Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
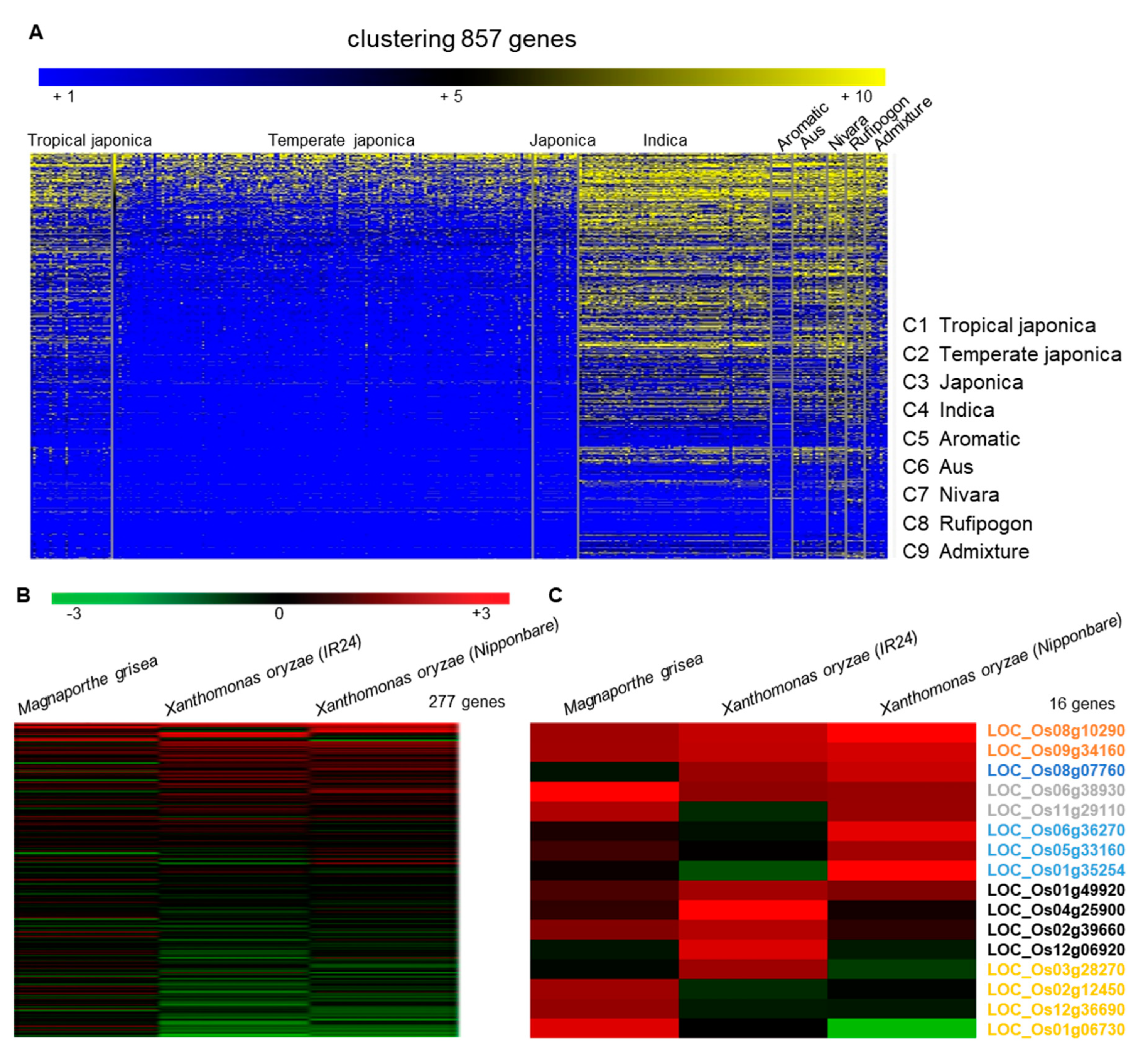
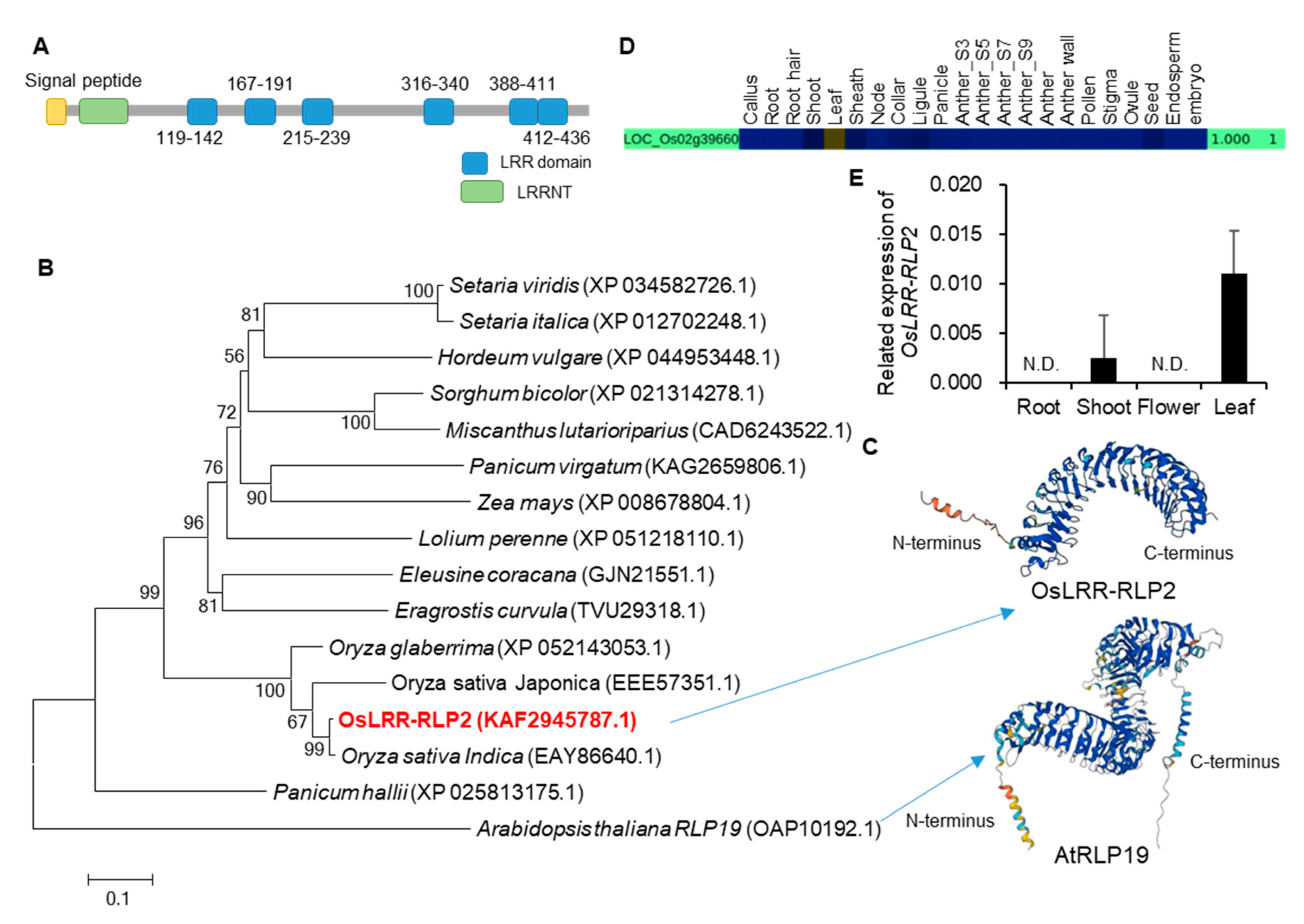
2.1. Selection of LRR Candidates and Phylogenetic Analysis
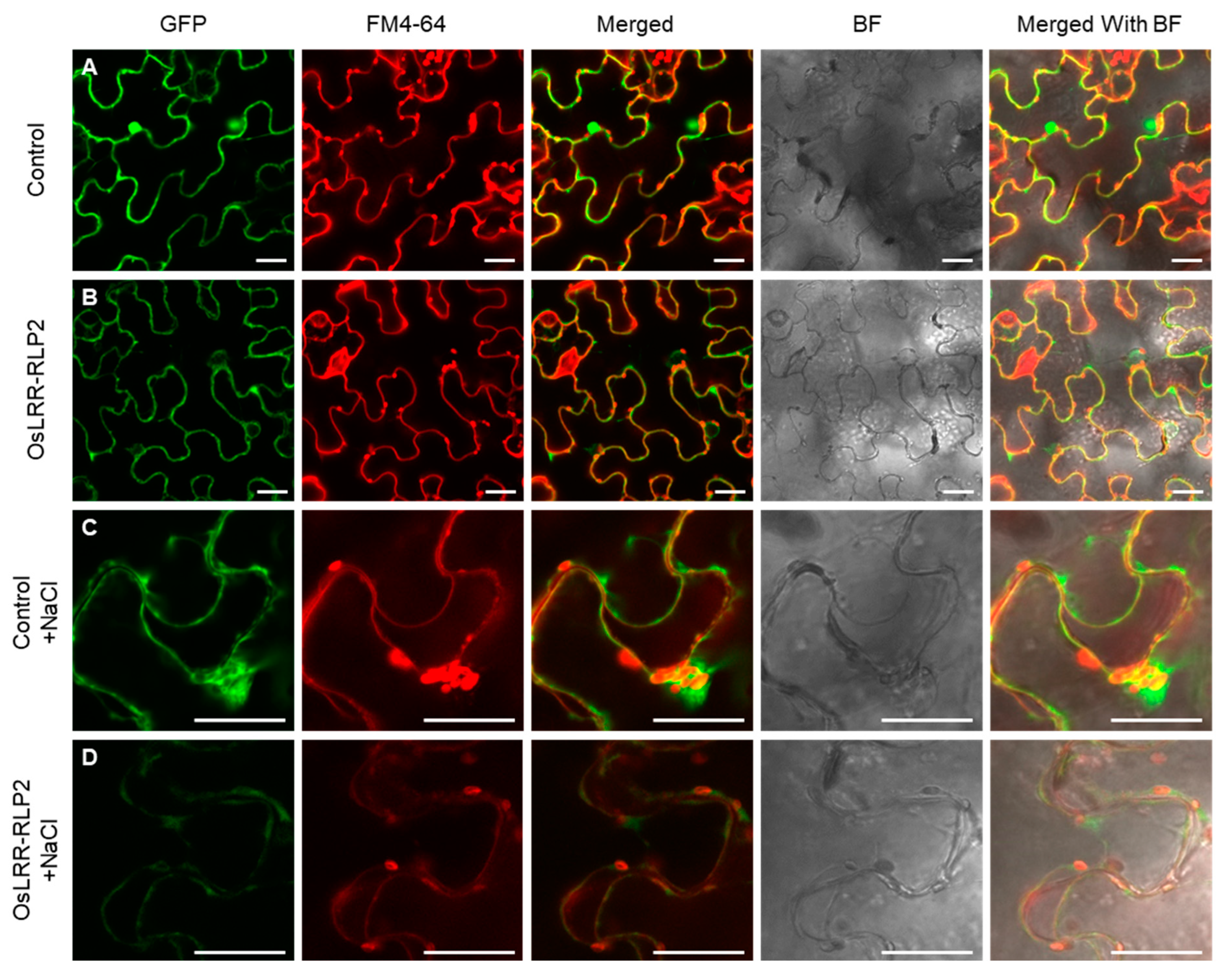
2.2. Expression and Localization Analysis of OsLRR-RLP
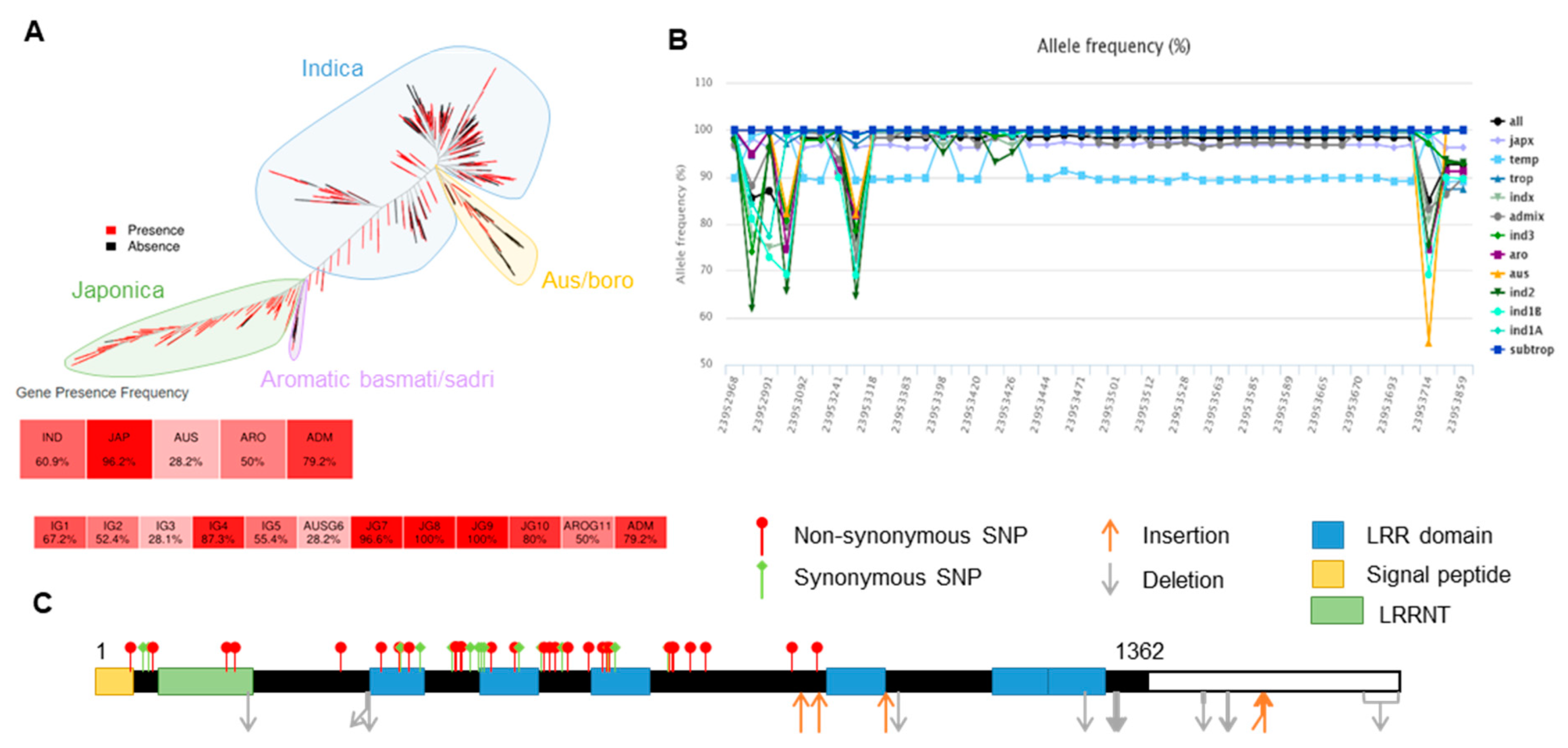
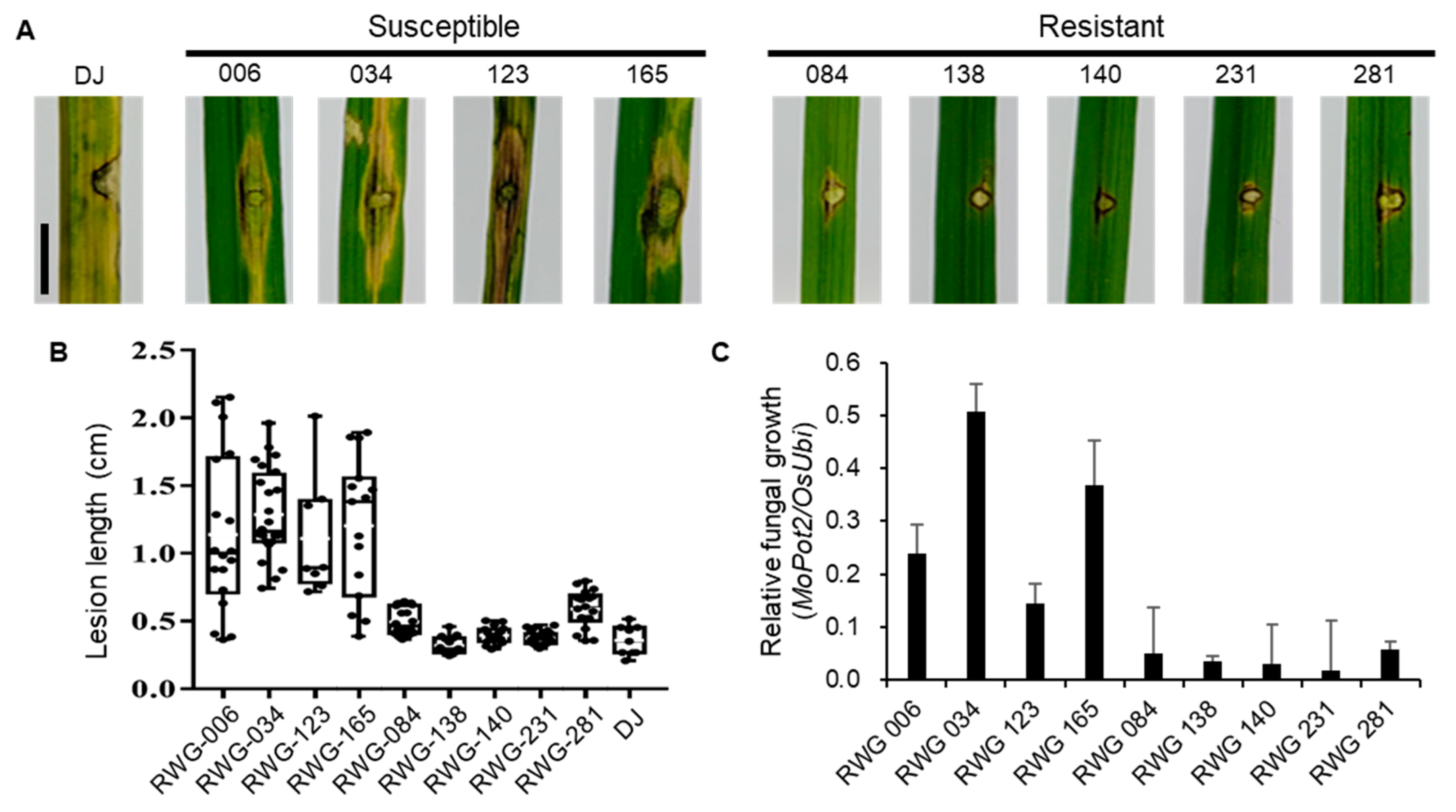
2.3. Haplotype Analysis of OsLRR-RLP2 Sequence and Validation of M. oryzae Response
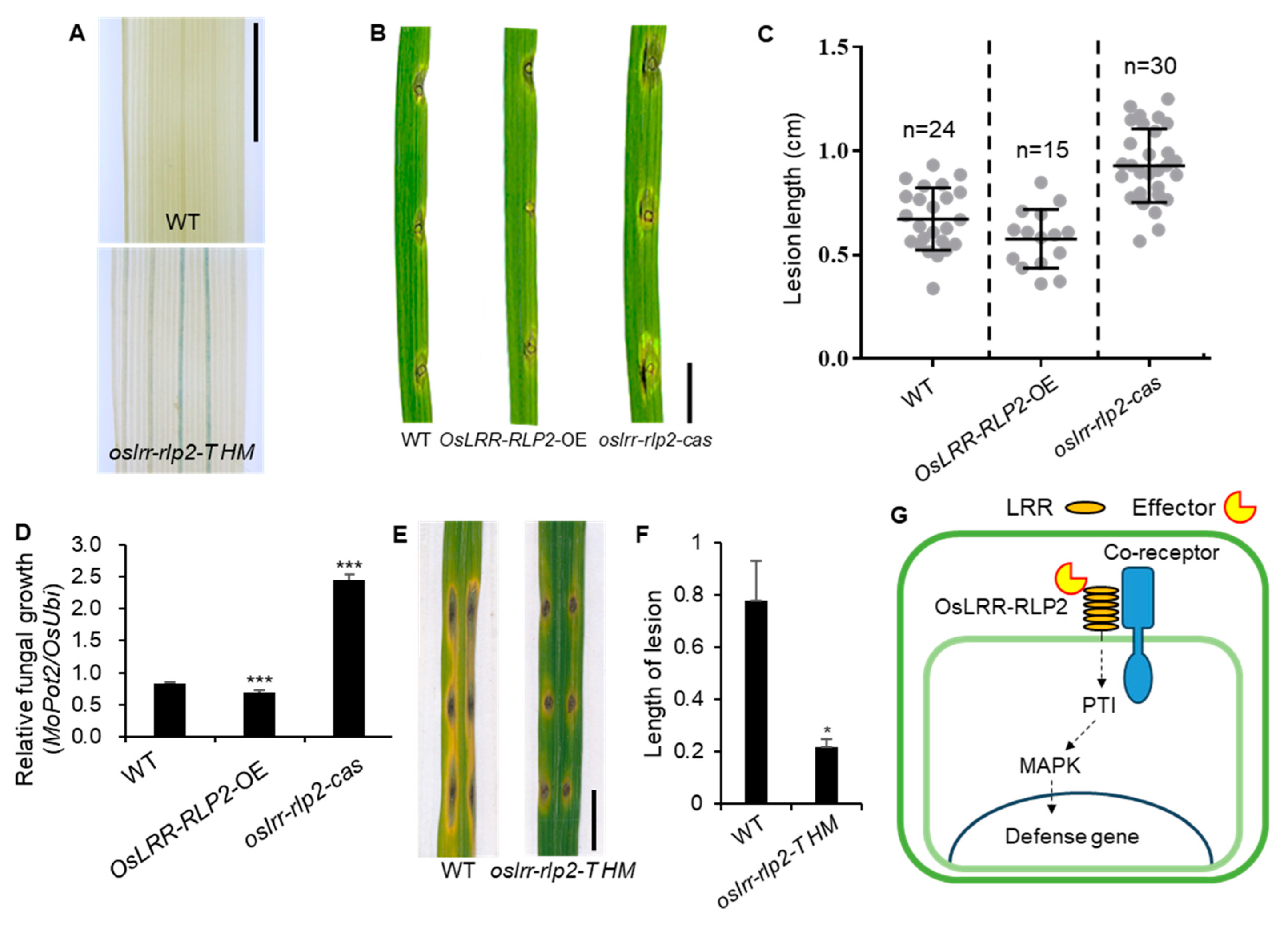
2.4. Functional Analysis of OsLRR-RLP2 Involving Rice Blast Disease
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Blast Inoculation
4.2. Gene Identification and Sequence Analysis
4.3. Construction of Vectors and Plant Transformation
4.4. DNA Isolation and Genotypic Analysis
4.5. RNA Isolation, cDNA Synthesis, and qRT-PCR
4.6. Generation of Fusion Proteins and Subcellular Localization Analysis
4.7. Histochemical GUS Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dean, R.; Van Kan, J. A.; Pretorius, Z. A.; Hammond-Kosack, K. E.; Di Pietro, A.; Spanu, P. D.; Rudd, J. J.; Dickman, M.; Kahmann, R.; Ellis, J., The Top 10 fungal pathogens in molecular plant pathology. Molecular plant pathology 2012, 13, 414-430. [CrossRef]
