Submitted:
02 January 2024
Posted:
03 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Vegetative and reproductive traits
2.2. Vitality of orchid individuals and populations
2.3. Environmental threats
3. Discussion
4. Materials and methods
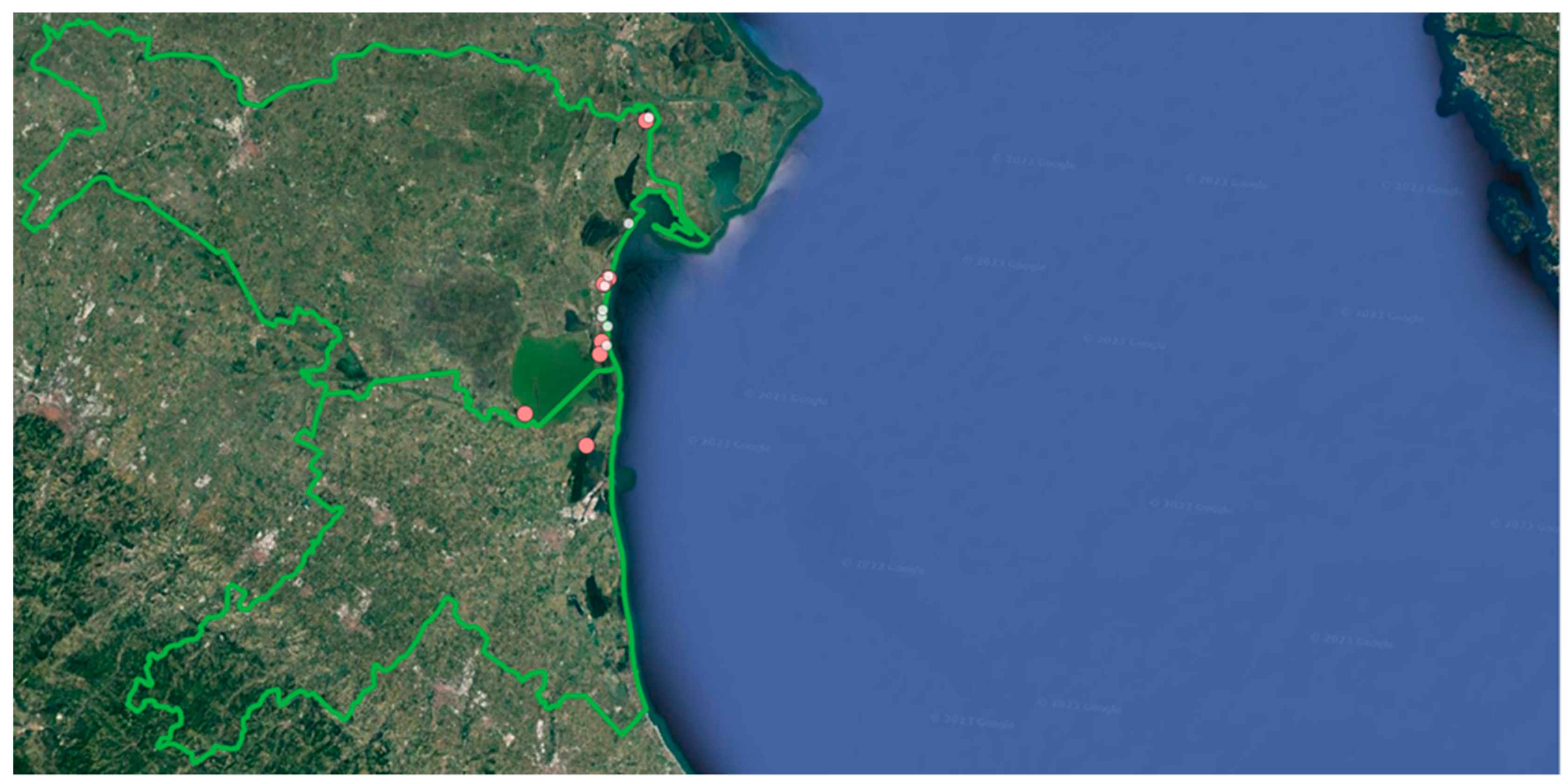
4.1. Study area
4.2. Sampling and laboratory analyses
4.3. Data compilation and statistical analyses
- Vegetative traits: stem height (measured in the field); number of leaves (counted in the field); rosette diameter (measured in the field); specific leaf area (SLA, the ratio between leaf area and leaf fresh mass as obtained by laboratory scanning and weighing); leaf dry matter content (LDMC, the ratio between dry mass and fresh mass as obtained by laboratory weighings).
