Submitted:
27 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
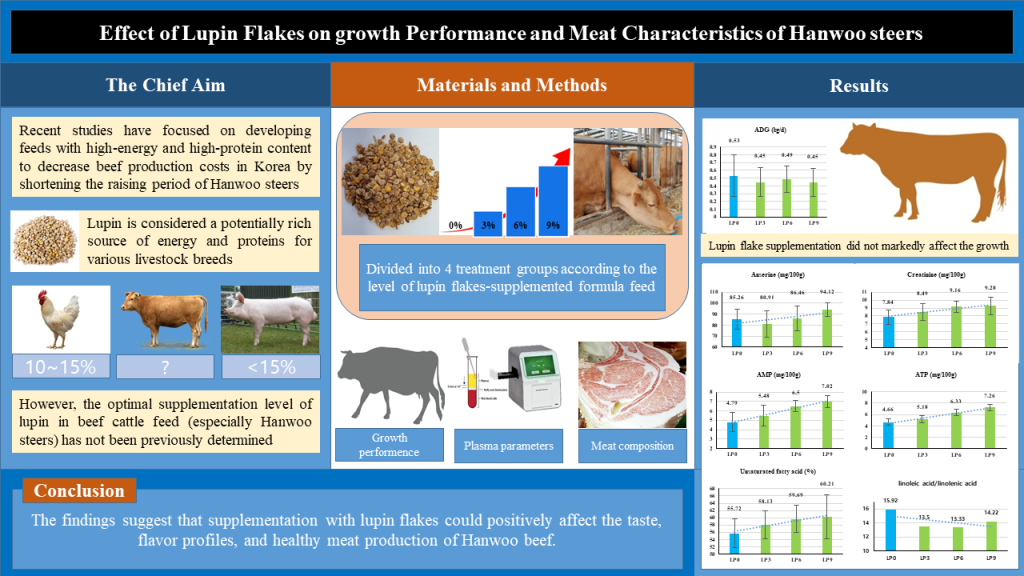
2. Materials and Methods
2.1. Animals, treatment, and management
2.2. Growth Performance
2.3. Plasma Parameters
2.4. Carcass Characteristics
2.5. Meat Composition
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Plasma Parameters
3.3. Carcass characteristics
3.4. Meat Composition
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chung, K.Y.; Lee, S.H.; Cho, S.H.; Kwon, E.G.; Lee, J.H. Current situation and future prospects for beef production in South Korea-A review. Asian-Australas J. Anim. Sci. 2018, 31, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jung, J.S.; Choi, K.C. A preliminary study on effects of fermented feed supplementation on growth performance, carcass characteristics, and meat quality of Hanwoo steers during the early and late fattening period. Applied Sciences 2021, 11, 5202–5215. [Google Scholar] [CrossRef]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and nutritional evaluation of several lupin seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Chiofalo, B.; Presti, V.L.; Chiofalo, V.; Gresta, F. The productive traits, fatty acid profile and nutritional indices of three lupin (Lupinus spp.) species cultivated in a Mediterranean environment for the livestock. Anim. Feed Sci. Technol. 2012, 171, 230–239. [Google Scholar] [CrossRef]
- Calabrò, S.; Cutrignelli, M.I.; Lo Presti, V.; Tudisco, R.; Chiofalo, V.; Grossi, M.; Infascelli, F.; Chiofalo, B. Characterization and effect of year of harvest on the nutritional properties of three varieties of white lupine (Lupinus albus L.). J. Sci. Food Agric. 2015, 95, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Bähr, M.; Fechner, A.; Hasenkopf, K.; Mittermaier, S.; Jahreis, G. Chemical composition of dehulled seeds of selected lupin cultivars in comparison to pea and soya bean. LWT - Food Sci. Technol. 2014, 59, 587–590. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Korczak, J.; Nogala-Kalucka, M.; Zawirska-Wojtasiak, R. Antioxidant properties of lupin seed products. Food Chem. 2003, 83, 279–285. [Google Scholar] [CrossRef]
- Bertoglio, J.C.; Calvo, M.A.; Hancke, J.L.; Burgos, R.A.; Riva, A.; Morazzoni, P.; Ponzone, C.; Magni, C.; Duranti, M. Hypoglycemic effect of lupin seed γ-conglutin in experimental animals and healthy human subjects. Fitoterapia 2011, 82, 933–938. [Google Scholar] [CrossRef]
- Wang, S.; Errington, S.; Yap, H.H. Studies on carotenoids from lupin seeds. In Lupins for Health and Wealth’ Proceedings of the 12th International Lupin Conference. Canterbury, New Zealand, September 2008; pp. 14–18.