- Skamnioti, P.; Gurr, S. J., Against the grain: safeguarding rice from rice blast disease. Trends in biotechnology 2009, 27, 141-150. [CrossRef]
- Couto, D.; Zipfel, C., Regulation of pattern recognition receptor signalling in plants. Nature Reviews Immunology 2016, 16, 537-552. [CrossRef]
- Cui, H.; Tsuda, K.; Parker, J. E., Effector-triggered immunity: from pathogen perception to robust defense. Annual review of plant biology 2015, 66, 487-511. [CrossRef]
- Li, D.; Wu, M., Pattern recognition receptors in health and diseases. Signal transduction and targeted therapy 2021, 6, 291. [CrossRef]
- Boutrot, F.; Zipfel, C., Function, discovery, and exploitation of plant pattern recognition receptors for broad-spectrum disease resistance. Annual review of phytopathology 2017, 55, 257-286. [CrossRef]
- Wang, G.; Ellendorff, U.; Kemp, B.; Mansfield, J. W.; Forsyth, A.; Mitchell, K.; Bastas, K.; Liu, C.-M.; Woods-Tör, A.; Zipfel, C., A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant physiology 2008, 147, 503-517. [CrossRef]
- Jones, D. A.; Thomas, C. M.; Hammond-Kosack, K. E.; Balint-Kurti, P. J.; Jones, J. D., Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science 1994, 266, 789-793. [CrossRef]
- Nadeau, J. A.; Sack, F. D., Control of stomatal distribution on the Arabidopsis leaf surface. Science 2002, 296, 1697-1700. [CrossRef]
- Zipfel, C., Plant pattern-recognition receptors. Trends in immunology 2014, 35, 345-351. [CrossRef]
- Fritz-Laylin, L. K.; Krishnamurthy, N.; Tör, M.; Sjölander, K. V.; Jones, J. D., Phylogenomic analysis of the receptor-like proteins of rice and Arabidopsis. Plant physiology 2005, 138, 611-623. [CrossRef]
- Jehle, A. K.; Lipschis, M.; Albert, M.; Fallahzadeh-Mamaghani, V.; Fürst, U.; Mueller, K.; Felix, G., The receptor-like protein ReMAX of Arabidopsis detects the microbe-associated molecular pattern eMax from Xanthomonas. The Plant Cell 2013, 25, 2330-2340. [CrossRef]
- Thomas, C. M.; Jones, D. A.; Parniske, M.; Harrison, K.; Balint-Kurti, P. J.; Hatzixanthis, K.; Jones, J. D., Characterization of the tomato Cf-4 gene for resistance to Cladosporium fulvum identifies sequences that determine recognitional specificity in Cf-4 and Cf-9. The Plant Cell 1997, 9, 2209-2224. [CrossRef]
