6, 39–46. [Google Scholar] [CrossRef]
- Álvarez, E.; Barba, E. Nest quality in relation to adult bird condition and its impact on reproduction in great tits. Acta Ornithol. 2008, 43, 3–9. [Google Scholar] [CrossRef]
- Hurtrez-Boussès, S.; de Garine-Wichatitsky, M.; Perret, P.; Blondel, J.; Renaud, F. Variations in prevalence and intensity of blow fly infestations in an insular Mediterranean population of blue tits. Can. J. Zool. 1999, 77, 337–341. [Google Scholar] [CrossRef]
- Glądalski, M.; Kaliński, A.; Markowski, M.; Skwarska, J.; Wawrzyniak, J.; Bańbura, J. Nest size parameter of great tits and blue tits: a long-term study. J. Ornithol. 2023. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Aimé, C.; Midamegbe, A.; Galan, M.-J.; Perret, P.; Grégoire, A.; Doutrelant, C. Nest size and breeding success in first and replacement clutches: an experimental study in blue tits Cyanistes caeruleus. J. Ornithol. 2012, 153, 173–179. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Blondel, J.; Bernard, C.; Caro, S.P.; Charmantier, A.; Demeyrier, V.; Doutrelant, C.; Dubuc-Messier, G.; Fargevieille, A.; de Franceschi, C.; et al. Exploring biotic and abiotic determinants of nest size in Mediterranean great tits (Parus major) and blue tits (Cyanistes caeruleus). Ethology 2016, 122, 492–501. [Google Scholar] [CrossRef]
- Weduwen, D. der; Keogan, K.; Samplonius, J.M.; Phillimore, A.B.; Shut, J.D. The correlates of intraspecific variation in nest height and nest building duration in the Eurasian blue tit Cyanistes caeruleus. J. Avian Biol. 2021, 52, e02528. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Marrot, P.; Fargevieille, A.; Giovannini, P.; Lucas, A.; Demeyrier, V.; Midamegbe, A.; Perret, P.; Grégoire, A.; Charmantier, A.; Doutrelant, C. Nest size is not closely related to breeding success in blue tits: a long-term nest-box study in a Mediterranean oak habitat. Auk 2016, 133, 198–204. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Blondel, J.; de Franceschi, C.; Doutrelant, C. Nest size is positively correlated with fledging success in Corsican blue tits (Cyanistes caeruleus) in an insular oak-dominated habitat mosaic. J. Ornithol. 2017, 158, 125–132. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Haurez, J.; Bodineau, G.; Gagliardi, G.; Maistre, M.; Perret, P.; Pihan, P.; Wilhelm, B.; Wilhelm, J.; Bernard, C.; Blondel, J. Coal tits Periparus ater build larger nests than blue tits Cyanistes caeruleus and great tits Parus major living in the same Mediterranean coniferous woodland habitat. Acta Ornithol. 2016, 51, 123–129. [Google Scholar] [CrossRef]
- Sonnenberg, B.R.; Branch, C.L.; Benedict, L.M.; Pitera, A.M.; Pravosudov, V.V. Nest construction, ambient temperature and reproductive success in a cavity-nesting bird. Anim. Behav. 2020, 165, 43–58. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Charmantier, A.; Demeyrier, V.; Lucas, A.; Perret, S.; Abouladzé, M.; Bonnet, M.; Canonne, C.; Faucon, V.; Grosset, S.; et al. Nest design in a changing world: great tit Parus major nests from a Mediterranean city environment as a case study. Urban Ecosyst. 2017, 20, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.G. Aspects of the breeding biology of great tits Parus major L. and blue tits Parus caeruleus L. in Belfast. Ir. Nat. J. 1998, 26, 99–103. [Google Scholar]
- Britt, J.; Deeming, D.C. First-egg date and air temperature affect nest construction in blue tits (Cyanistes caeruleus), but not in great tits (Parus major). Bird Study 2011, 58, 78–89. [Google Scholar] [CrossRef]
- Cruz, Á.; Álvarez, E.; Barba, E. Nest insulation capacity during incubation and after fledging are related. Avian Biol. Res. 2016, 9, 22–27. [Google Scholar] [CrossRef]
- Briggs, K.B. , Deeming D.C. Use of materials in nest construction by pied flycatchers Ficedula hypoleuca reflects localized habitat and geographical location. Bird Study 2016, 63, 516–524. [Google Scholar] [CrossRef]
- Harnist, I.; Dubiec, A.; Mazgajski, T.D. Changes in nest mass in relation to nesting stages in the great tit Parus major. Bird Study 2020, 67, 292–299. [Google Scholar] [CrossRef]
- Lombardo, M.P. Nest architecture and reproductive performance in tree swallows (Tachycineta bicolor). Auk 1994, 111, 814–824. [Google Scholar] [CrossRef]
- Soler, J.J.; Møller, A.P.; Soler, M. 1998. Nest building, sexual selection and parental investment. Evol. Ecol. 1998, 12, 427–441. [Google Scholar] [CrossRef]
- Deeming, D.C.; Pike, T.W. Nest surface temperature predicts fledging success of blue tits Cyanistes caeruleus but not great tits Parus major. Acta Ornithol. 2015, 50, 247–251. [Google Scholar] [CrossRef]
- Tomás, G.; Merino, S.; Martínez-de la Puente, J.; Moreno, J.; Morales, J.; Lobato, E. Determinants of abundance and effects of blood-sucking flying insects in the nest of a hole-nesting bird. Oecologia 2008, 156, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Vázquez, F.; Merino, S.; Cuezva, S.; Sánchez-Moral, S. Nest gasses as a potential attraction cue for biting flying insects and other ectoparasites of cavity nesting birds. Front. Ecol. Evol. 2020, 8, 258. [Google Scholar] [CrossRef]
- Slagsvold, T.; Amundsen, T. Do great tits adjust hatching spread, egg size and offspring sex ratio to changes in clutch size? J. Anim. Ecol. 1992, 61, 249–258. [Google Scholar] [CrossRef]
- Mazgajski, T.D.; Rykowska, Z. Dependence of nest mass on nest hole depth in the great tit Parus major. Acta Ornithol. 2008, 43, 49–55. [Google Scholar] [CrossRef]
- Stanback, M.T.; Mercadante, A.N.; Cline, E.L.; Burke, T.H.; Roth, J.E. Cavity depth, not experience, determines nest height in Easthern bluebirds. Wilson J. Ornithol. 2013, 125, 301–306. [Google Scholar] [CrossRef]
- Deeming, D.C.; Morton, F.E.M.; Laverack, K.L. Nest box size affects mass and proportions of materials used in blue tit Cyanistes caeruleus nests. Bird Study 2019, 66, 130–135. [Google Scholar] [CrossRef]
- Holveck, M.-J.; Grégoire, A.; Doutrelant, C.; Lambrechts, M.M. Nest height is affected by lamppost lighting proximity in addition to nestbox size in urban great tits. J Avian. Biol. 2019, 50, e01798.