- Boschin, G.; Arnoldi, A. Legumes are valuable sources of tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef]
- Elbandy, M.; Rho, J.R. New flavone-di-C-glycosides from the seeds of Egyptian lupin (Lupinus termis). Phytochem. Lett. 2014, 9, 127–131. [Google Scholar] [CrossRef]
- Nalle, C.L.; Ravindran, V.; Ravindran, G. Nutritional value of narrow-leafed lupin (Lupinus angustifolius) for broilers. Br. Poult. Sci. 2011, 52, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Kocher, A.; Choct, M.; Hughes, R.J.; Broz, J. Effect of food enzymes on utilisation of lupin carbohydrates by broilers. Br. Poult. Sci. 2000, 41, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Marquardt, R.R.; Guenter, W.; Viveros, A. Effect of enzyme addition on the performance and gastrointestinal tract size of chicks fed lupin seed and their fractions. Poult. Sci. 2002, 81, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Steenfeldt, S.; González, E.; Knudsen, K.B. Effects of inclusion with blue lupins (Lupinus angustifolius) in broiler diets and enzyme supplementation on production performance, digestibility and dietary AME content. Anim. Feed Sci. Technol. 2003, 110, 185–200. [Google Scholar] [CrossRef]
- Kim, J.C.; Pluske, J.R.; Mullan, B.P. Nutritive value of yellow lupins (Lupinus luteus L.) for weaner pigs. Aust. J. Exp. Agric. 2008, 48, 1225–1231. [Google Scholar] [CrossRef]
- van Barneveld, R.J. Understanding the nutritional chemistry of lupin (Lupinus spp.) seed to improve livestock production efficiency. Nutr. Res. Rev. 1999, 12, 203–230. [Google Scholar] [CrossRef] [PubMed]
- White, C.L.; Staines, V.E. A review of the nutritional value of lupins for dairy cows. Aust. J. Agric. Res. 2007, 58, 185–202. [Google Scholar] [CrossRef]
- Petterson, D.S. The use of lupins in feeding systems-Review. Asian-Aust. J. Anim. Sci. 2000, 13, 861–882. [Google Scholar] [CrossRef]
- Nowak, W.; Wylegala, S. The effect of rapeseed oil on the ruminal degradability and intestinal protein digestibility of rapeseed meal, soyabean and lupin seed. J. Anim. Feed Sci. 2005, 14, 295–298. [Google Scholar] [CrossRef]
- Jeon, S.; Lee, M.; Seo, J.; Kim, J.H.; Kam, D.K.; Seo, S. High-level dietary crude protein decreased back fat thickness and increased carcass yield score in finishing Hanwoo beef cattle (Bos taurus coreanae). J. Anim. Sci. Technol. 2021, 63, 1064–1075. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed. USA: Association of official analytical chemists. Washington, DC, 1995.
- Goering, H.K.; Van Soest, P.J. Forage fiber analysis: Apparatus, reagents, procedures, and some applications. Washington, DC: Agricultural Research Service, U.S. Department of Agriculture. Agriculture Handbook. No. 379. 1979.
- NRC. Nutrient Requirements of Beef Cattle: Seventh Revised Edition. National Research Council. National Academy Press: Washington, DC, USA, 2001.
- Trout, G.R. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. J. Food Sci. 1989, 54, 536–540. [Google Scholar] [CrossRef]
- MAFRA (Ministry of Agriculture, Food and Rural Affairs). Degree of Hanwoo self-support 2018. Accessed in http://kass.mafra.go.kr/kass/phone/kass.htm on 1 December 2019.