- Reproductive traits: number of flowers (counted in the field); number of fruits (counted in the field); seed mass (as obtained by laboratory weighing); number of embryos (counted in the laboratory under the stereo microscope).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPBES. Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 2019. Available online: https://www.ipbes.net/global-assessment (accessed on 27 December 2023).
- Christenhusz, M.J.M.; Byng, J.W. The number of known plant species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [CrossRef]
- Fay, M.F.; Chase, M.W. Orchid biology: from Linnaeus via Darwin to the 21st century. Ann. Bot. 2009, 104, 359–364. [CrossRef]
- Khapugin, A. A global systematic review of publications concerning the invasion biology of four tree species. Hacquetia 2019, 18, 233–270. [CrossRef]
- Ticktin, T.; Charitonidou, M.; Douglas, J.; Halley, J.M.; Hernández-Apolinar, M.; Liu, H.; Mondragón, D.; Pérez-García, E.A.; Tremblay, R.L.; Phelps, J. Wild orchids: A framework for identifying and improving sustainable harvest. Biol. Conserv. 2023, 277, 109816. [CrossRef]
- Fay, M.F. Orchid conservation: how can we meet the challenges in the twenty-first century? Bot. Stud. 2018, 59, 16. [CrossRef]
- Reiter, N.; Whitfield, J.; Pollard, G.; Bedggood, W.; Argall, M.; Dixon, K.; Davis, B.; Swarts, N. Orchid re-introductions: an evaluation of success and ecological considerations using key comparative studies from Australia. Plant Ecol. 2016, 217, 81–95. [CrossRef]
- Kull, T. Population Dynamics of North Temperate Orchids. In Orchid biology VIII: Reviews and Perspectives, 1st ed.; Kull, T.; Arditti, J., Eds.; Kluwer Academic Publishers: Dordrecht, 2002; pp. 139–165, ISBN 978-1-4020-0580-0.
- Štípková, Z.; Kindlmann, P. Factors determining the distribution of orchids – a review with examples from the Czech Republic. Eur. J. Environ. Sci. 2021, 11, 21–30. [CrossRef]
- Wraith, J.; Pickering, C. Quantifying anthropogenic threats to orchids using the IUCN Red List. Ambio 2018, 47, 307–317. [CrossRef]
- Bianco, P.M. Le orchidee come indicatori di qualità degli habitat. Biol. Ital. 2012, 42, 35–48.
- Gale, S.W.; Fischer, G.A.; Cribb, P.J.; Fay, M.F. Orchid conservation: bridging the gap between science and practice. Bot. J. Linn. Soc. 2018, 186, 425–434. [CrossRef]
- Newman, B.J.; Ladd, P.; Brundrett, M.; Dixon, K.W. Effects of habitat fragmentation on plant reproductive success and population viability at the landscape and habitat scale. Biol. Conserv. 2013, 159, 16–23. [CrossRef]
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 2013, 161, 230–238. [CrossRef]
- Abeli, T.; D’Agostino, M.; Orsenigo, S. et al. IDPlanT: the Italian database of plant translocation. Plant Biosyst. 2021. [CrossRef]
- Orlikowska, E.H.; Roberge, J.M.; Blicharska, M.; Mikusiński, G. Gaps in ecological research on the world's largest internationally coordinated network of protected areas: A review of Natura 2000. Biol. Conserv. 2016, 200, 216–227. [CrossRef]
- European Commission Natura 2000. 2020. Available online: https://ec.europa.eu/environment/nature/natura2000/index_en.htm (accessed on 27 December 2023).
- Hill, R.; Miller, C.; Newell, B.; Dunlop, M.; Iain, J.G. Why biodiversity declines as protected areas increase: the effect of the power of governance regimes on sustainable landscapes. Sustain. Sci. 2015, 10, 357–369. [CrossRef]
- Gray, C.; Hill, S.; Newbold, T.; Hudson, L.N; Borger, L.; Contu, S.; Hpskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [CrossRef]
- Pulido-Chadid, K.; Virtanen, E.; Geldmann, J. How effective are protected areas for reducing threats to biodiversity? A systematic review protocol. Environ. Evid. 2023, 12, 18. [CrossRef]
- Olmeda, C.; Šefferová, V.; Underwood, E.; Millan, L.; Gil, T.; Naumann, S. (compilers). EU Action plan to maintain and restore to favourable conservation status the habitat type 6210 Semi-natural dry grasslands and scrubland facies on calcareous substrates (Festuco-Brometalia) (*important orchid sites). 2019. Available online: https://www.ecologic.eu/17536 (accessed on 27 December 2023).
