Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Overview
1.2. Epidemiology of Duchenne muscular dystrophy (DMD)
1.3. Genetics
1.4. Dystrophin and membrane instability
1.5. Preclinical models of DMD
1.6. Clinical manifestations of DMD
2. Cardiomyopathy in DMD: Functional and Histological Manifestations
2.1. Echocardiography
2.2. Limitations of echocardiography
2.3. Cardiac magnetic resonance imaging
2.4. Limitations of cardiac magnetic resonance imaging
2.5. Hemodynamic biomarkers
2.6. Systolic and diastolic dysfunction
2.7. Cardiac fibrosis
2.8. Dilated cardiomyopathy
2.9. Arrhythmia
3. Mechanisms of Cardiomyopathy in DMD
3.1. Inflammatory signaling and immune response
3.2. Calcium handling dysregulation and cell death
3.3. Mitochondrial permeability transition pore and apoptosis
3.4. Oxidative phosphorylation and substrate catabolism
3.5. Reactive oxygen species (ROS) and mitochondrial H2O2 emission
3.6. Altered mitochondrial autophagy (mitophagy)
3.7. Altered mitochondrial content and structure
4. Current interventional targets and approved non-genetic therapies for cardiomyopathy in DMD
4.1. Glucocorticoid therapy
4.2. Angiotensin-inhibiting therapies
4.3. Beta-adrenergic receptor blockers
4.4. Mineralocorticoid receptor antagonists
4.5. Gene-targeted therapies
4.6. Other therapies
5. Chamber-specific cardiomyopathy in DMD
5.1. Human Duchenne muscular dystrophy
5.2. Dystrophin-deficient rodent models
5.3. Limitations and future directions of chamber-specific cardiomyopathy
6. Mitochondria as a potential therapeutic target
6.1. Targeting calcium handling
6.2. Mitochondrial calcium overload
6.3. Targeting cellular/mitochondrial antioxidant systems
6.4. Cardiolipin and membrane stability
7. Summary and future directions
Author Contributions
Funding
Acknowledgments
Competing interests
References
- Aartsma-Rus A,Van Deutekom JCT, Fokkema IF, Van Ommen GJB & den Dunnen JT Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 2006, 34, 135–144.
- Aartsma-Rus A & den Dunnen, JT. Phenotype predictions for exon deletions/duplications: A user guide for professionals and clinicians using Becker and Duchenne muscular dystrophy as examples. Hum Mutat 2019, 40, 1630–1633. [Google Scholar]
- Abutaleb ARA, McNally EM, Khan SS, Anderson AS, Carr JC & Wilcox JE. Myocarditis in Duchenne muscular dystrophy after changing steroids. JAMA Cardiol 2018, 3, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Adorisio R, Mencarelli E, Cantarutti N, Calvieri C, Amato L, Cicenia M, Silvetti M, D’Amico A, Grandinetti M, Drago F & Amodeo A. Duchenne dilated cardiomyopathy: cardiac management from prevention to advanced cardiovascular therapies. J Clin Med 2020, 9, 3186. [Google Scholar] [CrossRef] [PubMed]
- Aikawa T, Takeda A, Oyama-Manabe N, Naya M, Yamazawa H, Koyanagawa K, Ito YM & Anzai T. Progressive left ventricular dysfunction and myocardial fibrosis in Duchenne and Becker muscular dystrophy: a longitudinal cardiovascular magnetic resonance study. Pediatr Cardiol 2018, 40, 384–392. [Google Scholar]
- Aliev M, Guzun R, Karu-Varikmaa M, Kaambre T, Wallimann T & Saks V. Molecular system bioenergics of the heart: experimental studies of metabolic compartmentation and energy fluxes versus computer modeling. Int J Mol Sci 2011, 12, 9296–9331. [Google Scholar] [CrossRef] [PubMed]
- Allen DG, Whitehead NP & Yeung EW. Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol 2005, 567, 723–735. [Google Scholar] [CrossRef]
- Allen DG, Gervasio OL, Yeung EW & Whitehead NP. Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 2010, 88, 83–91. [Google Scholar]
- Allen DG & Whitehead, NP. Duchennz muscular dystrophy—What causes the increased membrane permeability in skeletal muscle. Int J Biochem Cell Biol 2010, 43, 290–294. [Google Scholar]
- Allen DG, Whitehead NP, & Froehner SC. Absence of Dystrophin Disrupts Skeletal Muscle Signaling: Roles of Ca2+, Reactive Oxygen Species, and Nitric Oxide in the Development of Muscular Dystrophy. Physiol Rev 2016, 96, 253–305. [Google Scholar] [CrossRef]
- 11. Allen HD, Flanigan KM, Thrush PT, Dvorchik I, Yin H, Canter C, Connolly AM, Parrish M, McDonald CM, Braunlin E, Colan SD, Day J, Darras B & Mendell JR. A randomized, double-blind trial of Lisinopril and Losartan for the treatment of cardiomyopathy in Duchenne muscular dystrophy. PLoS Curr, 2013; 5.
- Allen ME, Pennington ER, Perry JB, Dadoo S, Makrecka-Kuka M, Dambrova M, Moukdar F, Patel HD, Han X, Kidd GK, Benson EK, Raisch TB, Poelzing S, Brown D A & Shaikh SR. The cardiolipin-binding peptide elamipretide mitigates fragmentation of cristae networks following cardiac ischemia reperfusion in rats. Commun Biol 2020, 3, 389. [Google Scholar]
- Amedro P, Vincenti M, De La Villeon G, Lavastre K, Barrea C, Guillaumont S, Bredy C, Gamon L, Meli AC, Cazorla O, Fauconnier J; Meyer P; Rivier F, Adda J, Mura T & Lacampagne A. Speckle-tracking echocardiography in children with Duchenne muscular dystrophy: a prospective multicenter controlled cross-sectional study. J Am Soc Echocardiogr 2019, 32, 412–422. [Google Scholar] [CrossRef]
- Amoasii L, Li H, Sanchez-Ortiz E, Caballero D, Harron R, Massey C, Shelton J, Piercy R & Olson EN. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 2018, 362, 86–91. [Google Scholar] [CrossRef]
- Amoasii L, Long C, Li H, Mireault AA, Shelton JM, Sanchez-Ortiz E, McAnally JR, Bhattacharyya S, Schmidt F, Grimm D, Hauschka SD, Bassel-Duby R & Olson EN. Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci Transl Med 2017, 9, 1–11. [Google Scholar]
- Andersson DC & Marks, AR. Fixing ryanodine receptor Ca2+ leak—A novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech 2010, 7, e151–e157. [Google Scholar]
- Angelini C & Peterle, E. Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myol 2012, 31, 9–15. [Google Scholar]
- Archer JE, Gardner AC, Roper HP, Chikermane AA & Tatman AJ. Duchenne muscular dystrophy: the management of scoliosis. J Spine Surg 2016, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ascah A, Khairallah M, Daussin F, Bourcier-Lucas C, Godin R, Allen BG, Petrof BJ, Des Rosiers C & Burelle Y. Stress-induced opening of the permeability transition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am J Physiol Heart Circ Physiol 2011, 300, H144–H153. [Google Scholar] [CrossRef] [PubMed]
- Ashford MW, Liu W, Lin SJ, Abraszewski P, Caruthers SD, Connolly AM, Yu X & Wickline SA. Occult cardiac contractile dysfunction in dystrophin-deficient children revealed by cardiac magnetic resonance strain imaging. Circulation 2005, 112, 2462–2467. [Google Scholar] [CrossRef]
- Bach JR, O’Brien J, Krotenberg R & Alba AS. Management of end stage respiratory failure in Duchenne muscular dystrophy. Muscle Nerve 1987, 10, 177–182. [Google Scholar] [CrossRef]
- Bahler RC, Mohyuddin T, Finkelhor RS & Jacobs IB. Contribution of Doppler tissue imaging and myocardial performance index to assessment of left ventricular function in patients with Duchenne’s muscular dystrophy. J Am Soc Echocardiogr 2005, 18, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Banihani R, Smile S, Yoon G, Dupuis A, Mosleh M, Snider A & McAdam L. Cognitive and neurobehavioral profile in boys with Duchenne muscular dystrophy. J Child Neurol 2015, 30, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Barber BJ, Andrews JG, Lu Z, West NA, Meaney FJ, Price ET, Gray A, Sheehan DW, Pandya S, Yang M & Cunniff C. Oral corticosteroids and onset of cardiomyopathy in Duchenne muscular dystrophy. J Pediatr 2013, 163, 1080–1084e1. [Google Scholar] [CrossRef]
- Batra A, Barnard AM, Lott DJ, Willcocks RJ, Forbes SC, Chakraborty S, Daniels MJ, Arbogast J, Triplett W, Henricson EK, Dayan JG, Schmalfuss C, Sweeney L, Byrne BJ, McDonald CM, Vandenborne K & Walter GA. Longitudinal changes in cardiac function in Duchenne muscular dystrophy population as measured by magnetic resonance imaging. BMC Cardiovasc Disord 2022, 22, 260. [Google Scholar]
- Bauer R, Straub V, Blain A, Bushby K & MacGowan GA. Contrasting effects of steroids and angiotensin-converting-enzyme inhibitors in a mouse model of dystrophin-deficient cardiomyopathy. Eur J Heart Fail 2009, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Belanto JJ, Mader TL, Eckhoff MD, Strandjord DM, Banks GB, Gardner MK, Lowe DA, & Ervasti JM. Microtubule binding distinguishes dystrophin from utrophin. Proc Natl Acad Sci USA 2014, 111, 5723–5728. [Google Scholar] [CrossRef] [PubMed]
- Belanto JJ, Olthoff JT, Mader TL, Chamberlain CM, Nelson DM, McCourt PM, Talsness DM, Gundersen GG, Lowe DA, & Ervasti JM. Independent variability of microtubule perturbations associated with dystrophinopathy. Hum Mol Genet 2016, 25, 4951–4961. [Google Scholar]
- Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A & Marks AR. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 2009, 15, 325–330. [Google Scholar] [CrossRef]
- Bellissimo CA, Garibotti MC & Perry CGR. Mitochondria stress responses in Duchenne muscular dystrophy: metabolic dysfunction or adaptive reprogramming? Am J Physiol Cell Physiol 2022, 323, C718–C730. [Google Scholar] [CrossRef]
- Bellissimo CA, Delfinis LJ, Hughes MC, Turnbull PC, Gandhi S, DiBenedetto SN, Rahman FA, Tadi P, Amaral CA, Dehghani A, Cobley JN, Quadrilatero J, Schlattner U & Perry CGR. Mitochondrial creatine sensitivity is lost in the D2mdx model of Duchenne muscular dystrophy and rescued by the mitochondrial-enhancing compound. Olesoxime Am J Physiol Cell Physiol 2023, 324, C1141–C1157.
- Bengtsson NE, Hall JK, Odom GL, Phelps MP, Andrus CR, Hawkins RD, Hauschka SD, Chamberlain JR & Chamberlain JS. Muscle-specific CRISPR/Cas9 dystrophin gene editing ameliorates pathophysiology in a mouse model for Duchenne muscular dystrophy. Nat Commun 2017, 8, 1–9. [Google Scholar]
- Bernardi P & von Stockum, S. The permeability transition pore as a Ca(2þ) release channel: new answers to an old question. Cell Calcium 2012, 52, 22–27. [Google Scholar]
- Bernardi P, Carraro M & Lippe G. The mitochondrial permeability transition: recent progress and open questions. FEBS J 2022, 289, 7051–7074. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi P, Torchiana E, Confalonieri P, Brugnoni R, Barresi R, Mora M, Cornelio F, Morandi L & Mantegazza R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis: pathogenetic role of a fibrogenic cytokine. J Clin Invest 1995, 96, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, Seshan SV, Pardee JD, & Szeto HH. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 2013, 24, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Bilchick KC, Salerno M, Plitt D, Dori Y, Crawford TO, Drachman D & Thompson WR. Prevalence and distribution of regional scar in dysfunctional myocardial segments in Duchenne muscular dystrophy. J Cardiovasc Magn Reson 2011, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell 1989, 57, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, Case LE, Cripe L, Hadjiyannakis S, Olson AK, Sheehan DW, Bolen J, Weber DR, Ward LM & DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018, 17, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Bladen CL, Rafferty K, Straub V, Monges S, Moresco A, Dawkins H, Roy A, Chamova T, Guergueltcheva V, Korngut L, Campbell C, Dai Y, Barišić N, Kos T, Brabec P, Rahbek J, Lahdetie J, Tuffery-Giraud S, Claustres M,… Lochmüller H. The TREAT-NMD Duchenne 22 muscular dystrophy registries: conception, design, and utilization by industry and academia. Hum Mutat 2013, 34, 1449–1457. [Google Scholar] [CrossRef]
- Bladen CL, Salgado D, Monges S, Foncuberta ME, Kekou K, Kosma K, Dawkins H, Lamont L, Roy AJ, Chamova T, Guergueltcheva V, Chan S, Korngut L, Campbell C, Dai Y, Wang J, Barišić N, Brabec P, Lahdetie J, Walter MC,… Lochmüller H. The TREAT-NMD DMD Global Database: analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat 2015, 36, 395–402. [Google Scholar] [CrossRef]
- Blain A, Greally E, Laval S, Blamire A, Straub V & MacGowan GA. Beta-blockers, left and right ventricular function, and in vivo calcium influx in muscular dystrophy cardiomyopathy. PLOS One 2013, 8, e57260. [Google Scholar]
- Bodor GS, Porterfield D, Voss EM, Smith S & Apple FS. Cardiac troponin-I is not expressed in fetal and healthy or diseased adult human skeletal muscle tissue. Clin Chem 1995, 41, 1710–1715. [Google Scholar] [CrossRef]
- Borthwick LA, Wynn TA & Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Bosser G, Lucron H, Lethor JP, Burger G, Beltramo F, Marie PY & Marçon F. Evidence of early impairments in both right and left ventricular inotropic reserves in children with Duchenne’s muscular dystrophy. Am J Cardiol 2004, 93, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Boyman L, Chikando AC, Williams GS, Khairallah RJ, Kettlewell S, Ward CW, Smith GL, Kao JP & Lederer WJ. Calcium movement in cardiac mitochondria. Biophys J 2014, 107, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Brown DA, Vining A, Mackinnon A, Elustondo P, McKenna M & Nagaraju K (2022). The Mitochondria-targeting Peptide Elamipretide Potentiates Dystrophin Expression Induced by an Exon-skipping Morpholino in the mdx Mouse Model. Proceedings of Muscular Dystrophy Association (MDA) Clinical & Scientific Conference; Muscular Dystrophy Association: Nashville, TN, USA.
