Submitted:
01 February 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
- understand complex reaction pathways (with the aid of appropriate additional characterisation), and
- provide learning into how to suppress TR following the onset of rapid deterioration of electrodes and electrolyte.
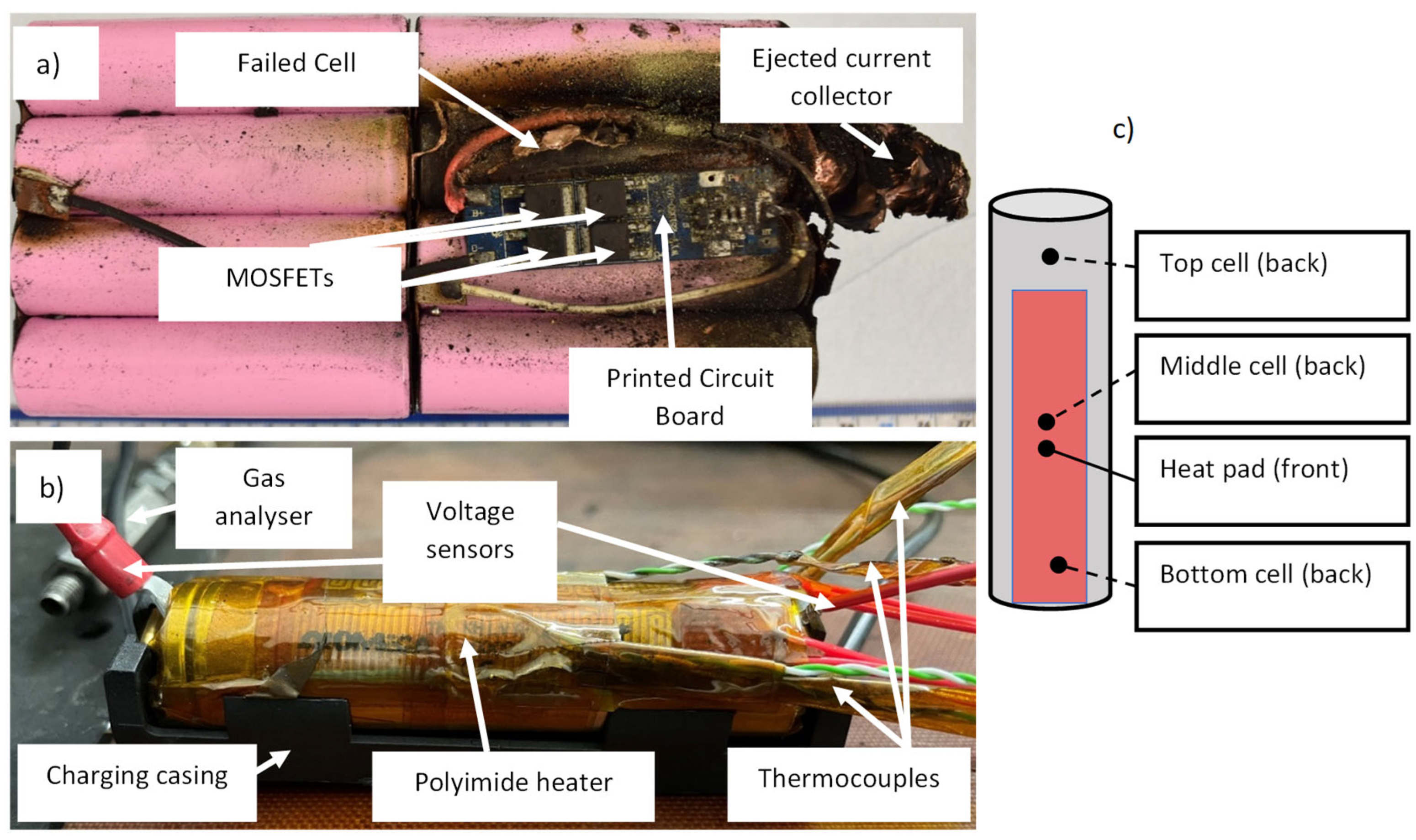
2. Experimental
3. Results and Discussion
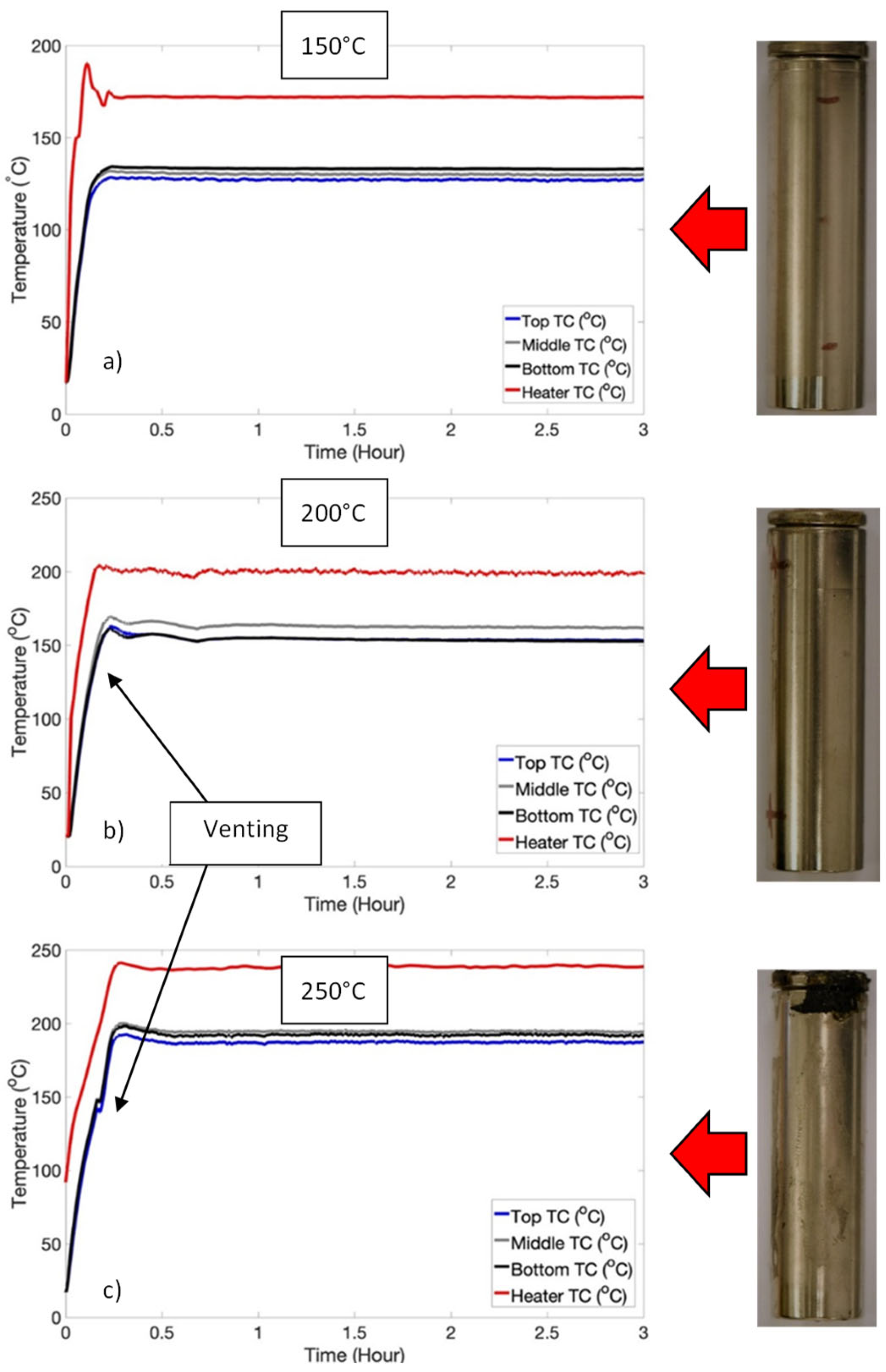
3.1. Temperature-Induced Venting Without Thermal Runaway: Low SOC Regime