- Sakraoui, W.; Bouslama, Z.; Belabed, A.I. Does increasing nestboxes size affects breeding success of blue tits (Cyanistes caeruleus)? Study in a locality of Seraidi (Northeast, Algeria). Eco. Env. Cons. 2019, 25, 609–613. [Google Scholar]
- Briggs, K.B.; Deeming, D.C. Effects of year and box size on construction of Eurasian Nuthatch Sitta europaea nests. Ardea, 2022; 110, 61–74. [Google Scholar]
- Järvinen, P.H.; Kluen, E.; Tiiri, M.; Brommer, J.E. Experimental manipulation of blue tit nest height does not support the thermoregulation hypothesis. Ornis Fenn. 2017, 94, 82–91. [Google Scholar] [CrossRef]
- Järvinen, P.H.; Brommer, J.E. Lining the nest with more feathers increases offspring recruitment probability: selection on an extended phenotype in the blue tit. Ecol. Evol. 2020, 10, 13327–13333. [Google Scholar] [CrossRef] [PubMed]
- Deeming, D.C.; Mainwaring, M.C.; Hartley, I.R.; Reynolds, S.J. Local temperature and not latitude determines the design of blue tit and great tit nests. Avian Biol. Res. 2012, 5, 203–205. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Caro, S.P. Egg cooling associated with nest size in a passerine bird. J. Therm. Biol. 2022, 110, 103383. [Google Scholar] [CrossRef]
- Mainwaring, M.C.; Hartley, I.R.; Bearhop, S.; Brulez, K.; du Feu, C.R.; Murphy, G.; Plummer, K.E.; Webber, S.L.; Reynolds, S.J.; Deeming, C.D. Latitudinal variation in blue tit and great tit nest characteristics indicates environmental adjustment. J. Biogeogr. 2012, 39, 1669–1677. [Google Scholar] [CrossRef]
- Kaliński, A.; Wawrzyniak, J.; Bańbura, M.; Skwarska, J.; Zielinski, P.; Gladalski, M.; Bańbura, J. Does the threat of European pine marten (Martes martes) predation influence the height of nests built by blue tits (Cyanistes caeruleus) and great tits (Parus major)? Avian Biol. Res. 2014, 7, 83–90. [Google Scholar] [CrossRef]
- Perrins, C.M. British Tits. Collins, 1979.
- Tomás, G.; Merino, S.; Martínez-de la Puente, J.; Moreno, J.; Morales, J.; Rivero-de Aguilar, J. Nest size and aromatic plants in the nest as sexually selected female traits in blue tits. Behav. Ecol. 2013, 24, 926–934. [Google Scholar] [CrossRef]
- Alworth, T. An experimental test of the function of sticks in the nests of house wrens. Condor 1996, 98, 841–844. [Google Scholar] [CrossRef]
- Slagsvold, T. Experiments on clutch size and nest size in passerine birds. Oecologia 1989, 80, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Glądalski, M.; Kaliński, A.; Wawrzyniak, J.; Bańbura, M.; Markowski, M.; Skwarska, J.; Bańbura, J. Physiological condition of nestling great tits Parus major in response to experimental reduction in nest micro- and macro-parasites. Conserv. Physiol. 2018, 6, coy062. [Google Scholar] [CrossRef] [PubMed]
- Remeš, V.; Krist, M. Nest design and the abundance of parasitic Protocalliphora blow flies in two hole-nesting passerines. Écoscience 2005, 12, 549–553. [Google Scholar] [CrossRef]
- Moreno, J.; Merino, S.; Lobato, E.; et al. Nest-dwelling ectoparasites of two sympatric hole-nesting passerines in relation to nest composition. An experimental study. Écoscience 2009, 16, 418–427. [Google Scholar] [CrossRef]
- Cantarero, A.; López-Arrabé, J.; Saavedra-Garcés, I.; Rodríguez-García, V.; Palma, A.; Moreno, J. The significance of nest structure and nesting material for hole-nesting passerines: an experimental study with Nuthatches Sitta europaea. Acta Ornithol. 2014, 49, 143–155. [Google Scholar] [CrossRef]
- Surgey, J.; du Feu, C.R.; Deeming, D.C. Opportunistic use of a wool-like artificial material as lining of tit (Paridae) nests. Condor 2012, 114, 385–392. [Google Scholar]
- Deeming, D.C.; Mainwaring, M.C. Functional properties of nests. In: Deeming, D.C. and Reynolds, S.J. (Eds.), Nests, eggs, and incubation: new ideas about avian reproduction, pp. 29–49. Oxford University Press, Oxford, 2015.
- Aasen, M.; Slagsvold, T. No cultural transmission of use of nest materials in titmice Paridae. Anim. Behav. 2020, 170, 27–32. [Google Scholar] [CrossRef]
- Winkler, D.W. Use and importance of feathers as nest lining in tree swallows (Tachycineta bicolor). Auk 1993, 110, 29–36. [Google Scholar]
- Lombardo, M.P.; Bosman, R.M.; Faro, C.A.; Houtteman, S.G.; Kluisza, T.S. Effect of feathers as nest insulation on incubation behavior and reproductive performance of tree swallows (Tachycineta bicolor). Auk 1995, 112, 973–981. [Google Scholar] [CrossRef]
- Veiga, J.P.; Polo, V. Feathers in the spotless starlig nests: a sexually selected trait? Behaviour 2011, 148, 1359–1375. [Google Scholar] [CrossRef]
- Sanz, J.J.; García-Navas, V. Nest ornamentation in blue tits: is feather carrying ability a male status symbol? Behav. Ecol. 2011, 22, 240–247. [Google Scholar] [CrossRef]
- García-Navas, V.; Ortega, J.; Ferrer, E.S.; Sanz, J.J. Feathers, suspicions, and infidelities: an experimental study of parental care and certainty of paternity in the blue tit. Biol. J. Linn. Soc. 2013, 109, 552–561. [Google Scholar] [CrossRef]
- Briggs, K.B.; Biddle, L.E.; Deeming, D.C. Geographical location affects size and materials used in the construction of European pied flycatcher (Ficedula hypoleuca) nests. Avian Res. 2019, 10, 17. [Google Scholar] [CrossRef]
- Briggs, K.B.; Deeming, D.C. Localized habitat affects size and materials used in the construction of Common Redstart Phoenicurus phoenicurus nests. Bird Study 2021, 68, 9–20. [Google Scholar] [CrossRef]
- Møller, A.P. On the use of feathers in birds’ nests: Predictions and tests. Ornis Scand. 1984, 15, 38–42. [Google Scholar] [CrossRef]
- White, D.W.; Kennedy, E.D. Effect of egg covering and habitat on nest destruction by house wrens. Condor 1997, 99, 873–879. [Google Scholar] [CrossRef]
- Slagsvold, T. , Wiebe, K.L. Egg covering in cavity nesting birds may prevent nest usurpation by other species. Behav. Ecol. Sociobiol. 2021, 75, 116. [Google Scholar] [CrossRef]
- Reynolds, S.J.; Davies, C.S.; Elwell, E.; Tasker, P.J.; Williams, A.; Sadler, J.P.; Hunt, D. Does the urban gradient influence the composition and ectoparasite load of nests of an urban bird species? Avian Biol. Res. 2016, 9, 224–234. [Google Scholar] [CrossRef]
- Hanmer, H.J.; Thomas, R.L.; Beswick, G.J.F.; Collins, B.P.; Fellowes, M.D.E. Use of anthropogenic material affects bird nest arthropod community structure: influence of urbanisation, and consequences for ectoparasites and fledging success. J. Ornithol. 2017, 158, 1045–1059. [Google Scholar] [CrossRef]
- Girão, J.; Bessa, F.; Garrido-Bautista, J.; Ferreira, B.; Santos-Baena, C.; Marques, M.P.M.; Batista de Carvalho, L.A.E.; Ramos, J.A.; Norte, A.C. Variation in the use of anthropogenic materials in tit nests: influence of human activities and pandemic restrictions. Urban Ecosyst. 2024. [Google Scholar] [CrossRef]
- Jagiello, Z.; Corsini, M.; Dylewski, L.; Ibáñez-Álamo, J.D.; Szulkin, M. The extended avian urban phenotype: anthropogenic solid waste pollution, nest design, and fitness. Science Total Environ. 2022, 838, 156034. [Google Scholar] [CrossRef] [PubMed]
- Clark, L. The nest protection hypothesis: the adaptive use of plant secondary compounds by European starlings. In: Loye, J. E. and Zuk, M. (Eds.). Bird-parasite interactions: ecology, evolution, and behaviour. Oxford, Oxford University Press, pp. 205-221, 1991.