- De Brito, G.F.; McGrath, S.R.; Holman, B.W.; Friend, M.A.; Fowler, S.M.; Van De Ven, R.J.; Hopkins, D.L. The effect of forage type on lamb carcass traits, meat quality and sensory traits. Meat Sci. 2016, 119, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.R.G.; de Moura, A.P.R.; Ramos, A.L.S.; Ramos, E.M. Comparison of Warner-Bratzler shear force values between round and square cross-section cores for assessment of beef Longissimus tenderness. Meat Sci. 2017, 125, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Utama, D.T.; Lee, C.W.; Park, Y.S.; Jang, A.; Lee, S.K. Comparison of meat quality, fatty acid composition and aroma volatiles of Chikso and Hanwoo beef. Asian-Aust. J. Anim. Sci. 2018, 31, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.; Purslow, P.P. The effect of ageing on the water-holding capacity of pork: Role of cytoskeletal proteins. Meat Sci. 2001, 58, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Mora, L.; Sentandreu, M.A.; Toldrá, F. Hydrophilic chromatographic determination of carnosine, anserine, balenine, creatine, and creatinine. J. Agric. Food Chem. 2007, 55, 4664–4669. [Google Scholar] [CrossRef]
- Mora, L.; Hernández-Cázares, A.S.; Aristoy, M.C.; Toldrá, F. Hydrophilic interaction chromatographic determination of adenosine triphosphate and its metabolites. Food Chem. 2010, 123, 1282–1288. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Vicenti, A.; Toteda, F.; Di Turi, L.; Cocca, C.; Perrucci, M.; Melodia, L.; Ragni, M. Use of sweet lupin (Lupinus albus L. var. Multitalia) in feeding for Podolian young bulls and influence on productive performances and meat quality traits. Meat Sci. 2009, 82, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.S.; Schuster, M.; Schwarz, F.J. Performance, carcass characteristics and chemical composition of beef affected by lupine seed, rapeseed meal and soybean meal. J. Anim. Physiol. Anim. Nutr. 2010, 94, 465–473. [Google Scholar] [CrossRef]
- Kwak, B.O.; Kim, C. The effect of different flaked lupin seed inclusion levels on the growth of growing Korean native bulls. Asian-Aust. J. Anim. Sci. 2001, 14, 1129–1132. [Google Scholar] [CrossRef]
- Robinson, P.H.; McNiven, M.A. Nutritive value of raw and roasted sweet white lupins (Lupinus albus) for lactating dairy cows. Anim. Feed Sci. Technol. 1993, 43, 275–290. [Google Scholar] [CrossRef]
- Prandini, A.; Morlacchini, M.; Moschini, M.; Fusconi, G.; Masoero, F.; Piva, G. Raw and extruded pea (Pisum sativum) and lupin (Lupinus albus var. Multitalia) seeds as protein sources in weaned piglets’ diets: Effect on growth rate and blood parameters. Ital. J. Anim. Sci. 2005, 4, 385–394. [Google Scholar] [CrossRef]
- Bayourthe, C.; Moncoulon, R.; Enjalbert, F. Effect of extruded lupin seeds as a protein source on lactational performance of dairy cows. Anim. Feed Sci. Technol. 1998, 72, 121–131. [Google Scholar] [CrossRef]
- Cutrignelli, M.I.; Piccolo, G.; Bovera, F.; Calabrò, S.; D’Urso, S.; Tudisco, R.; Infascelli, F. Effects of two protein sources and energy level of diet on the performance of young Marchigiana bulls. 1. Infra vitam performance and carcass quality. Ital. J. Anim. Sci. 2008, 7, 259–270. [Google Scholar] [CrossRef]
- Roth-Maier, D.A.; Böhmer, B.M.; Roth, F.X. Effects of feeding canola meal and sweet lupin (L. luteus, L. angustifolius) in amino acid balanced diets on growth performance and carcass characteristics of growing-finishing pigs. Anim. Res. 2004, 53, 21–34. [Google Scholar] [CrossRef]
- Laudadio, V.; Tufarelli, V. Dehulled-micronised lupin (Lupinus albus L. cv. Multitalia) as the main protein source for broilers: Influence on growth performance, carcass traits and meat fatty acid composition. J. Anim. Sci. Technol. 2011, 91, 2081–2087. [Google Scholar] [CrossRef]
- Bhardwaj, H.L.; Hamama, A.A.; van Santen, E. White lupin performance and nutritional value as affected by planting date and row spacing. Agron. J. 2004, 96, 580–583. [Google Scholar] [CrossRef]
- Kang, D.H.; Chung, K.Y.; Park, B.H.; Kim, U.H.; Jang, S.S.; Smith, Z.K.; Kim, J. Effects of feeding high-energy diet on growth performance, blood parameters, and carcass traits in Hanwoo steers. Anim. Biosci. 2022, 35, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.C.; Kwak, H.J.; Oh, Y.K.; Lee, Y.K.; Jang, S.S.; Lee, S.S.; Park, K.K. Characteristics of wet distillers grains on in vitro ruminal fermentation and its effects on performance and carcass characteristics of finishing Hanwoo steers. Asian-Aust. J. Anim. Sci. 2016, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.D. Carcass, sensory, and adipose tissue traits of Brangus steers fed casein-formaldehyde-protected starch and (or) lipid. Texas A&M University 2002.
