Submitted:
22 November 2023
Posted:
01 December 2023
You are already at the latest version
Abstract
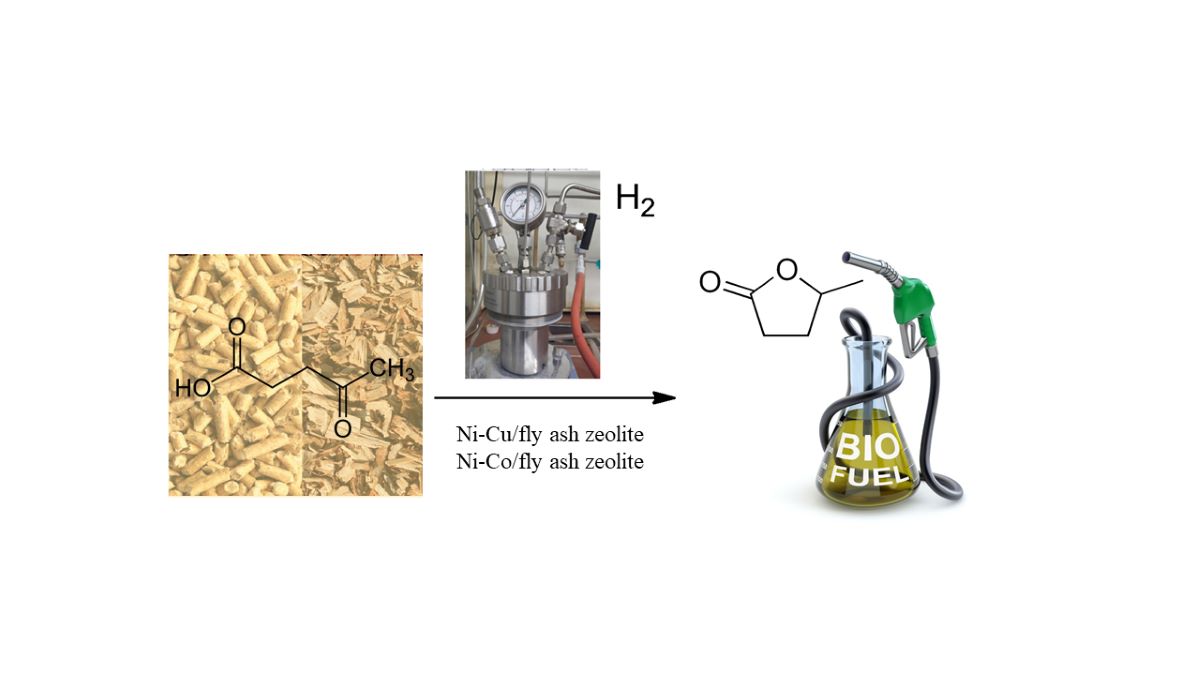
Keywords:
1. Introduction
2. Experimental Part
2.1. Materials
2.2. Synthesis of Coal Fly Ash Zeolite
2.3. Impregnation of Coal Fly Ash Zeolite with Ni, Co and Cu
2.3.1. Preparation of Monometallic Fly Ash Zeolite Catalysts
2.3.2. Preparation of Bimetallic Fly Ash Zeolite Catalysts
2.4. Characterization
2.5. Catalytic Experiments
3. Results and Discussion
4. Conclusions
Funding
References
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, Á. Catalytic conversion of carbohydrates to initial platform chemicals: chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Li, H.; Fang, Z.; Smith, R.L., Jr.; Yang, S. Efficient valorization of biomass to biofuels with bifunctional solid catalytic materials. Prog. Energy Combust. Sci. 2016, 55, 98–194. [Google Scholar] [CrossRef]
- Catalán-Martínez, D.; Domine, M.E.; Serra, J.M. Liquid fuels from biomass: An energy self-sustained process integrating H2 recovery and liquid refining. Fuel 2018, 212, 353–363. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; and Ragauskas, A.J. Sustainable energy and fuels from biomass: a review focusing on hydrothermal biomass processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Xuan, J.; Leung, M.K.H.; Leung, D.Y.C.; Ni, M. A review of biomass-derived fuel processors for fuel cell systems. Renew. Sustain. Energy Rev. 2009, 13, 1301–1313. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass-derived platform molecules: from high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic biomass valorization for bioethanol production: a circular bioeconomy approach. BioEnergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone–a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 14, 688–694. [Google Scholar] [CrossRef]
