Submitted:
01 March 2024
Posted:
04 March 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Patient Population
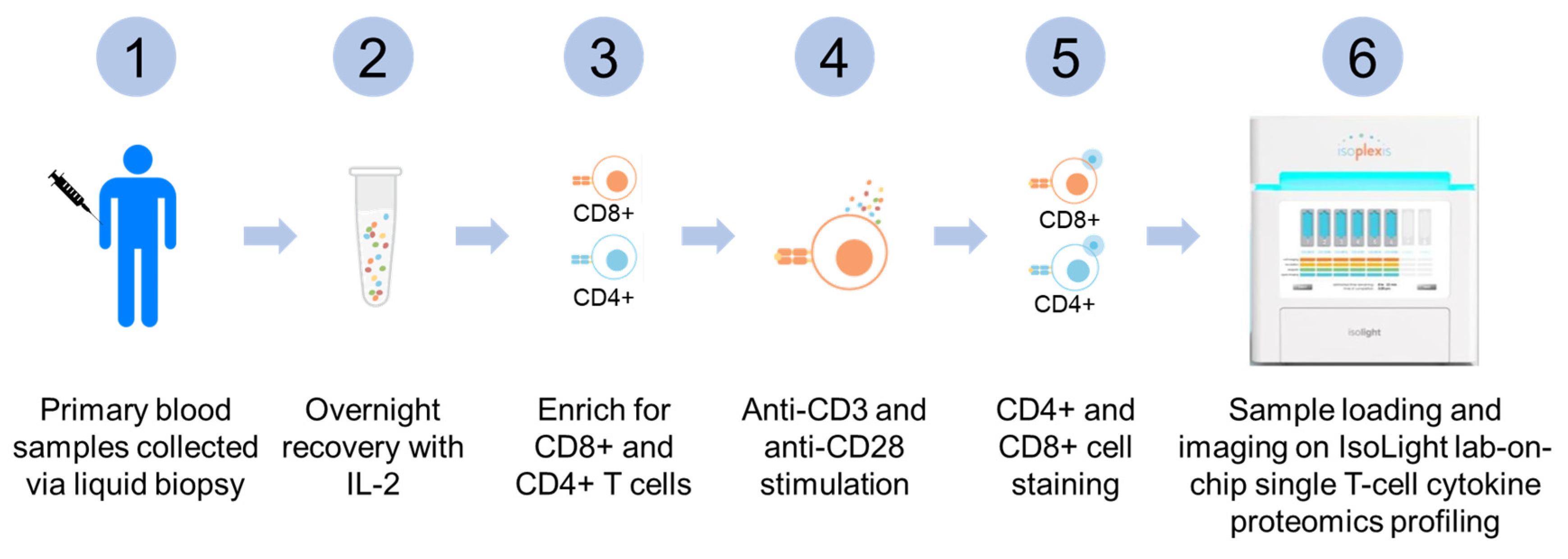
2.2. Collection, Processing, and Selection of T-Lymphocytes from Peripheral Blood Samples
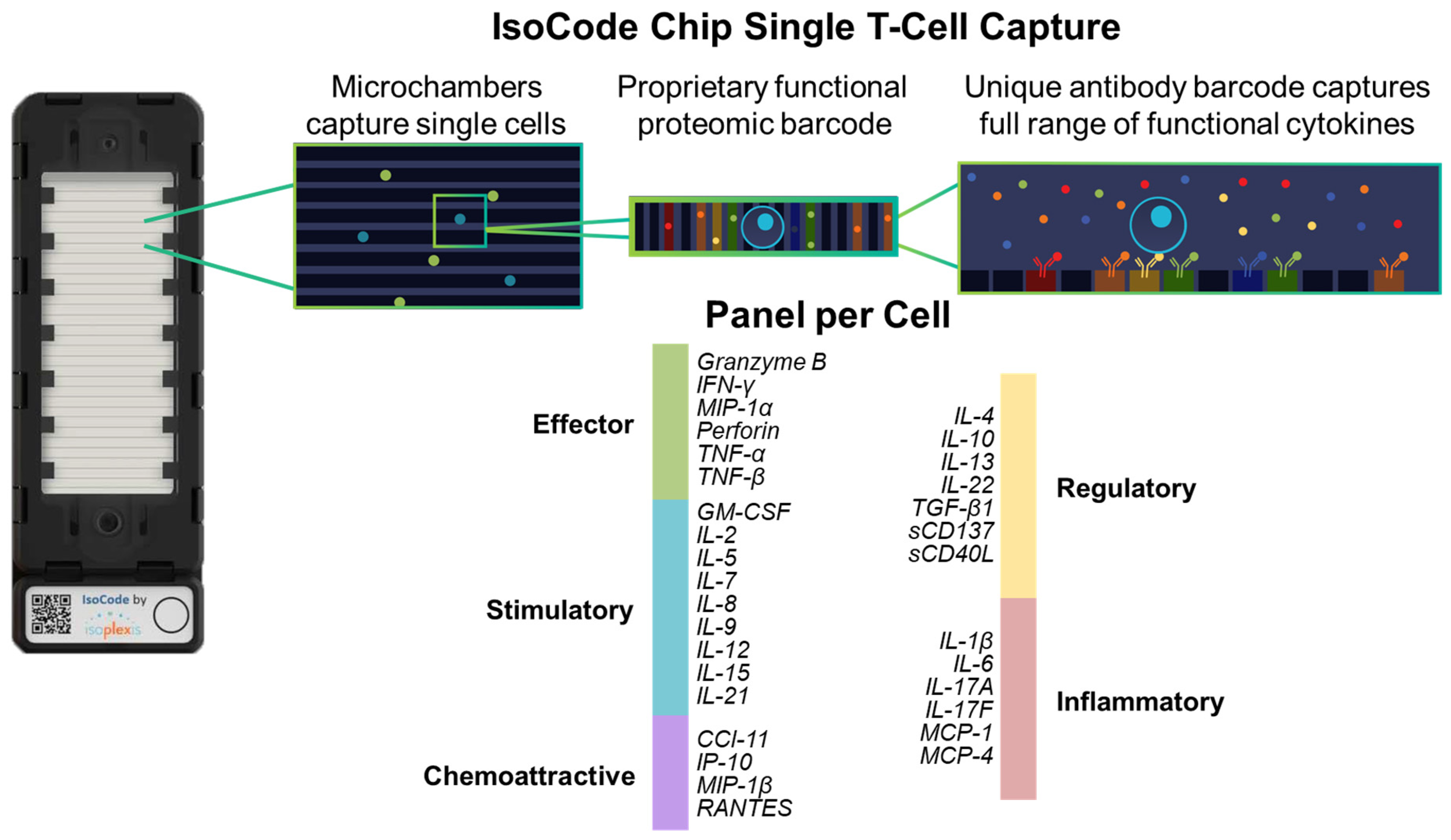
2.3. Live T-Lymphocytes Single Cell Proteomic Cytokines Profiling Using Automated Microfluidics Lab-On-Chip Isolight Platform
2.4. Polyfunctional Strength Index (PSI)
2.5. Biostatistics and Bioinformatics Analysis
3. Results
3.1. Study Cohort and Clinical Characteristics
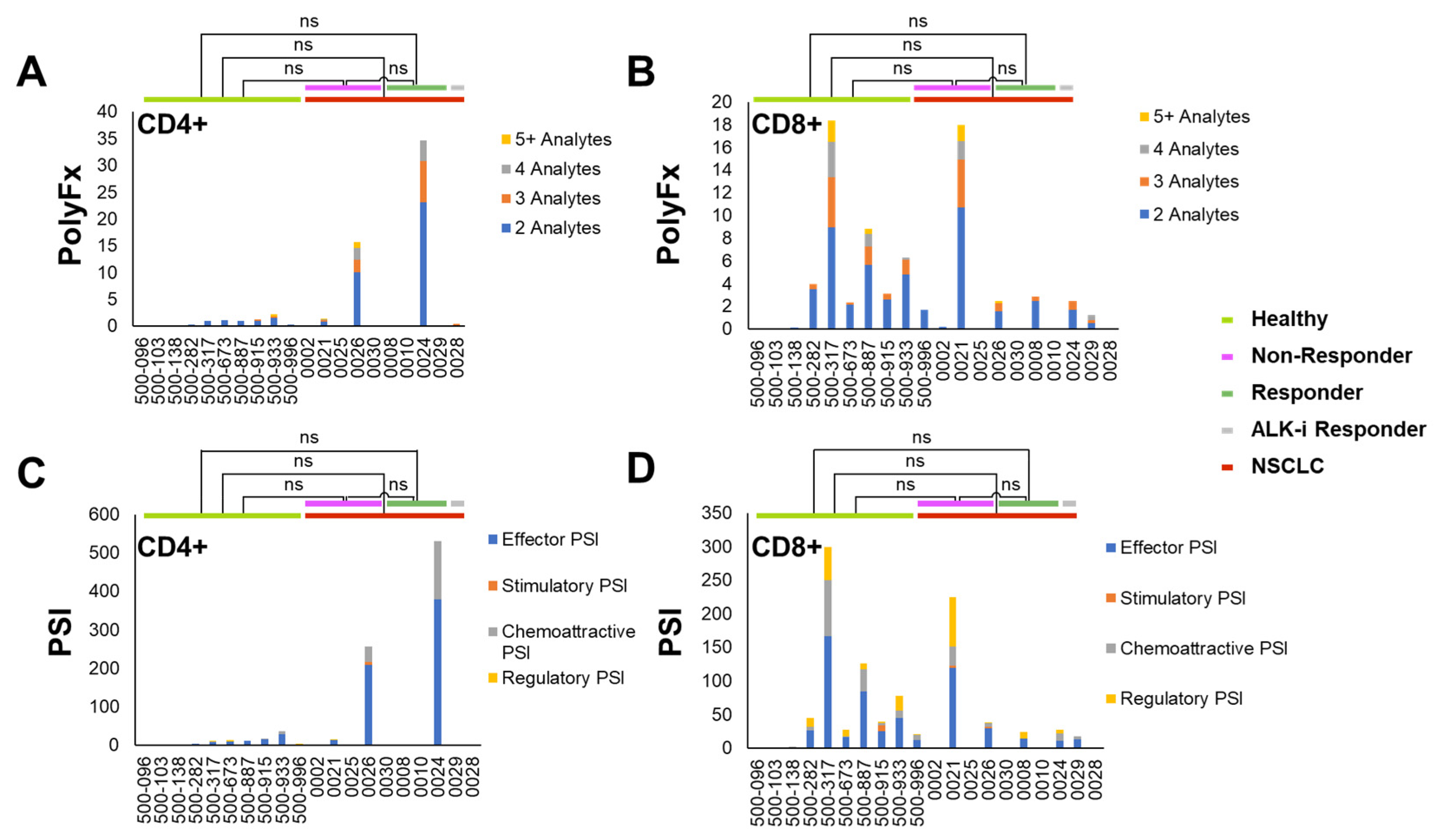
3.2. Pre-Treatment Baseline Single T-Lymphocytes Overall Polyfunctionality (PolyFx) And Polyfunctional Strength Index (PSI): Healthy Donors versus NSCLC
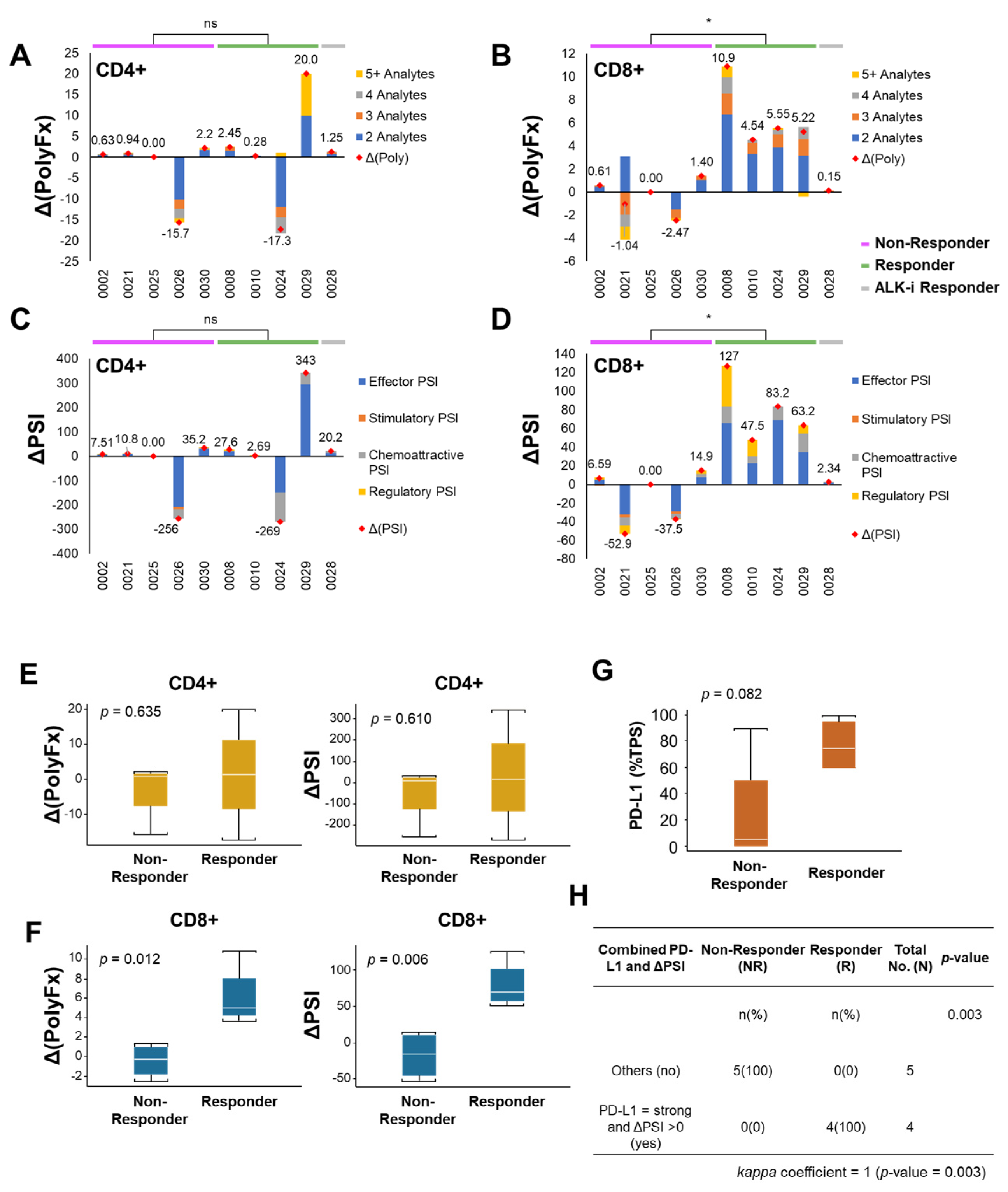
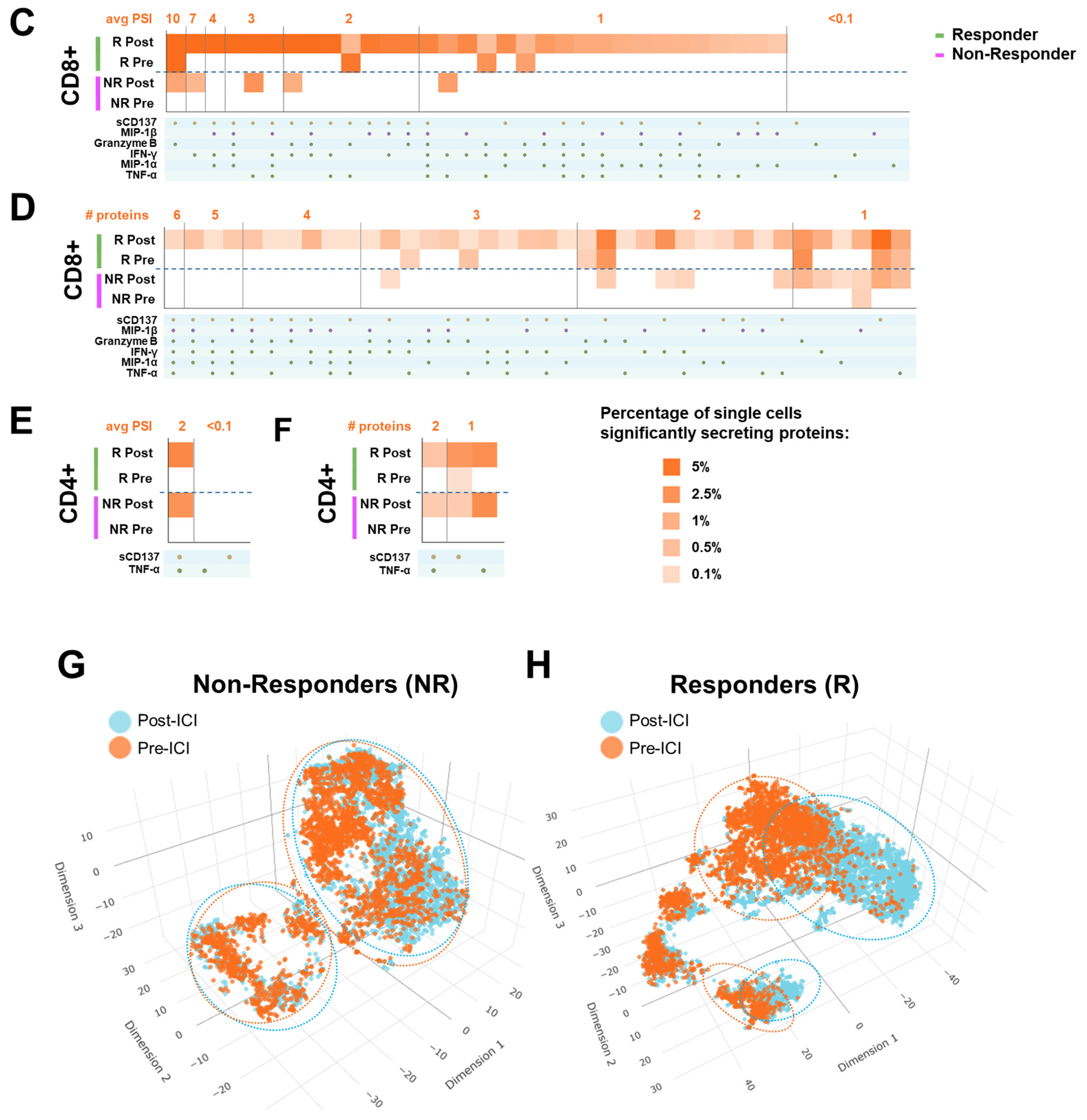
3.3. Early Changes in Peripheral CD8+ T-Lymphocytes Single Cell Overall Polyfunctionality (ΔPolyFx) and PSI (ΔPSI) as Novel Predictors of ICI Treatment Response
3.4. CD8+ ΔPolyFx and ΔPSI Perform Better than PD-L1 Tumor Proportion Score (TPS) Alone as a Predictive Biomarker for ICI Treatment Response in NSCLC
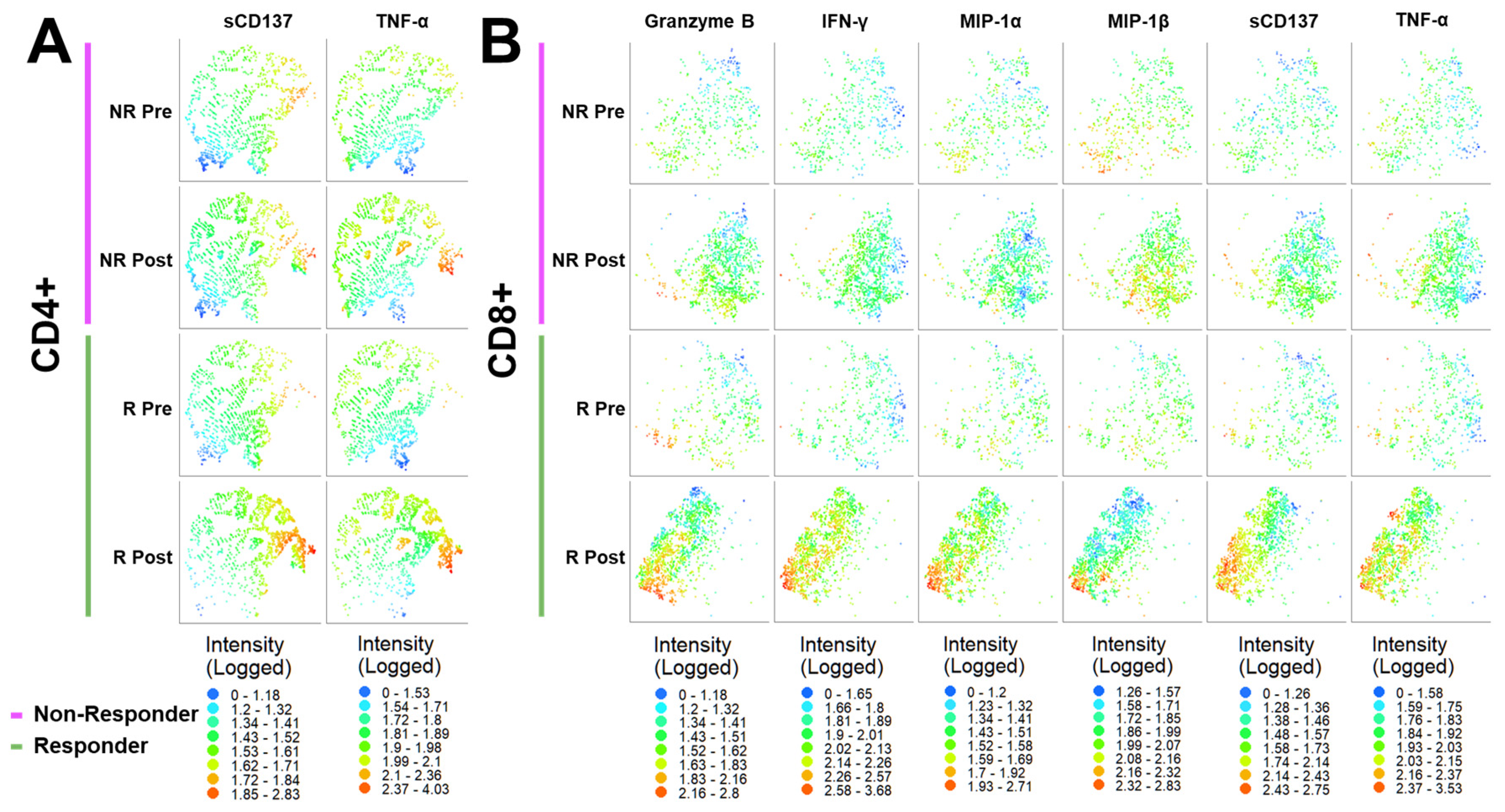
3.5. 2D t-SNE and 3D t-SNE Bioinformatics Analysis of Responder (R) and Non-Responder (NR) Treatment Response Groups
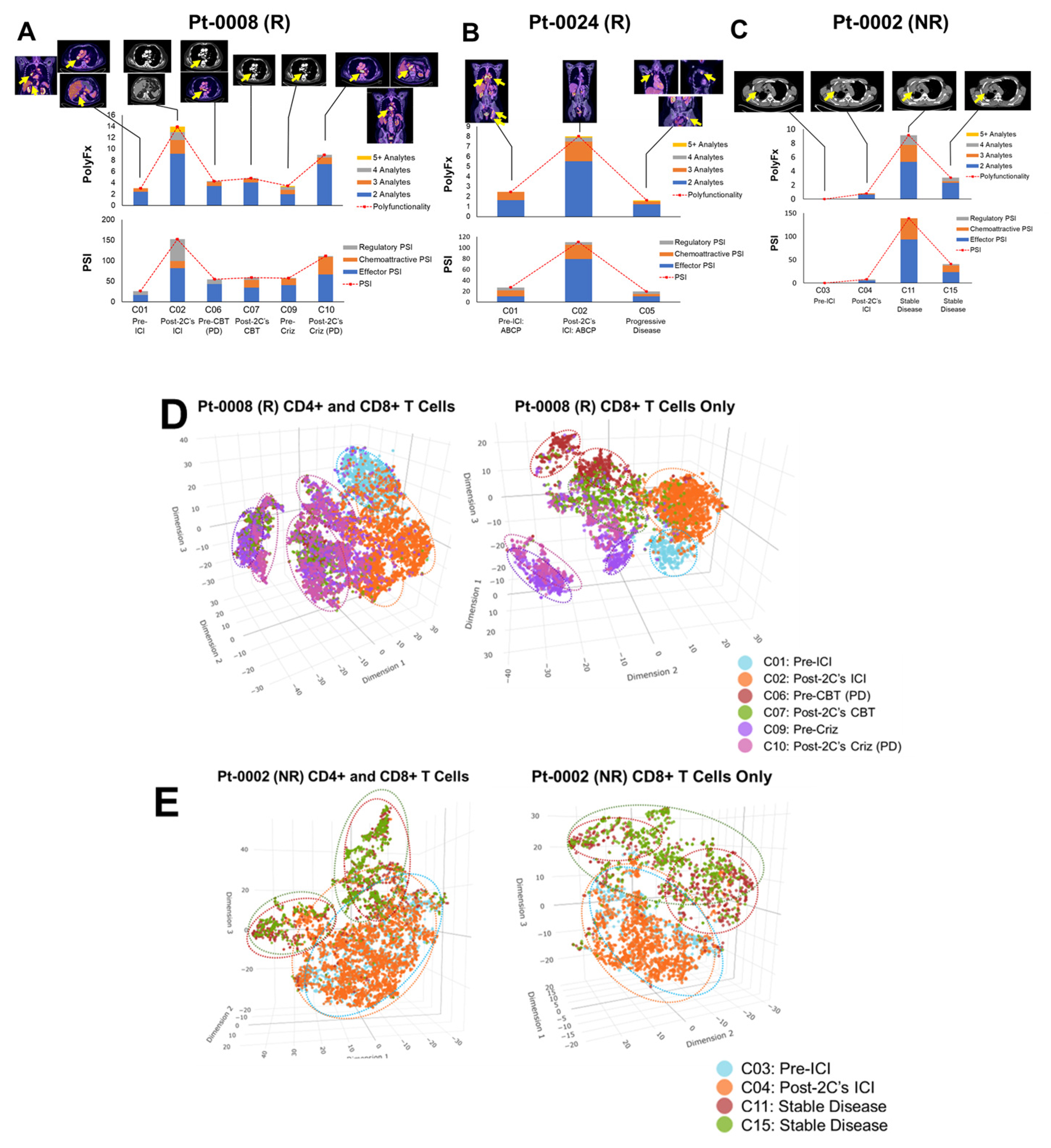
3.6. ΔPSI in Predicting Treatment Response of Oncogene-Addicted NSCLC under Targeted Therapies versus ICI Therapy
3.7. Longitudinal Monitoring of ICI Treatment Response Using Peripheral Single Cell T-Lymphocytes Proteomic Cytokines Profiling On Treatment