- Dubiec, A.; Gózdz, I.; Mazgajski, T.D. Green plant material in avian nests. Avian Biol. Res. 2013, 6, 133–146. [Google Scholar] [CrossRef]
- Wimberger, P.H. The use of green plant material in bird nests to avoid ectoparasites. Auk 1984, 101, 615–618. [Google Scholar] [CrossRef]
- Gwinner, H.; Oltrogge, M.; Trost, L.; Nienaber, U. Green plants in starling nests: effects on nestlings. Anim. Behav. 2000, 59, 301–309. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Hossaert-McKey, M. Olfaction, volatile compounds and reproduction in birds. Acta Zool. Sinica 2006, 52, 284–287. [Google Scholar]
- Veiga, J.P.; Polo, V.; Vinuela, J. Nest green plants as a male status signal and courtship display in the spotless starling. Ethology 2006, 112, 196–204. [Google Scholar] [CrossRef]
- Garrido-Bautista, J.; Ramos, J.A.; Arce, S.I.; Melero-Romero, P.; Ferreira, R.; Santos-Baena, C.; Guímaro, H.R.; Martín-Villegas, C.; Moreno-Rueda, G.; Norte, A.C. Is there a role for aromatic plants in blue tits (Cyanistes caeruleus) nests? Behav. Ecol. Sociobiol. 2023, 77, 118. [Google Scholar] [CrossRef]
- Eens, M.; Pinxten, R.; Verheyen, R.F. On the function of singing and wing-waving in the European starling Sturnus vulgaris. Bird Study 1990, 37, 48–52. [Google Scholar] [CrossRef]
- Cowie, R.J.; Hinsley, S.A. Timing and return with green vegetation by nesting blue tits Parus caeruleus. Ibis 1988, 130, 553–555. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Dos Santos, A. Aromatic herbs in Corsican blue tit nests: the ‘Potpourri’ hypothesis. Acta Oecol. 2000, 21, 175–178. [Google Scholar] [CrossRef]
- Gwinner, H. The function of green plants in nests of European starlings. Behaviour 1997, 134, 337–351. [Google Scholar] [CrossRef]
- Brouwer, L.; Komdeur, J. Green nest material has a function in mate attraction in the European starling. Anim. Behav. 2004, 67, 539–548. [Google Scholar] [CrossRef]
- Gwinner, H.; Yohannes, E.; Schwabl, H. Nest composition and yolk hormones: do female European starlings adjust yolk androgens to nest quality? Avian Biol. Res. 2013, 6, 307–312. [Google Scholar] [CrossRef]
- Polo, V.; Rubalcaba, J.G.; Veiga, J.P. Green plants in nests reduce offspring recruitment rates in the spotless starling. Behav. Ecol. 2015, 26, 1131–1137. [Google Scholar] [CrossRef]
- Rubalcaba, J.G.; Fuentes, D.; Veiga, J.P.; Polo, V. Nest decoration as social signals by males and females: greenery and feathers in starling colonies. Behav. Ecol. 2017, 28, 1369–1375. [Google Scholar] [CrossRef]
- Soler, J.J.; Ruiz-Castellano, C.; Figuerola, J.; Martín-Vivaldi, M.; Martínez-de la Puente, J.; Ruiz-Rodríguez, M.; Tomás, G. Telomere length and dynamics of spotless starling nestlings depend on nest-building materials used by parents. Anim. Behav. 2017, 126, 89–100. [Google Scholar] [CrossRef]
- Gwinner, H.; Capilla-Lasheras, P.; Cooper, C.; Helm, B. ‘Green incubation’: avian offspring benefit from aromatic nest herbs through improved parental incubation behaviour. Proc. R. Soc. Lond. Biol. Sci. 2018, 285, 20180376. [Google Scholar] [CrossRef]
- Tomás, G.; Merino, S.; Martínez-de la Puente, J.; Moreno, J.; Morales, J.; Lobato, E.; Rivero-de Aguilar, J.; del Cerro, S. Interacting effects of aromatic plants and female age on nest-dwelling ectoparasites and blood-sucking flies in avian nests. Behav. Proc. 2012, 90, 246–253. [Google Scholar] [CrossRef]
- Mennerat, A.; Perret, P.; Bourgault, P.; Blondel, J.; Gimenez, O.; Thomas, D.W.; Heeb, P.; Lambrechts, M.M. Aromatic plants in nests of blue tits: positive effects on nestlings. Anim. Behav. 2009, 77, 569–574. [Google Scholar] [CrossRef]
- Sugasawa, S.; Edwards, S.C.; Stanforth, R.; Bruton, E.; Hansell, M.; Reilly, M.; Healy, S.D. A non-destructive approach to collect nest material data using photographs. Ibis 2021, 163, 1457–1462. [Google Scholar] [CrossRef]
- Bańbura, J.; Blondel, J.; de Wilde-Lambrechts, H.; Perret, P. Why do female blue tits (Parus caeruleus bring fresh plants to their nests? J. Ornithol. 1994, 136, 217–221. [Google Scholar] [CrossRef]
- Rubalcaba, J.G.; Polo, V.; Maia, R.; Rubenstain, D.R.; Veiga, J.P. Sexual and natural selection in the evolution of extended phenotypes: the use of green nesting material in starlings. J. Evol. Biol. 2016, 29, 1585–1592. [Google Scholar] [CrossRef]
- Rendell, W.B.; Robertson, R.J. Nest-site characteristics, reproductive success and cavity availability for tree swallows breeding in natural cavities. Condor 1989, 91, 875–885. [Google Scholar] [CrossRef]
- Wesołowski, T. Clutch size and breeding performance of marsh tits Parus palustris in relation to hole size in a primeval forest. Acta Ornithol. 2003, 38, 65–72. [Google Scholar] [CrossRef]
- Mennerat, A.; Perret, P.; Caro, S.P.; Heeb, P.; Lambrechts, M.M. Aromatic plants in blue tit Cyanistes caeruleus nests: no negative effect on blood-sucking Protocalliphora blow fly larvae. J. Avian Biol. 2008, 39, 127–132. [Google Scholar] [CrossRef]
- Pires, B.A.; Belo, A.D.F.; Rabaça, J.E. Aromatic plants in Eurasian blue tit nests: the ‘nest Protection Hypothesis’ revisited. Wilson J. Ornithol. 2012, 124, 162–165. [Google Scholar] [CrossRef]
- Dawson, R.D. Does fresh vegetation protect avian nests from ectoparasites? An experiment with tree swallows. Can. J. Zool. 2004, 82, 1005–1010. [Google Scholar] [CrossRef]