- Zhang, H.; Chen, C.; Li, L.; Tan, X.; Wei, Z.; Li, Y.; Li, J.; Yan, F.; Chen, J.; Sun, Z., A rice LRR receptor-like protein associates with its adaptor kinase OsSOBIR1 to mediate plant immunity against viral infection. Plant Biotechnology Journal 2021, 19, 2319-2332. [CrossRef]
- Chen, E.; Huang, X.; Tian, Z.; Wing, R. A.; Han, B., The genomics of Oryza species provides insights into rice domestication and heterosis. Annual review of plant biology 2019, 70, 639-665. [CrossRef]
- Garris, A. J.; Tai, T. H.; Coburn, J.; Kresovich, S.; McCouch, S., Genetic structure and diversity in Oryza sativa L. Genetics 2005, 169, 1631-1638. [CrossRef]
- Escolà, G.; González-Miguel, V. M.; Campo, S.; Catala-Forner, M.; Domingo, C.; Marqués, L.; San Segundo, B., Development and Genome-Wide Analysis of a Blast-Resistant japonica Rice Variety. Plants 2023, 12, 3536. [CrossRef]
- Enkhbayar, P.; Kamiya, M.; Osaki, M.; Matsumoto, T.; Matsushima, N., Structural principles of leucine-rich repeat (LRR) proteins. Proteins: Structure, Function, and Bioinformatics 2004, 54, 394-403. [CrossRef]
- Lee, D.; Zhang, H.; Zeng, Y.; Kim, B.; Kwon, S.-W., Discovery of Genomic Regions and Candidate Genes for Awn Length Using QTL-seq in Rice (Oryza sativa L.). 2023. [CrossRef]