- Long, X.; Sun, P.; Li, Z.; Lang, R.; Xia, C.; Li, F. Magnetic Co/Al2O3 catalyst derived from hydrotalcite for hydrogenation of levulinic acid to γ-valerolactone. Chin. J. Catal. 2015, 36, 1512–1518. [Google Scholar] [CrossRef]
- Adeleye, A.T.; Louis, H.; Akakuru, O.U.; Joseph, I.; Enudi, O.C.; Michae, D.P. A review on the conversion of levulinic acid and its esters to various useful chemicals. AIMS Energy 2019, 7, 165–185. [Google Scholar] [CrossRef]
- Sosa, L.F.; da Silva, V.T.; de Souza, P.M. Hydrogenation of levulinic acid to γ-valerolactone using carbon nanotubes supported nickel catalysts. Cat. Tod. 2021, 381, 86–95. [Google Scholar] [CrossRef]
- Yanase, D.; Hara, T.; Sato, F.; Yamada, Y.; Sato, S. Vapor-phase hydrogenation of levulinic acid to γ-valerolactone over Cu-Ni alloy catalysts. Appl. Cat. A 2021, 616, 118093. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Xiong, J.; Li, X.; Bai, H.; Ji, N. Heterogeneous catalytic hydrogenation of levulinic acid to γ-valerolactone with formic acid as internal hydrogen source. Chem. Sus. Chem. 2020, 13, 2916–2930. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, H.; Jia, Z. Hydrogen production by ethanol reforming on Supported Ni–Cu catalysts. ACS Omega 2022, 7, 4577–4584. [Google Scholar] [CrossRef]
- Li, T.; Su, H.; Zhu, L.; Xu, D.; Ji, N.; Wang, S. Hydrogen production from steam reforming of biomass-derived levulinic acid over highly stable spinel-supported Ni catalysts. Waste Dispos. Sustain. Energy 2023. [Google Scholar] [CrossRef]