- Shutler, D.; Campbell, A.A. Experimental addition of greenery reduces flea loads in nests of a non-greenery using species, the tree swallow Tachycineta bicolor. J. Avian Biol. 2007, 38, 7–12. [Google Scholar] [CrossRef]
- Glądalski, M.; Bańbura, M.; Kaliński, A.; Markowski, M.; Skwarska, J.; Wawrzyniak, J.; Zieliński, P.; Bańbura, J. Consequences of experimental addition of fresh, aromatic plants into nests of blue tits (Cyanistes caeruleus) on the physiological condition of nestlings. Behav. Ecol. Sociobiol. 2020, 74, 29. [Google Scholar] [CrossRef]
- Holland, E.R.; Shutler, D. Nest feathering responses by tree swallows (Tachycineta bicolor). J. Ornithol. 2018, 159, 991–998. [Google Scholar] [CrossRef]
- Slagsvold, T. Nest site preference and clutch size in the pied flycatcher Ficedula hypoleuca. Ornis Scand. 1987, 18, 189–197. [Google Scholar] [CrossRef]
- Lens, L.; Wauters, L.A.; Dhondt, A.A. Nest-building by crested tit Parus cristatus males: an analysis of costs and benefits. Behav. Ecol. Sociobiol. 1994, 35, 431–436. [Google Scholar] [CrossRef]
- Mainwaring, M.C.; Benskin, C.; Mc, W.H.; Hartley, I.R. The weight of female-built nests correlates with female but not male quality in the blue tit Cyanistes caeruleus. Acta Ornithol. 2008, 43, 43–48. [Google Scholar] [CrossRef]
- Broggi, J.; Senar, J.C. Brighter great tit parents build bigger nests. Ibis 2009, 151, 588–591. [Google Scholar] [CrossRef]
- Mainwaring, M.C.; Hartley, I. R The energetic costs of nest building in birds. Avian Biol. Res. 2013, 6, 12–17. [Google Scholar] [CrossRef]
- Löhrl, H. Einfluβ des Brutraumfläche auf die Gelegegröβe des Kohlmeise (Parus major). J. Ornithol. 1973, 114, 339–347. [Google Scholar] [CrossRef]
- Löhrl, H. Weitere Versuche zur Frage „Brutraum und Gelegegröβe“ bei des Kohlmeise, Parus major. J. Ornithol. 1980, 121, 403–405. [Google Scholar] [CrossRef]
- Löhrl, H. Experimente zur Bruthöhlenwahl der Kohlmeise (Parus major). J. Ornithol. 1986, 127, S51–S59. [Google Scholar] [CrossRef]
- Karlsson, J.; Nilsson, S.G. The influence of nest-box area on clutch size in some hole-nesting passerines. Ibis 1977, 119, 207–211. [Google Scholar] [CrossRef]
- Van Balen, J.H. The relationship between nest-box size, occupation and breeding parameters of the great tit Parus major and some other hole-nesting species. Ardea 1984, 72, 163–175. [Google Scholar]
- Nilsson, S.G. The evolution of nest-site selection among hole-nesting birds: the importance of nest predation and competition. Ornis Scand. 1984, 15, 167–175. [Google Scholar] [CrossRef]
- Gustafsson, L.; Nilsson, S.G. Clutch size and breeding success of pied and collared flycatchers Ficedula spp. in nest-boxes of different sizes. Ibis 1985, 127, 380–385. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Abouladzé, M.; Bonnet, M.; Demeyrier, V.; Doutrelant, C.; Faucon, V.; le Prado, G.; Lidon, F.; Noell, T.; Pagano, P.; et al. Nest-box size influences where secondary-cavity exploiters roost and nest: a choice experiment. J. Ornithol. 2013, 154, 563–566. [Google Scholar] [CrossRef]
- Slagsvold, T.; Kleiven, K.W.; Eriksen, A.; Johannessen, L.E. Vertical and horizontal transmission of nest-site preferences in titmice. Anim. Behav. 2013, 85, 323–328. [Google Scholar] [CrossRef]
- Demeyrier, V.; Lambrechts, M.M.; Perret, P.; Grégoire, A. Experimental demonstration of an ecological trap for a wild bird in a man-transformed environment. Anim. Behav. 2016, 118, 181–190. [Google Scholar] [CrossRef]
- Maziarz, M.; Wesołowski, T.; Hebda, G.; Cholewa, M. Natural nest sites of Great Tits (Parus major) in a primeval temperate forest (Białowiez_a National Park, Poland). J. Ornithol. 2015, 156, 613–623. [Google Scholar] [CrossRef]
- Deeming, D.C. How does the bird-nest incubation unit work? Avian Biol. Res. 2016, 9, 103–113. [Google Scholar] [CrossRef]
- Kluijver, H.N. The population ecology of the great tit, Parus m. major L. Ardea 1951, 39, 1–135. [Google Scholar]
- Erbelding-Denk, C.; Trillmich, F. Das Mikroklima im Nistkasten und seine Auswikungen auf die Nestlinge beim Star (Sturnus vulgaris). J. Ornithol. 1990, 131, 73–84. [Google Scholar] [CrossRef]
- Wesołowski, T.; Czeszczewik, D.; Rowinski, P.; Walankiewicz, W. Nest soaking in natural holes – a serious cause of breeding failure? Ornis Fenn. 2002, 79, 132–138. [Google Scholar]
- Glądalski, M.; Bańbura, M.; Kaliński, A.; Markowski, M.; Skwarska, J.; Wawrzyniak, J.; Zieliński, P.; Cyżewska, I.; Bańbura, J. Effects of nest characteristics on reproductive performance in Blue Tits Cyanistes caeruleus and Great Tits Parus major. Avian Biol. Res. 2016, 9, 37–43. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Demeyrier, V.; Fargevieille, A.; Giovannini, P.; Lucas, A.; Marrot, P.; Midamegbe, A.; Perret, P.; Charmantier, A.; Doutrelant, C.; Grégoire, A. Great tits build shallower nests than blue tits. Avian Biol. Res. 2014, 7, 251–254. [Google Scholar] [CrossRef]
- Lambrechts, M.M.; Caizergues, A.E.; Perrier, C.; Charmantier, A.; Caro, S. Surface temperatures of non-incubated eggs in great tits (Parus major) are strongly associated with ambient temperature. Int. J. Biometeorol. 2020, 64, 1767–1775. [Google Scholar] [CrossRef]
- Mainwaring, M.C. Nest construction and incubation in a changing climate. In: Deeming DC, Reynolds SJ (Eds.) Nests, eggs, and incubation: new ideas about avian reproduction. Oxford University Press, Oxford, pp. 65–74, 2015.