- Mane, S.P.; Johnson, S.K.; Duranti, M.; Pareek, V.K.; Utikar, R.P. Lupin seed γ-conglutin: Extraction and purification methods-A review. Trends Food Sci. Technol. 2018, 73, 1–11. [Google Scholar] [CrossRef]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Kim, J.H.; Kim, K.H.; Choi, C.W.; Kang, S.W.; Nam, I.S.; Kim, D.H.; Song, M.K.; Kim, C.W.; Park, K.K. Effects of level and degradability of dietary protein on ruminal fermentation and concentrations of soluble non-ammonia nitrogen in ruminal and omasal digesta of Hanwoo steers. Asian-Aust. J. Anim. Sci. 2008, 21, 392–403. [Google Scholar] [CrossRef]
- Lestingi, A.; Toteda, F.; Vicenti, A.; De Marzo, D.; Facciolongo, A.M. The use of faba bean and sweet lupin seeds alone or in combination for growing lambs. 1. Effects on growth performance, carcass traits, and blood parameters. Pakistan J. Zool. 2015, 47, 989–996. [Google Scholar]
- Lestingi, A.; Facciolongo, A.M.; Jambrenghi, A.C.; Ragni, M.; Toteda, F. The use of peas and sweet lupin seeds alone or in association for fattening lambs: Effects on performance, blood parameters and meat quality. Small Rumin. Res. 2016, 143, 15–23. [Google Scholar] [CrossRef]
- Steiger, M.; Senn, M.; Altreuther, G.; Werling, D.; Sutter, F.; Kreuzer, M.; Langhans, W. Effect of a prolonged low-dose lipopolysaccharide infusion on feed intake and metabolism in heifers. J. Anim. Sci. 1999, 77, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, J.; Shukla, A.; Brandsch, C.; Hirche, F.; Stangl, G.I.; Eder, K. Dietary lupin protein lowers triglyceride concentrations in liver and plasma in rats by reducing hepatic gene expression of sterol regulatory element-binding protein-1c. Ann. Nutr. Metab. 2007, 51, 387–392. [Google Scholar] [CrossRef]
- Choi, C.W. Changes in in vivo ruminal fermentation patterns and blood metabolites by different protein fraction-enriched feeds in Holstein steers. Korean J. Agric. Sci. 2017, 44, 392–399. [Google Scholar]
- Oomah, B.D.; Bushuk, W. Characterization of lupine proteins. J. Food Sci. 1983, 48, 38–41. [Google Scholar] [CrossRef]
- González, F.D.; Muiño, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Vet. Sci. 2011, 12, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, W.; Erdene, K.; Du, H.; Ao, C. Effects of dietary supplementation with Allium mongolicum Regel extracts on growth performance, serum metabolites, immune responses, antioxidant status, and meat quality of lambs. Anim. Nutr. 2021, 7, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Ciurescu, G.; Vasilachi, A.; Ropota, M.; Palade, M.; Dragomir, C. Beneficial effects of increasing dietary levels of raw lentil seeds on meat fatty acid and plasma metabolic profile in broiler chickens. Indian J. Anim. Sci. 2017, 87, 1385–1390. [Google Scholar] [CrossRef]
- Pilkington, M. Characterisation of lupin-derived lupeol with a metabolomics study of the impact and potential neuroprotection of luepol. Ph.D. Thesis, Murdoch University, Perth, Western Australia, 2013.
- Lee, J.S. Effects of Stage of Lactation and Parity on Milk Component and Blood Metabolite Profile of Lactating Dairy Cows: A Case Study. Ph.D. Thesis, Chungnam National University. Daejeon, Korea, 2011.