- Buddhe S, Lewin M, Olson A, Ferguson M & Soriano BD. Comparison of left ventricular function assessment between echocardiography and MRI in Duchenne muscular dystrophy. Pediatr Radiol 2016, 46, 1399–1408. [Google Scholar] [CrossRef]
- Buddhe S, Cripe L; Friedland-Little J, Kertesz N, Eghtesady P, Finder J, Hor K, Judge DP, Kinnett K, McNally EM, Raman S, Thompson WR, Wagner KR & Olson AK. Cardiac management of the patient with Duchenne muscular. dystrophy Pediatrics 2018, 142, S72–S81.
- Budihardjo I, Oliver H, Lutter M, Luo X & Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999, 15, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Bulfield G, Siller WG & Wight PA. Moore KJ X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A 1984, 81, 1189–1192. [Google Scholar] [CrossRef]
- Burelle Y, Khairallah M, Ascah A, Allen BG, Deschepper CF, Petrof BJ & Des Rosiers C. Alterations in mitochondrial function as a harbinger of cardiomyopathy: lessons from the dystrophic heart. J Mol Cell Cardiol 2010, 48, 310–321. [Google Scholar] [CrossRef]
- Burkhoff D, Weiss RG, Schulman SP, Kalil-Filho R, Wannenburg T & Gerstenblith G. Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am J Physiol 1991, 261, H741–H750. [Google Scholar]
- Bushby K & Connor, E. Clinical outcome measures for trials in Duchenne muscular dystrophy: report from International Working Group meetings. Clin Investig 2011, 1, 1217–1235. [Google Scholar]
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J & Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Buyse GM, Goemans N, van den Hauwe M, Thijs D, de Groot IJ, Schara U, Ceulemans B, Meier T & Mertens L. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: Results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul Disord 2011, 21, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Buyse GM, Van der Mieren G, Erb M, D’Hooge J, Herijgers P, Verbeken E, Jara A, Van Den Bergh A, Mertens L, Courdier-Fruh I, Barzaghi P & Meier T. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: cardiac protection and improved exercise performance. Eur Heart J 2009, 30, 116–124. [Google Scholar]
- Buyse GM, Voit T, Schara U, Straathof CS, D’Angelo MG, Bernert G, Cuisset JM, Finkel RS, Goemans N, McDonald CM, Rummey C & Meier T. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): a double-blind randomised placebo-controlled phase 3 trial. Lancet 2015, 385, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Buyse GM, Voit T, Schara U, Straathof CS, D’Angelo MG, Bernert G, Cuisset JM, Finkel RS, Goemans N, Rummey C, Leinonen MS, Mayer OH, Spagnolo P, Meier T & McDonald CM. Treatment effect of idebenone on inspiratory function in patients with Duchenne muscular dystrophy. Pediatr Pulmonol 2017, 52, 508–515. [Google Scholar] [CrossRef]
- Bylo M, Farewell R, Coppenrath VA & Yogaratnam D. A review of deflazacort for patients with Duchenne muscular dystrophy. Ann Pharmacother 2020, 54, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Cai J, Yang J & Jones D. Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta 1998, 1366, 139–149. [Google Scholar] [CrossRef]
- Cai B, Spencer MJ, Nakamura G, Tseng-Ong L & Tidball JG. Eosinophilia of dystrophin-deficient muscle is promoted by perforin-mediated cytotoxicity by T cell effectors. Am J Pathol 2000, 156, 1789–1796. [Google Scholar] [CrossRef]
- Capitanio D, Moriggi M, Torretta E, Barbacini P, De Palma S, Vigano A, Lochmüller H, Muntoni F, Ferlini A, Mora M & Gelfi C. Comparative proteomic analyses of Duchenne muscular dystrophy and Becker muscular dystrophy muscles: changes contributing to preserve muscle function in Becker muscular dystrophy patients. J Cachexia Sarcopenia Muscle 2020, 11, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Chadwick JA, Swager SA, Lowe J, Welc SS, Tidball JG, Gomez-Sanchez CE, Gomez-Sanchez EP & Rafael-Fortney JA. Myeloid cells are capable of synthesizing aldosterone to exacerbate damage in muscular dystrophy. Hum Mol Genet 2016, 25, 5167–5177. [Google Scholar]
- Chamberlain JR & Chamberlain, JS. Progress toward gene therapy for Duchenne muscular dystrophy. Mol Ther 2017, 25, 1125–1131. [Google Scholar] [PubMed]
- Chemello F, Wang Z, Li H, McAnally JR, Liu N, Bassel-Duby R & Olson EN. Degenerative and regenerative pathways underlying Duchenne muscular dystrophy revealed by single-nucleus RNA sequencing. Proc Natl Acad Sci U S A 2020, 117, 29691–29701. [Google Scholar] [CrossRef] [PubMed]
- Chiang DY, Allen HD, Kim JJ, Valdes SO, Wang Y, Pignatelli RH, Lotze TE & Miyake CY. Relation of cardiac dysfunction to rhythm abnormalities in patients with Duchenne or Becker muscular dystrophies. Am J Cardiol 2016, 117, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- CHMP Assessment Report for Sovrima; European Medicines Agency: London, UK, 2008. Available at: https://wwwemaeuropaeu/en/documents/assessment-report/sovrima-epar-public-assessment-report_enpdf.
- Cho MJ, Lee JW, Lee J & Shin YB. Evaluation of early left ventricular dysfunction in patients with Duchenne muscular dystrophy using two-dimensional speckle tracking echocardiography and tissue Doppler imaging. Pediatr Cardiol 2018, 39, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Cinteza E, Stoicescu C, Butoianu N, Balgradean M, Nicolescu A & Angrés M. Acut myocardial injury in a child with Duchenne muscular dystrophy: pulse steroid therapy? Maedica 2017, 12, 180–183. [Google Scholar]
- Coley WD, Bogdanik L, Vila MC, Yu Q, Van Der Meulen JH, Rayavarapu S, Novak JS, Nearing M, Quinn JL, Saunders A, Dolan C, Andrews W, Lammert C, Austin A, Partridge TA, Cox GA, Lutz C & Nagaraju K. Effect of genetic background on the dystrophic phenotype in mdx mice. Hum Mol Genet 2016, 25, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Connolly AM Zaidman CM, Golumbek PT, Cradock MM, Flanigan KM, Kuntz NL, Finkel RS, McDonald CM, Iannaccone ST, Anand P, Siener CA, Florence JM, Lowes LP, Alfano LN, Johnson LB, Nicorici A, Nelson LL, Mendell JR & MDA DMD Clinical Research Network. Twice-weekly glucocorticosteroids in infants and young boys with Duchenne muscular dystrophy. Muscle Nerve 2019, 59, 650–657. [Google Scholar] [CrossRef]
- Connuck DM, Sleeper LA, Colan SD, Cox GF, Towbin JA, Lowe AM, Wilkinson JD, Orav EJ, Cuniberti L, Salbert BA & Lipshultz SE. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: a comparative study from the Pediatric Cardiomyopathy Registry. Am Heart J 2008, 155, 998–1005. [Google Scholar] [CrossRef]
- Corrado G, Lissoni A, Beretta S, Terenghi L, Tadeo G, Foglia-Manzillo G, Tagliagambe LM, Spata M & Santarone M. Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with Duchenne muscular dystrophy. Am J Cardiol 2002, 89, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli S, Sultana J, Fontana A, Salvo F, Messina S & Trifirò G. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet J Rare Dis 2020, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Cserne Szappanos H, Muralidharan P, Ingley E, Petereit J, Millar AH & Hool LC. Identification of a novel cAMP dependent protein kinase, A phosphorylation site on the human cardiac calcium channel. Sci Rep 2017, 7, 15118. [Google Scholar] [CrossRef] [PubMed]
- Cuccia C, Benedini G, Leonzi O, Pagnoni N & Giubbini R. [Acut myocardial infarction in a 13-year-old boy with Duchenne’s disease.] G Ital Cardiol 1984, 14, 817–820.
- Cui W, Jang A, Zhang P, Thompson B, Townsend D, Metzger JM & Zhang J. Early detection of myocardial bioenergetic deficits: a 94 tesla complete non invasive 31P MR spectroscopy study in mice with muscular dystrophy. PLOS One 2015, 10, e0135000. [Google Scholar]
- D’Amario D, Amodeo A, Adorisio R, Tiziano FD, Leone AM, Perri G, Bruno P, Massetti M, Ferlini A, Pane M, Niccoli G, Porto I, D’Angelo GA, Borovac JA, Mercuri E & Crea F. A current approach to heart failure in Duchenne muscular dystrophy. Heart 2017, 103, 1770–1779. [Google Scholar] [CrossRef]
- Dai DF, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF & Rabinovitch PS. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol 2011, 58, 73–82. [Google Scholar] [CrossRef]
- Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Des Rosiers C & Petrof BJ. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J 2001, 15, 1655–1657. [Google Scholar] [CrossRef]
- Dasgupta C & Zhang, L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov Today 2011, 16, 22–34. [Google Scholar]
- de Kermadec JM, Bécane HM, Chénard A, Tertrain F & Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J 1994, 127, 618–623. [Google Scholar] [CrossRef]
- De Luca A, Nico B, Liantonio A, Didonna MP, Fraysse B, Pierno S, Burdi R, Mangieri D, Rolland JF, Camerino C, Zallone A, Confalonieri P, Andreetta F, Arnoldi E, Courdier-Fruh I, Magyar JP, Frigeri A, Pisoni M, Svelto M & Conte Camerino D. A multidisciplinary evaluation of the effectiveness of cyclosporine A in dystrophic mdx mice. Am J Pathol 2005, 166, 477–489. [Google Scholar] [CrossRef] [PubMed]
- De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M & Clementi E. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis 2012, 3, e418. [Google Scholar] [CrossRef] [PubMed]
- Delfin DA, Xu Y, Peterson JM, Guttridge DC, Rafael-Fortney JA & Janssen PM. Improvement of cardiac contractile function by peptide-based inhibition of NF-kappaB in the utrophin/dystrophin-deficient murine model of muscular dystrophy. J Transl Med 2011, 9, 68. [Google Scholar] [CrossRef]
- Demachi J, Kagaya Y, Watanabe J, Sakuma M, Ikeda J, Kakuta Y, Motoyoshi I, Kohnosu T, Sakuma H, Shimazaki S, Sakai H, Kimpara T, Takahashi T, Omura K, Okada M, Saito H & Shirato K. Characteristics of the increase in plasma brain natriuretic peptide level in left ventricular systolic dysfunction, associated with muscular dystrophy in comparison with idiopathic dilated cardiomyopathy. Neuromuscul Disord 2004, 14, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Dikalov SI & Nazarewicz, RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal 2013, 19, 1085–1094. [Google Scholar]
- Dittrich S, Tuerk M, Haaker G, Greim V, Buchholz A, Burkhardt B, Fujak A, Trollmann R, Schmid A & Schroeder R. Cardiomyopathy in Duchenne muscular dystrophy: current value of clinical, electrophysiological and imaging findings in children and teenagers. Klin Padiatr 2015, 227, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Dolder M, Wendt S & Wallimann T. Mitochondrial creatine kinase in contact sites: interaction with porin and adenine nucleotide translocase, role in permeability transition and sensitivity to oxidative damage. Neurosignals 2001, 10, 93–111. [Google Scholar] [CrossRef]
- Dorchies OM, Reutenauer-Patte J, Dahmane E, Ismail HM, Petermann O, Patthey-Vuadens O, Comyn SA, Gayi E, Piacenza T, Handa RJ, Décosterd LA, & Ruegg UT. The Anticancer Drug Tamoxifen Counteracts the Pathology in a Mouse Model of Duchenne Muscular Dystrophy. Am. J. Pathol. 2013, 182, 485–504. [Google Scholar] [CrossRef]
- Dual SA, Maforo NG, McElhinney DB, Prosper A, Wu HH, Maskatia S, Renella P, Halnon N & Ennis DB. Right ventricular function and T1-mapping in boys with Duchenne muscular dystrophy. J Magn Reson Imaging 2021, 54, 1503–1513. [Google Scholar] [CrossRef]
- Duan, D. Systemic delivery of adeno-associated viral vectors. Curr Opin Virol 2016, 21, 16–25. [Google Scholar] [CrossRef]
- Duan, D. Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol Ther 2018, 26, 2337–2356. [Google Scholar] [CrossRef] [PubMed]
- Duan D, Goemans N, Takeda S, Mercuri E & Aartsma-Rus A. Duchenne muscular dystrophy. Nat Rev Dis Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Dubinin MV, Talanov EY, Tenkov KS, Starinets VS, Belosludtseva NV & Belosludtsev KN. The effect of deflazacort treatment on the functioning of skeletal muscle mitochondria in Duchenne muscular dystrophy. Int J Mol Sci 2020, 21, 8763. [Google Scholar] [CrossRef] [PubMed]
- Dubinin MV, Talanov EY, Tenkov KS, Starinets VS, Mikheeva IB, & Belosludtsev KN. Transport of Ca2+ and Ca2+-dependent permeability transition in heart. Biochim. Biophys. Acta 2020, 1861, 148250. [Google Scholar]
- mitochondria in the early stages of Duchenne muscular dystrophy.
- Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G & Bécane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol 2005, 45, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Duboc D, Meune C, Pierre B, Wahbi K, Eymard B, Toutain A, Berard C, Vaksmann G, Weber S & Bécane HM. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am Heart J 2007, 154, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Dunn JF, Burton KA & Dauncey MJ. Ouabain sensitive Na+ K+-ATPase content is elevated in mdx mice: Implications for the regulation of ions in dystrophic muscle. J Neurol Sci 1995, 133, 11–5. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Fakhari D, Dillmann U, Flotats-Bastardas M, Poryo M, Abdul-Khaliq H, Shamdeen MG, Mischo B, Zemlin M & Meyer S. Off-label use of ataluren in four non-ambulatory patients with nonsense mutation Duchenne muscular dystrophy: effects on cardiac and pulmonary function and muscle strength. Front Pediatr 2018, 6, 316. [Google Scholar] [CrossRef] [PubMed]
- Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A & Lerman L O. Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 2014, 103, 461–472. [Google Scholar] [CrossRef]
- Eisner DA, Caldwell JL, Kistamás K & Trafford AW. Calcium and excitation-contraction coupling in the heart. Circ Res 2017, 121, 181–195. [Google Scholar] [CrossRef]
- El Refaey M, Xu L, Gao Y, Canan BD, Adesanya TMA, Warner SC, Akagi K, Symer DE, Mohler PJ, Ma J; Janssen PML & Han R. In Vivo Genome Editing Restores Dystrophin Expression and Cardiac Function in Dystrophic. Mice Circ Res 2017, 121, 923–929.