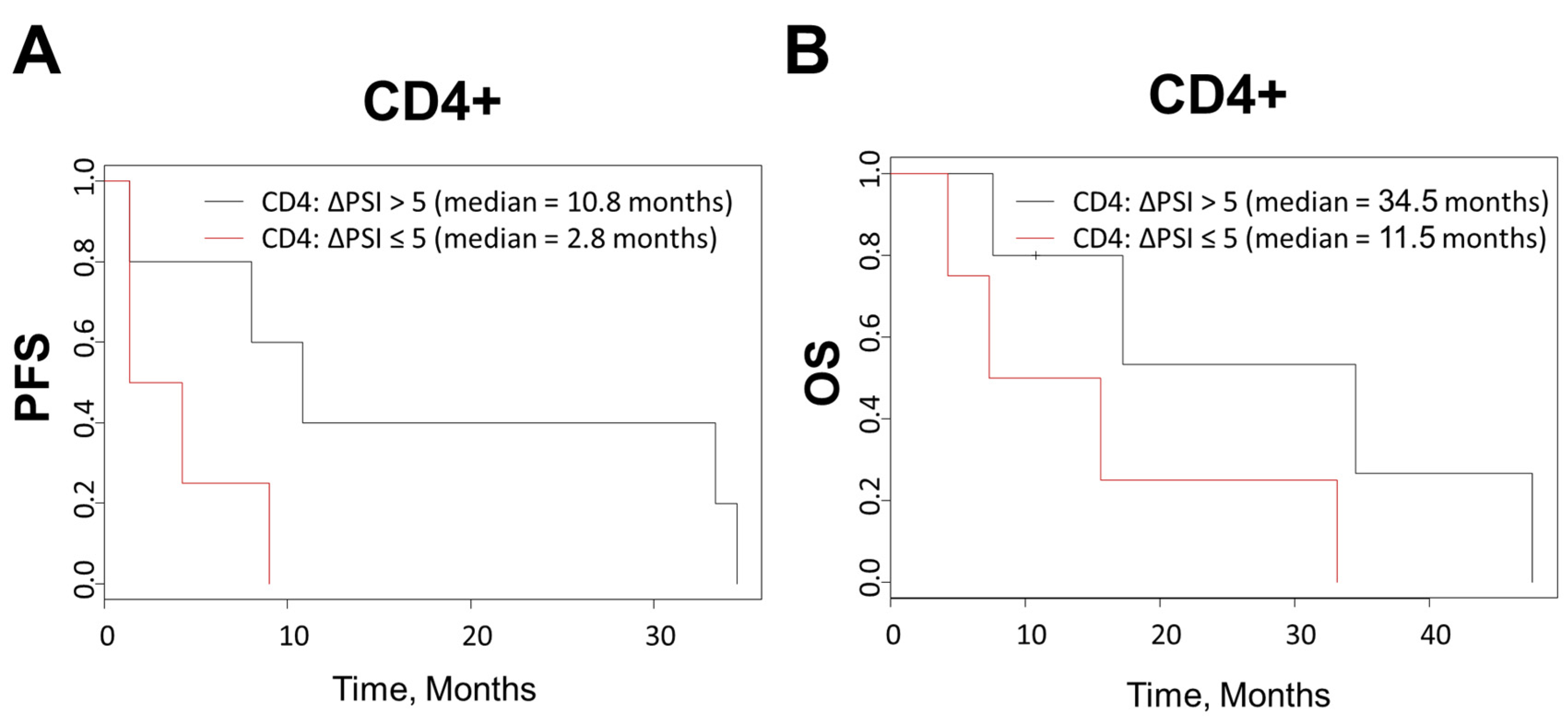
3.8. Kaplan-Meier Clinical Survival Outcomes Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, R.; Zhou, Y.; Wang, Y.; Du, C.; Wu, Y. Trends in Cancer Incidence and Mortality Rates in the United States from 1975 to 2016. Ann Transl Med 2020, 8, 1671. [Google Scholar] [CrossRef]
- Marrone, K.A.; Brahmer, J.R. Immune Checkpoint Therapy in Non-Small Cell Lung Cancer. Cancer J 2016, 22, 81–91. [Google Scholar] [CrossRef]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for Immune Checkpoint Inhibition in Non-Small Cell Lung Cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef]
- Won, S.E.; Park, H.J.; Byun, S.; Pyo, J.; Kim, J.H.; Choi, C.M.; Lee, J.C.; Lee, D.H.; Kim, S.W.; Yoon, S.; et al. Impact of Pseudoprogression and Treatment beyond Progression on Outcome in Patients with Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Oncoimmunology 2020, 9, 1776058. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune Checkpoint Inhibitors for Patients with Advanced Lung Cancer and Oncogenic Driver Alterations: Results from the IMMUNOTARGET Registry. Ann Oncol 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Akbay, E.A.; Koyama, S.; Carretero, J.; Altabef, A.; Tchaicha, J.H.; Christensen, C.L.; Mikse, O.R.; Cherniack, A.D.; Beauchamp, E.M.; Pugh, T.J.; et al. Activation of the PD-1 Pathway Contributes to Immune Escape in EGFR-Driven Lung Tumors. Cancer Discov 2013, 3, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Arcila, M.E.; Oxnard, G.R.; Nafa, K.; Riely, G.J.; Solomon, S.B.; Zakowski, M.F.; Kris, M.G.; Pao, W.; Miller, V.A.; Ladanyi, M. Rebiopsy of Lung Cancer Patients with Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin Cancer Res 2011, 17, 1169–1180. [Google Scholar] [CrossRef]