- Ray, D. K.; Mueller, N. D.; West, P. C.; Foley, J. A., Yield trends are insufficient to double global crop production by 2050. PloS one 2013, 8, e66428. [CrossRef]
- van Ooijen, G.; van den Burg, H. A.; Cornelissen, B. J.; Takken, F. L., Structure and function of resistance proteins in solanaceous plants. Annu. Rev. Phytopathol. 2007, 45, 43-72. [CrossRef]
- Gust, A. A.; Felix, G., Receptor like proteins associate with SOBIR1-type of adaptors to form bimolecular receptor kinases. Current opinion in plant biology 2014, 21, 104-111. [CrossRef]
- Sun, Y.; Wang, Y.; Zhang, X.; Chen, Z.; Xia, Y.; Wang, L.; Sun, Y.; Zhang, M.; Xiao, Y.; Han, Z., Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature 2022, 610, 335-342. [CrossRef]
- Albert, I.; Zhang, L.; Bemm, H.; Nürnberger, T., Structure-function analysis of immune receptor at RLP23 with its ligand nlp20 and coreceptors at SOBIR1 and At BAK1. Molecular Plant-Microbe Interactions 2019, 32, 1038-1046. [CrossRef]
- Zhang, W.; Fraiture, M.; Kolb, D.; Löffelhardt, B.; Desaki, Y.; Boutrot, F. F.; Tör, M.; Zipfel, C.; Gust, A. A.; Brunner, F., Arabidopsis receptor-like protein30 and receptor-like kinase suppressor of BIR1-1/EVERSHED mediate innate immunity to necrotrophic fungi. The Plant Cell 2013, 25, 4227-4241. [CrossRef]
- Min, C. W.; Jang, J. W.; Lee, G. H.; Gupta, R.; Yoon, J.; Park, H. J.; Cho, H. S.; Park, S. R.; Kwon, S.-W.; Cho, L.-H., TMT-based quantitative membrane proteomics identified PRRs potentially involved in the perception of MSP1 in rice leaves. Journal of Proteomics 2022, 267, 104687. [CrossRef]
- Singh, J.; Gupta, S. K.; Devanna, B.; Singh, S.; Upadhyay, A.; Sharma, T. R., Blast resistance gene Pi54 over-expressed in rice to understand its cellular and sub-cellular localization and response to different pathogens. Scientific reports 2020, 10, 5243. [CrossRef]
- Wang, G.-F.; Balint-Kurti, P. J., Cytoplasmic and nuclear localizations are important for the hypersensitive response conferred by maize autoactive Rp1-D21 protein. Molecular Plant-Microbe Interactions 2015, 28, 1023-1031. [CrossRef]
- Bai, S.; Liu, J.; Chang, C.; Zhang, L.; Maekawa, T.; Wang, Q.; Xiao, W.; Liu, Y.; Chai, J.; Takken, F. L., Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS pathogens 2012, 8, e1002752. [CrossRef]