- Lambrechts, M.M.; Rieux, A.; Galan, M.-J.; Cartan-Son, M.; Perret, P.; Blondel, J. Double-brooded great tits (Parus major) in Mediterranean oak habitats: do first broods always perform better than second broods? Russ. J. Ecol. 2008, 39, 516–522. [Google Scholar] [CrossRef]
- Stephenson, S.; Hannon, S.; Proctor, H. The function of feathers in tree swallow nests: insulation or ectoparasite barrier? Condor 2009, 111, 479–487. [Google Scholar] [CrossRef]
- Dawson, R.D.; O’Brien, E.L.; Mlynowski, T.J. The price of insulation: costs and benefits of feather delivery to nests for male tree swallows Tachycineta bicolor. J. Avian Biol. 2011, 42, 93–102. [Google Scholar] [CrossRef]
- Mainwaring, M.C.; Wolfenden, A.; Read, J.E.; Robson, J.M.A.; Tomlinson, C.J.; Hartley, I.R. Feathering the nest: the effects of feather supplementation to blue tit nests. Avian Biol. Res. 2016, 9, 89–95. [Google Scholar] [CrossRef]
- Scott-Baumann, J.F.; Morgan, E.R. A review of the nest protection hypothesis: does inclusion of fresh green plant material in birds’ nests reduce parasite infestation? Parasitology 2015, 142, 1016–1023. [Google Scholar] [CrossRef]
- Pires, B.A.; Belo, A.D.F.; Diamantino, F.; Rabaça, J.E.; Merino, S. Development of nestling blue tits (Cyanistes caeruleus) is affected by experimental addition of aromatic plants. Avian Biol. Res. 2020, 13, 44–48. [Google Scholar] [CrossRef]
- Fauth, P.T.; Krementz, D.G.; Hines, J.E. Ectoparasitism and the role of green nesting material in the Europena starling. Oecologia 1991, 88, 22–29. [Google Scholar] [CrossRef]
- McCarty, J.P.; Secord, A.L. Nest-building behavior in PCB-contaminated tree swallows. Auk 1999, 116, 55–63. [Google Scholar] [CrossRef]
- Pettifor, R.A.; Perrins, C.M.; McCleery, R.H. Individual optimization of clutch size in great tits. Nature 1988, 336, 160–162. [Google Scholar] [CrossRef]
- Tinbergen, J.M.; Both, C. Is clutch size individually optimized? Behav. Ecol. 1999, 10, 504–509. [Google Scholar] [CrossRef]
- Tinbergen, J.M.; Boerlijst, M.C. Nestling weight and survival in individual great tits (Parus major). J. Anim. Ecol. 1990, 59, 1113–1127. [Google Scholar] [CrossRef]
- Both, C.; Visser, M.E.; Verboven, N. Density-dependent recruitment rates in great tits: the importance of being heavier. Proc. R. Soc. Lond. Biol. Sci. 1999, 266, 465–469. [Google Scholar] [CrossRef]
- Naef-Daenzer, B.; Widmer, F.; Nuber, M. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J. Anim. Ecol. 2001, 70, 730–738. [Google Scholar] [CrossRef]
- Perrins, C.M.; McCleery, R.H. The effect of fledgling mass on the lives of great tits Parus major. Ardea 2001, 89, 135–142. [Google Scholar]
- Rodríguez, S.; Álvarez, E.; Barba, E. Factors affecting fledgling output of great tits, Parus major, in the long term. Anim. Biodiv. Cons. 2016, 39, 147–154. [Google Scholar] [CrossRef]
- Rodríguez, S.; van Noordwijk, A.J.; Álvarez, E.; Barba, E. A recipe for post fledgling survival in great tits Parus major: be large and be early (but not too much). Ecol. Evol. 2016, 6, 4458–4467. [Google Scholar] [CrossRef]
- Curio, E. Why do young birds reproduce less well? Ibis 1982, 125, 400–404. [Google Scholar] [CrossRef]
- O’Neill, L.G.; Parker, T.H.; Griffith, S.C. Nest size is predicted by female identity and the local environment in the blue tit (Cyanistes caeruleus), but is not related to the nest size of the genetic or foster mother. R. Soc. open sci. 2018, 5, 172036. [Google Scholar] [CrossRef]
- Lack, D. Family-size in titmice of the genus Parus. Evolution 1950, 4, 279–290. [Google Scholar] [CrossRef]
- Blondel, J.; Gosler, A.; Lebreton, J.D.; McCleery, R. (Eds.) Population biology of passerine birds: an integrated approach. NATO ASI Series G, volume 24. Springer-Verlag, Berlin and Heidelberg, 1990.
- Payevsky, V.A. Mortality rate and population density regulation in the great tit, Parus major L.: a review. Russ. J. Ecol. 2006, 37, 180–187. [Google Scholar] [CrossRef]
- Chamberlain, D.E.; Cannon, A.R.; Toms, M.P.; Leech, D.I.; Hatchwell, B.J.; Gaston, K.J. Avian productivity in urban landscapes: a review and meta-analysis. Ibis 2009, 151, 1–18. [Google Scholar] [CrossRef]
- Mägi, M.; Mänd, R.; Tamm, H.; Sisask, E.; Kilgas, P.; Tilgar, V. Low reproductive success of great tits in the preferred habitat: a role of food availability. Écoscience 2009, 16, 145–157. [Google Scholar] [CrossRef]
- Dawson, A. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 12, 363, 1621–1633. [Google Scholar] [CrossRef]
- Williams, T.D. Physiological adaptations for breeding in birds; Princeton University Press: Princeton, New Jersey, USA, 2012. [Google Scholar]
- Nager, R.G.; van Noordwijk, A.J. Energetic limitations in the egg-laying period of great tits. Proc. R. Soc. Lond. B Biol. Sci. 1992, 249, 259–263. [Google Scholar]
- Stanback, M.T.; Bartholomew, J.E.; Bergner, L.M.; Cline, E.L.; Helms, P.I.; McGovern, P.G.; Millican, D.M.; Roth, J.E. House wrens alter nest architecture to compensate for cavity vulnerability. Wilson J. Ornithol. 2013, 125, 174–178. [Google Scholar] [CrossRef]
- Maziarz, M.; Wesołowski, T. Does darkness limit the use of tree cavities for nesting birds? J. Ornithol. 2014, 155, 793–799. [Google Scholar] [CrossRef]
