Submitted:
03 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
Morphological examinations
DNA extraction
DNA amplification and sequencing
DNA taxonomy
Molecular phylogenetic analyses
3. Results
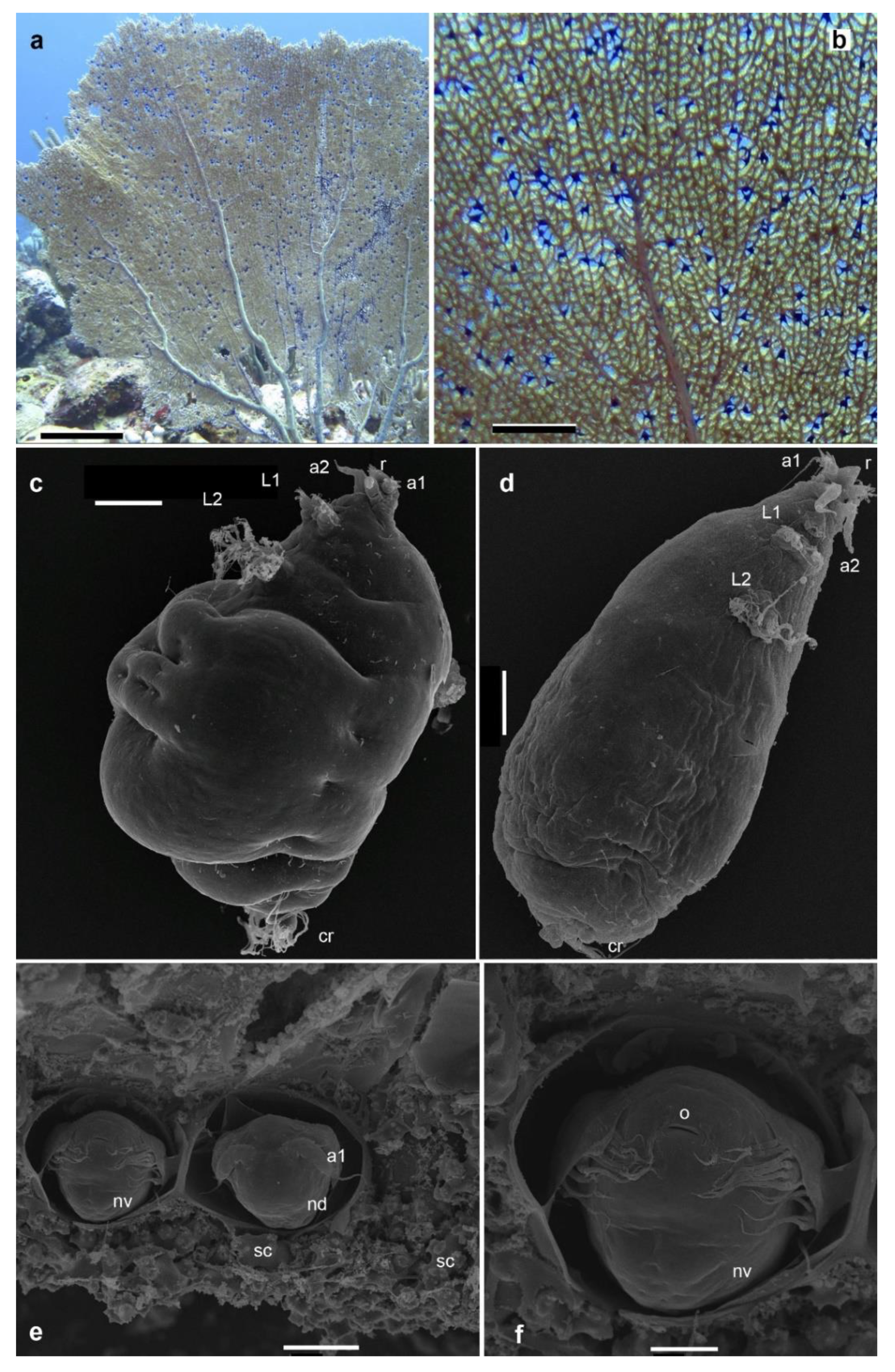
Observation of the purple galls on Gorgonia ventalina
Morphological features of Sphaerippe spp. from the purple galls
Interspecies molecular diversity
Intraspecific molecular diversity
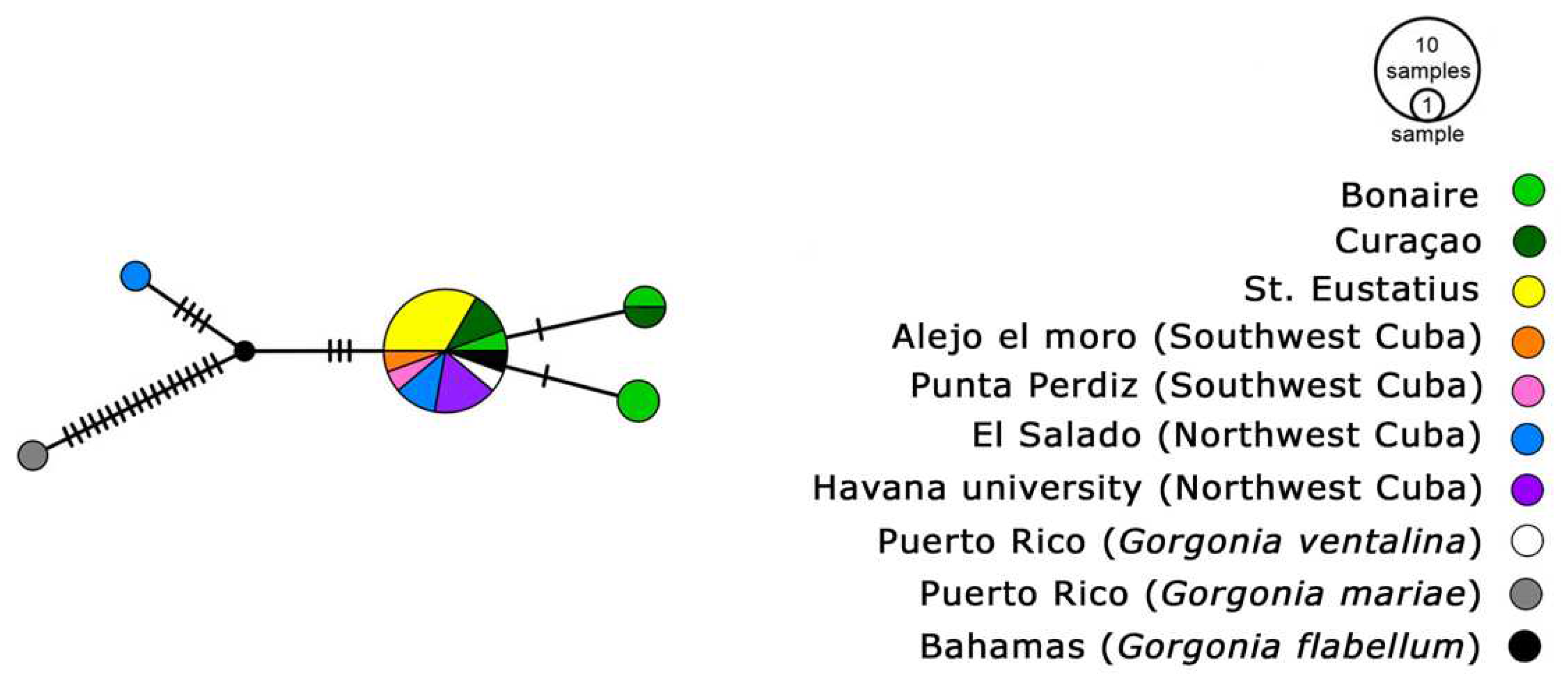
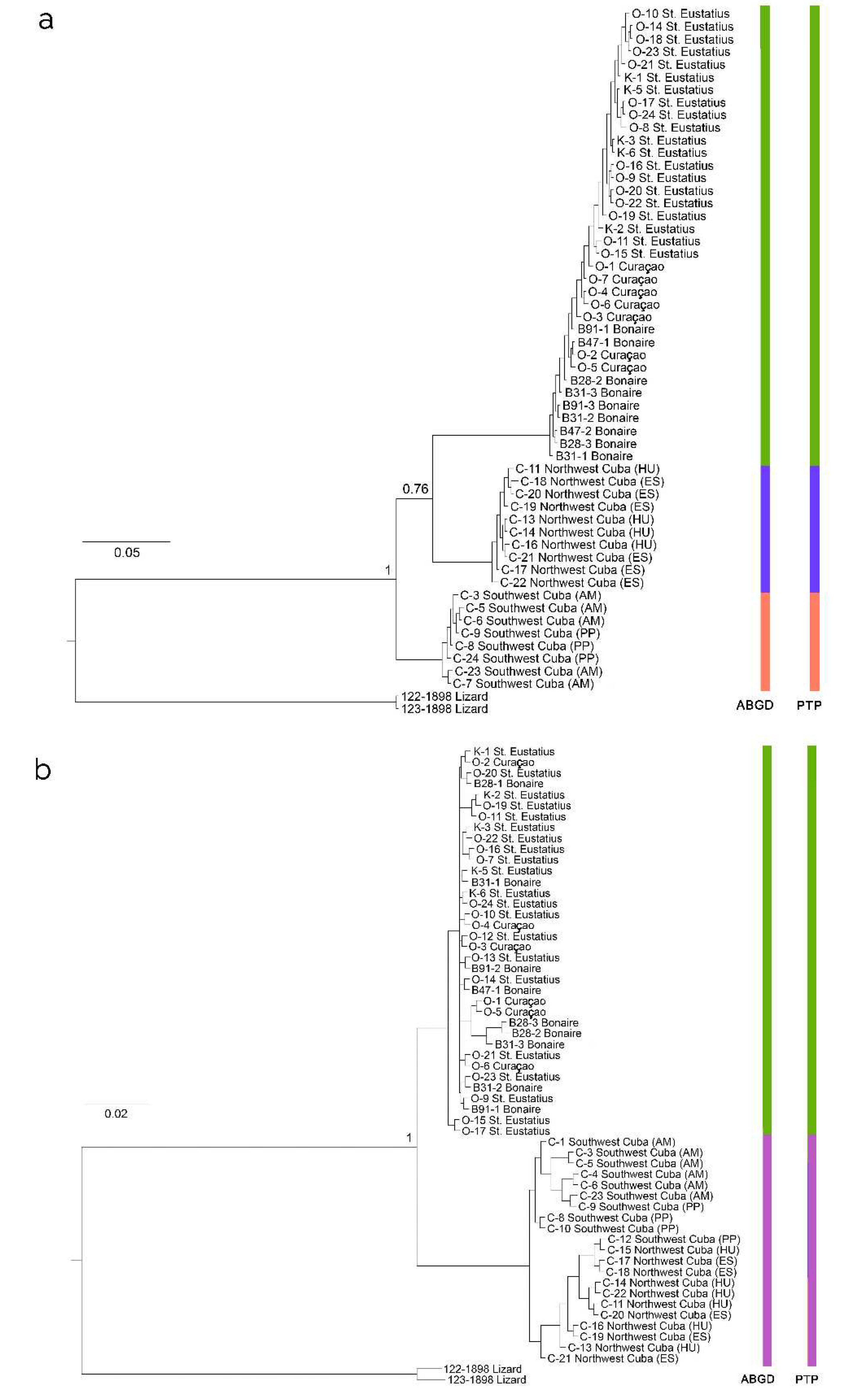
Phylogeny reconstruction

4. Discussion
Morphological examination of copepod specimens
Molecular phylogenetic divergence
Geographical heterogeneity of parasite and host populations
Coral diseases and the multifocal purple spot syndrome (MFPS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aguilar, C.; Sánchez, J. A. Phylogenetic hypotheses of gorgoniid octocorals according to ITS2 and their predicted RNA secondary structures. Molecular Phylogenetics and Evolution 2007, 43, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S. F.; Gish, W.; Miller, W.; Myers, E. W.; Lipman, D. J. Basic local alignment search tool. Journal of Molecular Biology 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Andras, J. P.; Kirk, N. L.; Harvell, C. D. Range–wide population genetic structure of Symbiodinium associated with the Caribbean Sea fan coral, Gorgonia ventalina. Molecular Ecology 2011, 20, 2525–42. [Google Scholar] [CrossRef]
- Andras, J. P.; Rypien, K. L.; Harvell, C. D. Range–wide population genetic structure of the Caribbean sea fan coral, Gorgonia ventalina. Molecular Ecology 2013, 22, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Bayer, F. M. The shallow–water Octocorallia of the West Indian region. Studies on the Fauna of Curaçao and other Caribbean Islands.
- Becker, A. A. M. J.; Freeman, M. A.; Dennis, M. M. A combined diagnostic approach for the investigation of lesions resembling aspergillosis in Caribbean sea fans (Gorgonia spp.). Veterinary Pathology. [CrossRef]