- Wu, Y.; Xun, Q.; Guo, Y.; Zhang, J.; Cheng, K.; Shi, T.; He, K.; Hou, S.; Gou, X.; Li, J., Genome-wide expression pattern analyses of the Arabidopsis leucine-rich repeat receptor-like kinases. Molecular plant 2016, 9, 289-300. [CrossRef]
- Rodrigues, F. Á.; Benhamou, N.; Datnoff, L. E.; Jones, J. B.; Bélanger, R. R., Ultrastructural and cytochemical aspects of silicon-mediated rice blast resistance. Phytopathology 2003, 93, 535-546. [CrossRef]
- Dolatabadian, A.; Bayer, P. E.; Tirnaz, S.; Hurgobin, B.; Edwards, D.; Batley, J., Characterization of disease resistance genes in the Brassica napus pangenome reveals significant structural variation. Plant biotechnology journal 2020, 18, 969-982. [CrossRef]
- Petre, B.; Hacquard, S.; Duplessis, S.; Rouhier, N., Genome analysis of poplar LRR-RLP gene clusters reveals RISP, a defense-related gene coding a candidate endogenous peptide elicitor. Frontiers in plant science 2014, 5, 111. [CrossRef]
- Wu, Y.; Xiao, N.; Yu, L.; Pan, C.; Li, Y.; Zhang, X.; Liu, G.; Dai, Z.; Pan, X.; Li, A., Combination patterns of major R genes determine the level of resistance to the M. oryzae in rice (Oryza sativa L.). PLoS One 2015, 10, e0126130. [CrossRef]