- Robertson, S.D.; McNicol, B.D.; De Baas, J.H.; Kloet, S.C.; Jenkins, J.W. Determination of reducibility and identification of alloying in copper-nickel-on-silica catalysts by temperature-programmed reduction. J. Cat. 1975, 37, 424–431. [Google Scholar] [CrossRef]
- López-Fonseca, R.; Jiménez-González, C.; de Rivas, B.; Gutiérrez-Ortiz, J.I. Partial oxidation of methane to syngas on bulk NiAl2O4 catalyst. Comparison with alumina supported nickel, platinum, and rhodium catalysts. Appl. Catal. A 2012, 437, 53–62. [Google Scholar] [CrossRef]
- Popova, M.; Djinović, P.; Ristić, A.; Lazarova, H.; Dražić, G.; Pintar, A.; Balu, A.M.; Tušar, N.N. Vapor-phase hydrogenation of levulinic acid to γ-valerolactone over bi-functional Ni/HZSM-5 catalyst. Front. Chem. 2018, 6, 285. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.-M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.-S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Indust. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Xue, Z.; Liu, Q.; Wang, J.; Mu, T. Valorization of levulinic acid over non-noble metal catalysts: challenges and opportunities. Green Chem. 2018, 20, 4391–4408. [Google Scholar] [CrossRef]
- Hengne, A.M.; Kadu, B.S.; Biradar, N.S.; Chikate, R.C.; Rode, C.V. Transfer hydrogenation of biomass-derived levulinic acid to γ-valerolactone over supported Ni catalysts. RSC Adv. 2016, 6, 59753–59761. [Google Scholar] [CrossRef]
- Song, S.; Yao, S.; Cao, J.; Di, L.; Wu, G.; Guan, N.; Li, L. Heterostructured Ni/NiO composite as a robust catalyst for the hydrogenation of levulinic acid to –valerolactone, 2017, 217, 115–124. [CrossRef]