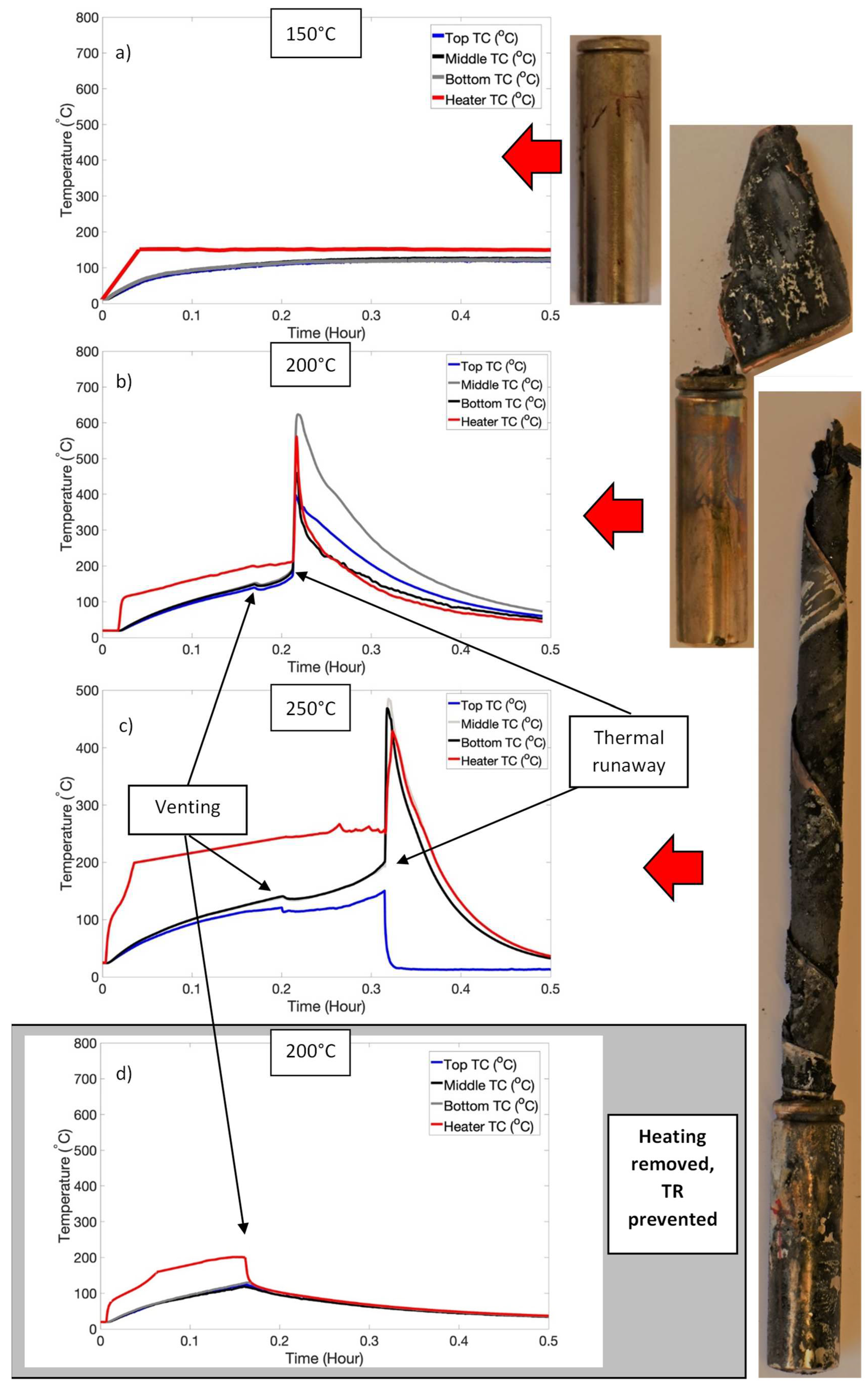
3.2. Triggering of Thermal Runaway: High SOC Regime
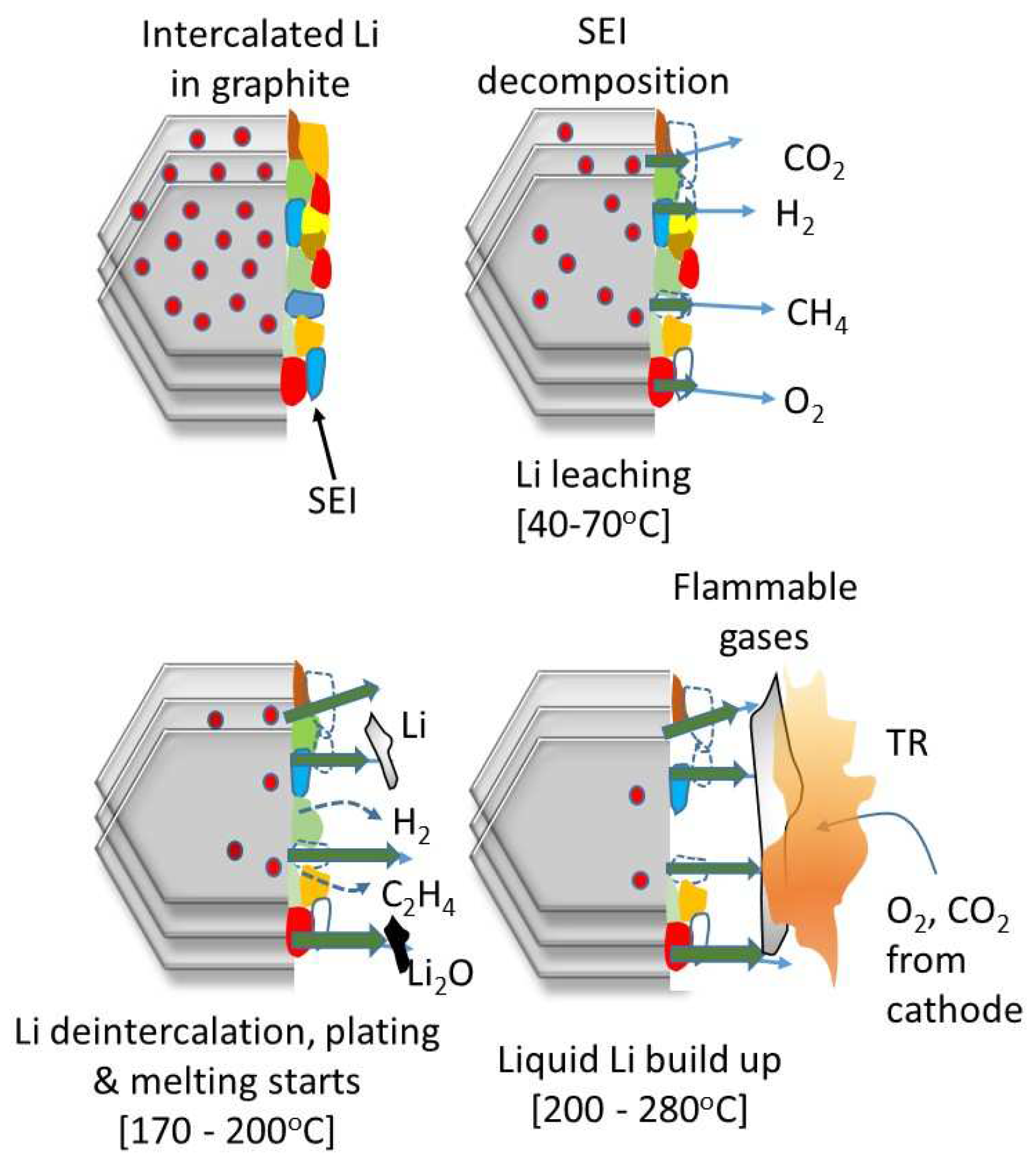
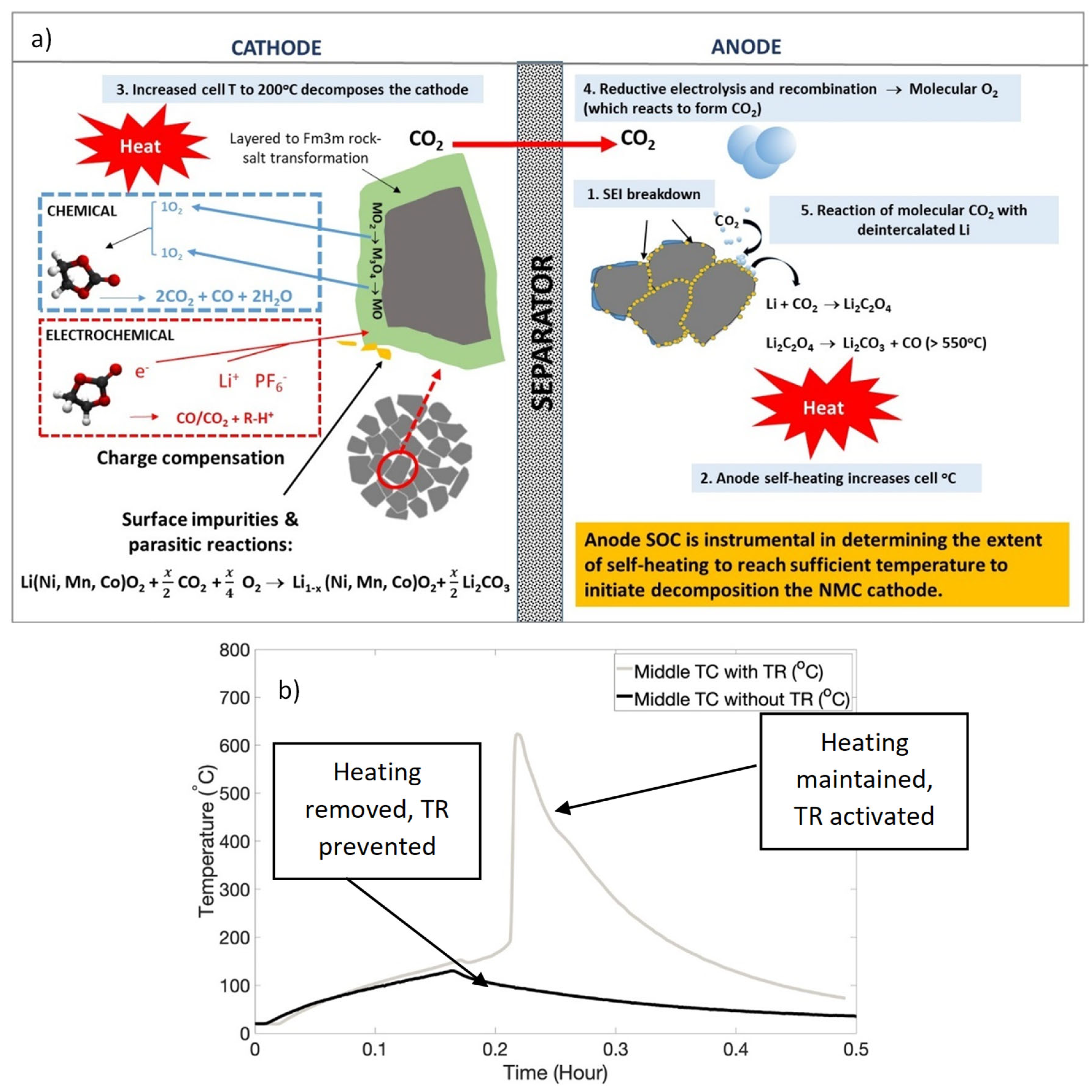
3.3. Implications of NMC Cathode Chemistry on TR
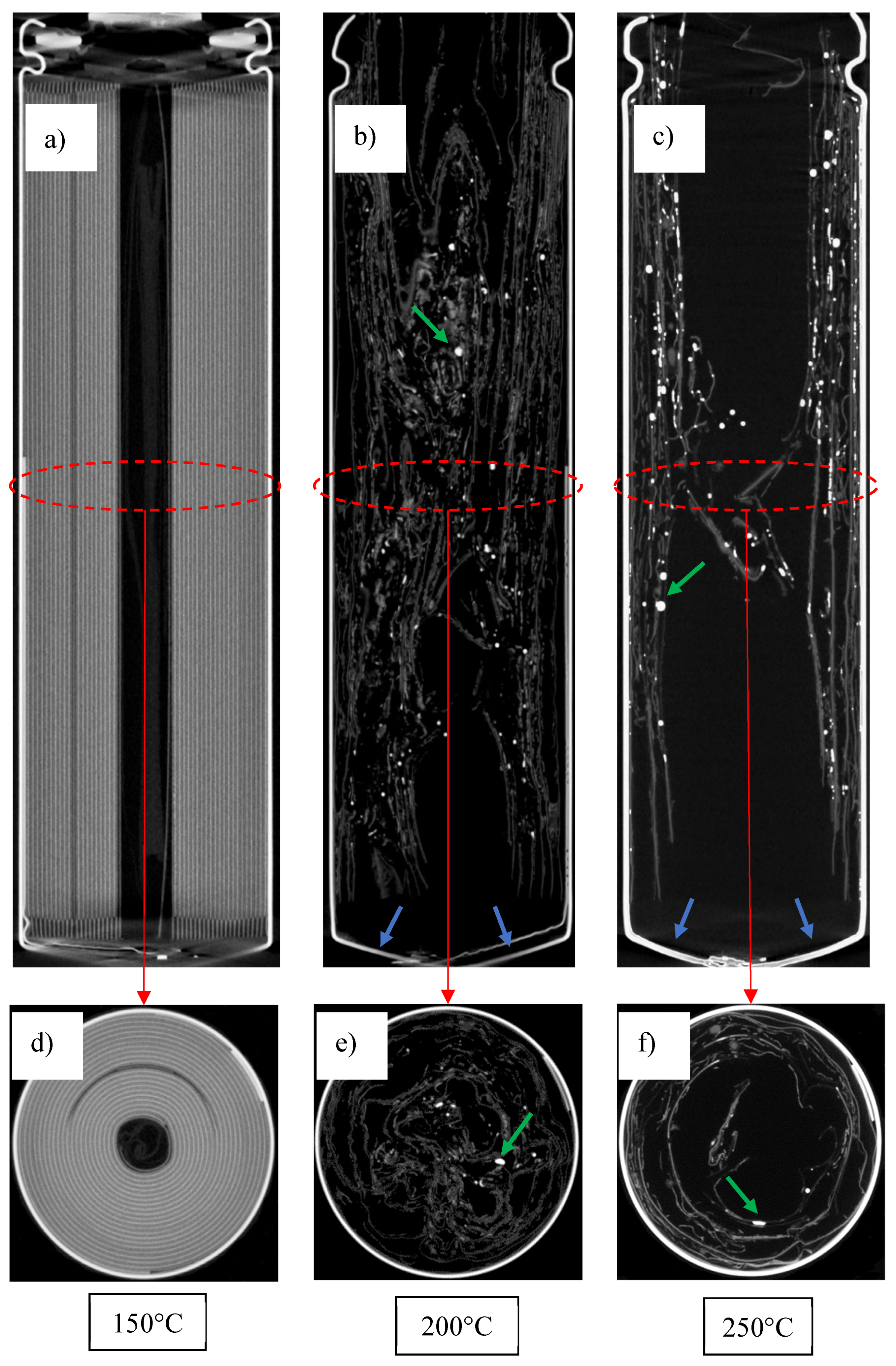
3.4. Examination of Cells Following Thermal Runaway

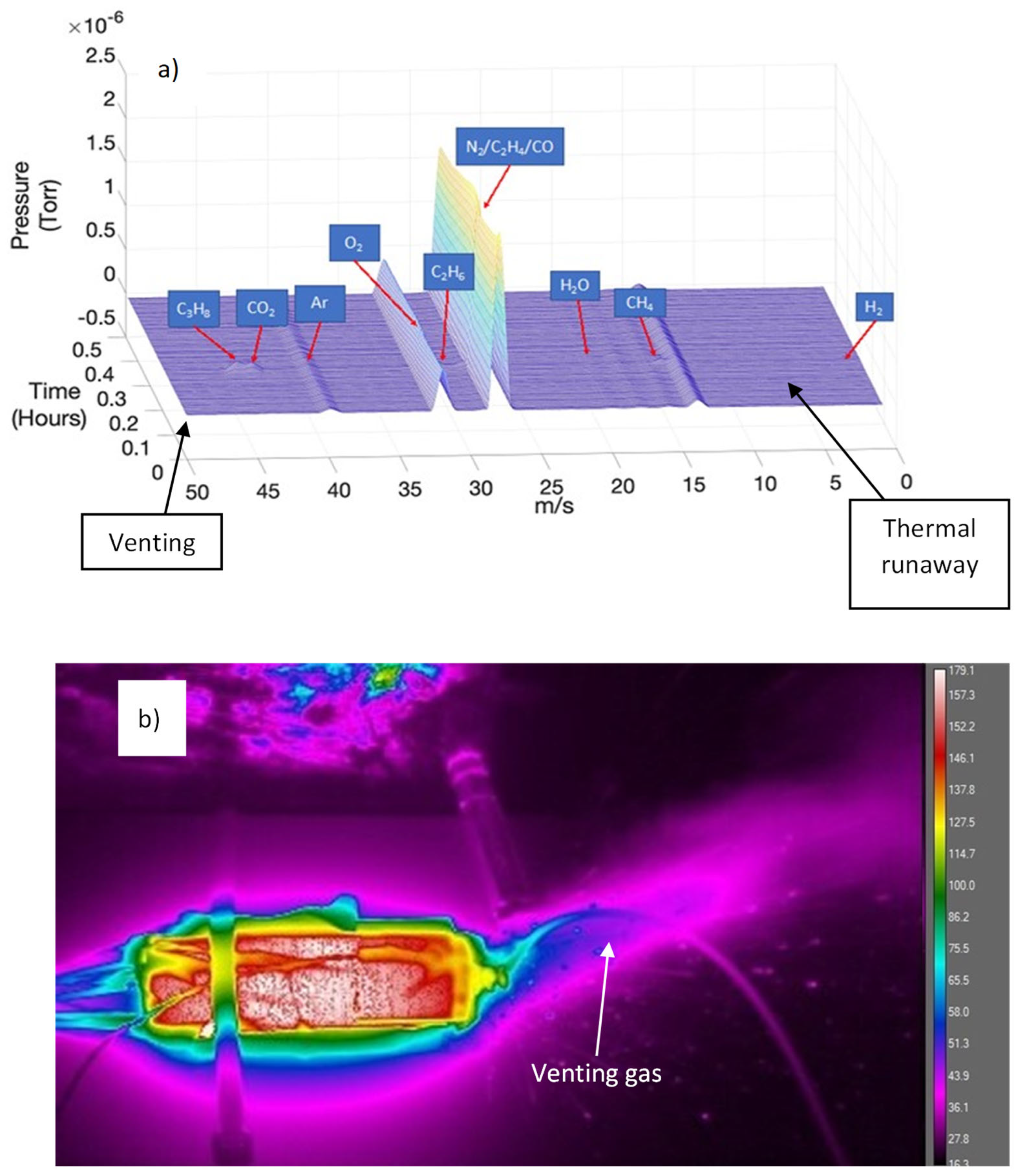
3.5. Released Gas Analysis
4. Conclusions
Author Contributions
Acknowledgments
Declaration of Competing Interest
Glossary
| CC = constant current |