- Benedettini, E.; Sholl, L.M.; Peyton, M.; Reilly, J.; Ware, C.; Davis, L.; Vena, N.; Bailey, D.; Yeap, B.Y.; Fiorentino, M.; et al. Met Activation in Non-Small Cell Lung Cancer Is Associated with de Novo Resistance to EGFR Inhibitors and the Development of Brain Metastasis. Am J Pathol 2010, 177, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Calles, A.; Riess, J.W.; Brahmer, J.R. Checkpoint Blockade in Lung Cancer With Driver Mutation: Choose the Road Wisely. Am. Soc. Clin. Oncol. Educ. Book 2020, 372–384. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Rizvi, H.; Bandlamudi, C.; Sauter, J.L.; Travis, W.D.; Rekhtman, N.; Plodkowski, A.J.; Perez-Johnston, R.; Sawan, P.; Beras, A.; et al. Clinical and Molecular Correlates of PD-L1 Expression in Patients with Lung Adenocarcinomas. Ann Oncol 2020, 31, 599–608. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat Genet 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Veldore, V.H.; Choughule, A.; Routhu, T.; Mandloi, N.; Noronha, V.; Joshi, A.; Dutt, A.; Gupta, R.; Vedam, R.; Prabhash, K. Validation of Liquid Biopsy: Plasma Cell-Free DNA Testing in Clinical Management of Advanced Non-Small Cell Lung Cancer. Lung Cancer Targets Ther. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J. Current and Future Perspectives of Cell-Free DNA in Liquid Biopsy. Curr. Issues Mol. Biol. 2022, 44, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8(+) T Cell States in Human Cancer: Insights from Single-Cell Analysis. Nat Rev Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef]