- Elliott EI & Sutterwala, FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 2015, 265, 35–52. [Google Scholar]
- Emery AEH. The muscular dystrophies. Lancet 2002, 359, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Ervasti JM & Campbell, KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 1993, 122, 809–823. [Google Scholar]
- Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, Viswanathan V, Kornberg AJ, Bertorini TE, Nevo Y, Lotze T, Pestronk A, Ryan MM, Monasterio E, Day JW, Zimmerman A, Arrieta A, Henricson E, Mayhew J, Florence J,… Connolly AM. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology 2011, 77, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Esposito G & Carsana, A. Metabolic alterations in cardiomyocytes of patients with Duchenne and Becker muscular dystrophies. J Clin Med 2019, 8, 2151. [Google Scholar]
- Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J & Grange RW. Immune-mediated mechanisms potentially regulate the disease time-course of Duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R 2009, 1, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Fauconnier J, Thireau J, Reiken S, Cassan C, Richard S, Matecki S, Marks AR & Lacampagne A. Leaky RyR2 trigger ventricular arrhythmias in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 2010, 107, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Fayssoil A, Abasse S & Silverston K. Cardiac involvement classification and therapeutic management in patients with Duchenne muscular dystrophy. J Neuromuscul Dis 2017, 4, 17–23. [Google Scholar] [CrossRef]
- Felsburg, PJ. Overview of immune system development in the dog: comparison with humans. Hum Exp Toxicol 2002, 21, 487–492. [Google Scholar] [CrossRef]
- Feng J, Schaus BJ, Fallavollita JA, Lee TC & Canty JM. Preload induces troponin I degradation independently of myocardial ischemia. Circulation 2001, 103, 2035–2037. [Google Scholar] [CrossRef]
- Finsterer J, Cripe L. Treatment of dystrophin cardiomyopathies. Nat Rev Cardiol 2014, 11, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Finsterer J, Stöllberger C. The heart in human dystrophinopathies. Cardiology 2003, 99, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fischer Y, Thomas J, Sevilla L, Muñoz P, Becker C, Holman G, Kozka IJ, Palacín M, Testar X, Kammermeier H & Zorzano A. Insulin-induced recruitment of glucose transporter 4 (GLUT4) and GLUT1 in isolated rat cardiac myocytes: evidence of the existence of different intracellular GLUT4 vesicle populations. J Biol Chem 1997, 272, 7085–7092. [Google Scholar] [CrossRef]
- Flanigan KM (2017). Duchenne and Becker muscular dystrophies. In Swaiman’s Pediatric Neurology, 6th edn, ed. Elsevier Inc, pp. e2482–e2492. Edinburgh, UK.
- Florczyk-Soluch U, Polak K & Dulak J. The multifaceted view of heart problem in Duchenne muscular dystrophy. Cell Mol Life Sci 2021, 78, 5447–5468. [Google Scholar] [CrossRef] [PubMed]
- Florian A, Ludwig A, Rösch S, Yildiz H, Sechtem U & Yilmaz A. Myocardial fibrosis imaging based on T1- mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging 2014, 15, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Florian A-R, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U & Yilmaz A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson 2014, 16, 81. [Google Scholar] [CrossRef]
- Florian A, Rösch S, Bietenbeck M, Engelen M, Stypmann J, Waltenberger J, Sechtem U & Yilmaz A. Cardiac involvement in female Duchenne and Becker muscular dystrophy carriers in comparison to their first-degree male relatives: a comparative cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging 2016, 17, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Frankel KA & Rosser, RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol 1976, 7, 375–386. [Google Scholar]
- Gale EM, Caravan P, Rao AG, McDonald RJ, Winfeld M, Fleck RJ & Gee MS. Gadolinium-based contrast agents in pediatric magnetic resonance imaging. Pediatr Radiol 2017, 47, 507–521. [Google Scholar] [CrossRef]
- Gartz M, Haberman M, Prom MJ, Beatka MJ, Strande JL & Lawlor MW. A Long-Term Study Evaluating the Effects of Nicorandil Treatment on Duchenne Muscular Dystrophy-Associated Cardiomyopathy in mdx Mice. J Cardiovasc Pharmacol Ther 2022, 27, 10742484221088655. [Google Scholar]
- Gardner-Medwin, D. Clinical features and classification of the muscular dystrophies. Br Med Bull 1980, 36, 109–15. [Google Scholar] [CrossRef]
- Giatrakos N, Kinali M, Stephens D, Dawson D, Muntoni F & Nihoyannopoulos P. Cardiac tissue velocities and strain rate in the early detection of myocardial dysfunction of asymptomatic boys with Duchenne’s muscular dystrophy: relationship to clinical outcome. Heart 2006, 92, 840–842. [Google Scholar]
- Gillis JC, Benfield P & McTavish D. Idebenone. Drugs & Aging 1994, 5, 133–152. [Google Scholar]
- Giorgio V, Guo L, Bassot C, Petronilli V, & Bernardi P. Calcium and regulation of the mitochondrial permeability transition. Cell Calcium 2018, 70, 56–63. [Google Scholar] [CrossRef]
- Glancy B & Balaban, RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 2012, 51, 2959–7293. [Google Scholar]
- Glatz JFC, Nabben M, Young ME, Schulze PC, Taegtmeyer H & Luiken JJFP. Re-balancing cellular energy substrate metabolism to mend the failing heart. Biochimica et Biophysica Acta: Molecular Basis of Disease 2020, 1866, 165579. [Google Scholar]
- Gloss D, Moxley RT III; Ashwal S & Oskoui M. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: report of the guideline development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez DR, Treuer AV, Lamirault G, Mayo V, Cao Y, Dulce RA & Hare JM. NADPH oxidase-2 inhibition restores contractility and intracellular calcium handling and reduces arrhythmogenicity in dystrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 2014, 307, H710–H721. [Google Scholar] [CrossRef]
- Goodwin FC & Muntoni, F. Cardiac involvement in muscular dystrophies: molecular mechanisms. Muscle & Nerve, 2005; 32, 577–588. [Google Scholar]
- Gorospe JR, Tharp M, Demitsu T & Hoffman EP. Dystrophin-deficient myofibers are vulnerable to mast cell granule-induced necrosis. Neuromusc Disord 1994, 4, 325–333. [Google Scholar] [CrossRef]
- Götte MJ, Germans T, Rüssel IK, Zwanenburg JJ, Marcus JT, van Rossum AC & van Veldhuisen DJ. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. J Am Coll Cardiol 2006, 48, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Greally E, Davison BJ, Blain A, Laval S, Blamire A, Straub V & MacGowan GA. Heterogeneous abnormalities of in vivo left ventricular calcium influx and function in mouse models of muscular dystrophy cardiomyopathy. J Cardiovasc Magn Reson 2013, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, BH. Role of angiotensin receptor blockers in heart failure: not yet resolved. Circulation 1999, 100, 1032–1034. [Google Scholar] [CrossRef]
- Griffin JL & Des Rosiers, C. Applications of metabolomics and proteomics to the mdx mouse model of Duchenne muscular dystrophy: lessons from downstream of the transcriptome. Genome Med 2009, 1, 32. [Google Scholar]
- Groh, WJ. Arrhythmias in the muscular dystrophies. Heart Rhythm 2012, 9, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Guerron AD, Rawat R, Sali A, Spurney CF, Pistilli E, Cha HJ Pandey GS, Gernapudi R, Francia D, Farajian V, Escolar DM, Bossi L, Becker M, Zerr P, de la Porte S, Gordish-Dressman H, Partridge T, Hoffman EP & Nagaraju K. Functional and molecular effects of arginine butyrate and prednisone on muscle and heart in the mdx mouse model of Duchenne muscular dystrophy. PLOS One 2010, 5, e11220. [Google Scholar]
- Gueven N, Ravishankar P, Eri R & Rybalka E. Idebenone: When an antioxidant is not an antioxidant. Redox Biol 2021, 38, 101812. [Google Scholar]
- Guglieri M, Clemens PR, Perlman SJ, Smith EC, Horrocks I, Finkel RS, Mah JK, Deconinck N, Goemans N, Haberlova J, Straub V, Mengle-Gaw LJ, Schwartz BD, Harper AD, Shieh PB, De Waele L, Castro D, Yang ML, Ryan MM, McDonald CM, Tulinius M, Webster R, McMillan HJ, Kuntz NL, Rao VK, Baranello G, Spinty S, Childs A-M, Sbrocchi AM, Selby KA, Monduy M, Nevo Y, Vilchez-Padilla JJ, Nascimento-Osorio A, Niks EH, de Groot IJM, Katsalouli M, James MK, van den Anker J, Damsker JM, Ahmet A, Ward LM, Jaros M, Shale P, Dang UJ, & Hoffman EP. Efficacy and Safety of Vamorolone vs Placebo and Prednisone Among Boys With Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA Neurol 2022, 79, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Guzun R, Gonzalez-Granillo M, Karu-Varikmaa M, Grichine A, Usson Y, Kaambre T, Guerrero-Roesch K, Kuznetsov A, Schlattner U & Saks V. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within Mitochondrial Interactosome. Biochim Biophys Acta 2012, 1818, 1545–1554. [Google Scholar] [CrossRef]
- Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP & Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson 2016, 18, 89. [Google Scholar] [CrossRef]
- Hagenbuch SC, Gottliebson WM, Wansapura J, Mazur W, Fleck R, Benson DW & Hor KN. Detection of progressive cardiac dysfunction by serial evaluation of circumferential strain in patients with Duchenne muscular dystrophy. Am J Cardiol 2010, 105, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Hakim CH, Wasala NB, Nelson CE, Wasala LP, Yue Y, Louderman JA, Lessa TB, Dai A, Zhang K, Jenkins GJ, Nance ME, Pan X, Kodippili K, Yang NN, Chen SJ, Gersbach CA & Duan D. AAV CRISPR editing rescues cardiac and muscle function for 18 months in dystrophic mice. JCI Insight 2018, 3, 1–13. [Google Scholar]
- Hakim CH, Wasala NB, Pan X, Kodippili K, Yue Y, Zhang K, Yao G, Haffner B, Duan SX, Ramos J, Schneider JS, Yang NN, Chamberlain JS & Duan D. A five-repeat micro-dystrophin gene ameliorated dystrophic phenotype in the severe DBA/2J-mdx model of Duchenne muscular dystrophy. Mol Ther Methods Clin Dev 2017, 6, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Hart CC, il Lee Y, Xie J & Gao G, Hammers DW, Sweeney L (2022). Potential limitations of micro-dystrophin gene therapy for Duchenne muscular dystrophy bioRxiv DOI: 1002510519. [CrossRef]
- Hayes HM, Angerosa J, Piers AT, White JD, Koleff J, Thurgood M, Moody J, Cheung MM, & Pepe S. Preserved Left Ventricular Function despite Myocardial Fibrosis and Myopathy in the Dystrophin-Deficient D2.B10-Dmdmdx/J Mouse. Oxid Med Cell Longev, 2022; 5362115.