- Burge, C. A.; Douglas, N.; Conti–Jerpe, I.; Weil, E.; Roberts, S.; Friedman, C. S.; Harvell, C. D. Friend or foe: the association of Labyrinthulomycetes with the Caribbean sea fan Gorgonia ventalina. Diseases of Aquatic Organisms 2012, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Burge, C. A.; Mouchka, M. E.; Harvell, C. D.; Roberts, S. Immune response of the Caribbean seafan, Gorgonia ventalina exposed to an Aplanochytrium parasite as revealed by transcriptome sequencing. Frontiers in Physiology 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calderón–Hernández, A.; Urbina–Villalobos, A.; Mora–Barboza, C. , Lesions in octocorals of the Costa Rican Caribbean During The 2015–2016 El Niño. Coral Reefs 2021, 40, 1167–1179. [Google Scholar] [CrossRef]
- Cheng, Y. R.; Lin, C. Y.; Yu, J. K. Embryonic and post–embryonic development in the parasitic copepod Ive ptychoderae (Copepoda: Iviidae): Insights into its phylogenetic position. PLoS One 2023, 18, e0281013. [Google Scholar] [CrossRef] [PubMed]
- Chollett, I.; Müller–Karger, F. E.; Heron, S. F.; Skirving, W.; Mumby, P. J. Seasonal and spatial heterogeneity of recent sea surface temperature trends in the Caribbean Sea and southeast Gulf of Mexico. Marine Pollution Bulletin 2012, 64, 956–965. [Google Scholar] [CrossRef]
- Conradi, M.; Bandera, E.; Mudrova, S. V.; Ivanenko, V. N. Five new coexisting species of copepod crustaceans of the genus Spaniomolgus (Poecilostomatoida: Rhynchomolgidae), symbionts of the stony coral Stylophora pistillata (Scleractinia). ZooKeys 2018, 791, 71–95. [Google Scholar] [CrossRef]
- Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Phylogenies and the comparative method. The American Naturalist 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Ferrari, F. D.; Ivanenko, V. N.; Dahms, H. –U. Body architecture and relationships among basal copepods. Journal of Crustacean Biology 2010, 30, 465–477. [Google Scholar] [CrossRef]