- Osorio, J.C.; Arbour, K.C.; Le, D.T.; Durham, J.N.; Plodkowski, A.J.; Halpenny, D.F.; Ginsberg, M.S.; Sawan, P.; Crompton, J.G.; Yu, H.A.; et al. Lesion-Level Response Dynamics to Programmed Cell Death Protein (PD-1) Blockade. J Clin Oncol 2019, 37, 3546–3555. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, T.; Ye, J.; Li, H.; Huang, J.; Li, X.; Wu, B.; Huang, X.; Hou, J. Tumor-Infiltrating Lymphocytes Predict Response to Chemotherapy in Patients with Advance Non-Small Cell Lung Cancer. Cancer Immunol Immunother 2012, 61, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.R.; Ribas, A.; Mischel, P.S. Single-Cell Analysis Tools for Drug Discovery and Development. Nat Rev Drug Discov 2016, 15, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, P.K.; Gierahn, T.M.; Roederer, M.; Love, J.C. Single-Cell Technologies for Monitoring Immune Systems. Nat Immunol 2014, 15, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Stubbington, M.J.T.; Rozenblatt-Rosen, O.; Regev, A.; Teichmann, S.A. Single-Cell Transcriptomics to Explore the Immune System in Health and Disease. Science 2017, 358, 58–63. [Google Scholar] [CrossRef]
- Xue, Q.; Bettini, E.; Paczkowski, P.; Ng, C.; Kaiser, A.; McConnell, T.; Kodrasi, O.; Quigley, M.F.; Heath, J.; Fan, R.; et al. Single-Cell Multiplexed Cytokine Profiling of CD19 CAR-T Cells Reveals a Diverse Landscape of Polyfunctional Antigen-Specific Response. J Immunother Cancer 2017, 5, 85. [Google Scholar] [CrossRef]