- Heier CR, Yu Q, Fiorillo AA, Tully CB, Tucker A, Mazala DA, Uaesoontrachoon K, Srinivassane S, Damsker JM, Hoffman EP, Nagaraju K & Spurney CF. Vamorolone targets dual nuclear receptors to treat inflammation and dystrophic cardiomyopathy. Life Sci Alliance 2019, 2, e201800186. [Google Scholar] [CrossRef] [PubMed]
- Hoffman EP, Reeves E, Damsker J, Nagaraju K, McCall JM, Connor EM & Bushby K. Novel approaches to corticosteroid treatment in Duchenne muscular dystrophy. Phys Med Rehabil Clin N Am 2012, 23, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, EP. The discovery of dystrophin, the protein product of the Duchenne muscular dystrophy gene. FEBS J 2020, 287, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Hor KN, Mah ML, Johnston P, Cripe TP & Cripe LH. Advances in the diagnosis and management of cardiomyopathy in Duchenne muscular dystrophy. Neuromuscul Disord 2018, 28, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Hor KN, Kissoon N, Mazur W, Gupta R, Ittenbach RF, Al-Khalidi HR, Cripe LH, Raman SV, Puchalski MD, Gottliebson WM & Benson DW. Regional circumferential strain is a biomarker for disease severity in Duchenne muscular dystrophy heart disease: A cross-sectional study. Pediatr Cardiol 2015, 36, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hor KN, Taylor MD, Al-Khalidi HR, LH, Raman SV, Jefferies JL, O’Donnell R, Benson DW & Mazur W. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: Effect of age and left ventricular systolic function. J Cardiovasc Magn Reson 2013, 15, 107. [Google Scholar] [CrossRef]
- Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, Benson DW & Gottliebson WM. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: A cardiac magnetic resonance tagging study. J Am Coll Cardiol 2009, 53, 1204–1210. [Google Scholar] [CrossRef]
- Houde S, Filiatrault M, Fournier A, Dubé J, D'Arcy S, Bérubé D, Brousseau Y, Lapierre G & Vanasse M. Deflazacort use in Duchenne muscular dystrophy: An 8-year follow-up. Pediatr Neurol 2008, 38, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Howard ZM, Dorn LE, Lowe J, Gertzen MD, Ciccone P, Rastogi N, Odom GL, Accornero F, Chamberlain JS & Rafael-Fortney JA. Micro-dystrophin gene therapy prevents heart failure in an improved Duchenne muscular dystrophy cardiomyopathy mouse model. JCI Insight 2021, 6, e146511. [Google Scholar] [CrossRef] [PubMed]
- Howell JM, Fletcher S, Kakulas BA, O’Hara M, Lochmuller H & Karpati G. Use of the dog model for Duchenne muscular dystrophy in gene therapy trials. Neuromuscul Disord 1997, 7, 325–328. [Google Scholar] [CrossRef]
- Hughes MC, Ramos SV, Turnbull PC, Edgett BA, Huber JS, Polidovitch N, Schlattner U, Backx PH, Simpson JA & Perry CGR. Impairments in left ventricular mitochondrial bioenergetics precede overt cardiac dysfunction and remodelling in Duchenne muscular dystrophy. J Physiol 2020, 598, 1377–1392. [Google Scholar] [CrossRef] [PubMed]
- Hughes MC, Ramos SV, Turnbull PC, Rebalka IA, Cao A, Monaco CMF, Varah NE, Edgett BA, Huber JS, Tadi P, Delfinis LJ, Schlattner U, Simpson JA, Hawke TJ & Perry CGR. Early myopathy in Duchenne muscular dystrophy is associated with elevated mitochondrial H2O2 emission during impaired oxidative phosphorylation. J Cachexia Sarcopenia Muscle 2019, 10, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Iwata Y, Ohtake H, Suzuki O, Matsuda J, Komamura K & Wakabayashi S. Blockade of sarcolemmal TRPV2 accumulation inhibits progression of dilated cardiomyopathy. Cardiovasc Res 2013, 99, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Ionăsescu V, Luca N, & Vuia O. Respiratory control and oxidative phosphorylation in the dystrophic muscle. Acta Neurol Scand 1967, 43, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, Eisenbarth SC, Nauseef WM, Cassel SL & Sutterwala FS. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 2013, 39, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Jacobs PA, Hunt PA, Mayer M & Bart RD. Duchenne muscular dystrophy (DMD) in a female with an X/autosome translocation: further evidence that the DMD locus is at Xp21. Am J Hum Genet 1981, 33, 513–518. [Google Scholar]
- Janssen PML, Murray JD, Schill KE, Rastogi N, Schultz EJ, Tran T, Raman SV & Rafael-Fortney JA. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PLOS One 2014, 9, e88360. [Google Scholar]
- Jefferies JL, Eidem BW, Belmont JW, Craigen WJ, Ware SM, Fernbach SD, Neish SR, Smith EO & Towbin J. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation 2005, 112, 2799–2804. [Google Scholar] [CrossRef]
- Johnston JR & McNally, EM. Genetic correction strategies for Duchenne muscular dystrophy and their impact on the heart. Prog Pediatr Cardiol 2021, 63, 101460. [Google Scholar]
- Johnstone VP, Viola HM & Hool LC. Dystrophic cardiomyopathy—potential role of calcium in pathogenesis, treatment and novel therapies. Genes (Basel) 2017, 8, 108. [Google Scholar] [CrossRef]
- Judge DP, Kass DA, Thompson WR & Wagner KR. Pathophysiology and therapy of cardiac dysfunction in Duchenne muscular dystrophy. Am J Cardiovasc Drugs 2011, 11, 287–294. [Google Scholar] [CrossRef]
- Jung C, Martins AS, Niggli E & Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovas Res 2007, 77, 766–773. [Google Scholar]
- Kajimoto H, Ishigaki K, Okumura K, Tomimatsu H, Nakazawa M, Saito K, Osawa M & Nakanishi T. Beta-blocker therapy for cardiac dysfunction in patients with muscular dystrophy. Circulation 2006, 70, 991–994. [Google Scholar] [CrossRef]
- Kamdar F, Das S, Gong W, Kamdar AK, Meyers TA, Shah P, Ervasti JM, Townsend D, Kamp TJ, Wu JC, Garry MG, Zhang J & Garry DJ. Stem cell-derived cardiomyocytes and beta-adrenergic receptor blockade in Duchenne muscular dystrophy cardiomyopathy. J Am Coll Cardiol 2020, 75, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Kamdar F & Garry, DJ. Dystrophin-deficient cardiomyopathy. J Am Coll Cardiol 2016, 67, 2533–2546. [Google Scholar]
- Kang C, Badr MA, Kyrychenko V, Eskelinen EL & Shirokova N. Deficit in PINK1/PARKIN-mediated mitochondrial autophagy at late stages of dystrophic cardiomyopathy. Cardiovasc Res 2018, 114, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, Lin SCJ, Aronow BJ, Tallquist MD & Molkentin JD. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 2016, 7, 12260. [Google Scholar] [CrossRef] [PubMed]
- Kaspar RW, Allen HD & Montanaro F. Current understanding and management of dilated cardiomyopathy in Duchenne and Becker muscular dystrophy. J Am Acad Nurse Pract 2009, 21, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Kawai T, Forrester SJ, O’Brien S, Baggett A, Rizzo V & Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res 2017, 125, 4–13. [Google Scholar] [CrossRef]
- Kellman P, Chefd’hotel C, Lorenz CH, Mancini C, Arai AE & McVeigh ER. High spatial and temporal resolution cardiac cine MRI from retrospective reconstruction of data acquired in real time using motion correction and resorting. Magn Reson Med 2009, 62, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Kellman P, Bandettini WP, Mancini C, Hammer-Hansen S, Hansen MS & Arai AE. Characterization of myocardial T1-mapping bias caused by intramyocardial fat in inversion recovery and saturation recovery techniques. J Cardiovasc Magn Reson 2015, 17, 1–11. [Google Scholar]
- Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG & Ward CW. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci Signal 2012, 5, ra56. [Google Scholar]
- Khairallah M; Khairallah R, Young ME, Dyck JR, Petrof BJ & Des Rosiers C. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol 2007, 43, 119–129. [Google Scholar] [CrossRef]
- Koenig X, Rubi L, Obermair GJ, Cervenka R, Dang XB, Lukacs P, Kummer S, Bittner RE, Kubista H, Todt H & Hilber K. Enhanced currents through L-type calcium channels in cardiomyocytes disturb the electrophysiology of the dystrophic heart. Am J Physiol Heart Circ Physiol 2014, 306, H564–H573. [Google Scholar] [CrossRef]
- Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C & Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef]
- Koufen P, Rück A, Brdiczka D, Wendt S, Wallimann T & Stark G. Free radical-induced inactivation of creatine kinase: influence on the octameric and dimeric states of the mitochondrial enzyme (Mib-CK). Biochem J 1999, 344, 413–417. [Google Scholar] [CrossRef]
- Kranias EG & Hajjar, RJ. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ Res 2012, 110, 1646. [Google Scholar]
- Krumenacker JS, Hanafy KA & Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull 2004, 62, 505–515. [Google Scholar] [CrossRef]
- Krumenacker JS, Hyder SM & Murad F. Estradiol rapidly inhibits soluble guanylyl cyclase expression in rat uterus. Proc Natl Acad Sci U S A 2001, 98, 717–722. [Google Scholar] [CrossRef]
- Kumar A & Boriek, AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB Jo 2003, 17, 386–396. [Google Scholar]
- Kuno A, Hosoda R, Sebori R, Hayashi T, Sakuragi H, Tanabe M & Horio Y. Resveratrol ameliorates mitophagy disturbance and improves cardiac pathophysiology of dystrophin-deficient mdx mice. Sci Rep 2018, 8, 15555. [Google Scholar] [CrossRef]
- Kyrychenko V, Poláková E, Janíček R & Shirokova N. Mitochondrial dysfunctions during progression of dystrophic cardiomyopathy. Cell Calcium 2015, 58, 186–195. [Google Scholar] [CrossRef]
- Kyrychenko S, Poláková E, Kang C, Pocsai K, Ullrich ND, Niggli E & Shirokova N. Hierarchical accumulation of RyR post-translational modifications drives disease progression in dystrophic cardiomyopathy. Cardiovasc Res 2013, 97, 666–675. [Google Scholar] [CrossRef]
- LaCorte JC, Cabreriza SE, Rabkin DG, Printz BF, Coku L, Weinberg A, Gersony WM & Spotnitz HM. Correlation of the Tei index with invasive measurements of ventricular function in a porcine model. J Am Soc Echocardiogr 2003, 16, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Laing NG, Siddique T, Bartlett R, Yamaoka LH, Hung WY, Pericak-Vance MA & Roses AD. Duchenne muscular dystrophy: detection of deletion carriers by spectrophotometric densitometry. Clin Genet 1989, 35, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Larcher T, Lafoux A, Tesson L, Remy S, Thepenier V, François V, Le Guiner C, Goubin H, Dutilleul M, Guigand L, Toumaniantz G, De Cian A, Boix C, Renaud JB, Cherel Y, Giovannangeli C, Concordet JP, Anegon I & Huchet C. Characterization of dystrophin deficient rats: a new model for Duchenne muscular dystrophy. PLoS One 2014, 9, e110371. [Google Scholar]
- Law ML, Prins KW, Olander ME & Metzger JM. Exacerbation of dystrophic cardiomyopathy by phospholamban deficiency mediated chronically increased cardiac Ca(2+) cycling in vivo. Am J Physiol Heart Circ Physiol 2018, 315, H1544–H1552. [Google Scholar] [CrossRef]
- Law ML, Cohen H, Martin AA, Angulski ABB & Metzger JM. Dysregulation of calcium handling in Duchenne muscular dystrophy-associated dilated cardiomyopathy: mechanisms and experimental therapeutic strategies. J Clin Med 2020, 9, 520. [Google Scholar] [CrossRef]
- Lu QL, Rabinowitz A, Chen YC, Yokota T, Yin H, Alter J, Jadoon A, Bou-Gharios G & Partridge T. Systemic delivery of antisense oligoribonucleotide restores dystrophin expression in body-wide skeletal muscles. Proc Natl Acad Sci U S A 2005, 102, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Le S, Yu M, Hovan L, Zhao Z, Ervasti J & Yan J. Dystrophin as a molecular shock absorber. ACS Nano 2018, 12, 12140–12148. [Google Scholar] [CrossRef] [PubMed]
- Le Guiner C Servais L, Montus M, Larcher T, Fraysse B, Moullec S, Allais M, François V, Dutilleul M, Malerba A, Koo T, Thibaut JL, Matot B, Devaux M, Le Duff J, Deschamps JY, Barthelemy I, Blot S, Testault I,… Dickson G. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat Commun 2017, 8, 16105. [Google Scholar] [CrossRef] [PubMed]
- Lee S, Lee M & Hor KN. The role of imaging in characterizing the cardiac natural history of Duchenne muscular dystrophy. Pediatr Pulmonol 2021, 56, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Lemasters JJ, Theruvath TP, Zhong Z & Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 2009, 1787, 1395–1401. [Google Scholar] [CrossRef]
- Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, Yaeger L, Hardi A, Holland MR, Hamvas A & Singh GK. Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr 2016, 29, 209–225e6. [Google Scholar] [CrossRef]
- Li X, Zhang W, Cao Q, Wang Z, Zhao M, Xu L & Zhuang Q. Mitochondrial dysfunction in fibrotic diseases. Cell Death Discov 2020, 6, 80. [Google Scholar] [CrossRef]
- Lin B, Li Y, Han L, Kaplan AD, Ao Y, Kalra S, Bett GC, Rasmusson RL, Denning C & Yang L. Modeling and study of the mechanism of dilated cardiomyopathy using induced pluripotent stem cells derived from individuals with Duchenne muscular dystrophy. Dis Model Mech 2015, 8, 457–466. [Google Scholar] [CrossRef]
- Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, Brown JP, Cohen A & Kim H. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013, 9, 30. [Google Scholar] [CrossRef]
- Lorin C, Vögeli I & Niggli E. Dystrophic cardiomyopathy: role of TRPV2 channels in stretch-induced cell damage. Cardiovasc Res 2015, 106, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Luan P, D’Amico D, Andreux PA, Laurila PP, Wohlwend M, Li H, Imamura de Lima T, Place N, Rinsch C, Zanou N & Auwerx J. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci Transl Med 2021, 13, eabb0319. [Google Scholar] [CrossRef] [PubMed]
- MacLennan D & Kranias, E. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol 2003, 4, 566–577. [Google Scholar]
- Magrath P, Maforo N, Renella P, Nelson SF, Halnon N & Ennis DB. Cardiac MRI biomarkers for Duchenne muscular dystrophy. Biomark Med 2018, 12, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Magri F, Govoni A, D’Angelo MG, Del Bo R, Ghezzi S, Sandra G, Turconi AC, Sciacco M, Ciscato P, Bordoni A, Tedeschi S, Fortunato F, Lucchini V, Bonato S, Lamperti C, Coviello D, Torrente Y, Corti S, Moggio M,… Comi, GP. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J Neurol 2011, 258, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Mah JK, Korngut L, Dykeman J, Day L, Pringsheim T & Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord 2014, 24, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Markham LW, Spicer RL, Khoury PR, Wong BL, Mathews KD & Cripe LH. Steroid therapy and cardiac function in Duchenne muscular dystrophy. Pediatr Cardiol 2005, 26, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Markham LW, Kinnett K, Wong BL, Benson DW & Cripe LH. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord 2008, 2008, 18–365. [Google Scholar]
- Markham LW, Michelfelder EC, Border WL, Khoury PR, Spicer RL, Wong BL, Benson DW & Cripe LH. Abnormalities of diastolic function precede dilated cardiomyopathy associated with Duchenne muscular dystrophy. J Am Soc Echocardiogr 2006, 19, 865–871. [Google Scholar] [CrossRef]
- Masubuchi N, Shidoh Y, Kondo S, Takatoh J & Hanaoka K. Subcellular localization of dystrophin isoforms in cardiomyocytes and phenotypic analysis of dystrophin-deficient mice reveal cardiac myopathy is predominantly caused by a deficiency in full-length dystrophin. Exp Anim 2013, 62, 211–217. [Google Scholar] [CrossRef]
- Matsumura T, Saito T, Fujimura H & Shinno S. Cardiac troponin I for accurate evaluation of cardiac status in myopathic patients. Brain Dev 2007, 29, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Matsumura T, Tamura T, Kuru S, Kikuchi Y & Kawai M. Carvedilol can prevent cardiac events in Duchenne muscular dystrophy. Intern Med 2010, 49, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni S, Bratis K, Papavasiliou A, Skouteli E, Karanasios E, Georgakopoulos D, Kolovou G & Papadopoulos G. CMR detects subclinical cardiomyopathy in mother-carriers of Duchenne and Becker muscular dystrophy. JACC Cardiovasc Imaging 2013, 6, 26–28. [Google Scholar]