- An, S.; Park, S.; Jeong, D.-H.; Lee, D.-Y.; Kang, H.-G.; Yu, J.-H.; Hur, J.; Kim, S.-R.; Kim, Y.-H.; Lee, M., Generation and analysis of end sequence database for T-DNA tagging lines in rice. Plant physiology 2003, 133, 2040-2047. [CrossRef]
- Xie, K.; Minkenberg, B.; Yang, Y., Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proceedings of the National Academy of Sciences 2015, 112, 3570-3575. [CrossRef]
- Lee, S.; Jeon, J.-S.; Jung, K.-H.; An, G., Binary vectors for efficient transformation of rice. Journal of Plant Biology 1999, 42, 310-316. [CrossRef]
- Livak, K. J.; Schmittgen, T. D., Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 2001, 25, 402-408. [CrossRef]
- Sparkes, I. A.; Runions, J.; Kearns, A.; Hawes, C., Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature protocols 2006, 1, 2019-2025. [CrossRef]
- Moon, S.; Chandran, A. K. N.; Kim, Y.-J.; Gho, Y.; Hong, W.-J.; An, G.; Lee, C.; Jung, K.-H., Rice RHC encoding a putative cellulase is essential for normal root hair elongation. Journal of Plant Biology 2019, 62, 82-91. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