- Huang, X.; Liu, K.; Vrijburg, W.L.; Ouyang, X.; Dugulan, A.I.; Liu, Y.; Verhoeven, M.W.G.M.Т.; Kosinov, N.A.; Pidko, E.A.; Hensen, E.J.M. Hydrogenation of levulinic acid to γ-valerolactone over Fe-Re/TiO2 catalysts. Appl. Cat. B: Environmental 2020, 278, 119314. [Google Scholar] [CrossRef]
- Gebresillase, M.N.; Raguindin, R.Q.; Kim, H.; Seo, J.G. Supported bimetallic catalysts for the solvent-free hydrogenation of levulinic acid to γ-valerolactone: effect of metal combination (Ni-Cu, Ni-Co, Cu-Co). Catalysts 2020, 10, 1354. [Google Scholar] [CrossRef]
- Yanase, D.; Yoshida, R.; Kanazawa, S.; Yamada, Y.; Sato, S. Efficient formation of γ-valerolactone in the vapor-phase hydrogenation of levulinic acid over Cu-Co/alumina catalyst. Cat. Commun. 2020, 139, 105967. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Yu, X.; Zhang, W.; Zhang, G.; Liu, M.; Shen, J.; Yang, C.; Jin, X. Non-noble metal catalysts for transfer hydrogenation of levulinic acid: The role of surface morphology and acid-base pairs. Mat. Tod. Energy 2020, 18, 100501. [Google Scholar] [CrossRef]
- Derle, S.N.; Parikh, P.A. Hydrogenation of levulinic acid and γ-valerolactone: steps towards biofuels. Biomass Conv. Bioref. 2014, 4, 293–299. [Google Scholar] [CrossRef]
- Hattori, H.; Ono, Y. Catalysts and catalysis for acid-base reactions. In Metal Oxides in Heterogeneous Catalysts; Vedrine, J.C., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 133–209. [Google Scholar] [CrossRef]
- Boycheva, S.; Szegedi, Á.; Lázár, K.; Popov, C.; Popova, M. Advanced high-iron coal fly ash zeolites for low-carbon emission catalytic combustion of VOCs. Cat. Today 2023, 418, 114109. [Google Scholar] [CrossRef]