- McDonald CM, Meier T, Voit T, Schara U, Straathof CS, D’Angelo MG, Bernert G, Cuisset JM, Finkel RS, Goemans N, Rummey C, Leinonen M, Spagnolo P, Buyse GM & DELOS Study Group. Idebenone reduces respiratory complications in patients with Duchenne muscular dystrophy. Neuromuscul Disord 2016, 26, 473–480. [Google Scholar] [CrossRef]
- McNally EM, Kaltman JR, Benson DW, Canter CE, Cripe LH, Duan D, Finder JD, Hoffman EP, Judge DP, Kertesz N, Kinnett K, Kirsch R, Metzger JM, Pearson GD, Rafael-Fortney JA, Raman SV, CF, Targum SL, Wagner KR & Markham LW. Contemporary cardiac issues in Duchenne muscular dystrophy. Circulation 2015, 131, 1590–1598. [Google Scholar] [CrossRef]
- Mehmood M, Hor KN, Al-Khalidi HR, Benson DW, Jefferies JL, Taylor MD, Egnaczyk GF, Raman SV, Basu SK, Cripe LH, Germann J & Mazur W. Comparison of right and left ventricular function and size in Duchenne muscular dystrophy. Eur J Radiol 2015, 84, 1938–1942. [Google Scholar] [CrossRef]
- Mehmood M, Ambach SA, Taylor MD, Jefferies JL, Raman SV, Taylor RJ, Sawani H, Mathew J, Mazur W, Hor KN & Al-Khalidi HR. Relationship of right ventricular size and function with respiratory status in Duchenne muscular dystrophy. Pediatr Cardiol 2016, 37, 878–883. [Google Scholar] [CrossRef]
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J; Kneile K, Dunn DM, Duval B, Aoyagi A, Hamil C, Mahmoud M, Roush K, Bird L, Rankin C, Lilly H, Street N, Chandrasekar R & Weiss RB. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012, 71, 304–313. [Google Scholar] [CrossRef]
- Menon SC, Etheridge SP, Liesemer KN, Williams RV, Bardsley T, Heywood MC & Puchalski MD. Predictive value of myocardial delayed enhancement in Duchenne muscular dystrophy. Pediatr Cardiol 2014, 35, 1279–1285. [Google Scholar] [CrossRef]
- Mercuri E, Bonnemann CG & Muntoni F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Mertens L, Ganame J, Claus P, Goemans N, Thijs D, Eyskens B, Van Laere D, Bijnens B, D’hooge J, Sutherland GR & Buyse G. Early regional myocardial dysfunction in young patients with Duchenne muscular dystrophy. J Am Soc Echocardiogr 2008, 21, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Meyer LE, Machado LB, Santiago APSA, da-Silva WS, Felice FGD, Holub O, Oliveira MF, & Galina A. Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem 2006, 281, 37361–37371. [Google Scholar] [CrossRef] [PubMed]
- Meyer RA & Sweeney HL, Kushmerick MJ. A simple analysis of the “phosphocreatine shuttle”. Am J Physiol 1984, 246, C365–C377. [Google Scholar] [CrossRef] [PubMed]
- Meyers TA & Townsend, D. Early right ventricular fibrosis and reduction in biventricular cardiac reserve in the dystrophin-deficient mdx heart. Am J Physiol Heart Circ Physiol 2015, 308, H303–H315. [Google Scholar]
- Meyers TA & Townsend, D. Cardiac pathophysiology and the future of cardiac therapies in Duchenne muscular dystrophy. Int J Mol Sci 2019, 20, E4098. [Google Scholar]
- Meyers TA, Heitzman JA & Townsend D. 2019. Acute myocardial injury in mdx hearts ameliorated by ARB but not ACE inhibitor treatment. bioRxiv. [CrossRef]
- Meyers TA, Heitzman JA, Krebsbach A, Aufdembrink LM, Hughes R, Bartolomucci A & Townsend D. Acute AT1R blockade prevents isoproterenol-induced injury in mdx hearts. J Mol Cell Cardiol 2019, 128, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Mijares A, Altamirano F, Kolster J, Adams JA & Lopez JR. Age-dependent changes in diastolic Ca2+ and Na+ concentrations in dystrophic cardiomyopathy: role of Ca2+ entry and IP3. Biochem Biophys Res Commun 2014, 452, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Millay DP, Sargent MA, Osinska H, Baines CP, Barton ER, Vuagniaux G, Sweeney HL, Robbins J & Molkentin JD. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med 2008, 14, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Mohyuddin T, Jacobs IB & Bahler RC. B-type natriuretic peptide and cardiac dysfunction in Duchenne muscular dystrophy. Int J Cardiol 2007, 119, 389–391. [Google Scholar] [CrossRef]
- Momose M, Iguchi N, Imamura K, Usui H, Ueda T, Miyamoto K & Inaba S. Depressed myocardial fatty acid metabolism in patients with muscular dystrophy. Neuromuscul Disord 2001, 11, 464–469. [Google Scholar] [CrossRef]
- Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zacà V, Ballo P, D’Andrea A, Muraru D, Losi M, Agricola E, D’Errico A, Buralli S, Sciomer S, Nistri S, Badano L & Echocardiography Study Group of the Italian Society of Cardiology (Rome, Italy). Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med 2011, 30, 71–83. [Google Scholar] [CrossRef]
- Mori K, Hayabuchi Y, Inoue M, Suzuki M, Sakata M, Nakagawa R, Kagami S, Tatara K, Hirayama Y & Abe Y. Myocardial strain imaging for early detection of cardiac involvement in patients with Duchenne’s progressive muscular dystrophy. Echocardiography 2007, 24, 598–608. [Google Scholar] [CrossRef]
- Muralidharan P, Cserne Szappanos H, Ingley E & Hool LC. The cardiac L-type calcium channel alpha subunit is a target for direct redox modification during oxidative stress-the role of cysteine residues in the alpha interacting domain. Clin Exp Pharmacol Physiol 2017, 44, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Murphy, MP. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta 2008, 1777, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Naik E & Dixit, VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 2011, 208, 417–420. [Google Scholar]
- Nakamura K, Fujii W Tsuboi M, Tanihata J, Teramoto N, Takeuchi S, Naito K, Yamanouchi K & Nishihara M. Generation of muscular dystrophy model rats with a CRISPR/Cas system. Sci Rep 2014, 4, 5635. [Google Scholar] [CrossRef]
- Nakamura A, Fueki N, Shiba N, Motoki H, Miyazaki D, Nishizawa H, Echigoya Y, Yokota T, Aoki Y & Takeda S. Deletion of exons 3-9 encompassing a mutational hot spot in the DMD gene presents an asymptomatic phenotype, indicating a target region for multiexon skipping therapy. J Exp Med 2016, 61, 663–667. [Google Scholar]
- Namgoog JH & Bertoni, C. Clinical potential of ataluren in the treatment of Duchenne muscular dystrophy. J Exp Med 2016, 6, 37–48. [Google Scholar]
- Naruse H, Miyagi J, Arii T, Ohyanagi M, Iwasaki T & Jinnai K. The relationship between clinical stage, prognosis and myocardial damage in patients with Duchenne-type muscular dystrophy: five-year follow-up study. Ann Nucl Med 2004, 18, 203–208. [Google Scholar] [CrossRef]
- Nelson CE, Wu Y, Gemberling MP, Oliver ML, Waller MA, Bohning JD, Robinson-Hamm JN, Bulaklak K, Castellanos Rivera RM, Collier JH, Asokan A & Gersbach CA. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat Med 2019, 25, 427–432. [Google Scholar] [CrossRef]
- Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX, Asokan A, Zhang F, Duan D & Gersbach CA. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 2016, 351, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Nelson DM, Lindsay A, Judge LM, Duan D, Chamberlain JS, Lowe DA, & Ervasti JM. Variable rescue of microtubule and physiological phenotypes in mdx muscle expressing different miniaturized dystrophins. Hum Mol Genet 2018, 27, 2090–2100. [Google Scholar] [CrossRef] [PubMed]
- Nicholls DG & Ferguson SJ (2013). Bioenergetics 4. Elsevier: p. 434.
- Nigro G, Comi LI, Limongelli FM, Giugliano MAM, Politano L, Petretta V, Passamano L & Stefanelli S. Prospective study of X-linked progressive muscular dystrophy in campania. Muscle & Nerve 1983, 6, 253–262. [Google Scholar]
- Nigro G, Comi LI, Politano L &, Bain RJI. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol 1990, 26, 271–277. [Google Scholar] [CrossRef]
- Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT & Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res 2004, 61, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Nisoli E, Falcone S, Tonello C, & Clementi E. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc. Natl. Acad. Sci. U.S.A 2004, 101, 16507–16512. [Google Scholar] [CrossRef]
- Obi C, Smith AT, Hughes GJ & Adeboye AA. Targeting mitochondrial dysfunction with elamipretide. Heart Fail Rev 2022, 27, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Ogata H, Ishikawa Y, Ishikawa Y & Minami R. Beneficial effects of beta-blockers and angiotensin-converting enzyme inhibitors in Duchenne muscular dystrophy. J Cardiol 2009, 53, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ogata H, Nakatani S, Ishikawa Y, Negishi A, Kobayashi M & Minami R. Myocardial strain changes in Duchenne muscular dystrophy without overt cardiomyopathy. Int J Cardiol 2007, 115, 190–195. [Google Scholar] [CrossRef]
- Olchowy C, Cebulski K, Łasecki M, Chaber R, Olchowy A, Kałwak K & Zaleska-Dorobisz U. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity: a systematic review. PLOS One 2017, 12, e0171704. [Google Scholar]
- Olivieri LJ, Kellman P, McCarter RJ, Cross RR, Hansen MS & Spurney CF. Native T1 values identify myocardial changes and stratify disease severity in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson 2016, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Olthoff JT, Lindsay A, Abo-Zahrah R, Baltgalvis KA, Patrinostro X, Belanto JJ, Yu D-Y, Perrin BJ, Garry DJ, Rodney GG, Lowe DA, & Ervasti JM. Loss of peroxiredoxin-2 exacerbates eccentric contraction-induced force loss in dystrophin-deficient muscle. Nat Commun 2018, 9, 5104. [Google Scholar] [CrossRef] [PubMed]
- Panovský R, Pešl M, Máchal J, Holeček T, Feitová V, Juříková L, Masárová L, Pešlová E, Opatřil L, Mojica-Pisciotti ML & Kincl V. Quantitative assessment of left ventricular longitudinal function and myocardial deformation in Duchenne muscular dystrophy patients. Orphanet J Rare Dis 2021, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Passamano L, Taglia A, Palladino A, Viggiano E, D’Ambrosio P, Scutifero M, Cecio MR, Torre V, De Luca F, Picillo E, Paciello O, Piluso G, Nigro G & Politano L. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol 2012, 31, 121–125. [Google Scholar]
- Patrianakos AP, Zacharaki A, Kalogerakis A, Solidakis G, Parthenakis F & Vardas P. Two-dimensiona global and segmental longitudinal strain: are the results from software in different high-end ultrasound systems comparable? Echo Res Pract 2015, 2, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, Koechlin-Ramonatxo C, Hugon G, Lacampagne A, Coisy-Quivy M, Liang F, Hussain S, Matecki S & Petrof BJ. AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol 2012, 181, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Perazella, MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol 2009, 4, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Perloff JK, Roberts WC, de Leon AC & O’Doherty D. The distinctive electrocardiogram of Duchenne’s progressive muscular dystrophy. Am J Med 1967, 42, 179–188. [Google Scholar] [CrossRef]
- Perloff JK, Henze E & Schelbert HR. Alterations in regional myocardial metabolism, perfusion, and wall motion in Duchenne muscular dystrophy studied by radionuclide imaging. Circulation 1984, 69, 33–42. [Google Scholar] [CrossRef]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM & Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A 1993, 90, 3710–3714. [Google Scholar] [CrossRef]
- Piga D, Salani S, Magri F, Brusa R, Mauri E, Comi GP, Bresolin N & Corti S. Human induced pluripotent stem cell models for the study and treatment of Duchenne and Becker muscular dystrophies. Ther Adv Neurol Disord 2019, 12, 175628641983347. [Google Scholar] [CrossRef] [PubMed]
- Pinniger GJ, Terrill JR, Assan EB, Grounds MD, & Arthur PG. Pre-clinical evaluation of N-acetylcysteine reveals side effects in the mdx mouse model of Duchenne muscular dystrophy. J Physiol 2017, 595, 7093–7107. [Google Scholar] [CrossRef] [PubMed]
- Politano L, Palladino A, Petretta VR, Mansi L, Passamano L, Nigro G, Comi LI & Nigro G. ST-segmen displacement in Duchenne muscular dystrophy: myocardial necrosis or apoptosis? Acta myol 2003, 22, 5–10. [Google Scholar]
- Porte-Thomé F, Nagaraju K, Yu Q, Tatem K, Bkaily G, Scholz W, Slade A, Bot N & Kant C. Development of Rimeporide, a sodium-hydrogen exchanger (NHE-1) inhibitor, for patients with Duchenne muscular dystrophy. Neuromuscul Disord 2015, 25, S259–S260. [Google Scholar] [CrossRef]
- Potter RA, Griffin DA, Heller KN, Peterson EL, Clark EK, Mendell JR & Rodino-Klapac LR. Dose-escalation study of systemically delivered rAAVrh74MHCK7 micro-dystrophin in the mdx mouse model of Duchenne muscular dystrophy. Hum Gene Ther 2021, 32, 375–389. [Google Scholar] [CrossRef]
- Power A, Poonja S, Disler D, Myers K, Patton DJ, Mah JK, Fine NM & Greenway SC. Echocardiographic image quality deteriorates with age in children and young adults with Duchenne muscular dystrophy. Front Cardiovasc Med 2017, 4, 82. [Google Scholar] [CrossRef]
- Prakash N, Suthar R, Sihag BK, Debi U, Kumar RM & Sankhyan N. Cardiac MRI and echocardiography for early diagnosis of cardiomyopathy among boys with Duchenne muscular dystrophy: a cross-sectional study. Front Pediatr 2022, 10, 818608. [Google Scholar] [CrossRef] [PubMed]
- Previtali SC, Gidaro T, Díaz-Manera J, Zambon A, Carnesecchi S, Roux-Lombard P, Spitali P, Signorelli M, Szigyarto CA, Johansson C, Gray J, Labolle D, Porte Thomé F, Pitchforth J, Domingos J & Muntoni F. Rimeporide as a first- in-class NHE-1 inhibitor: Results of a phase Ib trial in young patients with Duchenne Muscular Dystrophy. Pharmacol Res 2020, 159, 104999. [Google Scholar] [CrossRef] [PubMed]
- Prins KW, Humston JL, Mehta A, Tate V, Ralston E, & Ervasti JM. Dystrophin is a microtubule-associated protein. J Cell Biol 2009, 186, 363–369. [Google Scholar] [CrossRef]
- Prosser BL, Ward CW & Lederer WJ. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 2011, 333, 1440–1445. [Google Scholar] [CrossRef]
- Prosser BL, Khairallah RJ, Ziman AP, Ward CW & Lederer WJ. X-ROS signaling in the heart and skeletal muscle: stretch-dependent local ROS regulates [Ca2+]i. J Mol Cell Cardiol 2013, 58, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Puchalski MD, Williams RV, Askovich B, Sower CT, Hor KH, Su JT, Pack N, Dibella E & Gottliebson WM. Lat gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging 2009, 25, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli M, Zelikovich AS, Jiang Z, Peek CB, Demonbreun AR, Kuntz NL, Barish GD, Haldar SM & Bass J, McNally EM. Pulsed glucocorticoids enhance dystrophic muscle performance through epigenetic-metabolic reprogramming. JCI Insight 2019, 4, e132402. [Google Scholar] [CrossRef] [PubMed]
- Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfín DA, Janssen PML & Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation 2011, 124, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Raman SV, Hor KN, Mazur W, Cardona A, He X, Halnon N, Markham L, Soslow JH, Puchalski MD, Auerbach SR, Truong U, Smart S, McCarthy B, Saeed IM, Statland JM, Kissel JT & Cripe LH. Stabilization of early Duchenne cardiomyopathy with aldosterone inhibition: results of the multicenter AIDMD trial. J Am Heart Assoc 2019, 8, e013501. [Google Scholar] [CrossRef] [PubMed]
- Raman SV & Cripe, LH. Glucocorticoi therapy for Duchenne cardiomyopathy: a Hobson’s choice. J Am Heart Assoc 2015, 4, 1–3. [Google Scholar]
- 287. Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD, Jefferies JL, Rafael-Fortney JA, Lowe J, Roble SL & Cripe LH. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2015, 14, 153–161.