- Fontaneto, D.; Flot, J. F.; Tang, C. Q. Guidelines for DNA taxonomy, with a focus on the meiofauna. Marine Biodiversity 2015, 45, 1–19. [Google Scholar] [CrossRef]
- Fu, Y. –X. Statistical tests of neutrality against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all–taxa biotic surveys. Molecular Ecology Resources 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Grygier, M. J. An endoparasitic Lamippid Copepod in Acanella from the North Atlantic. Crustaceana 1983, 45, 176–182. [Google Scholar] [CrossRef]
- Grygier, M. J. Two new lamippid copepods parasitic on gorgonians from Hawaii and the Bahamas. Proceedings of the Biological Society of Washington 1980, 93, 662–673. [Google Scholar]
- Guindon, S.; Dufayard, J. F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum–likelihood phylogenies: assessing the performance of PhyML 3.0. 0. Systematic Biology 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Harvell, C. D.; Markel, S.; Jordan–Dahlgren, E.; Raymundo, L. J.; Rosenberg, E.; Smith, G. W.; Willis, B. L.; Weil, E. Coral disease, environmental drivers and the balance between coral and microbial associates. Oceanography 2007, 20, 36–59. [Google Scholar] [CrossRef]
- Hoeksema, B. W.; Reimer, J. D.; Vonk, R. Editorial: biodiversity of Caribbean coral reefs (with a focus on the Dutch Caribbean). Marine Biodiversity 2017, 47, 1–10. [Google Scholar] [CrossRef]
- Humes, A. G. Lamippe concinna sp. n., a copepod parasitic in a West African pennatulid coelenterate. Parasitology.
- Huys, R.; Llewellyn–Hughes, J.; Olson, P. D.; Nagasawa, K. Small subunit rDNA and Bayesian inference reveal Pectenophilus ornatus (Copepoda incertae sedis) as highly transformed Mytilicolidae, and support assignment of Chondracanthidae and Xarifiidae to Lichomolgoidea (Cyclopoida). Biological Journal of the Linnean Society 2006, 87, 403–425. [Google Scholar] [CrossRef]
- iNaturalist. Available from https://www.inaturalist.org. Accessed 15th 22. 20 December.