| CV = constant voltage |
| C = coulomb |
| NMC = lithium nickel cobalt manganese oxide |
| SEI = solid electrolyte interphase |
| SOC = state of charge |
| TR = thermal runaway |
| LIB = lithium ion battery |
| BMS = battery management system |
| TC = thermocouple |
| PID = proportional integral derivative controller |
| CID = current interrupt device |
| PTC = positive temperature coefficient device |
| QMS = quadrupole mass spectrometer |
| PCB = printed circuit board |
| ISC = internal short circuit |
| XRD = x-ray diffraction |
| MS = mass spectroscopy |
| TM = transition metal |
| PVdF = polyvinylidene fluoride |
| NDT = non-destructive testing |
References
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef]
- Lisbona, D.; Snee, T. A review of hazards associated with primary lithium and lithium-ion batteries. Process Saf. Environ. Prot. 2011, 89, 434–442. [Google Scholar] [CrossRef]
- Advanced Science - 2017 - Finegan - Identifying the Cause of Rupture of Li-Ion Batteries during Thermal Runaway.pdf.
- Hou, J.; et al. Thermal runaway of Lithium-ion batteries employing LiN(SO2F)2-based concentrated electrolytes. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; et al. Detection of Li-ion battery failure and venting with Carbon Dioxide sensors. eTransportation 2021, 7, 100100. [Google Scholar] [CrossRef]
- Gao, A.; Xu, F.; Dong, W. The Concept of early monitoring and warning of thermal runaway of lithium-ion power battery using parameter analysis. J. Phys. Conf. Ser. 2022, 2181. [Google Scholar] [CrossRef]
- Adv Energy and Sustain Res - 2021 - McKerracher - Advances in Prevention of Thermal Runaway in Lithium-Ion Batteries.pdf.
- Srinivasan, R.; et al. Review—Thermal Safety Management in Li-Ion Batteries: Current Issues and Perspectives. J. Electrochem. Soc. 2020, 167, 140516. [Google Scholar] [CrossRef]
- Golubkov, A.W.; et al. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Authorized NO, T.; Further FO, R.; Or, R.; Without, D.; From, P. Household and Commercial Batteries.