- Slaviero, A.; Del Vecchio, S.; Pierce, S.; Fantinato, E.; Buffa, G. Plant community attributes affect dry grassland orchid establishment. Plant Ecol. 2016, 217, 1533–1543. [CrossRef]
- Ignatieva, M.; Hedblom, M. An alternative urban green carpet. How can we move to sustainable lawns in a time of climate change? Science 2018, 362, 148–149. [CrossRef]
- Ignatieva, M.; Haase, D.; Dushkova, D.; Haase, A. Lawns in cities: from a globalised urban green space phenomenon to sustainable nature-based solutions. Land 2020, 9, 73. [CrossRef]
- Sehrt M.; Bossdorf O.; Freitag M.; Bucharova A. Less is more! Rapid increase in plant species richness after reduced mowing in urban grasslands. Basic Appl. Ecol. 2020, 42, 47–53. [CrossRef]
- Paudel, S.; States, S.L. Urban green spaces and sustainability: Exploring the ecosystem services and disservices of grassy lawns versus floral meadows. Urban For. Urban Green. 2023, 84, 127932. [CrossRef]
- Simeoni, U.; Corbau, C. A review of the Delta Po evolution (Italy) related to climatic changes ad human impacts. Geomorphology 2009, 107, 64–71. [CrossRef]
- Committee on the Environment, Agriculture and Local and Regional Affairs, Protection of European deltas Report. 2005. Available online: https://assembly.coe.int/nw/xml/XRef/X2H-Xref-ViewHTML.asp?FileID=10868&lang=EN (accessed on 27 December 2023).
- Jacquemyn, H.; Hutchings, M.J. Biological flora of the British Isles: Ophrys sphegodes. J. Ecol. 2015, 103, 1680-1696. [CrossRef]
- Teibert, C.F. Reproductive success of Anacamptis morio (Orchidaceae) in der Donau-Auen National Park, Austria. MSc Thesis, 2018. University of Natural Resources and Life Sciences, Vienna, Austria.
- Robbirt, K.M. Phenological responses of British orchids and their pollinators to climate change: an assessment using herbarium and museum collections. MSc Thesis, 2012. University of East Anglia, Norwich, UK.
- Lanzino, M.; Palermo, A.M.; Pellegrino, G. The effect of inflorescence display size and flower position on pollination success in two deceptive and one rewarding orchid. Plant Biol. 2023, 25, 396–402. [CrossRef]
- Willems, J.H. Population dynamics of Spiranthes spiralis in South Limburg, The Netherlands. Mém. Soc. Royale Bot. Belgium 1998, 11, 115–121.
- Moen, A.; Oien, D.I. Ecology and survival of Nigritella nigra, a threatened orchid species in Scandinavia. Nord. J. Bot. 2002, 22, 435–461. [CrossRef]
- Damgaard, C.; Moeslund, J.E.; Wind, P. Changes in the abundance of Danish orchids over the past 30 years. Diversity 2020, 12, 244. [CrossRef]
- Djordjević, V.; Aćić, S.; Kabaš, E.; Lazarević, P.; Tsiftsis, S.; Lakušić, D. The orchids of wetland vegetation in the Central Balkans. Diversity 2023, 15, 26. [CrossRef]
- Pierce, S.; Vagge, I.; Brusa, G.; Cerabolini, B.E.L. The intimacy between sexual traits and Grime’s CSR strategies for orchids coexisting in semi-natural calcareous grassland at the Olive Lawn. Plant Ecol. 2014, 215, 495–505. [CrossRef]
- Meekers, T.; Hutchings, M.J.; Honnay, O.; Jacquemyn, H. Biological flora of the British Isles: Gymnadenia conopsea s.l. J. Ecol. 2012, 100, 1269–1288. [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [CrossRef]
- Tonin, R.; Gerdol, R.; Tomaselli, M.; Petraglia, A.; Carbognani, M.; Wellstein, C. Intraspecific functional trait response to advanced snowmelt suggests increase of growth potential but decrease of seed production in snowbed plant species. Front. Plant Sci. 2019, 10, 289. [CrossRef]
- Ackerman, J.D.; Tremblay, R.L.; Pérez, M.E.; Madden, H.; Bechtold, M.; Boeken, M. Small populations on small islands: What chance does an orchid have? Int. J. Plant Sci. 2020, 181, 667–685. [CrossRef]
- Willems, J.H.; Melser, C. Population dynamics and life-history of Coeloglossum viride (L.) Hartm.: an endangered orchid species in The Netherlands. Bot. J. Linn. Soc. 1998, 126, 83–93. [CrossRef]
- Zhang, S.; Hu, H.; Zhou, Z.; Xu, K.; Yan, N.; Li, S. Photosynthesis in relation to reproductive success of Cypripedium flavum. Ann. Bot. – London 2005, 96, 43–49. [CrossRef]
- Landi, M.; Frignani, F.; Lazzeri, C.; Angiolini, C. Abundance of orchids on calcareous grasslands in relation to community species, environmental, and vegetational conditions. Russ. J Ecol 2009, 40, 486–494. [CrossRef]
- Catorci, A.; Cesaretti, S.; Gatti, R. Effect of long-term abandonment and spring grazing on floristic and functional composition of dry grasslands in a central Apennine farmland. Pol. J. Ecol. 2013, 61, 505–518.