- Ivanenko, V. N.; Hoeksema, B. W.; Mudrova, S. V.; Nikitin, M. A.; Martínez, A.; Rimskaya–Korsakova, N. N.; Berumen, M. L.; Fontaneto, D. Lack of host specificity of copepod crustaceans associated with mushroom corals in the Red Sea. Molecular Phylogenetics and Evolution 2018, 127, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, V. N.; Nikitin, M. A.; Hoeksema, B. W. Multiple purple spots in the Caribbean Sea fan Gorgonia ventalina caused by parasitic copepods at St. Eustatius, Dutch Caribbean. Marine Biodiversity 2017, 47, 79–80. [Google Scholar] [CrossRef]
- Ivanenko, V. N.; Ferrari, F. D. Redescription of adults and description of copepodid development of Dermatomyzon nigripes (Brady, Robertson, 1876) and of Asterocheres lilljeborgi Boeck, 1859 (Copepoda: Siphonostomatoida: Asterocheridae). Proceedings of the Biological Society of Washington 2003, 116, 661–691. [Google Scholar]
- Ivanenko, V. N.; Ferrari, F. D.; Smurov, A. V. Nauplii and copepodids of Scottomyzon gibberum (Copepoda: Siphonostomatoida: Scottomyzotidae, a new family), a symbiont of Asterias rubens (Asteroidea). Proceedings of the Biological Society of Washington 2001, 114, 237–61. [Google Scholar]
- Ivanenko, V. N.; Defaye, D. A new and primitive genus and species of deep–sea Tegastidae (Crustacea, Copepoda, Harpacticoida) from the Mid–Atlantic Ridge, 37°N (Azores Triple Junction, Lucky Strike). Cahiers de Biologie Marine 2004, 45, 255–268. [Google Scholar]
- Jossart, Q.; De Ridder, C.; Lessios, H. A.; Bauwens, M.; Sébastien, M.; Thierry, R.; Rémi, A. W.; Bruno, D. Highly contrasted population genetic structures in a host–parasite pair in the Caribbean Sea. Ecology and Evolution 2017, 7, 9267–9280. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K. D. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics 2017, bbx108, 1–7. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones–Havas, S.; Cheung, M.; Sturrock, S.; Drummond, A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Kim, K.; Rypien, K. Aspergillosis of Caribbean Sea Fan Corals, Gorgonia spp. In Diseases of Coral; 2015, 236–241.