- Bareño, J.; et al. Effect of overcharge on Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium ion cells with poly(vinylidene fluoride) binder. III — Chemical changes in the cathode. J. Power Sources 2018, 385, 165–171. [Google Scholar] [CrossRef]
- Liu, X.; et al. Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit. Joule 2018, 2, 2047–2064. [Google Scholar] [CrossRef]
- Mendoza-Hernandez, O.S.; Ishikawa, H.; Nishikawa, Y.; Maruyama, Y.; Umeda, M. Cathode material comparison of thermal runaway behavior of Li-ion cells at different state of charges including over charge. J. Power Sources 2015, 280, 499–504. [Google Scholar] [CrossRef]
- Gilbert, J.A.; et al. Cycling Behavior of NCM523/Graphite Lithium-Ion Cells in the 3–4.4 V Range: Diagnostic Studies of Full Cells and Harvested Electrodes. J. Electrochem. Soc. 2017, 164, A6054–A6065. [Google Scholar] [CrossRef]
- Wu, L.; et al. Structural origin of overcharge-induced thermal instability of Ni-containing layered-cathodes for high-energy-density lithium batteries. Chem. Mater. 2011, 23, 3953–3960. [Google Scholar] [CrossRef]
- Shu, J.; et al. In-situ X-ray diffraction study on the structural evolutions of LiNi 0.5Co0.3Mn0.2O2 in different working potential windows. J. Power Sources 2014, 245, 7–18. [Google Scholar] [CrossRef]
- Zheng, Y.; et al. Influence of over-discharge on the lifetime and performance of LiFePO4/graphite batteries. RSC Adv. 2016, 6, 30474–30483. [Google Scholar] [CrossRef]
- Li, T.; et al. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries. Electrochemical Energy Reviews vol. 3 (Springer Singapore, 2020).
- Zeng, X.; Zhan, C.; Lu, J.; Amine, K. Stabilization of a High-Capacity and High-Power Nickel-Based Cathode for Li-Ion Batteries. Chem 2018, 4, 690–704. [Google Scholar] [CrossRef]
- Shizuka, K.; Kiyohara, C.; Shima, K.; Takeda, Y. Effect of CO2 on layered Li1+zNi1-x-yCoxMyO2 (M = Al, Mn) cathode materials for lithium ion batteries. J. Power Sources 2007, 166, 233–238. [Google Scholar] [CrossRef]
- Wang, D.; et al. Effect of high Ni on battery thermal safety. Int. J. Energy Res. 2020, 44, 12158–12168. [Google Scholar] [CrossRef]
- Tan, C. C.; et al. Ageing analysis and asymmetric stress considerations for small format cylindrical cells for wearable electronic devices. J. Power Sources 2020, 472, 228626. [Google Scholar] [CrossRef]
- Lammer, M.; Königseder, A.; Hacker, V. Holistic methodology for characterisation of the thermally induced failure of commercially available 18650 lithium ion cells. RSC Adv. 2017, 7, 24425–24429. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; He, Y.; Yuen, R.; Wang, J. Study of the fire hazards of lithium-ion batteries at different pressures. Appl. Therm. Eng. 2017, 125, 1061–1074. [Google Scholar] [CrossRef]
- Somandepalli, V.; Marr, K.; Horn, Q. Quantification of combustion hazards of thermal runaway failures in lithium-ion batteries. SAE Int. J. Altern. Powertrains 2014, 3, 98–104. [Google Scholar] [CrossRef]
- Finegan, D. P.; et al. Modelling and experiments to identify high-risk failure scenarios for testing the safety of lithium-ion cells. J. Power Sources 2019, 417, 29–41. [Google Scholar] [CrossRef]
- Finegan, D. P.; et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Doughty, D.; Roth, E.P. A general discussion of Li Ion battery safety. Electrochem. Soc. Interface 2012, 21, 37–44. [Google Scholar]
- Finegan, D. P.; et al. Characterising thermal runaway within lithium-ion cells by inducing and monitoring internal short circuits. Energy Environ. Sci. 2017, 10, 1377–1388. [Google Scholar] [CrossRef]
- Furushima, Y.; Yanagisawa, C.; Nakagawa, T.; Aoki, Y.; Muraki, N. Thermal stability and kinetics of delithiated LiCoO2. J. Power Sources 2011, 196, 2260–2263. [Google Scholar] [CrossRef]
- Bak, S.; et al. Structural Changes and Thermal Stability of Charged LiNi. Appl. Mater. Interfaces 2014, 6, 22594–22601. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; et al. The truth about the 1st cycle Coulombic efficiency of LiNi1/3Co1/3Mn1/3O2 (NCM) cathodes. Phys. Chem. Chem. Phys. 2016, 18, 3956–3965. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, G.; Liu, F.; Yue, X.; Chen, Z. Resolving the Compositional and Structural Defects of Degraded LiNixCoyMnzO2 Particles to Directly Regenerate High-Performance Lithium-Ion Battery Cathodes. ACS Energy Lett. 2018, 3, 1683–1692. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Röser, S.; Börner, M.; Winter, M. Do Increased Ni Contents in LiNi xMn yCo zO2 (NMC) Electrodes Decrease Structural and Thermal Stability of Li Ion Batteries? A Thorough Look by Consideration of the Li+ Extraction Ratio. ACS Appl. Energy Mater. 2019, 2, 7733–7737. [Google Scholar] [CrossRef]
- Adams, R.A.; Varma, A.; Pol, V.G. Mechanistic elucidation of thermal runaway in potassium-ion batteries. J. Power Sources 2018, 375, 131–137. [Google Scholar] [CrossRef]
- Mao, B.; Huang, P.; Chen, H.; Wang, Q.; Sun, J. Self-heating reaction and thermal runaway criticality of the lithium ion battery. Int. J. Heat Mass Transf. 2020, 149, 119178. [Google Scholar] [CrossRef]
- Advanced Energy Materials - 2019 - Sharifi-Asl - Oxygen Release Degradation in Li-Ion Battery Cathode Materials Mechanisms.pdf. Adv. Energy Mater. 2019, 9, 1900551.