- King, R.H. Lupin-seed meal (Lupinus albus cv. Hamburg) as a source of protein for growing pigs. Anim. Feed Sci. Technol. 1981, 6, 285–296. [Google Scholar] [CrossRef]
- Dawson, L.E.R. The effect of inclusion of lupins/triticale whole crop silage in the diet of winter finishing beef cattle on their performance and meat quality at two levels of concentrates. Anim. Feed Sci. Technol. 2012, 171, 75–84. [Google Scholar] [CrossRef]
- Muchenje, V.; Dzama, K.; Chimonyo, M.; Strydom, P.E.; Hugo, A.; Raats, J.G. Some biochemical aspects pertaining to beef eating quality and consumer health: A review. Food Chem. 2009, 112, 279–289. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Livingston, D.J.; La Mar, G.N.; Brown, W.D. Myoglobin diffusion in bovine heart muscle. Sci. 1983, 220, 71–73. [Google Scholar] [CrossRef]
- Wittenberg, J.B. Wittenberg, B.A. Myoglobin function reassessed. J. Exp. Biol. 2003, 206, 2011–2020. [Google Scholar] [CrossRef]
- Brewer, M.S.; Ikins, W.I.G.; Harbers, C.A.A. TBA values, sensory characteristics, and volatiles in ground pork during long-term frozen storage: Effects of packaging. J. Food Sci. 1992, 57, 558–563. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Dal Bello, F.; Meineri, G. Effect of cooking method on carnosine and its homologues, pentosidine and thiobarbituric acid-reactive substance contents in beef and turkey meat. Food Chem. 2012, 132, 80–85. [Google Scholar] [CrossRef]
- Aliani, M.; Ryland, D.; Williamson, J.; Rempel, N. The synergistic effect of ribose, carnosine, and ascorbic acid on the sensory and physico-chemical characteristics of minced bison meat. Food Sci. Nutr. 2013, 1, 172–183. [Google Scholar] [CrossRef]
- Decker, E.A.; Livisay, S.A.; Zhou, S. A re-evaluation of the antioxidant activity of purified carnosine. Biochem. 2000, 7, 901–906. [Google Scholar]
- Komatsu, T.; Komatsu, M.; Uemoto, Y. The NT5E gene variant strongly affects the degradation rate of inosine 5′-monophosphate under postmortem conditions in Japanese Black beef. Meat Sci. 2019, 158, 107893. [Google Scholar] [CrossRef] [PubMed]
- Tapadia, M.; Carlessi, R.; Johnson, S.; Utikar, R.; Newsholme, P. Lupin seed hydrolysate promotes G-protein-coupled receptor, intracellular Ca2+ and enhanced glycolytic metabolism-mediated insulin secretion from BRIN-BD11 pancreatic beta cells. Mol. Cell. Endocrinol. 2019, 480, 83–96. [Google Scholar] [CrossRef]
- Anderson, B.A.; Kisellan, J.A.; Watt, B.K. Comprehensive evaluation of fatty acids in foods. II. Beef products. J. Amer. Diet Assoc. 1975, 67, 35–41. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, C.N.; Ko, K.B.; Park, S.P.; Kim, H.K.; Kim, J.M.; Ryu, Y.C. Comparisons of beef fatty acid and amino acid characteristics between Jeju black cattle, Hanwoo, and Wagyu breeds. Food Sci. Anim. Resour. 2019, 39, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Dunshea, F.R.; Lisle, A.; Roura, E. Feeding a high oleic acid (C18: 1) diet improves pleasing flavor attributes in pork. Food Chem. 2021, 357, 129770. [Google Scholar] [CrossRef]
- Anderson, B.A. Comprehensive evaluation of fatty acids in foods, VII, Pork products. J. Amer. Diet Assoc. 1976, 69, 44–49. [Google Scholar] [CrossRef]