- Kirillova, I.A.; Kirillov, D.V. Impact of weather conditions on the population dynamics and reproductive success of Platanthera bifolia (L.) Rich. in the Komi Republic. Contemp. Probl. Ecol. 2023, 16, 819–830. [CrossRef]
- Wells, T.C.E.; Rothery, P.; Cox, R.; Bamford, S. Flowering dynamics of Orchis morio L. and Herminium monorchis (L.) R.Br. at two sites in eastern England. Bot. J. Linn. Soc. 1998, 126, 39–48. [CrossRef]
- Rafferty, N.E.; Ives, A.R. Effects of experimental shifts in flowering phenology on plant-pollinator interactions. Ecol. Lett. 2011, 14, 69–74. [CrossRef]
- Jersáková, J.; Kindlmann, P. Patterns of pollinator-generated fruit set in Orchis morio (Orchidaceae). Folia Geobot. 1998, 33, 377–390. [CrossRef]
- Neiland, M.R.M.; Wilcock, C.C. Fruit set, nectar reward, and rarity in the Orchidaceae. Am. J. Bot. 1998, 85, 1657–1671. [CrossRef]
- Pellissier, L.; Vittoz, P.; Internicola, A.I; Gigord, L.D.B. Generalized food-deceptive orchid species flower earlier and occur at lower altitudes than rewarding ones. J. Plant Ecol. 2010, 3, 243–250. [CrossRef]
- Hornemann, G.; Michalski, S.G.; Durka, W. Short-term fitness and long-term population trends in the orchid Anacamptis morio. Plant Ecol. 2012, 213, 1583–1595. [CrossRef]
- Arditti, J. Fundamentals of Orchid Biology. John Wiley & Sons Ltd.: New York, USA, 1992; 691 p.
- Nazarov, V.V., Efetov, K.A. On the role of Zygaenidae (Lepidoptera) in pollination of Anacamptis pyramidalis (Orchidaceae). Zool. Zhurn. 1993, 72, 54–67.
- Taylor, R.; Allen, A. In Protecting Groundwater for Health: Managing the Quality of Drinking-water Sources; Schmoll, O.; Howard, G.; Chilton, J.; Chorus, I., Eds.; IWA: London, UK, 2006; p. 697.
- Gijbels, P.; De Hert, K.; Jacquemyn, H.; Honnay, O. Reduced fecundity and genetic diversity in small populations of rewarding versus deceptive orchid species: a meta-analysis. Plant Ecol. Evol. 2015, 148, 153–159. [CrossRef]
- Barbaro, L.; Dutoit, T.; Grossi, J.L. Influence des facteurs agro-écologiques sur les assemblages d'orchidées dans les pelouses calcicoles du Vercors (Préalpes, France). Bot. Helv. 2003, 113, 63–79.
- Metera, E.; Sakowski, T.; Sloniewski, K.; Romanowicz, B. Grazing as a tool to maintain biodiversity of grassland - a review. Anim. Sci. Pap. Rep. 2010, 28, 315–334.