- Podkowa, P.; Surmacki, A. The importance of illumination in nest site choice and nest characteristics of cavity nesting birds. Scientific Reports 2017, 7, 1329. [Google Scholar] [CrossRef] [PubMed]
- Tollenaar, D. Legperioden en eierproductie bij eenige wilde vogelsoorten, vergeleken met die bij hoenderrassen. Mededeelingen Landbouwhoogeschool Wageningen 1922, 23, 1–46. [Google Scholar]
- Caro, S.P.; Schaper, S.V.; Hut, R.A.; Ball, G.F.; Visser, M.E. The case of the missing mechanism: how does temperature influence seasonal timing in endotherms? PLoS Biol. 2013, 11, e1001517. [Google Scholar] [CrossRef] [PubMed]
- Bonamour, S.; Chevin, L.-M.; Charmantier, A.; Teplitsky, C. Phenotypic plasticity in response to climate change: the importance of cue variation. Phil. Trans. R. Soc. Lond. B 2019, 374, 20180178. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Parasites, predators and nest boxes: facts and artefacts in nest box studies of birds? Oikos 1989, 56, 421–423. [Google Scholar] [CrossRef]
- Breen, A.J.; Healy, S.D.; Guillette, L.M. Reproductive consequences of material use in avian nest construction. Behav. Proc. 2021, 193, 104507. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.J.; Ibáñez-Álamo, J.D.; Sumasgutner, P.; Mainwaring, M.C. Urbanisation and nest building in birds: a review of threats and opportunities. J. Ornithol. 2019, 160, 841–860. [Google Scholar] [CrossRef]
- Slagsvold, T. On the evolution of clutch size and nest size in passerine birds. Oecologia 1989, 79, 300–305. [Google Scholar] [CrossRef]
- Tomás, G.; Merino, S.; Moreno, J.; Sanz, J.J.; Morales, J.; Garcia-Fraílle, S. Nest weight and female health in the Blue Tit (Cyanistes caeruleus). Auk 2006, 123, 1013–1021. [Google Scholar] [CrossRef]
- Smith, J.A.; Harrison, T.J.E.; Martin, G.R.; Reynolds, S.J. Feathering the nest: food supplementation influences nest construction by blue (Cyanistes caeruleus) and great tits (Parus major). Avian Biol. Res. 2013, 6, 18–25. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Merino, S.; Lobato, E.; Moreno, J.; Tomás, G.; Morales, J. Male nest-building activity influences clutch mass in pied flycatchers Ficedula hypoleuca. Bird Study 2009, 56, 264–267. [Google Scholar] [CrossRef]
- Hilton, G.M.; Hansell, M.H.; Ruxton, G.D.; Reid, J.; Monaghan, P. Using artificial nests to test importance of nesting material and nest shelter for incubation energetics. Auk 2004, 121, 777–787. [Google Scholar] [CrossRef]
- Polo, V.; Veiga, J.P. Nest ornamentation by female spotless starlings in response to a male display: an experimental study. J. Anim. Ecol. 2006, 75, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.D.; Doherty, P.F.; Piaggio, A.J.; Huyvaert, K.P. Sex and nest type influence avian blood parasite prevalence in a high-elevation bird community. Parasites Vectors 2021, 14, 145. [Google Scholar] [CrossRef]
- Clark, L.; Mason, J.R. Effect of biologically active plants used as nest material and the derived benefits to starling nestlings. Oecologia 1988, 77, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.J.; Cuervo, J.J.; Møller, A.P.; de Lope, F. Nest building is a sexually selected behaviour in the Barn Swallow. Anim. Behav. 1998, 56, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Mainwaring, M.C.; Deeming, D.C.; Jones, C.I.; Hartley, I.R. Adaptive latitudinal variation in common blackbird Turdus merula nest characteristics. Ecol. Evol. 2014b, 4, 841–851. [Google Scholar] [CrossRef]
- Crossman, C.A.; Rohwer, V.G.; Martin, P.R. Variation in the structure of bird nests between northern Manitoba and southeastern Ontario. PLoS ONE 2011, 6, e19086. [Google Scholar] [CrossRef]
- Deeming, D.C.; Merrils-Brown, M. 2024 Architecture differentially affects thermal properties of Song Thrush Turdus philomelos nest walls. Bird Study 2024, in press. [Google Scholar]
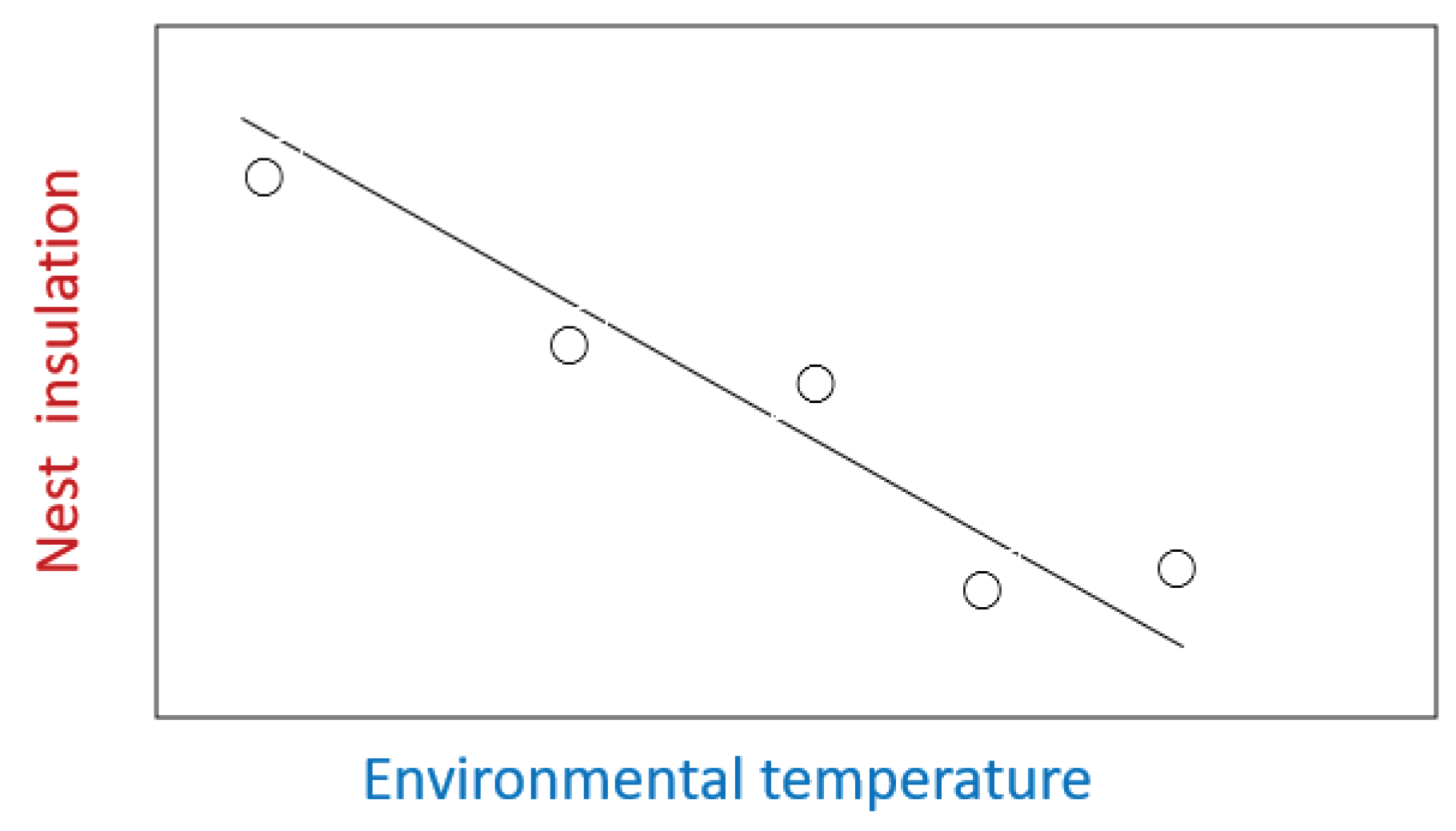
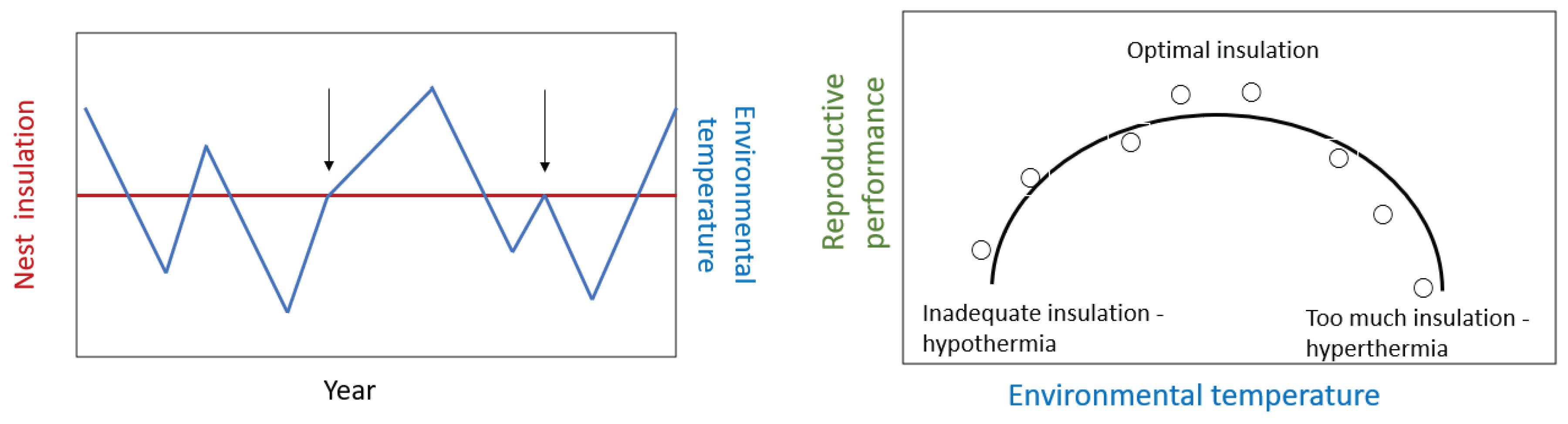
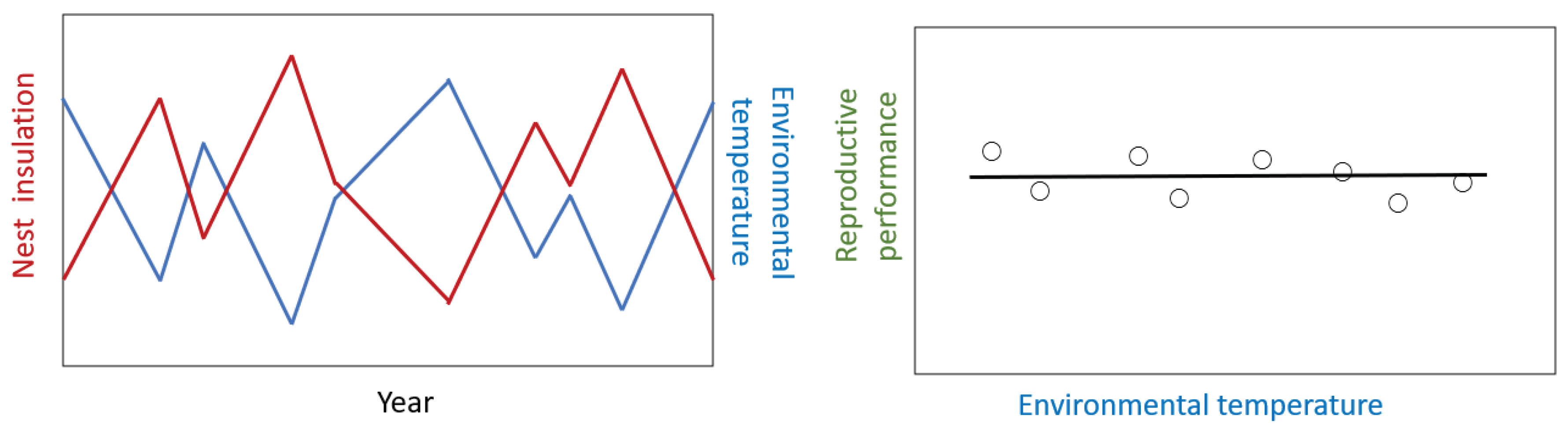
| Hypothesis | Prediction | Species studied | Analysis and conditions tested | Conditions of expression or selective factors | Reference |
|---|---|---|---|---|---|
| Nest-size components | |||||
| Direct effects | |||||
| Bigger cavity nests reduce egg breaking risks | Less nest desertion or more hatchlings in bigger nests | GT | Not tested | Cavities with irregular floor and wall surfaces | 54 |
| Bigger nests can physically support heavier or larger broods | Larger clutches and larger broods in larger nests | Cavity-nesting passerines GT, BT, PF, CF |
Correlative Experimental |
Cavity size (depth, floor area) | 4, 143, 149, 202 |
| Bigger nests allow nest cup expansion reducing competition for space or overheating in crowded nest cups | More fledglings in nest cups that expand more | BT, PF, GT, TS | Experiments with artificial nests in BT and PF or very small boxes in GT | Bigger nestlings and larger broods in larger cavities, also depending on ambient temperatures | 18, 70, 91, 152, 202 |
| Thicker nests reduce negative effects of nest compression due to growing nestlings | Bigger or heavier broods in thicker nests | GT, BT | Not tested | Cavities with irregular floor and wall surfaces, and nest bases mainly built from soft nest material, like moss | 4, 72 |