- Kimes, N. E.; Van Nostrand, J. D.; Weil, E.; Zhou, J.; Morris, P. J. Microbial functional structure of Montastraea faveolata, an important Caribbean reef–building coral, differs between healthy and Caribbean yellow–band diseased colonies. Environmental Microbiology 2010, 12, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, N.; Rohwer, F. Multispecies microbial mutualisms on coral reefs: the host as a habitat. The American Naturalist 2003, 162, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Korein, E.; Vega–Rodriguez, M.; Metz Estrella, T. Developing recommendations for coral disease management in Puerto Rico using key informant interviews and participatory mapping. Ocean and Coastal Management 2023, 236, 106488. [Google Scholar] [CrossRef]
- Korzhavina, O.A.; Grishina, D.Y.; Chen, X.; Fontaneto, D.; Ivanenko, V.N. Diving into diversity: copepod crustaceans in octocoral association. Diversity 2023, 15, 1140. [Google Scholar] [CrossRef]
- Korzhavina, O.A.; Hoeksema, B.W.; Ivanenko, V.N. A review of Caribbean Copepoda associated with reef–dwelling cnidarians, echinoderms, and sponges. Contributions to Zoology 2019, 88, 297–349. [Google Scholar] [CrossRef]
- Korzhavina, O.A.; Reimer, J.D.; Ehrlich, H.; Ivanenko, V.N. Global diversity and distribution of Lamippidae copepods symbiotic on Octocorallia. Symbiosis 2021, 83, 265–277. [Google Scholar] [CrossRef]
- Kuraku, S.; Zmasek, C. M.; Nishimura, O.; Katoh, K. aLeaves facilitates on–demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Research 2013, 41, W22–W28. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S. Y.; Guindon, S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P. B.; Wright, A. M.; Senfeld, T.; Calcott, B. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Laubier, L. Lamippe (Lamippe) bouligandi sp. nov., copépode parasite d'Octocoralliaire de la Mer du Labrador. Crustaceana 1972, 22, 285–293. [Google Scholar] [CrossRef]
- Leigh, J. W.; Bryant, D. POPART: full–feature software for haplotype network construction. Methods in Ecology and Evolution 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Lesser, M. P.; Bythell, J. C.; Gates, R. D.; Johnstone, R. W.; Hoegh–Guldberg, O. Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. Journal of Experimental Marine Biology and Ecology 2007, 34, 36–44. [Google Scholar] [CrossRef]
- Martin, S. W.; Meek, A. H.; Willerberg, P. Veterinary epidemiology, principles and methods. Iowa State University Press, Ames 1987, p. 343.
- McFadden, C. S.; France, S. C.; Sánchez, J. A.; Alderslade, P. A molecular phylogenetic analysis of the Octocorallia (Cnidaria: Anthozoa) based on mitochondrial protein–coding sequences. Molecular Phylogenetics and Evolution 2006, 41, 513–527. [Google Scholar] [CrossRef]
- McFadden, C. S.; Sánchez, J. A.; France, S. C. Molecular phylogenetic insights into the evolution of Octocorallia: a review. Integrative and Comparative Biology 2010, 50, 389–410. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H. J.; Stickel, S.; Sogin, M. L. The characterization of enzymatically amplified eukaryotic 16S–like rRNA–coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Mikhailov, K.V.; Ivanenko, V.N. Lack of reproducibility of molecular phylogenetic analysis of Cyclopoida. Molecular Phylogenetics and Evolution 2019, 139, 106574. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Appel, E.; Gorb, S.N. Functional diversity of resilin in Arthropoda. Beilstein J Nanotechnol. 2016, 1, 1241–1259. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, K.V.; Ivanenko, V.N. Low support values and lack of reproducibility of molecular phylogenetic analysis of Copepoda orders. Arthropoda Selecta 2021, 30, 39–42. [Google Scholar] [CrossRef]
- Montano, S.; Maggioni, D.; Liguori, G. , Morpho–molecular traits of Indo–Pacific and Caribbean Halofolliculina ciliate infections. Coral Reefs 2020, 39, 375–386. [Google Scholar] [CrossRef]
- Petes, L. E.; Harvell, C. D.; Peters, E. C.; Webb, M. A. H.; Mullen, K. M. Pathogens compromise reproduction and induce melanization in Caribbean Sea fans. Marine Ecology Progress Series 2003, 264, 167–171. [Google Scholar] [CrossRef]