- Michalev, T.; Petrov, I. The removal of heavy metal ions by synthetic zeolites: A review, Proceedings of the University of Ruse 2012, 51, 79–84. ISSN 2012, 2603–4123. [Google Scholar]
- Feng, W.; Lu, X.; Xiong, J.; Yu, Z.; Wang, Y.; Cui, J.; Zhang, R.; Weng, R. Solid–waste–derived geopolymer–type zeolite–like high functional catalytic materials catalyze efficient hydrogenation of levulinic acid. Catalysts 2022, 12, 1361. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, X.; Zhou, S.; Zhao, W.; Xu, Q.; Liu, X. Efficient synthesis of alkyl levulinates fuel additives using sulfonic acid functionalized polystyrene coated coal fly ash catalyst. J. Biores. Bioprod. 2023, 8, 198–213. [Google Scholar] [CrossRef]
- Gong, L.; Xu, Z.-Y.; Dong, J.-J.; Li, H.; Han, R.-Z.; Xu, G.-C.; Ni, Y. Composite coal fly ash solid acid catalyst in synergy with chloride for biphasic preparation of furfural from corn stover hydrolysate. Biores. Technol. 2019, 293, 122065. [Google Scholar] [CrossRef]
- Alterary, S.S.; Marei, N.H. Fly ash properties, characterization, and applications: A review. J. King Saud Uni. – Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Popova, M.; Boycheva, S.; Lazarova, H.; Zgureva, D.; Lázár, K.; Szegedi, Á. VOC oxidation and CO2 adsorption on dual adsorption/catalytic system based on fly ash zeolites. Cat. Today 2020, 357, 518–525. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Václavíková, M.; Kalvachev, Y.; Lazarova, H.; Popova, M. Studies on non-modified and copper-modified coal ash zeolites as heterogeneous catalysts for VOCs oxidation. J. Haz. Mat. 2019, 361, 374–382. [Google Scholar] [CrossRef]