- Enser, M.; Wood, J.D. Effect of time of year on fatty acid composition and melting point of UK lamb. Proceedings of the 39th International Congress of Meat Science and Technology, Bristol, UK, 1993; pp. 74.
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effect of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef] [PubMed]
| Item | Treatments1 | Lupin flake | Rice straw | |||||||
| LP0 | LP3 | LP6 | LP9 | |||||||
| ---------------- Ingredient composition (% of as-fed basis) ---------------- | ||||||||||
| Concentrated feed2 | 30.0 | 22.0 | 19.0 | 17.0 | - | |||||
| Lupin flake3 | - | 3.0 | 6.0 | 9.0 | - | |||||
| Corn flake | 25.0 | 27.3 | 30.0 | 31.0 | - | |||||
| Corn gluten feed | 21.0 | 20.0 | 20.0 | 19.0 | - | |||||
| Corn starch pulp | 9.5 | 11.0 | 8.2 | 6.0 | - | |||||
| Ground almond hull | 8.0 | 10.0 | 10.0 | 11.0 | - | |||||
| Cane molasses | 5.0 | 5.0 | 5.0 | 5.0 | - | |||||
| Limestone | 0.8 | 1.0 | 1.1 | 1.3 | - | |||||
| Salt dehydrate | 0.3 | 0.3 | 0.3 | 0.3 | - | |||||
| Sodium bicarbonate | 0.3 | 0.3 | 0.3 | 0.3 | - | |||||
| Vitamin-mineral mix4 | 0.1 | 0.1 | 0.1 | 0.1 | - | |||||
| ---------------- Chemical composition (% of DM5 basis) ---------------- | ||||||||||
| Dry matter | 88.88 | 88.93 | 89.25 | 89.33 | 90.40 | 88.15 | ||||
| Crude protein | 15.58 | 15.57 | 15.59 | 15.55 | 35.84 | 3.44 | ||||
| Ether extract | 4.10 | 4.30 | 4.80 | 4.60 | 6.28 | 0.52 | ||||
| NDF6 | 29.97 | 33.73 | 37.39 | 38.88 | 26.32 | 70.41 | ||||
| ADF7 | 12.28 | 12.43 | 11.02 | 10.80 | 20.94 | 21.79 | ||||
| Ca | 0.70 | 0.70 | 0.70 | 0.80 | 0.23 | 0.20 | ||||
| P | 0.50 | 0.50 | 0.40 | 0.40 | 0.33 | 0.10 | ||||
| TDN8 | 83.2 | 84.0 | 84.9 | 85.3 | 94.6 | 38.3 | ||||
| Item | LP0 | LP3 | LP6 | LP9 | Contrast P-value | |
| Linear | Quadratic | |||||
| Initial BW1 (kg) | 756.7±118.1 | 755.0±49.1 | 755.6±67.3 | 758.3±38.8 | 0.957 | 0.994 |
| Final BW (kg) | 818.4±93.9 | 810.7±52.5 | 813.0±57.7 | 811.4±39.2 | 0.860 | 0.960 |
| ADG2 (kg/day) | 0.53±0.27 | 0.45±0.19 | 0.49±0.17 | 0.45±0.18 | 0.793 | 0.679 |
| Intake (DM3 kg/steer/day) | ||||||
| Formula feed | 8.00±0.02 | 8.01±0.01 | 8.02±0.01 | 8.02±0.02 | 0.127 | 0.174 |
| Rice straw | 2.63±0.01 | 2.65±0.01 | 2.63±0.02 | 2.64±0.02 | 0.694 | 0.865 |
| DMI4 | 10.63±0.01 | 10.65±0.01 | 10.65±0.03 | 10.64±0.02 | 0.134 | 0.179 |
| Crude protein | 1.34±0.01 | 1.34±0.01 | 1.34±0.01 | 1.34±0.01 | 0.154 | 0.371 |
| TDN5 | 7.66±0.01 | 7.74±0.01 | 7.81±0.02 | 7.85±0.01 | <0.001 | <0.001 |
| FCR6 | 28.79±23.76 | 28.67±13.78 | 24.25±9.00 | 28.53±14.88 | 0.816 | 0.883 |
| Item | Initial (0 d) | Final (85 d) | |||||||||||
| LP0 | LP3 | LP6 | LP9 | Contrast P-value | LP0 | LP3 | LP6 | LP9 | Contrast P-value | ||||
| Linear | Quadratic | Linear | Quadratic | ||||||||||
| GLU1 | 78.30 ±9.09 |
91.50 ±14.94 |
92.10 ±21.34 |
92.70 ±11.22 |
0.051 | 0.063 | 76.10 ±5.93 |
84.50 ±16.98 |
89.20 ±34.32 |
70.60 ±10.39 |
0.691 | 0.106 | |
| (mg/dL) | |||||||||||||
| NEFA2 (uEq/L) |
233.8 ±94.47 |
240.6 ±84.24 |
226.8 ±90.39 |
217.6 ±36.88 |
0.575 | 0.813 | 174.8 ±55.73 |
272.7 ±90.70 |
161.2 ±64.81 |
195.4 ±49.45 |
0.592 | 0.577 | |
| BUN3 (mg/L) |
15.41 ±4.21 |
14.87 ±2.15 |
14.67 ±1.69 |
13.65 ±1.08 |