- Porco, D.; Rougerie, R.; Deharveng, L.; Hebert, P. Coupling non–destructive DNA extraction and voucher retrieval for small soft–bodied Arthropods in a high–throughput context: the example of Collembola. Molecular Ecology Resources 2010, 10, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A. J.; Xie, D.; Baele, G.; Suchard, M. A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. 7. Systematic Biology 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K. B. Regulation of microbial populations by mucus–associated bacteria. Marine Ecology Progress Series 2006, 322, 1–14. [Google Scholar] [CrossRef]
- Rocha, L. A.; Rocha, C. R.; Robertson, D. R.; Bowen, B. W. Comparative phylogeography of Atlantic reef fishes indicates both origin and accumulation of diversity in the Caribbean. BMC Evolutionary Biology 2008, 8, 157. [Google Scholar] [CrossRef]
- Rogers, C. S. Words matter: recommendations for clarifying coral disease nomenclature and terminology. Diseases of Aquatic Organisms 2010, 91, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D. L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M. A.; Huelsenbeck, J. P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Ben–Haim, Y. Microbial diseases of corals and global warming. Environmental Microbiology 2002, 4, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Ruiz–Moreno, D.; Willis, B. L.; Page, A. C.; Weil, E.; Croquer, A.; Vargas–Angel, B.; Jordan–Garza, A. G.; Jordán–Dahlgren, E.; Raymundo, L.; Harvell, C. D. Global coral disease prevalence associated with sea temperature anomalies and local factors. Diseases of Aquatic Organisms 2012, 100, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J. A.; Aguilar, C.; Dorado, D.; Manrique, N. Phenotypic plasticity and morphological integration in a marine modular invertebrate. BMC Evolutionary Biology 2007, 7, 122. [Google Scholar] [CrossRef]
- Sánchez, J. A.; McFadden, C. S.; France, S. C.; Lasker, H. R. Molecular phylogenetic analyses of shallow–water Caribbean octocorals. Marine Biology 2003, 142, 975–987. [Google Scholar] [CrossRef]
- Sanchez, J. A.; Wirshing, H.H. A field key to the identification of tropical western Atlantic zooxanthellate octocorals (Octocorallia: Cnidaria). Caribbean Journal of Science 2005, 41, 508–522. [Google Scholar]
- Shelyakin, P. V.; Garushyants, S. K.; Nikitin, M. A.; Mudrova, S. V.; Berumen, M.; Speksnijder, A. G. C. L.; Hoeksema, B. W.; Fontaneto, D.; Gelfand, M. S.; Ivanenko, V. N. Microbiomes of gall–inducing copepod crustaceans from the corals Stylophora pistillata (Scleractinia) and Gorgonia ventalina (Alcyonacea). Scientific Reports 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, G. W.; Weil, E. Aspergillosis of gorgonians. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: New York, 2004. [Google Scholar]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics Society of America 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M. S.; Hellberg, M. E. Comparative phylogeography in a genus of coral reef fishes: biogeographic and genetic concordance in the Caribbean. Molecular Ecology 2006, 15, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Tracy, A. M.; Weil, E.; Harvell, C. D. Octocoral co–infection as a balance between host immunity and host environment. Oecologia 2018, 186, 743. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L. T.; von Haeseler, A.; Minh, B. Q. Nucleic Acids Research 2016, 44 (W1), W232–W235.
- van de Water, J. A. J. M.; Allemand, D.; Ferrier–Pagès, C. Host–microbe interactions in octocoral holobionts – recent advances and perspectives. Microbiome 2018, 6, 64. [Google Scholar] [CrossRef]
- Weil, E.; Hooten, A. J. Underwater cards for assessing coral health on Caribbean reefs. GEF–CRTR Program, Center for Marine Sciences: University of Queensland, Brisbane, 2008.
- Weil, E. Coral reef diseases in the wider Caribbean. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer, New York, 2004, 35–68.
- Weil, E.; Rogers, C. S. Coral reef diseases in the Atlantic–Caribbean. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer, 2011, 465–491.
- Weil, E.; Rogers, C. S.; Croquer, A. Octocoral diseases in a changing ocean. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas Saco del Valle, C., Eds.; Springer International Publishing, 2017, 1–55.