- Raman SV, Hor KN, Mazur W, He X, Kissel JT, Smart S, McCarthy B, Roble SL & Cripe LH. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: results of a two-year open-label extension trial. Orphanet J Rare Dis 2017, 12, 1–5. [Google Scholar]
- Ramos SV, Hughes MC, Delfinis LJ, Bellissimo CA & Perry CGR. Mitochondrial bioenergetic dysfunction in the D2mdx model of Duchenne muscular dystrophy is associated with microtubule disorganization in skeletal muscle. PLOS One 2020, 15, e0237138. [Google Scholar]
- Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA & Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Reid AL & Alexander, MS. The interplay of mitophagy and inflammation in Duchenne muscular dystrophy. Life (Basel) 2021, 11, 648. [Google Scholar]
- Reutenauer J, Dorchies OM, Patthey-Vuadens O, Vuagniaux G, & Ruegg UT. Investigation of Debio 025, a cyclophilin inhibitor, in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. Br. J. Pharmacol. 2008, 155, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Rhodes J, Margossian R, Darras BT, Colan SD, Jenkins KJ, Geva T & Powell AJ. Safety and efficacy of carvedilol therapy for patients with dilated cardiomyopathy secondary to muscular dystrophy. Pediatr Cardiol 2008, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Rittoo D, Jones A, Lecky B & Neithercut D. Elevation of cardiac troponin T, but not cardiac troponin I, in patients with neuromuscular diseases: implications for the diagnosis of myocardial infarction. J Am Coll Cardiol 2014, 63, 2411–2420. [Google Scholar] [CrossRef]
- Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V & Pozzan T. Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem 2001, 276, 4647–4651. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues MV, Stoco-Oliveira MC, Silva TDD, Ferreira C, Valente HB, Vanzella LM & Vanderlei LCM. Autonomic modulation at rest and in response to postural change in adolescents with Duchenne muscular dystrophy: a cross-sectional study. Arq Neuropsiquiatr 2021, 79, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Rohman MS, Emoto N, Takeshima Y, Yokoyama M & Matsuo M. Decreased mAKAP, ryanodine receptor, and SERCA2a gene expression in mdx hearts. Biochem Biophys Res Commun 2003, 310, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Romfh A & McNally, EM. Cardiac assessment in Duchenne and Becker muscular dystrophies. Curr Heart Fail Rep 2010, 7, 212–218. [Google Scholar]
- Roujol S, Weingärtner S, Foppa M, Chow K, Kawaji K, Ngo LH, Kellman P, Manning WJ, Thompson R & Nezafat R. Accuracy, precision, and reproducibility of four T1 mapping sequences: a head-to-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 2014, 272, 683–689. [Google Scholar] [CrossRef]
- Ryan TD, Taylor MD, Mazur W, Cripe LH, Pratt J, King EC, Lao K, Grenier MA, Jefferies JL, Benson DW & Hor KN. Abnormal circumferential strain is present in young Duchenne muscular dystrophy patients. Pediatr Cardiol 2013, 34, 1159–1165. [Google Scholar] [CrossRef]
- Rybalka E, Timpani CA, Cooke MB, Williams AD & Hayes A. Defects in mitochondrial ATP synthesis in dystrophin-deficient mdx skeletal muscles may be caused by complex I insufficiency. PLOS One 2014, 9, e115763. [Google Scholar]
- Ryder S, Leadley RM, Armstrong N, Westwood M, de Kock S, Butt T, Jain M & Kleijnen J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J Rare Dis 2017, 12, 79. [Google Scholar] [CrossRef]
- Salva MZ, Himeda CL, Tai PWL, Nishiuchi E, Gregorevic P, Allen JM, Finn EE, Nguyen QG, Blankinship MJ, Meuse L, Chamberlain JS & Hauschkaet SD. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther 2007, 15, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Santos MA, Costa Fde A, Travessa AF, Bombig MTN, Fonseca FH, Filho BL, Mussi A, de Souza D, de Oliveira A & Povoa R. Duchenne muscular dystrophy: electrocardiographic analysis of 131 patients. Arq Bras Cardiol 2010, 94, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Sanyal SK, Johnson WW, Dische MR, Pitner SE & Beard C. Dystrophic degeneration of papillary muscle and ventricular myocardium: a basis for mitral valve prolapse in Duchenne’s muscular dystrophy. Circulation 1980, 62, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Sarma S, Li N, van Oort RJ, Reynolds C, Skapura DG & Wehrens XHT. Genetic inhibition of PKA phosphorylation of RyR2 prevents dystrophic cardiomyopathy. Proc Natl Acad Sci U S A 2010, 107, 13165–13170. [Google Scholar] [CrossRef] [PubMed]
- Schiavone M, Zulian A, Menazza S, Petronilli V, Argenton F, Merlini L, Sabatelli P, & Bernardi P. Alisporivir rescues defective mitochondrial respiration in Duchenne muscular dystrophy. Pharmacol. Res. 2017, 125, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Schlattner U, Kay L & Tokarska-Schlattner M. Mitochondrial Proteolipid Complexes of Creatine Kinase. Subcell Biochem 2018, 87, 365–408. [Google Scholar]
- Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, Asslaber M, Radl R, Beer M, Polacin M, Mair J, Szolar D, Berghold A, Quasthoff S, Binder JS & Rainer PP. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol 2018, 71, 1540–1549. [Google Scholar] [CrossRef]
- Schneider SM, Sridhar V, Bettis AK, Heath-Barnett H, Balog-Alvarez CJ, Guo LJ, Johnson R, Jaques S, Vitha S, Glowcwski AC, Kornegay JN & Nghiem PP. Glucose metabolism as a pre-clinical biomarker for the golden retriever model of Duchenne muscular dystrophy. Mol Imaging Biol 2018, 20, 780–788. [Google Scholar] [CrossRef]
- Schneider SM, Sansom GT, Guo LJ, Furuya S, Weeks BR & Kornegay JN. Natural history of histopathologic changes in cardiomyopathy of golden retriever muscular dystrophy. Front Vet Sci 2022, 8, 759585. [Google Scholar] [CrossRef] [PubMed]
- Schoeffler M, Wallet F & Robert MO. Elévation de troponine I coronarographie normale chez un patient porteur d’une myopathie de Duchenne [Increased troponin I level in a Duchenne muscular dystrophy patient with normal coronarography]. Ann Fr Anesth Reanim 2008, 27, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Schram G, Fournier A, Leduc H, Dahdah N, Therien J, Vanasse M & Khairy P. All-cause mortality and cardiovascular outcomes with prophylactic steroid therapy in Duchenne muscular dystrophy. J Am Coll Cardiol 2013, 61, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Schultz TI, Raucci FJ Jr & Salloum FN. Cardiovascular disease in Duchenne muscular dystrophy: overview and insight into novel therapeutic targets. JACC Basic Transl Sci 2022, 7, 608–625. [Google Scholar] [CrossRef] [PubMed]
- Segawa K, Komaki H, Mori-Yoshimura M, Oya Y, Kimura K, Tachimori H, Kato N, Sasaki M & Takahashi Y. Cardiac conduction disturbances and aging in patients with Duchenne muscular dystrophy. Medicine (Baltimore) 2017, 96, e8335. [Google Scholar] [CrossRef] [PubMed]
- Shah AM, Jefferies JL, Rossano JW, Decker JA, Cannon BC & Kim JJ. Electrocardiographic abnormalities and arrhythmias are strongly associated with the development of cardiomyopathy in muscular dystrophy. Heart Rhythm 2010, 7, 1484–1488. [Google Scholar] [CrossRef] [PubMed]
- Shah MNA & Yokota, Y. Cardiac therapies for Duchenne muscular dystrophy. Ther Adv Neurol Disord 2023, 16, 17562864231182934. [Google Scholar]
- Shih JA, Folch A & Wong BL. Duchenne muscular dystrophy: the heart of the matter. Curr Heart Fail Rep 2020, 17, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Shin JH, Bostick B, Yue Y, Hajjar R & Duan D. SERCA2a gene transfer improves electrocardiographic performance in aged mdx mice. J Transl Med 2011, 9, 132. [Google Scholar] [CrossRef]
- Shirokova N & Niggli, E. Cardiac phenotype of Duchenne muscular dystrophy: insights from cellular studies. J Mol Cell Cardiol 2013, 58, 217–224. [Google Scholar]
- Siddiqui S, Alsaied T, Henson SE, Gandhi J, Patel P, Khoury P, Villa C, Ryan TD, Wittekind SG, Lang SM & Taylor MD. Left ventricular magnetic resonance imaging strain predicts the onset of Duchenne muscular dystrophy-associated cardiomyopathy. Circ Cardiovasc Imaging 2020, 13, e011526. [Google Scholar] [CrossRef] [PubMed]
- Siegel B, Olivieri L, Gordish-Dressman H & Spurney C. Myocardial strain using cardiac MR feature tracking and speckle tracking echocardiography in Duchenne muscular dystrophy patients. Pediatr Cardiol 2017, 39, 478–483. [Google Scholar]
- Siegel MP, Kruse SE, Percival JM, Goh J, White CC, Hopkins HC, Kavanagh TJ, Szeto HH, Rabinovitch PS & Marcinek DJ. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 2013, 12, 763–771. [Google Scholar] [CrossRef]
- Silva MC, Magalhães TA, Meira ZMA, Rassi CHRE, de Souza Andrade AC, Gutierrez PS, Azevedo CF, Gurgel-Giannetti J, Vainzof M, Zatz M, Kalil-Filho R & Rochitte CE. Myocardial fibrosis progression in Duchenne and Becker muscular dystrophy: a randomized clinical trial. JAMA Cardiol 2017, 2, 190–199. [Google Scholar] [CrossRef]
- Silversides CK, Webb GD, Harris VA & Biggar DW. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. A J Cardiol 2003, 91, 769–772. [Google Scholar] [CrossRef]
- Soslow JH, Xu M, Slaughter JC, Stanley M, Crum K, Markham LW & Parra DA. Evaluation of echocardiographic measures of left ventricular function in patients with Duchenne muscular dystrophy: assessment of reproducibility and comparison to cardiac magnetic resonance imaging. J Am Soc Echocardiogr 2016, 29, 983–991. [Google Scholar] [CrossRef]
- Spencer MJ, Croall DE & Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem 1995, 270, 10909–10914. [Google Scholar] [CrossRef] [PubMed]
- Spencer MJ, Walsh CM, Dorshkind KA, Rodriguez EM & Tidball JG. Myonuclear apoptosis in dystrophic mdx muscle occurs by perforin-mediated cytotoxicity. J Clin Invest 1997, 99, 2745–2751. [Google Scholar] [CrossRef]
- Spencer MJ, Montecino-Rodriguez E, Dorshkind K & Tidball JG. Helper (CD4(+)) and cytotoxic (CD8(+)) T cells promote the pathology of dystrophin-deficient muscle. Clin Immunol 2001, 98, 235–243. [Google Scholar] [CrossRef]
- Spurney C, Shimizu R, Morgenroth LP, Kolski H, Gordish-Dressman H, Clemens PR & CINRG Investigators. Cooperative International Neuromuscular Research Group Duchenne Natural History Study demonstrates insufficient diagnosis and treatment of cardiomyopathy in Duchenne muscular dystrophy. Muscle & Nerve 2014, 50, 250–256. [Google Scholar]
- Spurney CF, Ascheim D, Charnas L, Cripe L, Hor K, King N, Kinnett K, McNally EM, Sauer JM, Sweeney L, Villa C & Markham LW. Current state of cardiac troponin testing in Duchenne muscular dystrophy cardiomyopathy: review and recommendations from the Parent Project Muscular Dystrophy expert panel. Open Heart 2021, 8, e001592. [Google Scholar] [CrossRef] [PubMed]
- Spurney, CF. Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle & Nerve 2011, 44, 8–19. [Google Scholar]
- Stevens JA, Dobratz TC, Fischer KD, Palmer A, Bourdage K, Wong AJ, Chapoy-Villanueva H, Garry DJ, Liu JC, Kay MW, Kuzmiak-Glancy S, & Townsend D. Mechanisms of reduced myocardial energetics of the dystrophic heart. Am J Physiol Heart Circ Physiol 2024, 326, H396–H407. [Google Scholar] [CrossRef]
- Stocco A, Smolina N, Sabatelli P, Sileikyte J, Artusi E, Mouly V, Cohen M, Forte M, Schiavone M, & Bernardi P. Treatment with a triazole inhibitor of the mitochondrial permeability transition pore fully corrects the pathology of sapje zebrafish lacking dystrophin. Pharmacol. Res. 2021, 165, 105421. [Google Scholar] [CrossRef] [PubMed]
- Stanley WC, Recchia FA & Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 2005, 85, 1093–1129. [Google Scholar] [CrossRef]