- Boycheva, S.; Marinov, I.; Miteva, S.; Zgureva, D. Conversion of coal fly ash into nanozeolite Na-X by applying ultrasound assisted hydrothermal and fusion-hydrothermal alkaline activation. Sustain. Chem. Pharm. 2020, 15, 100217. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Lazarova, K.; Babeva, T.; Popov, C.; Lazarova, H.; Popova, M. Progress in the utilization of coal fly ash by conversion to zeolites with green energy applications. Materials 2020, 13, 2014. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Di Gennaro, S.; Palma, A.; Ragone, P.; Fiore, S. Mobility of trace elements in fly ash and in zeolitised coal fly ash. Fuel 2015, 144, 369–379. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva-Filipova, D.; Popov, C.; Lazarova, H.; Popova, M. Plasma-modified coal fly ash zeolites with enhanced catalytic efficiency toward the total oxidation of volatile organic compounds as low-cost substitutes for platinum group metals catalysts. Phys. Stat. Sol. 2022, 219, 2100632. [Google Scholar] [CrossRef]
- Boycheva, S.; Zgureva, D.; Lazarova, H.; Popova, M. Comparative studies of carbon capture onto coal fly ash zeolites Na-X and Na–Ca-X. Chemosphere 2021, 271, 129505. [Google Scholar] [CrossRef]
- Boycheva, S.; Miteva, S.; Zgureva, D.; Marinov, I. Characterization of fly ashes from thermal power plants in Bulgaria supplied by lignite coal, In proceedings of XXVIII Scientific symposium with international participation “Situation in Ecologically loaded regions of Slovakia and Central Europe, 2019, 24–25 October 2019, Slovakia, Hrádok, pp. 97–104. ISBN 978-80-89883-10-3.
- Tangcharoen, T.; Klysubun, W. Kongmark, Composition Dependence of Structural, Optical, Magnetic and Photodegradation Properties of Nanocrystalline NiO/CuO Heterostructured Powders. Ch. Phys. Status Solidi A 2022, 219, 2200072. [Google Scholar] [CrossRef]