- Williams, E. H. Jr.; Bunkley–Williams, L. Life Cycle and Life History Strategies of Parasitic Crustacea. Parasitic Crustacea 2019, 3, 179–266. [Google Scholar]
- Williams, J. D.; Anchaluisa, B.; Boyko, C. B.; McDaniel, N. Description of a new endoparasitic copepod genus and species (Lamippidae) that induces gall formation in leaves of the sea pen Ptilosarcus gurneyi (Octocorallia) from British Columbia. Marine Biodiversity 2018, 48, 1–11. [Google Scholar] [CrossRef]
- Work, T. M.; Aeby, G. S. Systematically describing gross lesions in corals. Diseases of Aquatic Organisms 2006, 70, 155–160. [Google Scholar] [CrossRef]
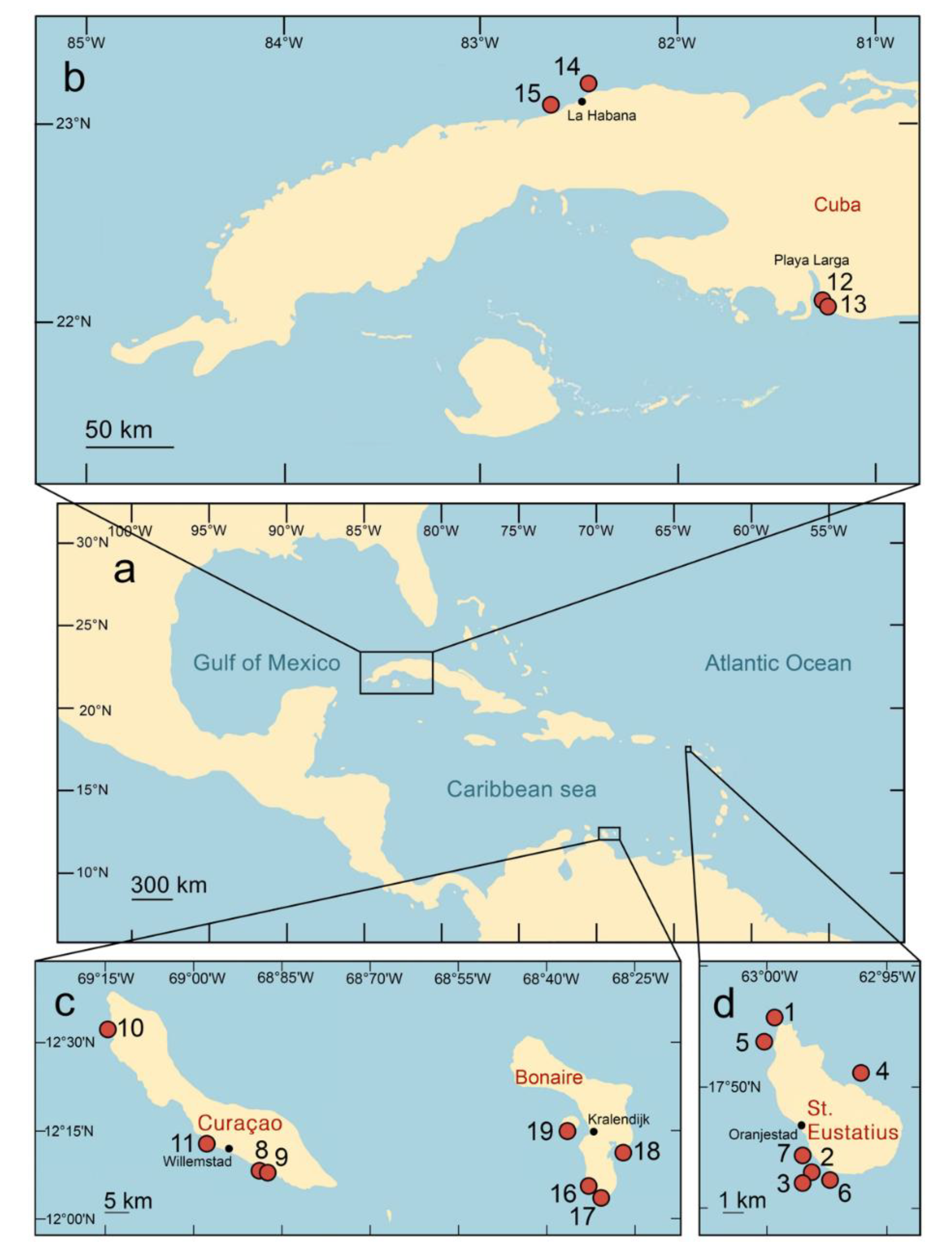

| Locality name | Coordinates | Date of sampling | Collector(s) | Name of specimens | Depth, m | Coral | Copepods |
|---|---|---|---|---|---|---|---|
| Gibraltar, St. Eustatius, (Fig. 1, point 1) | 17°31'36.5"N 62°59'57.5"W | 12.06.2015 | V.N.I. | Statia15-99 | 5-20 | + | + |
| Anchor Point North, St. Eustatius (Fig. 1, point 2) | 17°27'50.0"N 62°59'15.7"W | 17.06.2015 | V.N.I. | Statia15-134 | 15-20 | + | |
| Statia15-135 | 15-20 | + | |||||
| Anchor Reef, St. Eustatius (Fig. 1, point 3) | 17°27'44.8"N 62°59'07.7"W | 18.06.2015 | V.N.I. | Statia15-141 | 15.6 | + | + |
| Statia15-142 | 15.6 | + | + | ||||
| English Quarter, St. Eustatius (Fig. 1, point 4) | 17°30'18.2"N 62°57'46.3"W | 19.06.2015 | V.N.I. | Statia15-146 | 17.3 | + | |
| Twin Sisters, St. Eustatius (Fig. 1, point 5) | 17°30'59.6"N 63°00'10.8"W | 22.06.2015 | V.N.I. | Statia15-163 | 13.8 | + | |
| Blund Shoal, St. Eustatius (Fig. 1, point 6) | 17°27'52.6"N 62°58'38.7"W | 26.06.2015 | V.N.I. | Statia15-170 | 5.9 | + | |
| Gallows Bay, St. Eustatius (Fig. 1, point 7) | 17°28'30.3"N 62°59'10.3"W | 27.06.2015 | V.N.I. | Statia15-173 | 13.8 | + | |
| Statia15-174 | 2-3 | + | |||||
| Director's Bay, Curaçao, (Fig. 1, point 8) | 12°03'59"N 68°51'38"W | 13.06.2017 | V.N.I. | Cur17-39 | 4.1 | + | + |
| Tugboat 2, Curaçao (Fig. 1, point 9) | 12°04'05"N, 68°51'44"W | 19.06.2017 | V.N.I. | Cur17-81 | 5.2-5.5 | + | |
| Playa Lagun, Curaçao (Fig. 1, point 10) | 12°19'02"N, 69°09'09"W | 20.06.2017 | V.N.I. | Cur17-88 | 4.9 | + | |