- Stuckey DJ, Carr CA, Camelliti P, Tyler DJ, Davies KE & Clarke K. In vivo MRI characterization of progressive cardiac dysfunction in the mdx mouse model of muscular dystrophy. PLOS One 2012, 7, e28569. [Google Scholar]
- Su JA, Ramos-Platt L & Menteer J. Left ventricular tonic contraction as a novel biomarker of cardiomyopathy in Duchenne muscular dystrophy. Pediatr Cardiol 2016, 37, 678–685. [Google Scholar] [CrossRef]
- Subramanian S, Hor KN, Mazur W, Smart S, Tran T, Halnon N, Taylor MD, Cripe L & Raman SV. Effect of pulmonary function on right heart function in Duchenne muscular dystrophy patients. J Cardiovasc Magn Reson 2015, 17, P279. [Google Scholar] [CrossRef]
- Sui T, Lau YS, Liu D, Liu T, Xu L, Gao Y, Lai L, Li Z & Han R. A novel rabbit model of Duchenne muscular dystrophy generated by CRISPR/Cas9. Dis Model Mech 2018, 11, dmm032201. [Google Scholar] [CrossRef]
- Suno M, Nagaoka A. Inhibition of lipid peroxidation by a novel compound (CV-2619) in brain mitochondria and mode of action of the inhibition. Biochem Biophys Res Commun 1984, 125, 1046–52. [Google Scholar] [CrossRef]
- Suno M, Nagaoka A. Inhibition of lipid peroxidation by idebenone in brain mitochondria in the presence of succinate. Arch Gerontol Geriatr 1989, 8, 291–7. [Google Scholar] [CrossRef] [PubMed]
- Svobodova B, Jelinkova S, Pesl M, Beckerová D, Lacampagne A, Meli AC & Rotrekl V. Cellular pathology of the human heart in Duchenne muscular dystrophy (DMD): lessons learned from in vitro modeling. Pflügers Archiv 2021, 473, 1099–1115. [Google Scholar] [CrossRef] [PubMed]
- Swiderski K & Lynch, GS. Murine models of Duchenne muscular dystrophy: is there a best model? Am J Physiol 2021, 321, C409–C412. [Google Scholar]
- Szabo SM, Salhany RM, Deighton A, Harwood M, Mah J & Gooch KL. The clinical course of Duchenne muscular dystrophy in the corticosteroid treatment era: a systematic literature review. Orphanet J Rare Dis 2021, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Szeto HH & Schiller. PW Novel therapies targeting inner mitochondrial membrane--from discovery to clinical development. Pharm Res 2011, 28, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Szeto, HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 2014, 171, 2029–2050. [Google Scholar] [CrossRef] [PubMed]
- Szeto HH & Birk, AV. Serendipity and the discovery of novel compounds that restore mitochondrial plasticity. Clin Pharmacol Ther 2014, 96, 672–683. [Google Scholar]
- Taglietti V, Kefi K, Bronisz-Budzyńska I, Mirciloglu B, Rodrigues M, Cardone N, Coulpier F, Periou B, Gentil C, Goddard M, Authier FJ, Pietri-Rouxel F, Malfatti E, Lafuste P, Tiret L & Relaix F. Duchenne muscular dystrophy trajectory in R-DMDdel52 preclinical rat model identifies COMP as biomarker of fibrosis. Acta Neuropathol Commun 2022, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Takami Y, Takeshima Y, Awano H, Okizuka Y, Yagi M & Matsuo M. High incidence of electrocardiogram abnormalities in young patients with Duchenne muscular dystrophy. Pediatr Neurol 2008, 39, 399–403. [Google Scholar] [CrossRef]
- Tandon A, Villa CR, Hor KN, Jefferies JL, Gao Z, Towbin JA, Wong BL, Mazur W, Fleck RJ, Sticka JJ, Benson DW & Taylor MD. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in Duchenne muscular dystrophy. J Am Heart Assoc 2015, 4, 4. [Google Scholar]
- Taqatqa A, Bokowski J, Al-Kubaisi M, Khalil A, Miranda C, Alaksham H, Fughhi I, Kenny D & Diab KA. The use of speckle tracking echocardiography for early detection of myocardial dysfunction in patients with Duchenne muscular dystrophy. Pediatr Cardiol 2016, 37, 1422–1428. [Google Scholar] [CrossRef]
- Thrush PT, Allen HD, Viollet L & Mendell JR. Re-examination of the electrocardiogram in boys with Duchenne muscular dystrophy and correlation with its dilated cardiomyopathy. Am J Cardiol 2009, 103, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Torriani M, Townsend E, Thomas BJ, Bredella MA, Ghomi RH & Tseng BS. Lower leg muscle involvement in Duchenne muscular dystrophy: an MR imaging and spectroscopy study. Skeletal Radiol 2012, 41, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Townsend DW, Turner I, Yasuda S, Martindale J, Davis J, Shillingford M, Kornegay JN & Metzger JM. Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs. J Clin Invest 2010, 120, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Townsend D, Yasuda S, McNally E & Metzger JM. Distinct pathophysiological mechanisms of cardiomyopathy in hearts lacking dystrophin or the sarcoglycan complex. FASEB J 2011, 25, 3106–3114. [Google Scholar] [CrossRef] [PubMed]
- Townsend D, Yasuda S, Li S, Chamberlain JS & Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther 2008, 16, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Illarramendi A, Toral-Ojeda I, Aldanondo G & López de Munain A. Dysregulation of calcium homeostasis in muscular dystrophies. Expert Rev Mol Med 2014, 16, e16. [Google Scholar] [CrossRef] [PubMed]
- van Bockel EA, Lind JS, Zijlstra JG, Wijkstra PJ, Meijer PM, van den Berg MP, Slart RHJA, Aarts LPHJ & Tulleken JE. Cardiac assessment of patients with late stage Duchenne muscular dystrophy. Neth Heart J 2009, 17, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Van Erp C, Loch D, Laws N, Trebbin A & Hoey AJ. Timeline of cardiac dystrophy in 3– 18-month-old MDX mice. Muscle & Nerve 2010, 42, 504–513. [Google Scholar]
- van Westering TL, Betts CA & Wood MJ. Current understanding of molecular pathology and treatment of cardiomyopathy in Duchenne muscular dystrophy. Molecules, 2015, 20, 8823–8855. [Google Scholar] [CrossRef]
- Verhaert D, Richards K, Rafael-Fortney JA & Raman SV. Cardiac involvement in patients with muscular dystrophies: magnetic resonance imaging phenotype and genotypic considerations. Circ Cardiovasc Imaging 2011, 4, 67–76. [Google Scholar] [CrossRef]
- Villa CR, Czosek RJ, Ahmed H, Khoury PR, Anderson JB, Knilans TK, Jefferies JL, Wong B & Spar DS. Ambulatory monitoring and arrhythmic outcomes in pediatric and adolescent patients with Duchenne muscular dystrophy. J Am Heart Assoc 2015, 5, e002620. [Google Scholar]
- Viola HM, Davies SMK, Filipovska A & Hool LC. L-type Ca2+ channel contributes to alterations in mitochondrial calcium handling in the mdx ventricular myocyte. Am J Physiol Heart Circ Physiol 2013, 304, H767–H775. [Google Scholar] [CrossRef] [PubMed]
- Viola HM, Arthur PG & Hool LC. Evidence for regulation of mitochondrial function by the L-type Ca2+ channel in ventricular myocytes. J Mol Cell Cardiol 2009, 46, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Viola HM, Adams AM, Davies SMK, Fletcher S, Filipovska A & Hool LC. Impaired functional communication between the L-type calcium channel and mitochondria contributes to metabolic inhibition in the mdx heart. Proc Natl Acad Sci U S A 2014, 111, E2905–E2914. [Google Scholar]
- Viollet L, Thrush PT, Flanigan KM, Mendell JR & Allen HD. Effects of angiotensin-converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in Duchenne muscular dystrophy. Am J Cardiol 2012, 110, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Voit A, Patel V, Pachon R, Shah V, Bakhutma M, Kohlbrenner E, McArdle JJ, Dell’Italia LJ, Mendell JR, Xie LH, Hajjar RJ, Duan D, Fraidenraich D & Babu GJ. Reducing sarcolipin expression mitigates Duchenne muscular dystrophy and associated cardiomyopathy in mice. Nat Commun 2017, 8, 1068. [Google Scholar] [CrossRef] [PubMed]
- Voleti S, Olivieri L, Hamann K, Gordish-Dressman H & Spurney C. Troponin I levels correlate with cardiac MR LGE and native T1 values in Duchenne muscular dystrophy cardiomyopathy and identify early disease progression. Pediatr Cardiol 2020, 41, 1–7. [Google Scholar]
- Wagner S, Knipp S, Weber C, Hein S, Schinkel S, Walther A, Bekeredjian R, Müller OJ & Friedrich O. The heart in Duchenne muscular dystrophy: early detection of contractile performance alteration. J Cell Mol Med 2012, 16, 3028–3036. [Google Scholar] [CrossRef]
- Wagner KR, Lechtzin N & Judge DP. Current treatment of adult Duchenne muscular dystrophy. Biochi Biophys Acta 2007, 1772, 229–237. [Google Scholar] [CrossRef]
- Wagner S, Maier LS & Bers DM. Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ Res 2015, 16, 1956–1970. [Google Scholar]
- Wallimann T, Tokarska-Schlattner M & Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang H, Liu S, Xing YL & Chen R. [The limitation of MB isoenzyme of creatine kinase mass in assess myocardial injury with muscular disease]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue [Chinese Critical Care Medicine] 2011, 23, 723–726. [Google Scholar]
- Wansapura JP, Hor KN, Mazur W, Fleck R, Hagenbuch S, Benson DW & Gottliebson WM. Left ventricular T2 distribution in Duchenne muscular dystrophy. Cardiovasc Magn Reson 2010, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Wasala NB, Yue Y, Lostal W, Wasala LP, Niranjan N, Hajjar RJ, Babu GJ & Duan D. Single SERCA2a therapy ameliorated dilated cardiomyopathy for 18 months in a mouse model of Duchenne muscular dystrophy. Mol Ther 2020, 28, 845–854. [Google Scholar] [CrossRef]
- Wehling M, Spencer MJ & Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol 2001, 155, 123–131. [Google Scholar] [CrossRef]
- Wens SCA, Schaaf GJ, Michels M, Kruijshaar ME, van Gestel TJM, in 't Groen S, Pijnenburg J, Dekkers DHW, Demmers JAA, Verdijk LB, Brusse E, van Schaik RHN, van der Ploeg AT, van Doorn PA & Pim Pijnappel WWM. Elevated plasma cardiac troponin T levels caused by skeletal muscle damage in Pompe disease. Circ Cardiovasc Genet 2016, 9, 6–13. [Google Scholar] [CrossRef] [PubMed]
- 378. Wissing ER, Millay DP, Vuagniaux G, & Molkentin JD. Debio-025 is more effective than prednisone in reducing muscular pathology in mdx mice. Neuromuscul Disord, 20, 753–760.
- Willi L, Abramovich I, Fernandez-Garcia J, Agranovich B, Shulman M, Milman H, Baskin P, Eisen B, Michele DE, Arad M, Binah O & Gottlieb E. Bioenergetic and metabolic impairments in induced pluripotent stem cell-derived cardiomyocytes generated from Duchenne muscular dystrophy patients. Int J Mol Sci 2022, 23, 9808. [Google Scholar] [CrossRef] [PubMed]
- Williams GS, Boyman L, Chikando AC, Khairallah RJ & Lederer WJ. Mitochondrial calcium uptake. Proc Natl Acad Sci U S A 2013, 110, 10479–10486. [Google Scholar] [CrossRef]
- Williams IA & Allen, DG. Intracellular calcium handling in ventricular myocytes from mdx mice. Am J Physiol Heart Circ Physiol 2007, 292, H846–H855. [Google Scholar]
- Wong TWY, Ahmed A, Yang G, Maino E, Steiman S, Hyatt E, Chan P, Lindsay K, Wong N, Golebiowski D, Schneider J, Delgado-Olguin P, Ivakine EA, & Cohn, RD. A novel mouse model of Duchenne muscular dystrophy carrying a multi-exonic Dmd deletion exhibits progressive muscular dystrophy and early-onset cardiomyopathy. Dis Model Mech 2020, 13, dmm045369. [Google Scholar] [CrossRef] [PubMed]
- Wrogemann K, & Blanchaer, MC. Oxidative phosphorylation by muscle mitochondria of dystrophic mice. Can. J. Biochem 1967, 45, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Wrogemann K, & Blanchaer, MC. Respiration and oxidative phosphorylation by muscle and heart mitochondria of hamsters with hereditary myocardiopathy and polymyopathy. Can. J. Biochem 1968, 46, 323–329. [Google Scholar] [CrossRef] [PubMed]
- 385. Wrogemann K, Blanchaer, MC, & Jacobson, BE. A calcium-associated magnesium-responsive defect of respiration and oxidative phosphorylation by skeletal muscle mitochondria of BIO 14.6 dystrophic hamsters. Life Sci, 1970; II9, 1167–1173.