- Maron, J.L.; Crone, E. Herbivory: effects on plant abundance, distribution and population growth. Proc. R. Soc. B 2006, 273, 2575–2584. [CrossRef]
- Wooten, S.; Call, G.; Dattilo, A.; Cruse-Sanders, J.; Boyd, J.N. Impacts of forest thinning and white-tailed deer herbivory on translocation of the rare terrestrial orchid Platanthera integrilabia. Diversity 2020, 12, 412. [CrossRef]
- McGraw, J.B.; Furedi, M.A. Deer browsing and population viability of a forest understory plant. Science 2005, 307, 920–922. [CrossRef]
- Vallius, E. Factors affecting fruit and seed production in Dactylorhiza maculata (Orchidaceae), Bot. J. Linn. Soc. 2001, 135, 89–95. [CrossRef]
- Moreira, X.; Castagneyrol, B.; Abdala-Roberts, L.; Traveset, A. A meta-analysis of herbivore effects on plant attractiveness to pollinators. Ecology 2019, 100, e02707. [CrossRef]
- Vavra, M.; Parks, C.J.; Wisdom, M. J. Biodiversity, exotic plant species, and herbivory: The good, the bad, and the ungulate, Forest Ecol. Manag. 2007, 246, 66–72. [CrossRef]
- Whigham, D.E. Ecology of woodland herbs in temperate deciduous forests. Annu. Rev. Ecol. Evol. S. 2004, 35, 583–621. [CrossRef]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species' traits and environment. Glob. Change Biol. 2012, 18, 1725–1737. [CrossRef]
- Martín-Forés, I.; Bywaters, S.L.; Sparrow, B.; Guerin, G.R. Simultaneous effect of habitat remnancy, exotic species, and anthropogenic disturbance on orchid diversity in South Australia. Conserv. Sci. Pract. 2022, 4, e12652. [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: a meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [CrossRef]
- Kottawa-Arachchi, J.D.; Gunasekara, R.S. Research priorities and future directions in conservation of wild orchids in Sri Lanka: A review. Nat. Conserv. Res. 2020, 5, 34–45. [CrossRef]
- Bonnardeaux, Y.; Brundrett, M.; Batty, A.; Dixon, K.; Koch, J.; Sivasithamparam, K. Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycol. Res. 2007, 111, 51–61. [CrossRef]
- Li, T.; Wu, S.; Yang, W.; Selosse, M.A.; Gao, J. How mycorrhizal associations influence orchid distribution and population dynamics Front. Plant Sci. 2021, 12, 647114. [CrossRef]
- Foxcroft, L.C.; Jarošík, V.; Pyšek, P.; Richardson, D.M.; Mathieu, R. Protected-area boundaries as filters of plant invasions. Conserv. Biol. 2010, 25, 400–405. [CrossRef]
- Le, H.; Zhao, C.; Xu, W.; Deng, Y.; Xie, Z. Anthropogenic activities explained the difference in exotic plants invasion between protected and non-protected areas at a northern subtropics biodiversity hotspot. J. Environ. Manage. 2023, 345, 118939. [CrossRef]
- Jones, P. Aspects of the population biology of Liparis loeselii (L.) Rich. var. ovata Ridd. ex Godfery (Orchidaceae) in the dune slacks of South Wales, UK. Bot. J. Linn. Soc. 1998, 126, 123–139. [CrossRef]
- Huang, B.Q.; Yang, X.Q.; Yu, F.H.; Luo, Y.B.; Tai, Y.D. Surprisingly high orchid diversity in travertine and forest areas in the Huanglong valley, China, and implications for conservation. Biodivers. Conserv. 2008, 17, 2773–2786. [CrossRef]
- Heywood, W.H. Conserving plants within and beyond protected areas - still problematic and future uncertain. Plant Divers. 2019, 41, 36–49. [CrossRef]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction Ann. Bot. – London 2009, 104, 543–556. [CrossRef]
- Alessandrini, A.; Balboni, G.; Brancaleoni, L.; Gerdol, R.; Nobili, G.; Pellizzari, M.; Piccoli, F.; Ravaglioli, M. The Vascular Flora of the Bosco della Mesola Nature Reserve (Northern Italy). Springer Nature Switzerland AG: Cham, Switzerland, 2021; Geobotany Studies; 107 p. [CrossRef]
- Piccoli, F.; Pellizzari, M.; Alessandrini, A. Flora del Ferrarese. Istituto per i Beni Artistici Culturali e Naturali della Regione Emilia-Romagna, Longo Ed.: Ravenna, Italy, 2014; 321 p.