| Buoy 1, Curaçao (Fig. 1, point 11) | 12°07'23"N, 68°58'14"W | 21.06.2017 | V.N.I. | Cur17-96 | 8.2 | + | |
| Alejo el Moro, Cuba (Fig. 1, point 12) | 22°06'54.99"N 81°06'58.96"W | 04.02.2019 | V.N.I., O.A.K. | Cuba19-1 | 7.0 | + | + |
| Cuba19-2 | 8.5 | + | + | ||||
| Cuba19-3 | 4.5 | + | + | ||||
| Punta Perdiz, Cuba (Fig. 1, point 13) | 22°06'29.65"N 81°06'49.42"W | 04.02.2019 | V.N.I., O.A.K. | Cuba19-5 | 4.8-5.0 | + | + |
| Coast near Havana University, Cuba (Fig. 1, point 14) | 23°07'38.75"N82°25'21.68"W | 07.02.2019 | V.N.I., O.A.K. | Cuba19-21 | 11.6 | + | |
| Cuba19-22 | 8.5 | + | |||||
| Cuba19-23 | 11 | + | + | ||||
| Cuba19-25 | 8.1-8.2 | + | + | ||||
| El Salado, Cuba (Fig. 1, point 15) | 23°02'20.33"N 82°36'18.55"W | 08.02.2019 | V.N.I., O.A.K. | Cuba19-27 | 13.8 | + | + |
| Cuba19-28 | 10.0 | + | + | ||||
| Cuba19-30 | 12.6 | + | |||||
| Cuba19-32 | 8.3 | + | + | ||||
| Red Beryl, Bonaire (Fig. 1, point 16) | 12° 2' 49.14"N 68° 16' 4.38"W | 28.10.2019 | V.N.I. | Bonaire19-28 | 5 | + | + |
| Red Slave, Bonaire (Fig. 1, point 17) | 12° 1' 36.3"N 68° 15' 4.74"W | 29.10.2019 | V.N.I. | Bonaire19-31 | 14 | + | + |
| Cai (outside of lagoon), Bonaire (Fig. 1, point 18) | 12° 6' 10.98"N 68° 13' 19.98"W | 31.10.2019 | V.N.I. | Bonaire19-47 | 11 | + | + |
| Klein Bonaire: South Bay, Bonaire (Fig. 1, point 19) | 12° 9' 0.06"N 68° 19' 14.04"W | 08.11.2019 | V.N.I. | Bonaire19-91 | 3 | + | + |
| Species of sample | Localities | Gene | Number of sequences | Nucleotide diversity | Tajima’s D | Fu’s F |
|---|---|---|---|---|---|---|
| Sphaerippe spp. | Bonaire, Curaçao, St. Eustatius | COI | 36 | 0.00264 | 0.30709 | -0.850 |
| Sphaerippe spp. | Northwest Cuba | COI | 10 | 0.00250 | -1.49280 | -2.563 |
| Sphaerippe spp. | Southwest Cuba | COI | 8 | 0.00168 | -0.17740 | 0.390 |
| Sphaerippe spp. | Bonaire, Curaçao, St. Eustatius | ITS2 | 36 | All sequences are identical | ||
| Sphaerippe spp. | Cuba | ITS2 | 21 | 0.00635 | 1.03432 | -0.378 |
| Gorgonia ventalina (Linnaeus 1758) | Caribbean region | ITS2 | All sequences are identical | |||
| Gorgonia ventalina (Linnaeus 1758) | Caribbean region | msh1 | 25 | 0.00538 | -1.92207 | 2.449 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