- Yano, A.; Shikano, M.; Ueda, A.; Sakaebe, H.; Ogumi, Z. LiCoO 2 Degradation Behavior in the High-Voltage Phase Transition Region and Improved Reversibility with Surface Coating. J. Electrochem. Soc. 2017, 164, A6116–A6122. [Google Scholar] [CrossRef]
- Al, K.-W. N. Adv Func Combining In Situ Synchrotron X-Ray Diffraction and Absorption Techniques with.pdf. 23, 1047–1063 (2013).
- Sharifi-Asl, S.; Lu, J.; Amine, K.; Shahbazian-Yassar, R. Oxygen Release Degradation in Li-Ion Battery Cathode Materials: Mechanisms and Mitigating Approaches. Adv. Energy Mater. 2019, 9, 1900551. [Google Scholar] [CrossRef]
- Jung, R.; Strobl, P.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Temperature Dependence of Oxygen Release from LiNi 0.6 Mn 0.2 Co 0.2 O 2 (NMC622) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A2869–A2879. [Google Scholar] [CrossRef]
- Ellis, L.D.; et al. Quantifying, Understanding and Evaluating the Effects of Gas Consumption in Lithium-Ion Cells. J. Electrochem. Soc. 2017, 164, A3518–A3528. [Google Scholar] [CrossRef]
- Girgis, M.M.; El-Awad, A.M. Kinetics and mechanism of thermal decomposition of lithium oxalate catalysed by Cd1-xCoxFe2O4(x =0.0, 0.5 and 1.0) ferrospinel additives. Thermochim. Acta 1993, 214, 291–303. [Google Scholar] [CrossRef]
- Liu, X.; et al. In situ observation of thermal-driven degradation and safety concerns of lithiated graphite anode. Nat. Commun. 2021, 12, 4235. [Google Scholar] [CrossRef]
- Finegan, D. P.; et al. Modelling and experiments to identify high-risk failure scenarios for testing the safety of lithium-ion cells. J. Power Sources 2019, 417, 29–41. [Google Scholar] [CrossRef]
- Finegan, D. P.; et al. Identifying the Cause of Rupture of Li-Ion Batteries during Thermal Runaway. Adv. Sci. 2018, 5, 1700369. [Google Scholar] [CrossRef] [PubMed]
- Yufit, V.; et al. Investigation of lithium-ion polymer battery cell failure using X-ray computed tomography. Electrochem. commun. 2011, 13, 608–610. [Google Scholar] [CrossRef]
- Holloway, J.; et al. Determining the limits and effects of high-rate cycling on lithium iron phosphate cylindrical cells. Batteries 2020, 6, 1–18. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.I.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Golubkov, A.W.; et al. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes - Impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Jun, S.C. Mechanical Testing and Evaluation. Graphene-based Energy Devices (Wiley-VCH verlag GmbH & Co. KgaA, 2015).
- Baker, H. ASM Handbook, Volume 3: Alloy Phase Diagrams. (ASM International, 1992).
- Becker, W. et al. ASM Handbook, Volume 11: Failure Analysis and Prevention. (2002).
- Cartwright, R. Book Reviews: Book Reviews. Perspect. Public Health 2010, 130, 239–239. [Google Scholar] [CrossRef]
- Sun, J.; et al. Toxicity, a serious concern of thermal runaway from commercial Li-ion battery. Nano Energy 2016, 27, 313–319. [Google Scholar] [CrossRef]
- Yuan, L.; Dubaniewicz, T.; Zlochower, I.; Thomas, R.; Rayyan, N. Experimental study on thermal runaway and vented gases of lithium-ion cells. Process Saf. Environ. Prot. 2020, 144, 186–192. [Google Scholar] [CrossRef]
| Test | Temperature (°C) | Events |
|---|---|---|
| Low SOC | 100 | Nil |
| 150 | Nil | |
| 200 | Venting only | |
| 250 | Venting only |
| Test | Temperature (°C) | Events |
|---|---|---|
| High SOC | 100 | Nil |
| 150 | Nil | |
| 200 | Venting and TR | |
| 250 | Venting and TR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