| Bigger nests provide better insulation improving incubation or brooding when the incubator is on the eggs | More ectotherm nestlings, or better growing embryos or nestlings, in bigger nests | GT, BT | Correlative Experimental |
Cold weather | 72, 82, 84, 86 |
| Bigger or asymmetric nests can block air flows in damaged cavities containing cracks or slits | Improved incubation efficiency increasing survival probabilities of ectotherm embryos or nestlings | GT, BT, CT | Not tested | Cold and windy weather | 62 |
| Bigger nests hamper cooling of species-specific eggs or ectotherm nestlings when the incubator or brooder is off the nest | Better egg survival or improved embryo growth in bigger nests | GT | Laboratory | Cold weather | 85 |
| Smaller nest cups reduce heat loss during incubation | Higher hatching success in smaller nest cups | BT, PF | Experiments with artificial nests, but not tested | Cold weather | 91 |
| Bigger nests reduce nest soaking risks | Less nest desertion, and higher breeding success in bigger and thicker nests | PF, CF, MT, EN | Natural cavities Anecdotal |
Cavities exposed to heavy rain | 162 |
| Nest size determines exposure to nest parasites, depending on the types of parasites. Larger nests might harbor more non-flying nest parasites or produce more gasses that attract more flying nest parasites |
Breeding success associated with nest parasitism but depending on the types of parasites infesting the nest. Some nest parasites might vaccinate bird hosts potentially improving contributions to next generations. |
GT, BT | Correlative | Cavities exposed to nest parasites. Climate-dependent parasite activity and growth |
23, 24, 73 |
| Bigger nests reduce contact with cavity walls increasing sanitary conditions | More hatchlings or fledglings, and better growing embryos or nestlings, in bigger nests | GT | Not tested | Wetter cavities promoting micro-organism development | 52 |
| Nest size controls cavity illumination influencing predation risks or abilities to perceive nestling phenotypes | Darker nests suffer less from nest predation, and cavity illumination is associated with nestling phenotypes and brood characteristics | GT | Correlative Experimental, but not tested |
Light intensity combined with structure of predator community | 79, 194, 195 |
| Smaller nests with lower insulation properties prevent overheating of eggs prior to incubation | Higher egg hatching success in smaller, less insulated, nests | Cavity-nesting passerines TS |
Not tested | Hot conditions and direct sunlight exposure combined with larger clutches favour asynchronous hatching | 166 M. Lambrechts (Idea) |
| Smaller nests with lower insulation properties prevent overheating of nestlings | Higher fledging success in smaller, less insulated, nests | GT | Not tested | Hot conditions combined with larger broods | 18 |
| Smaller or thinner nests reduce nest predation risks | More eggs, hatchlings, or fledglings in smaller or thinner nests | GT, BT | Correlative, Experimental | High nest predation pressures | 60, 61, 76, 87 |
| Dry nests lower cooling of eggs or ectotherm nestlings. Smaller moss-based nests dry out more quickly and therefore built in conditions with more rainfall | More hatchlings and fledglings in smaller moss-based nests | GT, BT | Correlative Not tested |
Cavities frequently exposed to rainfall | 58, 91 |
| Indirect effects | |||||
| Better parents produce bigger nests | Less nest desertion, larger clutches, and more hatchlings or fledglings, in bigger nests | BT, GT | Correlative | Individual-specific physiology | 54, 144, 145, 146, 203 |
| Territories providing more resources contain bigger nests | Less nest desertion, larger clutches, and more hatchlings or fledglings, in bigger nests | BT | Food supplementation | Individual-specific resource availability | 54, 147, 204 |
| Bigger nests attract better mates or stimulate reproductive investment | Larger clutches, more hatchlings, and more fledglings in bigger nests | PF, BT | Correlative Experimental |
Individual-specific resource availability and post-mating investments in nest building | 71, 89, 205, 210 |
| Animal-derived nest material (ADNM) | |||||
| Direct effects | |||||
| Nests with more ADNM better hide clutches reducing interspecific competition or predation risks | Less nest desertion or more hatchlings in nests with more ADNM | HW, GT, BT | Correlative, Experimental |
Environments with more competitors | 51, 107, 108 |
| ADNM creates a physical barrier to keep incubators, eggs and nestlings dry in wet moss-based nests. Wet nests accelerate cooling | More hatchlings or ectotherm nestlings, or better growing embryos or nestlings in wet nests with more ADNM | Cavity-nesters mainly using moss GT, BT |
Not tested | Wet environments combined with use moss to build the foundation of the nest given that moss-based materials rapidly absorb rainwater and slowly dry out, whereas hair or fur dry out faster | 91 M. Lambrechts & D.C. Deeming (Idea) |
| Nests with more ADNM provide better insulation when the incubator is on the eggs or ectotherm nestlings | More hatchlings or ectotherm nestlings, or better growing embryos or nestlings, in nests with more ADNM | Small-bodied passerines, TS, BT, GT | Correlative Experiments |