0.130 | 0.309 | 18.41 ±2.20 |
18.64 ±3.50 |
18.90 ±2.81 |
16.81 ±1.92 |
0.240 | 0.334 | |
| ALB4 (g/dL) |
3.84 ±0.11 |
3.72 ±0.13 |
3.87 ±0.41 |
3.78 ±0.60 |
0.911 | 0.964 | 3.91 ±0.24 |
3.95 ±0.30 |
3.98 ±0.33 |
3.79 ±0.16 |
0.385 | 0.269 | |
| TP5 (g/dL) |
7.54 ±0.32 |
7.01 ±0.39 |
7.25 ±0.41 |
7.18 ±0.60 |
0.208 | 0.132 | 7.62 ±0.43 |
7.48 ±0.63 |
7.46 ±0.80 |
7.11 ±0.44 |
0.068 | 0.166 | |
| CHOL6 (mg/dL) |
152.70 ±46.08 |
170.90 ±45.85 |
187.0 ±29.12 |
173.40 ±27.15 |
0.155 | 0.155 | 97.6 ±22.92 |
118.50 ±30.69 |
118.60 ±16.37 |
110.30 ±16.95 |
0.251 | 0.068 | |
| TG7 (mg/dL) |
32.90 ±9.04 |
34.80 ±10.45 |
33.50 ±10.43 |
35.10 ±10.57 |
0.707 | 0.932 | 26.80 ±7.52 |
35.90 ±8.70 |
30.10 ±6.70 |
34.20 ±11.02 |
0.204 | 0.308 | |
| CREA8 (mg/dL) |
1.35 ±0.15 |
1.34 ±0.15 |
1.41 ±0.11 |
1.33 ±0.18 |
0.962 | 0.761 | 1.64 ±0.31 |
1.65 ±0.17 |
1.74 ±0.28 |
1.62 ±0.24 |
0.934 | 0.723 | |
| AST9 (U/L) |
76.00 ±17.31 |
71.00 ±8.10 |
73.30 ±11.20 |
79.60 ±20.73 |
0.542 | 0.414 | 70.90 ±18.00 |
69.60 ±8.81 |
69.30 ±15.85 |
79.10 ±12.75 |
0.234 | 0.234 | |
| ALT10 (U/L) |
21.20 ±2.57 |
21.00 ±2.54 |
22.70 ±3.74 |
22.20 ±3.97 |
0.309 | 0.593 | 9.30 ±4.64 |
16.50 ±5.97 |
21.40 ±12.52 |
19.60 ±7.95 |
0.005 | 0.005 | |
| GGT11 (U/L) |
34.40 ±14.90 |
30.50 ±4.62 |
33.20 ±11.56 |
42.80 ±25.75 |
0.227 | 0.202 | 22.80 ±4.83 |
24.90 ±4.31 |
24.30 ±5.23 |
25.50 ±9.81 |
0.404 | 0.692 | |
| IP12 (mg/dL) |
7.14 ±0.73 |
7.18 ±0.43 |
7.44 ±0.47 |
7.10 ±0.43 |
0.854 | 0.524 | 6.54 ±0.87 |
6.88 ±1.07 |
7.25 ±0.66 |
6.45 ±0.59 |
0.935 | 0.103 | |
| Ca13 (mg/dL) |
8.80 ±0.26 |
8.76 ±0.27 |
8.86 ±0.34 |
8.70 ±0.33 |
0.641 | 0.740 | 9.18 ±0.60 |
9.50 ±0.87 |
9.46 ±0.55 |
9.10 ±0.39 |
0.757 | 0.224 | |
| Mg14 (mg/dL) |
2.33 ±0.19 |
2.38 ±0.11 |
2.45 ±0.14 |
2.46 ±0.16 |
0.036 | 0.105 | 2.36 ±0.23 |
2.33 ±0.23 |
2.34 ±0.28 |
2.26 ±0.12 |
0.342 | 0.599 | |
| Item | LP0 | LP3 | LP6 | LP9 | Contrast P-value | |
| Linear | Quadratic | |||||
| Yield traits1 | ||||||
| CW2 (kg) | 448.5±67.0 | 460.4±36.9 | 460.1±41.7 | 453.6±29.3 | 0.819 | 0.802 |
| REA3 (cm2) | 95.00±12.92 | 96.10±6.95 | 97.20±5.41 | 94.50±4.91 | 0.972 | 0.760 |
| BFT4 (mm) | 12.50±5.78 | 16.90±3.63 | 13.80±3.12 | 16.40±4.38 | 0.186 | 0.347 |
| Yield index | 61.98±2.15 | 60.49±1.16 | 61.43±1.08 | 60.59±1.51 | 0.155 | 0.299 |
| Grade (A:B:C, %) | 40:10:50 | 10:30:60 | 10:70:20 | 10:40:50 | - | |
| Quality traits5 | ||||||
| Marbling score | 4.90±1.91 | 5.50±1.65 | 5.10±2.33 | 4.90±2.09 | 0.887 | 0.809 |
| Meat color | 4.80±0.42 | 4.80±0.40 | 4.80±0.42 | 4.80±0.42 | 1.000 | 1.000 |
| Fat color | 3.00±0.00 | 3.00±0.00 | 3.00±0.00 | 3.00±0.00 | NS | NS |
| texture | 2.60±1.07 | 2.40±0.97 | 2.50±1.08 | 2.60±1.17 | 0.947 | 0.903 |
| Maturity | 2.20±0.42 | 2.10±0.32 | 2.30±0.48 | 2.10±0.32 | 0.857 | 0.908 |
| Grade (1++:1+:1:2:3, %) | 10:30:40:10:10 | 20:30:40:10:0 | 20:30:30:20:0 | 30:0:50:20:0 | - | |
| Item | LP0 | LP3 | LP6 | LP9 | Contrast P-value | |
| Linear | Quadratic | |||||
| Chemical composition (%) | ||||||
| Moisture | 62.10±3.26 | 62.37±3.51 | 63.37±5.40 | 62.77±5.85 | 0.630 | 0.849 |
| Crude protein | 19.74±1.16 | 19.06±1.84 | 20.06±2.65 | 19.99±1.73 | 0.465 | 0.683 |