- Duchemann, B.; Friboulet, L.; Besse, B. Therapeutic Management of ALK+ Nonsmall Cell Lung Cancer Patients. Eur Respir J 2015, 46, 230–242. [Google Scholar] [CrossRef]
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Kibirova, A.; Mattes, M.D.; Smolkin, M.; Ma, P.C. The Journey of an EGFR-Mutant Lung Adenocarcinoma through Erlotinib, Osimertinib and ABCP Immunotherapy Regimens: Sensitivity and Resistance. Case Rep. Oncol. 2019, 12, 765–776. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant Atezolizumab after Adjuvant Chemotherapy in Resected Stage IB-IIIA Non-Small-Cell Lung Cancer (IMpower010): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Lond. Engl. 2021, 398, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Anagnostou, V.; Landon, B.V.; Medina, J.E.; Forde, P.; Velculescu, V.E. Translating the Evolving Molecular Landscape of Tumors to Biomarkers of Response for Cancer Immunotherapy. Sci. Transl. Med. 2022, 14, eabo3958. [Google Scholar] [CrossRef]
- Anagnostou, V.; Bardelli, A.; Chan, T.A.; Turajlic, S. The Status of Tumor Mutational Burden and Immunotherapy. Nat. Cancer 2022, 3, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Ho, C.; Nicholas, G.; Juergens, R.A.; Sacher, A.; Fung, A.S.; Wheatley-Price, P.; Laurie, S.A.; Levy, B.; Brahmer, J.R.; et al. ctDNA Response after Pembrolizumab in Non-Small Cell Lung Cancer: Phase 2 Adaptive Trial Results. Nat. Med. 2023, 29, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Oloomi, M.; Moazzezy, N.; Bouzari, S. Comparing Blood versus Tissue-Based Biomarkers Expression in Breast Cancer Patients. Heliyon 2020, 6, e03728. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, H.; Ahmed, S.; Armstrong, S.; Mok, T.; Jalal, S.I. Blood-Based Tumor Biomarkers in Lung Cancer for Detection and Treatment. Transl. Lung Cancer Res. 2017, 6, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Hou, Y.; Zhou, X.; Li, X.; Yong, J.; Wang, Y.; Wang, W.; Yan, J.; Hu, B.; Guo, H.; et al. Single-Cell Multiomics Sequencing and Analyses of Human Colorectal Cancer. Science 2018, 362, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Rovers, S.; Janssens, A.; Raskin, J.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.; Marcq, E. Recent Advances of Immune Checkpoint Inhibition and Potential for (Combined) TIGIT Blockade as a New Strategy for Malignant Pleural Mesothelioma. Biomedicines 2022, 10, 673. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT Co-Inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Kwek, S.S.; Raju, S.S.; Li, T.; McCarthy, E.; Chow, E.; Aran, D.; Ilano, A.; Pai, C.-C.S.; Rancan, C.; et al. Intratumoral CD4+ T Cells Mediate Anti-Tumor Cytotoxicity in Human Bladder Cancer. Cell 2020, 181, 1612–1625. [Google Scholar] [CrossRef]
- González-Navajas, J.M.; Elkord, E.; Lee, J. Editorial: CD4+ T Cells in Cancer Immunotherapies. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4+ T-Cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef]
- Chu, Y.; Dai, E.; Li, Y.; Han, G.; Pei, G.; Ingram, D.R.; Thakkar, K.; Qin, J.-J.; Dang, M.; Le, X.; et al. Pan-Cancer T Cell Atlas Links a Cellular Stress Response State to Immunotherapy Resistance. Nat. Med. 2023, 29, 1550–1562. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two Subsets of Memory T Lymphocytes with Distinct Homing Potentials and Effector Functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Imai, N.; Tawara, I.; Yamane, M.; Muraoka, D.; Shiku, H.; Ikeda, H. CD4+ T Cells Support Polyfunctionality of Cytotoxic CD8+ T Cells with Memory Potential in Immunological Control of Tumor. Cancer Sci. 2020, 111, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Earland, N.; Zhang, W.; Usmani, A.; Nene, A.; Bacchiocchi, A.; Chen, D.Y.; Sznol, M.; Halaban, R.; Chaudhuri, A.A.; Newman, A.M. CD4 T Cells and Toxicity from Immune Checkpoint Blockade. Immunol. Rev. 2023, 318, 96–109. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Tang, T.; Ali, F.S.; Johnson, D.H.; Qiao, W.; Diab, A.; Wang, Y. The Impact of Immune Checkpoint Inhibitor-Related Adverse Events and Their Immunosuppressive Treatment on Patients’ Outcomes. J. Immunother. Precis. Oncol. 2020, 1, 7–18. [Google Scholar] [CrossRef]
- Owen, D.H.; Wei, L.; Bertino, E.M.; Edd, T.; Villalona-Calero, M.A.; He, K.; Shields, P.G.; Carbone, D.P.; Otterson, G.A. Incidence, Risk Factors, and Effect on Survival of Immune-Related Adverse Events in Patients With Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, e893–e900. [Google Scholar] [CrossRef]
- Albandar, H.J.; Fuqua, J.; Albandar, J.M.; Safi, S.; Merrill, S.A.; Ma, P.C. Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not? Cancers 2021, 13, 989. [Google Scholar] [CrossRef] [PubMed]
| Patient ID | Age | Sex | Histology/Genotype | TNM Stage (8th Ed.) | PD-L1 (22C3) | Tx Response (s/p 2 Cycles) | ICI Regimen |
| 0002 | 66 | M | Adenocarcinoma | IIIC (T4N3M0) |
90% | SD 1 | Durvalumab |