- Bularzik, J.; Davies, P.K.; Navrotsky, A. Thermodynamics of Solid-Solution Formation in NiO-CuO. J. Am. Ceram. Soc. 1986, 69, 453–457. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Kukushki, R.G.; Yeletsky, P.M.; Bulavchenko, O.A.; Chesаlov, Y.A.; Yakovlev, V.A. Temperature-programmed reduction of model CuO, NiO and mixed CuO–NiO catalysts with hydrogen\. J. Alloys Compd. 2020, 844, 156135. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R. St. C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni, Applied Surface Science 257 (2011) 2717–2730. [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Khromova, S.A.; Bulavchenko, O.A.; Kaichev, V.V.; Saraev, A.A. Reshetnikov, S.I. Bykova, M.V. Trusov, L.I. and Yakovlev, V.A. Effect of the Ni/Cu ratio on the composition and catalytic properties of nickel-copper alloy in anisole hydrodeoxygenation. Kinet. Catal. 2014, 55, 69–78. [Google Scholar] [CrossRef]
- Smirnov, A.A.; Khromova, S.A.; Bulavchenko, O.A.; Kaichev, V.V.; Saraev, A.A.; Reshetnikov, S.I.; Bykova, M.V.; Trusov, L.I.; Yakovlev, V.A. Effect of the Ni/Cu Ratio on the Composition and Catalytic Properties of Nickel–Copper Alloy in Anisole Hydrodeoxygenation. Kinetika i Kataliz 2014, 55, 72–81. [Google Scholar] [CrossRef]
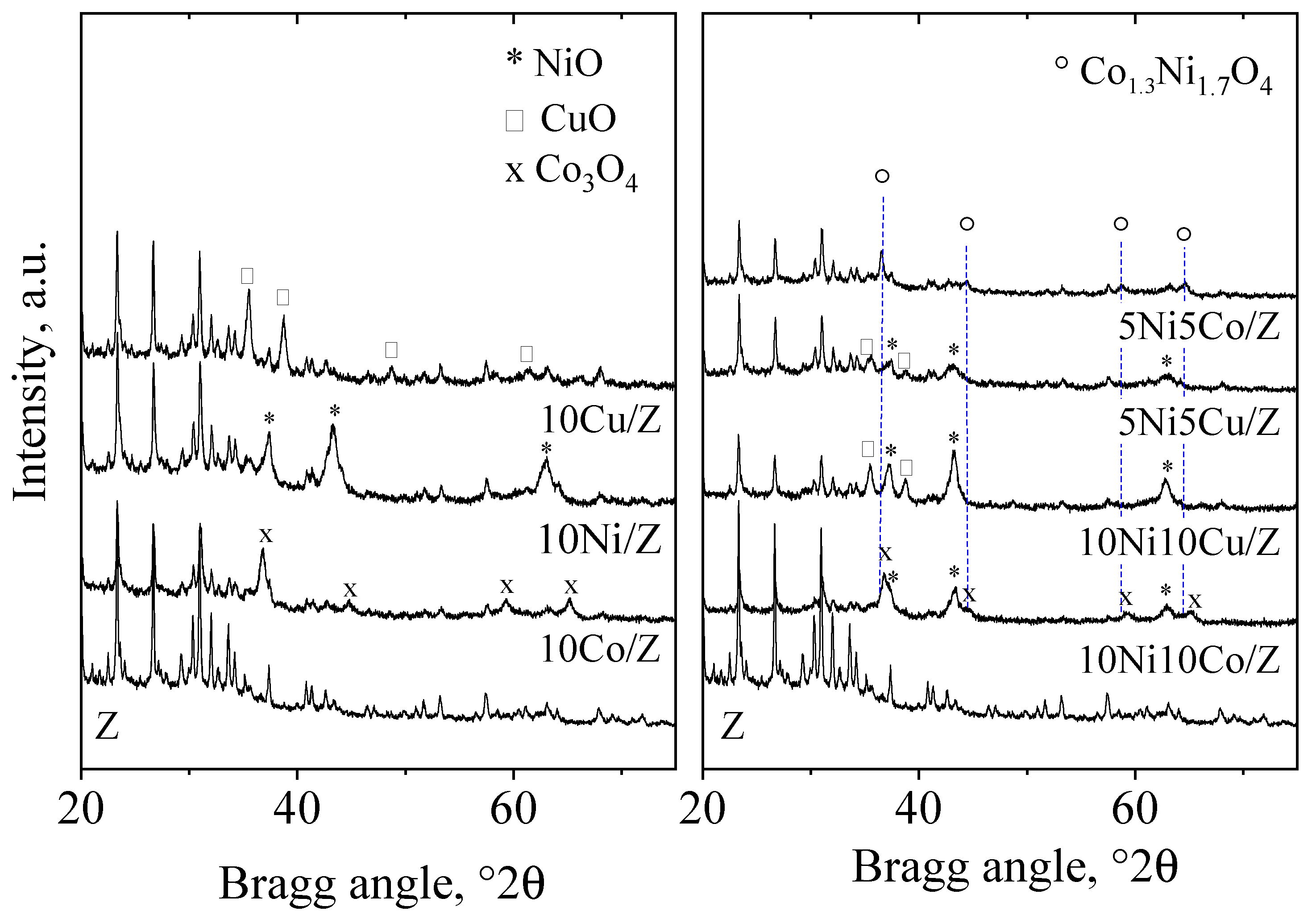
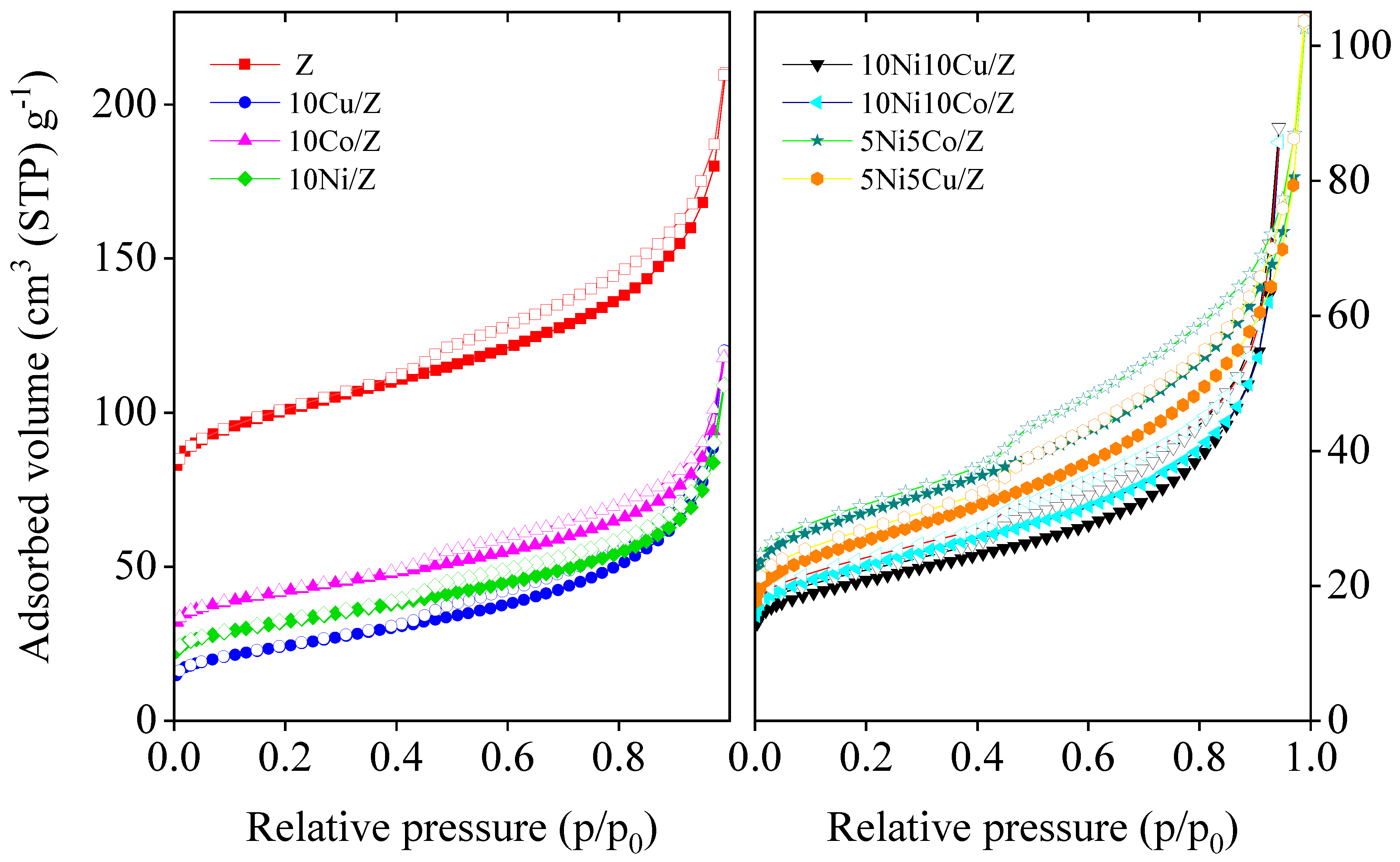
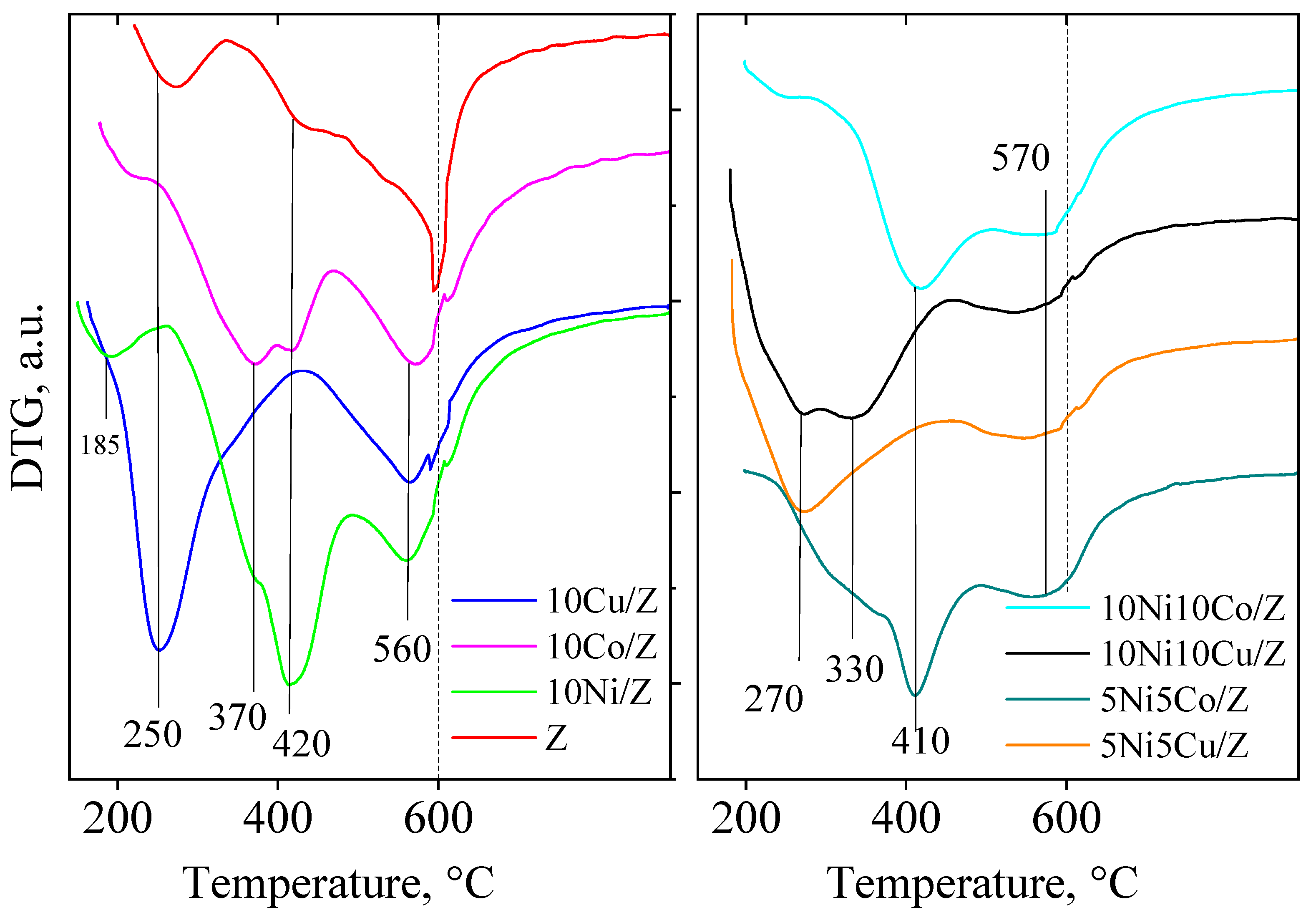
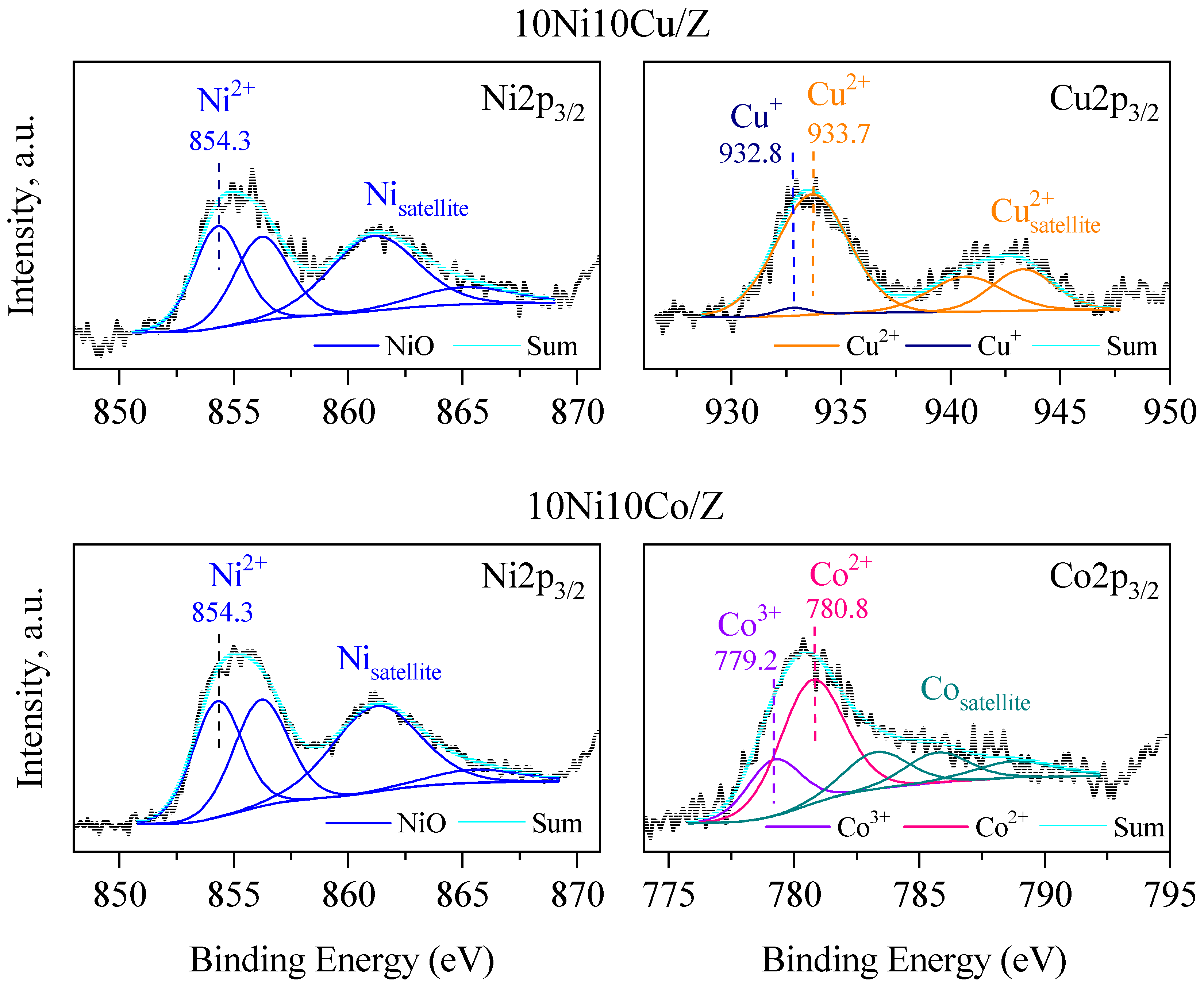
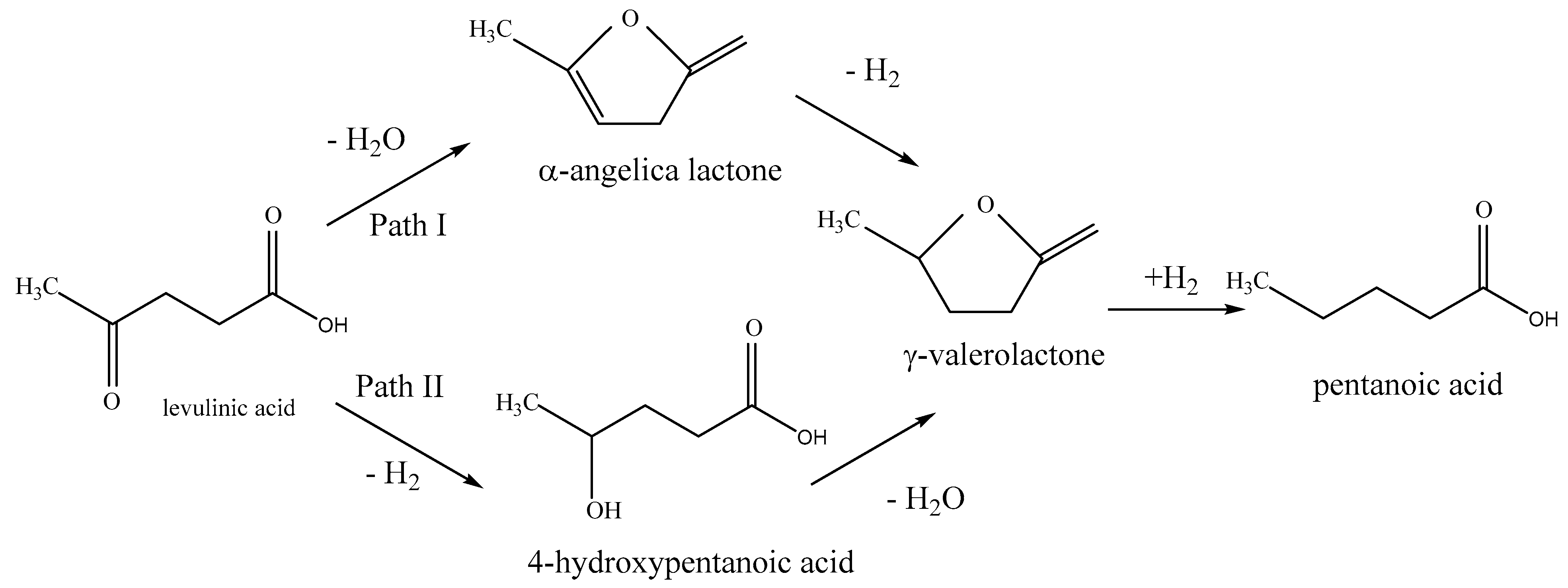
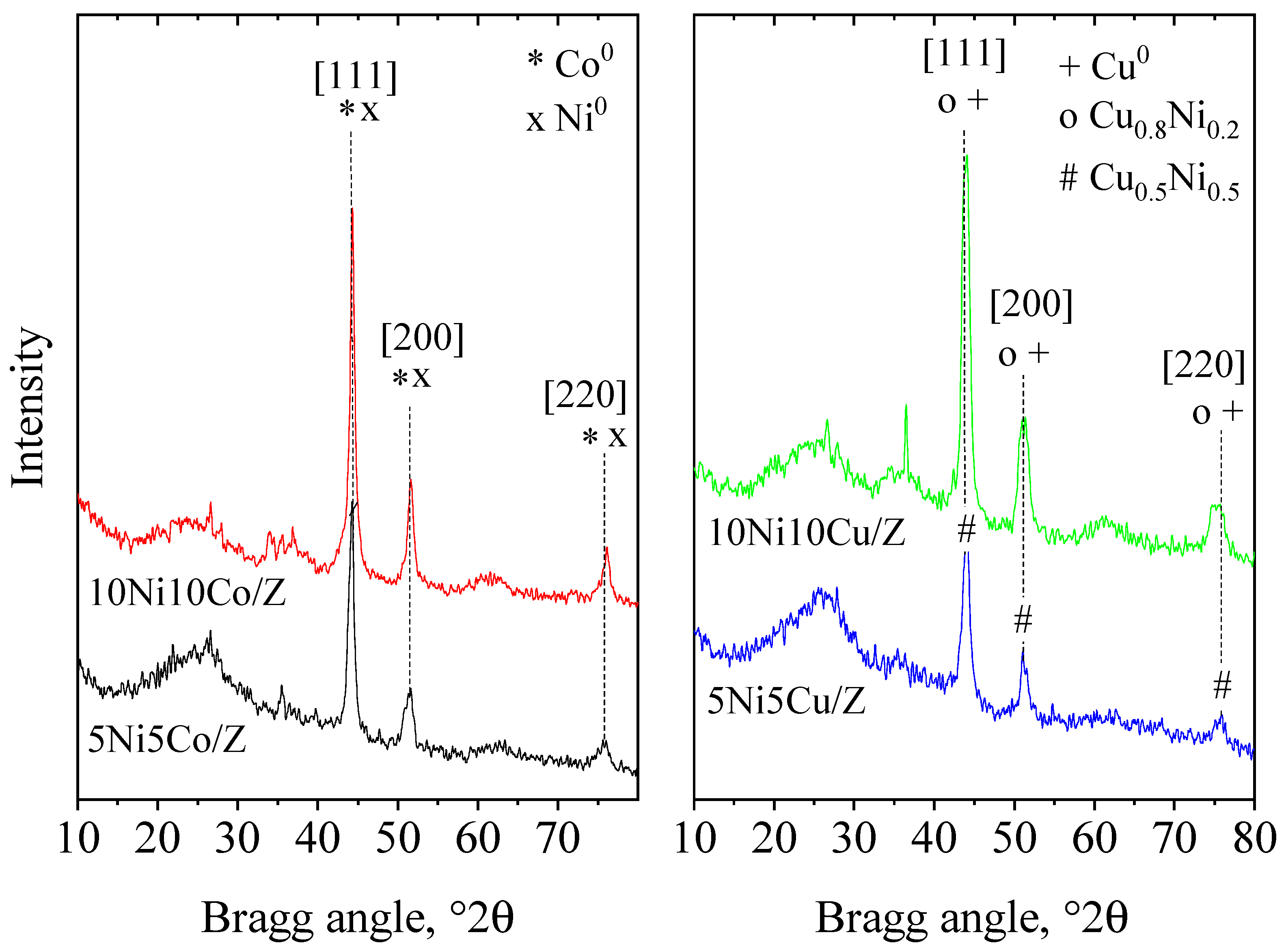
| Sample Name | Crystalline Phase | Crystallite Size, (nm) | Reducibility, % |
|---|---|---|---|
| 10 Ni/Z | NiO | 17 | 100 |
| 10 Co/Z | Co3O4 | 26 | 79 |
| 10Cu/Z | CuO | 35 | 100 |