| Ether extract | 19.82±4.68 | 20.30±3.85 | 19.03±5.46 | 18.71±6.31 | 0.502 | 0.774 |
| Crude ash | 0.79±0.07 | 0.82±0.14 | 0.87±0.11 | 0.83±0.08 | 0.191 | 0.275 |
| Surface color | ||||||
| L* (lightness) | 35.77±0.63 | 37.24±2.80 | 36.20±1.61 | 36.22±1.75 | 0.928 | 0.599 |
| a* (redness) | 19.59±1.66 | 19.64±2.65 | 19.44±1.07 | 19.88±1.65 | 0.849 | 0.953 |
| b* (yellowness) | 9.87±0.66 | 10.57±1.60 | 10.37±0.71 | 10.15±0.78 | 0.740 | 0.535 |
| Myoglobin (mg/g) | 9.53±0.76 | 9.03±1.27 | 9.26±1.27 | 10.13±0.95 | 0.312 | 0.075 |
| Physicochemical properties | ||||||
| pH | 5.35±0.04 | 5.38±0.05 | 5.37±0.02 | 5.35±0.03 | 0.700 | 0.065 |
| Shear force (N) | 45.87±5.41 | 48.06±10.89 | 51.13±11.53 | 46.57±7.28 | 0.705 | 0.506 |
| Cooking loss (%) | 24.32±1.48 | 25.66±1.74 | 26.39±2.86 | 26.76±2.90 | 0.032 | 0.085 |
| WHC1 (%) | 35.83±6.14 | 30.02±7.42 | 36.15±5.60 | 33.91±3.59 | 0.968 | 0.669 |
| TBARS2 (mg MA3/kg) |
0.31±0.04 | 0.26±0.02 | 0.27±0.06 | 0.25±0.03 | 0.006 | 0.017 |
| Item | LP0 | LP3 | LP6 | LP9 | Contrast P-value | |
| Linear | Quadratic | |||||
| Dipeptide (mg/100g) | ||||||
| Carnosine | 338.64±37.88 | 345.51±12.19 | 358.14±47.66 | 367.35±27.41 | 0.032 | 0.103 |
| Anserine | 85.26±9.18 | 80.91±11.93 | 86.46±11.33 | 94.12±6.19 | 0.025 | 0.012 |
| Creatine | 464.51±21.49 | 473.37±12.69 | 488.15±29.77 | 476.63±41.09 | 0.188 | 0.210 |
| Creatinine | 7.84±0.92 | 8.49±1.06 | 9.16±0.74 | 9.28±1.10 | <0.001 | 0.001 |
| Nucleic acid (mg/100g) | ||||||
| Hx1 | 16.34±0.82 | 16.88±0.67 | 16.61±0.85 | 16.21±1.81 | 0.674 | 0.394 |
| Inosine | 22.58±1.80 | 22.74±1.92 | 22.85±2.27 | 21.77±2.59 | 0.449 | 0.499 |
| IMP2 | 151.73±17.34 | 144.29±15.59 | 156.07±18.91 | 152.69±15.67 | 0.545 | 0.779 |
| AMP3 | 4.79±1.01 | 5.48±1.10 | 6.50±0.61 | 7.02±0.65 | <0.001 | <0.001 |
| ATP4 | 4.66±0.83 | 5.18±0.84 | 6.33±0.74 | 7.26±0.38 | <0.001 | <0.001 |
| Item | LP0 | LP3 | LP6 | LP9 | Contrast P-value | |
| Linear | Quadratic | |||||
| Octanoic (%) | 0.77±0.21 | 0.32±0.15 | 0.30±0.15 | 0.28±0.18 | <0.001 | <0.001 |
| Decanoic (%) | 0.78±0.20 | 0.33±0.20 | 0.32±0.20 | 0.31±0.22 | <0.001 | <0.001 |
| Lauric (%) | 0.37±0.30 | 0.23±0.12 | 0.41±0.55 | 0.55±0.69 | 0.270 | 0.352 |
| Myristic (%) | 5.39±1.84 | 5.43±2.15 | 4.61±2.30 | 5.75±2.24 | 0.929 | 0.717 |
| Palmitic (%) | 24.58±5.56 | 25.12±2.98 | 23.74±2.61 | 22.77±4.67 | 0.243 | 0.431 |
| Palmitoleic (%) | 7.54±1.73 | 11.13±1.87 | 10.73±2.36 | 10.52±2.91 | 0.019 | 0.003 |
| Stearic (%) | 11.63±1.32 | 10.05±2.25 | 10.40±1.64 | 9.64±2.63 | 0.056 | 0.134 |
| Oleic (%) | 41.65±2.24 | 42.26±3.53 | 43.60±3.93 | 43.14±3.73 | 0.229 | 0.433 |
| Linoleic (%) | 5.63±3.05 | 4.18±1.20 | 4.68±1.18 | 5.61±3.68 | 0.905 | 0.332 |
| Linolenic (%) | 0.90±1.16 | 0.57±0.46 | 0.68±0.56 | 0.94±1.03 | 0.835 | 0.536 |
| Arachdic (%) | 0.76±0.72 | 0.40±0.35 | 0.53±0.52 | 0.49±0.53 | 0.375 | 0.440 |
| SFA1 | 44.28±4.03 | 41.87±3.84 | 40.31±3.68 | 39.79±6.05 | <0.001 | <0.001 |
| UFA2 | 55.72±4.03 | 58.13±3.84 | 59.69±3.68 | 60.21±6.05 | <0.001 | <0.001 |
| n-6/n-33 | 15.92±8.61 | 13.50±9.41 | 13.33±9.47 | 14.22±9.84 | <0.001 | <0.001 |
| UFA/SFA | 1.26±0.23 | 1.39±0.21 | 1.50±0.21 | 1.58±0.50 | 0.003 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