- Zlobin, Y.; Kovalenko, I.; Klymenko, H.; Kyrylchuk, K.; Bondarieva, L.; Tykhonova, O.; Zubtsova, I. Vitality analysis algorithm in the study of plant individuals and populations. Open Agric. 2021, 15, 119–129. [CrossRef]
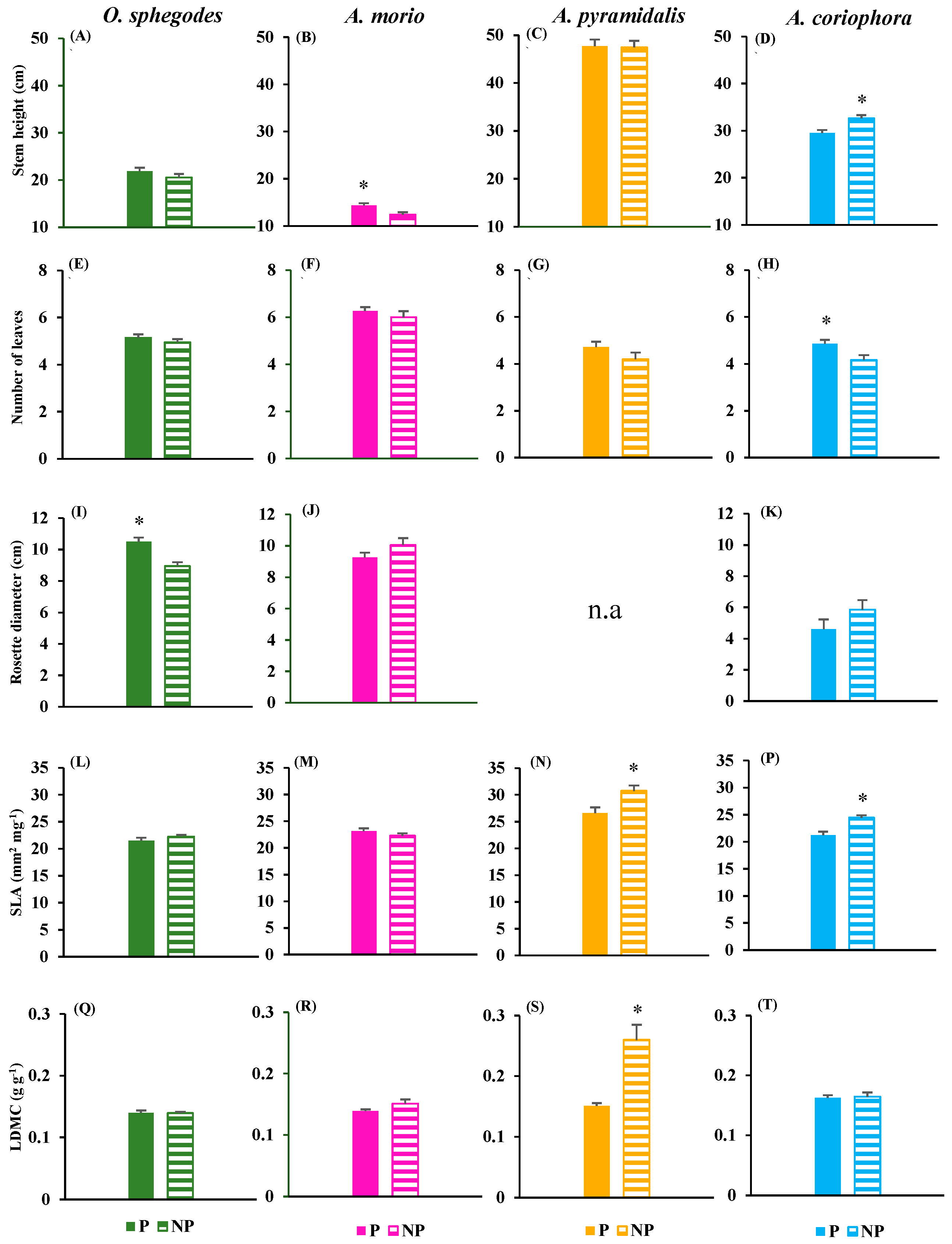
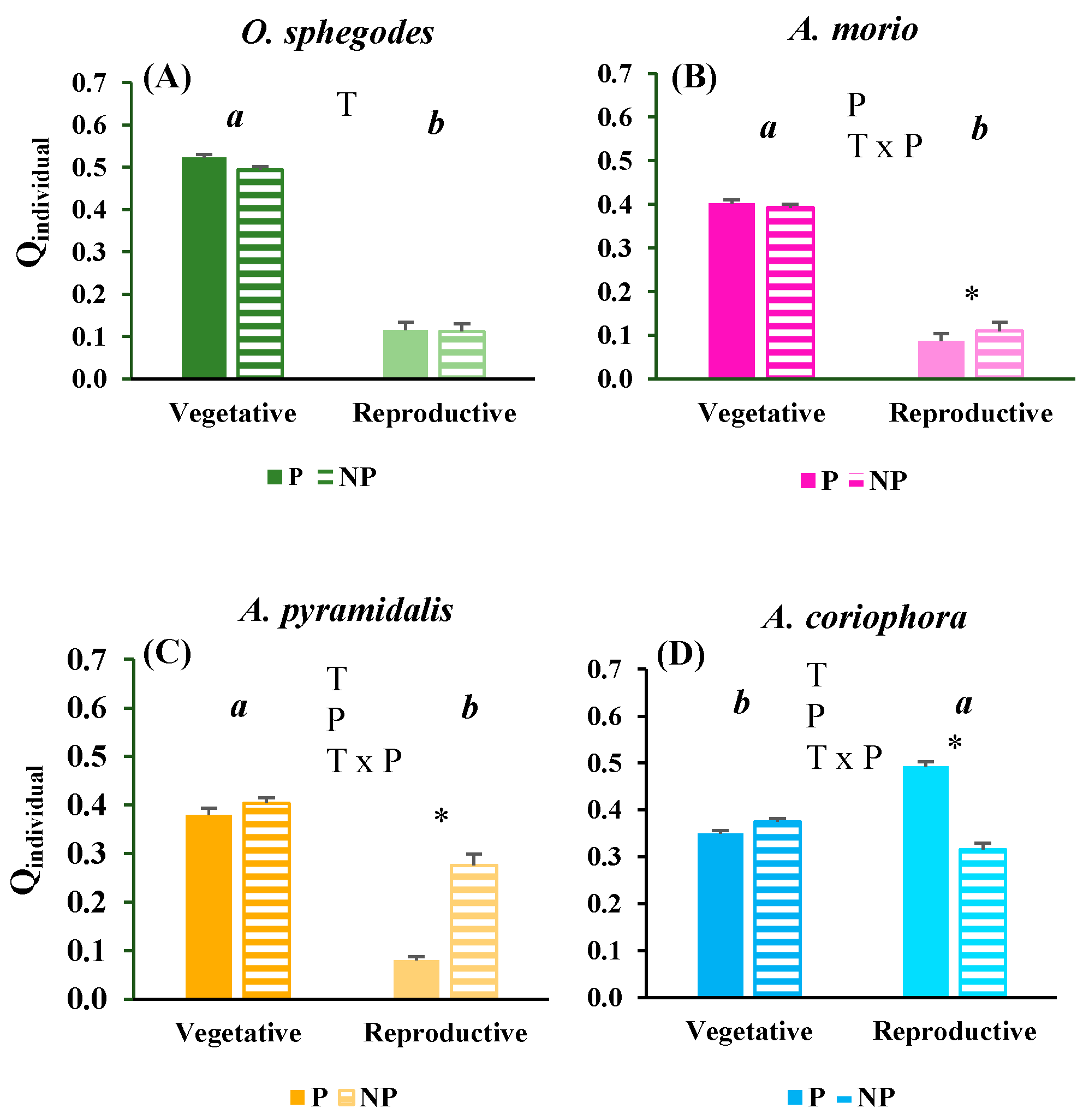
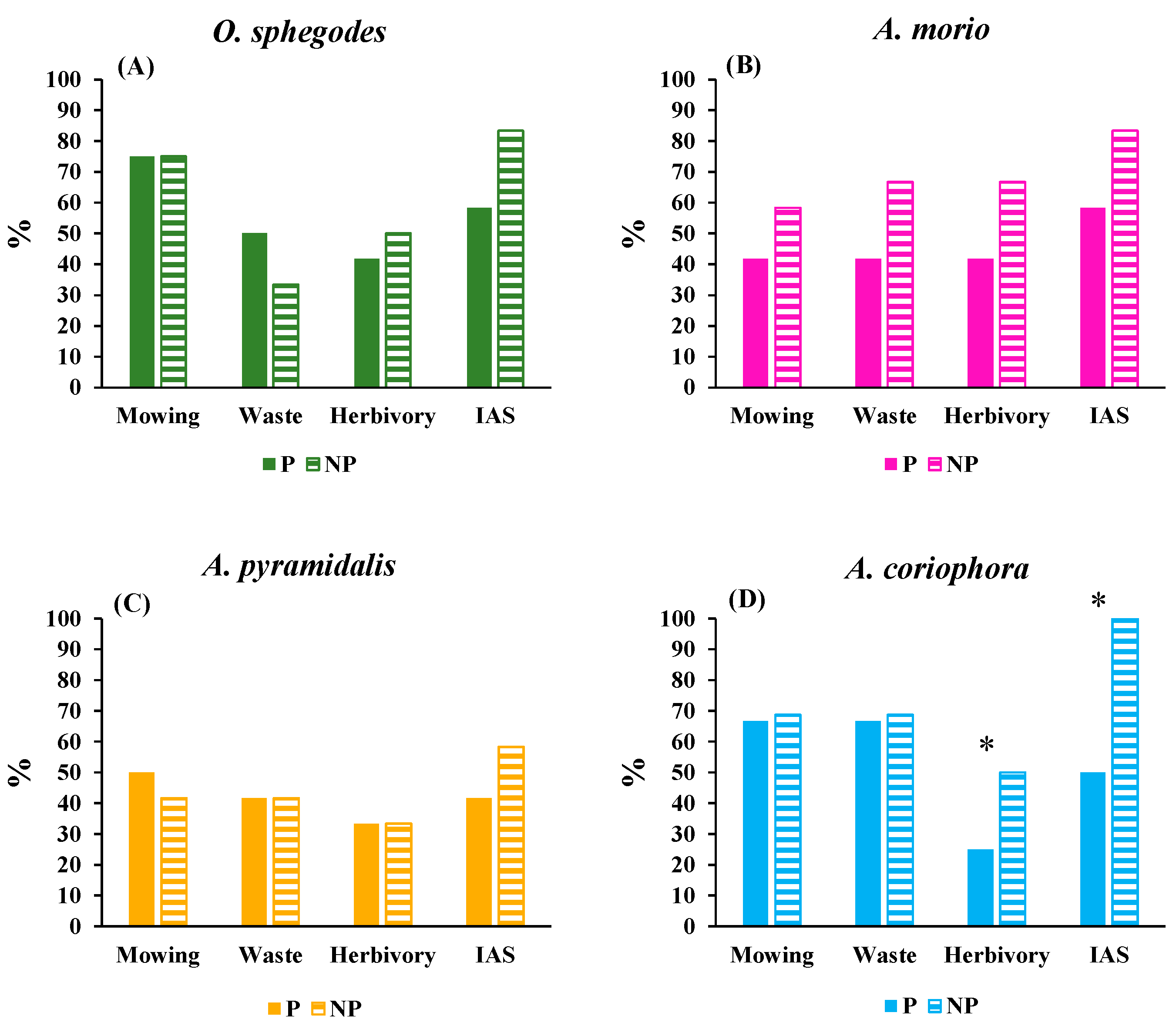
| Trait | O. sphegodes | A. morio | A. pyramidalis | A. coriophora | ||
|---|---|---|---|---|---|---|
| Number of flowers | P NP |