Colder climates and smaller bird species | 51, 66, 100, 106, 142, 147, 168, 170 |
| Nests with more ADNM hamper cooling of eggs or ectotherm nestlings when the incubator or brooder is off the eggs | More hatchlings or ectotherm nestlings, or better growing embryos or nestlings, in nests with more ADNM | GT, TS | Laboratory Not tested in the field |
Colder climates and smaller bird species | 99, 100, 106, 165, 206 |
| ADNM creates a physical barrier against nest parasites | Ectotherm and endotherm nestlings grow better in nests with more ADNM | TS, GT, BT | Correlative, Experimental |
Cavity nests with nest parasites | 92, 99, 100, 168, 170 |
| ADNM provides comfort during incubation or parental care stages | More hatchlings or fledglings in nests with more ADNM | SS | Not tested | Nest foundations built from hard nest material | 207 |
| Nests with less visible ADNM components attract less predators | Less nest desertion, and more hatchlings or fledglings, in nests with less ADNM | Passerines with nest rims close to the entrance hole | Not tested | Environments with nest predators. More likely in open nesters. | 106 |
| Nests with more mammal-derived material reduce risks of nest take overs or brood parasitism | Less nest desertion, and more hatchlings or nestlings, in nests with more mammal-derived material | GT | Correlative Experimental |
Environments with nest predators or competitors | 51, 108 |
| Nests with less ADNM decrease risks associated with premature egg heating or hyperthermia in nestlings | More hatchlings or fledglings in nests with less ADNM | Passerines with abundant ADNM | Not tested | Large broods combined with hot weather | 106 M. Lambrechts & D.C. Deeming (Idea) |
| Indirect effects | |||||
| Better parents deliver more ADNM used in status signaling | More hatchlings and fledglings in nests with more ADNM | SS, BT | Correlative Experimental |
Spatiotemporal variation in availability combined with increased intraspecific competition | 83, 102, 207 |
| Better territories result in more ADNM | More hatchlings and fledglings in nests with more ADNM | GT, BT | Food supplementation | Spatiotemporal variation in availability | 204 |
| Nests with more ADNM attract better mates or stimulate reproductive investment | More hatchlings and fledglings in nests with more ADNM | SS, BT | Experimental | Spatiotemporal variation in availability combined with increased intraspecific competition | 102, 207 |
| ADNM induces costly intraspecific competition | More nest desertion and lower breeding success or lower-quality nestlings in nests with more ADNM | BT | Experimental | High population densities promoting intraspecific competition | 103, 170 |
| Greenery | |||||
| Direct effects | |||||
| Greenery protects the roosting or incubating parents against pathogens or blood parasites | Breeders, and consequently nestlings, in better physical condition in nests with more greenery | BT | Not tested | Cavities increasing risks associated with pathogens or invertebrate vector exposure | 121, 208 |
| Greenery reduces nest parasitism reducing deleterious effects on breeders or nestlings | Breeders or nestlings in better physical condition in nests with more greenery | BT, ES, SS, TS | Correlative Experimental |
Cavities increasing risks associated with nest parasite exposure | 124, 131, 140, 172, 209 |
| Greenery produces more volatile compounds improving health status or immunocompetence | Breeders or nestlings grow better and have a higher body condition in nests with more greenery | ES | Experimental | All cavity types | 116, 129 |
| Greenery provides comfort during incubation | More hatchlings in nests with more greenery | ES | Correlative | All cavity types | 129 |
| Indirect effects | |||||
| Better parents deliver more greenery used in status signaling | Less nest desertion, and higher breeding success or higher-quality nestlings in nests with more greenery | SS, B | Experimental | Spatiotemporal variation in availability combined with increased intraspecific competition | 89, 126, 207 |
| Better territories result in more greenery delivered to the nest | Less nest desertion, and higher breeding success or higher-quality nestlings in nests with more greenery | Passerines from dry or heavily urbanized regions BT, ES, SS |
Not tested | Climate change reduces availability of nest material. Spatiotemporal variation in greenery availability perhaps covarying with other essential humidity-associated resources required for reproduction |
166 M. Lambrechts & D.C. Deeming (Idea) |
| More greenery improves mate attraction or stimulates reproductive investment in partners or offspring | Less nest desertion, and higher breeding success or higher-quality nestlings in nests with more greenery | ES, SS, BT | Experimental | Spatiotemporal variation in availability combined with increased intraspecific competition | 89, 118, 124, 207 |
| Greenery induces costly intraspecific competition | More nest desertion and lower breeding success or lower-quality nestlings in nests with more greenery | SS | Experimental | High population densities promoting intraspecific competition | 126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