- Wrogemann K, Jacobson, BE, & Blanchaer, MC. On the Mechanism of a Calcium-Associated Defect of Oxidative Phosphorylation in Progressive Muscular Dystrophy. Arch. Biochem. Biophys. 1973, 159, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Wrogemann K, & Pena, SDJ. Mitochondrial calcium overload: a general mechanism for cell-necrosis in muscle diseases. The Lancet 1976, 307, P672–P674. [Google Scholar] [CrossRef]
- Xu L, Lau YS, Gao Y, Li H & Han R. Life-long AAV-mediated CRISPR genome editing in dystrophic heart improves cardiomyopathy without causing serious lesions in mdx mice. Mol Ther 2019, 27, 1–8. [Google Scholar]
- Yamamoto T, Awano H, Zhang Z, Sakuma M, Kitaaki S, Matsumoto M, Nagai M, Sato I, Imanishi T, Hayashi N, Matsuo M, Iijima K & Saegusa J. Cardiac dysfunction in Duchenne muscular dystrophy is less frequent in patients with mutations in the dystrophin Dp116 coding region than in other regions. Circ Genom Precis Med 2018, 11, e001782. [Google Scholar]
- Yanagisawa A, Yokota N, Miyagawa M, Kawamura J, Ishihara T, Aoyagi T & Ishikawa K. Plasma levels of atrial natriuretic peptide in patients with Duchenne’s progressive muscular dystrophy. Am Heart J 1990, 120, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Yasuda S, Townsend D, Michele DE, Favre EG, Day SM & Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature 2005, 436, 1025–1029. [Google Scholar] [CrossRef]
- Yin H, Moulton HM, Seow Y, Boyd C, Boutilier J, Iverson P & Wood MJA. Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function. Hum Mol Genet 2008, 17, 3909–3918. [Google Scholar] [CrossRef]
- Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S & Hoffman E. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol 2009, 65, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Zambon AA, Ayyar Gupta V, Ridout D, Manzur AY, Baranello G, Trucco F, Muntoni F & UK Northstar Clinical Network. Peak functional ability and age at loss of ambulation in Duchenne muscular dystrophy. Dev Med Child Neurol 2022, 64, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW & Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 2004, 279, 34682–34690. [Google Scholar] [CrossRef] [PubMed]
- Zhao K, Luo G, Zhao G-M, Schiller PW, & Szeto HH. Transcellular transport of a highly polar 3+ net charge opioid tetrapeptide. J Pharmacol Exp Ther 2003, 304, 425–32. [Google Scholar] [CrossRef] [PubMed]
- Zorzano A, Sevilla L, Tomàs E, Gumà A, Palacín M & Fischer Y. GLUT4 trafficking in cardiac and skeletal muscle: isolation and characterization of distinct intracellular GLUT4-containing vesicle populations. Biochem Soc Trans 1997, 25, 968–974. [Google Scholar] [CrossRef]
- Zs-Nagy, I. Chemistry toxicology, pharmacology and pharmacokinetics of idebenone: a review. Arch Gerontol Geriatr 1990, 11, 177–186. [Google Scholar] [CrossRef]
- 399. Zulian A, Schiavone M, Giorgio V, & Bernardi P. Forty years later: Mitochondria as therapeutic targets in muscle diseases. Pharmacol Res, 2016; 113 (Pt A), 563–573.
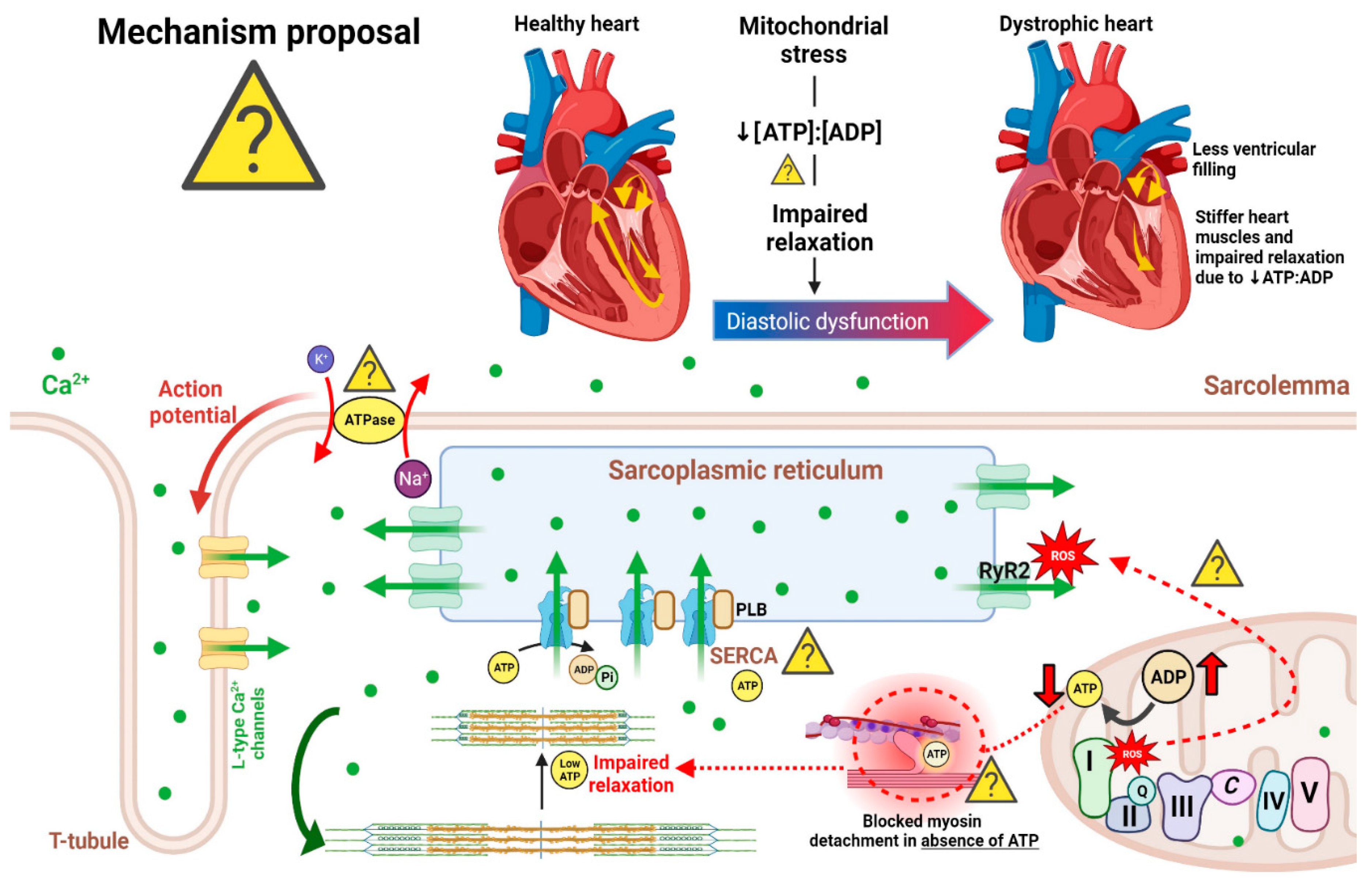
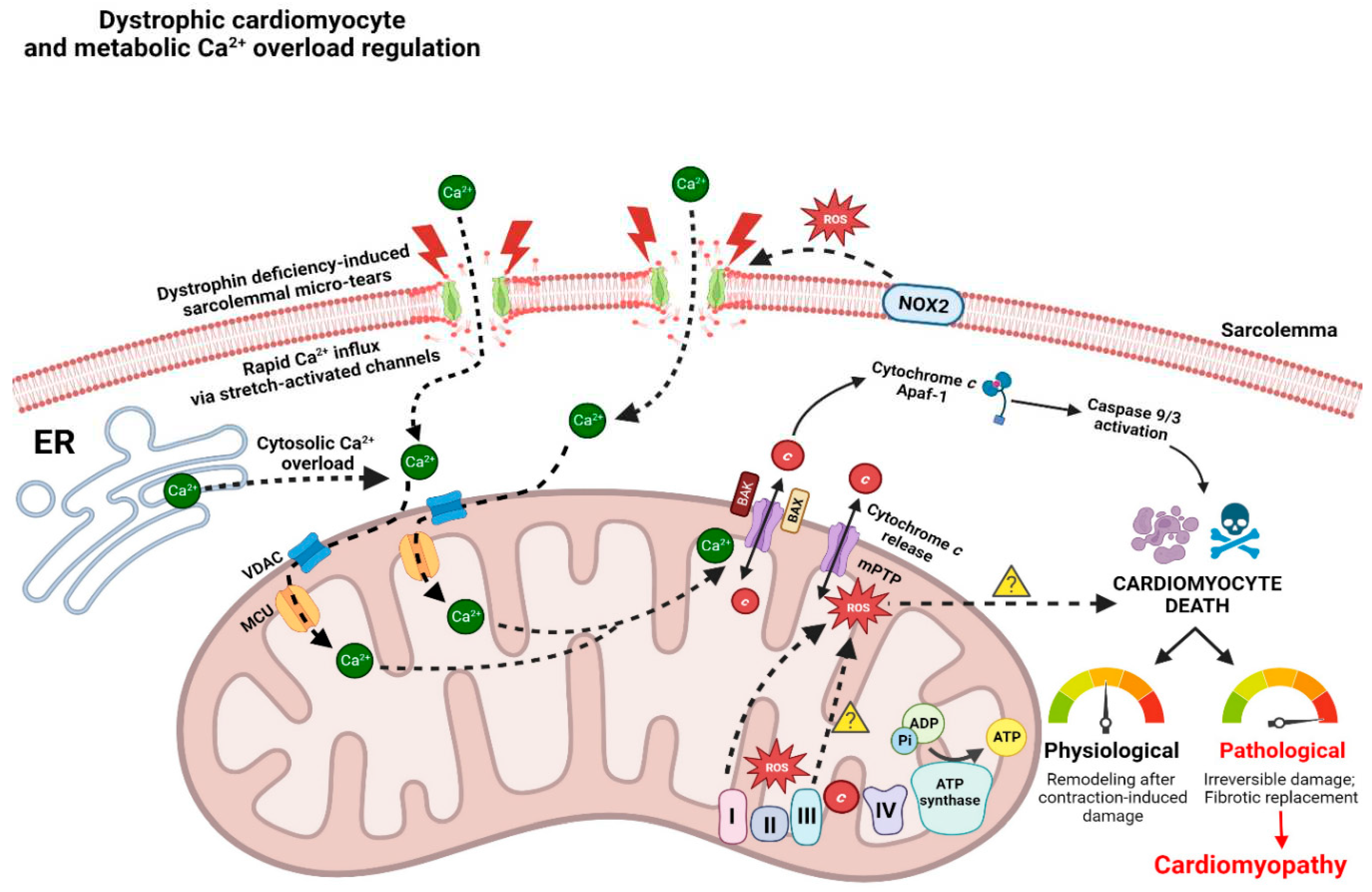
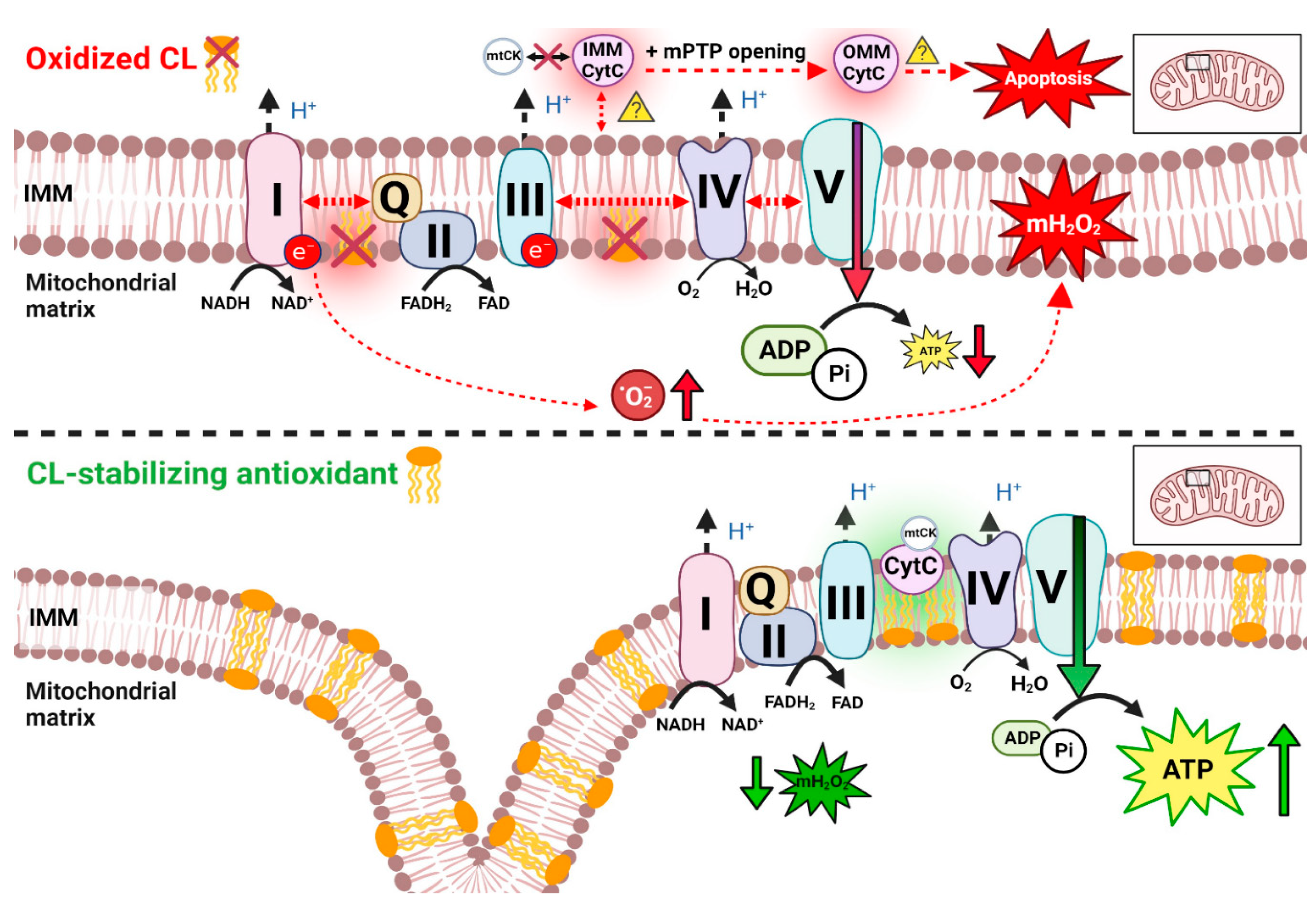
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