4.60±0.20 4.48±0.19 |
7.61±0.37 9.66±0.46* |
44.20±2.97 49.47±2.78 |
22.89±0.79 20.93±0.74 |
|
| Number of fruits | P | n.a | n.a | 2.79 ± 0.42 * | 15.80 ± 0.70 | |
| NP | n.a | n.a | 1.36 ± 0.22 | 13.86 ± 0.91 | ||
| Seed mass (mg) | P | n.a | n.a | 0.78 ± 0.12 | 5.30 ± 0.30 * | |
| NP | n.a | n.a | 0.69 ± 0.11 | 2.00 ± 0.20 | ||
| Number of embryos | P | n.a | n.a | 42.62 ± 5.98 | 96.37 ± 1.90 * | |
| NP | n.a | n.a | 39.78 ± 5.42 | 48.45 ± 4.52 | ||
| Species | Protection | Trait | Q |
|---|---|---|---|
| O. sphegodes | P | CV | 0.494 |
| NP | CV | 0.500 | |
| P | CR | 0.079 | |
| NP | CR | 0.082 | |
| A. morio | P | CV | 0.428 |
| NP | CV | 0.383 | |
| P | CR | 0.058 | |
| NP | CR | 0.076 | |
| A. pyramidalis | P | CV | 0.303 |
| NP | CV | 0.395 | |
| P | CR | 0.220 | |
| NP | CR | 0.211 | |
| A. coriophora | P | CV | 0.311 |
| NP | CV | 0.350 | |
| P | CR | 0.483 | |
| NP | CR | 0.267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