| 0008 | 79 | M | Adenocarcinoma, MET-amplified | IVB (T4N2M1c) | 95% | Excellent PR 2 | Pembrolizumab |
| 0010 | 68 | F | Adenocarcinoma, KRAS-G12C | IVA (T4N2M1a) |
60% | Excellent PR 2 | Carbo/Pem/Pembro 6 |
| 0021 | 71 | M | Squamous Cell | Recurrence IVA (T4N2M1a) |
5% | SD1 | Pembrolizumab |
| 0024 | 49 | F | Adenocarcinoma, EGFR-del19 (E746_A750del), T790M | IVB (T2N2M1c) |
60% | CR 3 | ABCP 7 |
| 0025 | 66 | F | Adenocarcinoma, KRAS-G12C, STK11+, TMB-High (11 muts/Mb) | Recurrence IVA (T1bN2M1b) | <1% | PD4 | Carbo/Pem/Pembro 6 |
| 0026 | 57 | F | Adenocarcinoma, EGFR-del19 (p.L747_P753 delinsS); PIK3CA-E545K | Recurrence IVA (T3N1M1a) |
50% | PD4 | ABCP 7 |
| 0028 | 78 | M | Adenocarcinoma, ALK-fusion | Recurrence IVA (TxN2M1a) |
2% | n/a (Alectinib Responder) | n/a |
| 0029 | 60 | F | Adenocarcinoma | IVA (T2aN1M1b) | 100% | PR 2 (also w/ later Dx 5 of Ovarian Ca) | Pembrolizumab |
| 0030 | 78 | F | Adenocarcinoma, KRAS-G12D; PTEN-R130G | IVB (TxN2M1c) |
<1% | Non-Responder | Carbo/Pem/Pembro 6 |
| 500-096 | 70 | F | Healthy | n/a | n/a | n/a | n/a |
| 500-103 | 53 | F | Healthy | n/a | n/a | n/a | n/a |
| 500-138 | 53 | F | Healthy | n/a | n/a | n/a | n/a |
| 500-282 | 61 | M | Healthy | n/a | n/a | n/a | n/a |
| 500-317 | 61 | M | Healthy | n/a | n/a | n/a | n/a |
| 500-673 | 62 | M | Healthy | n/a | n/a | n/a | n/a |
| 500-887 | 61 | F | Healthy | n/a | n/a | n/a | n/a |
| 500-915 | 58 | F | Healthy | n/a | n/a | n/a | n/a |
| 500-933 | 61 | M | Healthy | n/a | n/a | n/a | n/a |
| 500-996 | 56 | F | Healthy | n/a | n/a | n/a | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