| 10Ni10Co/Z | NiO*/-Co1.3Ni1.7O4*/-Co3O4 | 17*/-26*/-26 | 82 |
| 10Ni10Cu/Z | NiO/ Ni0.8Cu0.2O*/-CuO | 17*/-35 | 100 |
| 5Ni5Co/Z | Co1.3Ni1.7O4 | 35 | 100 |
| 5Ni5Cu/Z | NiO/Ni0.8Cu0.2O*/-CuO | 13*/-21 | 100 |
| Samples | SBET (m2/g) | Micropore Volume (cm3/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| 10Ni/Z | 115 | 0.019 | 0.169 |
| 10 Co/Z | 152 | 0.034 | 0.183 |
| 10Cu/Z | 86 | 0.006 | 0.186 |
| 10Ni10Co/Z | 82 | 0.015 | 0.133 |
| 10Ni10Cu/Z | 75 | 0.013 | 0.128 |
| 5Ni5Co/Z | 111 | 0.020 | 0.159 |
| 5Ni5Cu/Z | 96 | 0.015 | 0.160 |
| Z | 380 | 0.107 | 0.326 |
| Samples reduced at 600 °C | |||
| 10Ni10Co/Z | 181 | 0.047 | 0.130 |
| 5Ni5Co/Z | 228 | 0.061 | 0.152 |
| 10Ni/Z | 240 | 0.066 | 0.154 |
| Composition, at. % | Cu | Ni | Co | Si | Al | O | Fe | Na | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|---|
| 10Ni10Cu/Z | 3.2 | 7.3 | - | 10.4 | 4.5 | 61.4 | 3.9 | 6.5 | 1.4 | 1.4 |
| 10Ni10Co/Z | - | 11.4 | 3.9 | 9.1 | 3.5 | 60.3 | 5.0 | 4.0 | 1.3 | 1.5 |
| Catalysts | Conversion, % | HPA yield, % | GVL yield, % |
|---|---|---|---|
| 10Ni/Z | 35.0 | 8.0 | 27.0 |
| 10Co/Z | 2.7 | 0.8 | 1.9 |
| 10Cu/Z | 5.9 | 1.1 | 4.8 |
| 5Ni5Co/Z | 51.0 | 7.0 | 44.0 |
| 5Ni5Cu/Z | 63.0 | 10.0 | 53.0 |
| 10Ni10Co/Z | 99.5 | 14.8 | 84.7 |
| 10Ni10Cu/Z | 100.0 | 15.0 | 85.0 |
| Samples | SBET (m2/g) | Total Pore Volume (cm3/g) | Crystalline Phases | Crystallite Size, nm |
|---|---|---|---|---|
| 10Ni10Co/Z spent | 275 | 0.462 | Ni0/Co0 | 21 |
| 10Ni10Cu/Z spent | 271 | 0.422 | Cu0, Cu0.81Ni0.19 | 13/13 |
| 5Ni5Co/Z spent | 256 | 0.379 | Ni0/Co0 | 19 |
| 5Ni5Cu/Z spent | 279 | 0.400 | NixCu1-x (x~0.5) | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





