Submitted:
23 October 2023
Posted:
24 October 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. TBNC Subtype of Breast Cancer
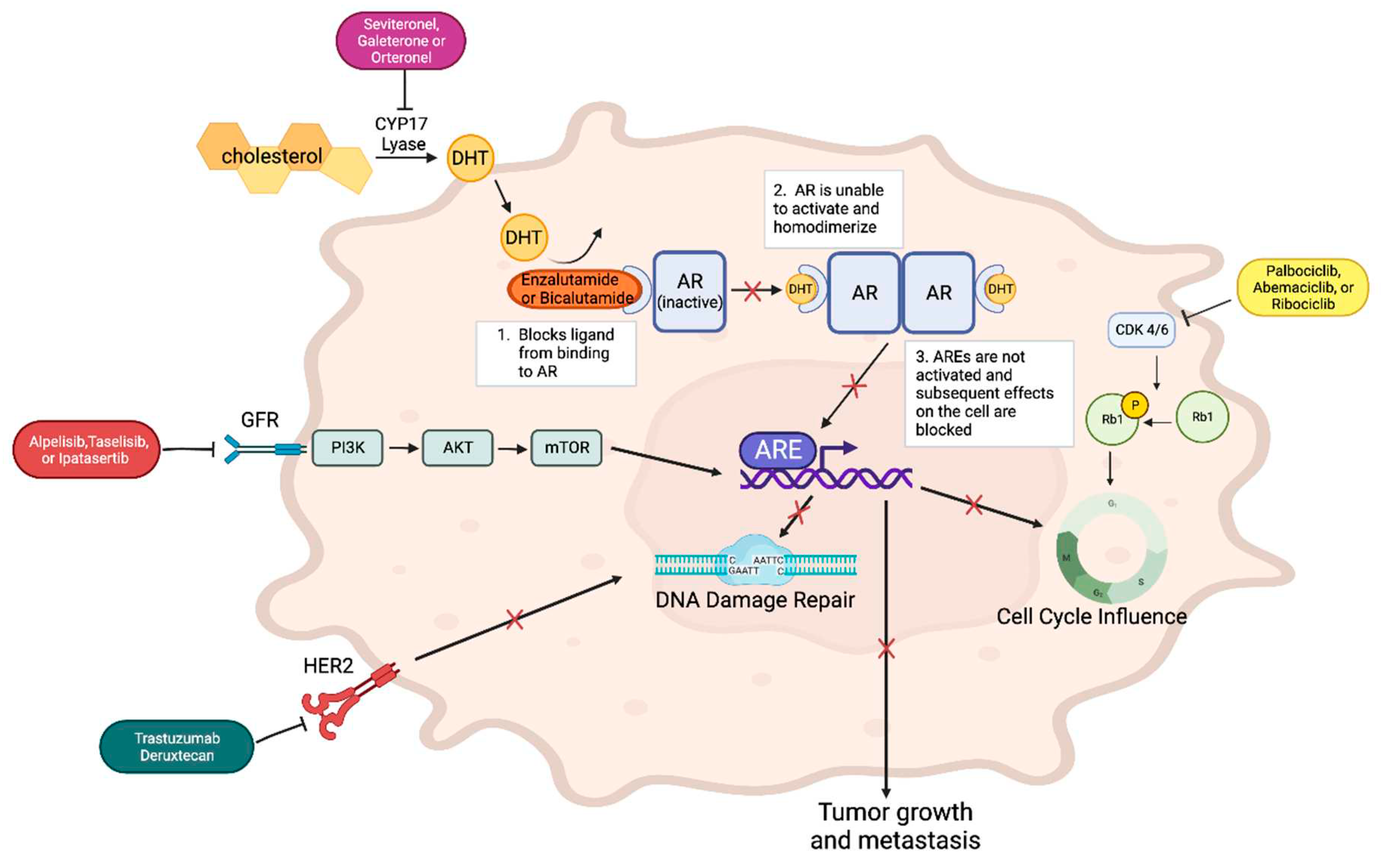
3. Why Target Androgen Receptor?
- AR in the Context of Hormone Dysregulation
- Why Co-target AR with Other Pathways?
- Co-targeting AR and CDK4/6
- Co-targeting AR and CYP17 Lyase
- Co-targeting AR and PI3K/AKT Pathway
- HER2 Low and Trastuzumab Deruxtecan
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: a view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus Chemotherapy in Advanced Triple-Negative Breast Cancer. New Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–33. [Google Scholar] [CrossRef] [PubMed]
- Traina, T.; Cadoo, K.; Gucalp, A. Palbociclib: an evidence-based review of its potential in the treatment of breast cancer. Breast Cancer: Targets Ther. 2014, 6, 123–133. [Google Scholar] [CrossRef]
- Lim, E.; Brufsky, A.; Rugo, H.S.; Vogel, C.L.; O'Shaughnessy, J.; Getzenberg, R.H.; Barnette, K.G.; Rodriguez, D.; Bird, G.; Steiner, M.S.; et al. Phase 3 ENABLAR-2 study to evaluate enobosarm and abemaciclib combination compared to estrogen-blocking agent for the second-line treatment of AR+, ER+, HER2- metastatic breast cancer in patients who previously received palbociclib and estrogen-blocking agent combination therapy. J. Clin. Oncol. 2022, 40, TPS1121. [Google Scholar] [CrossRef]
- Yin, L.; Hu, Q. CYP17 inhibitors—abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat. Rev. Urol. 2014, 11, 32–42. [Google Scholar] [CrossRef]
- Gomez, L.; Kovac, J.R.; Lamb, D.J. CYP17A1 inhibitors in castration-resistant prostate cancer. Steroids 2015, 95, 80–87. [Google Scholar] [CrossRef]
- Alex, A.B.; Pal, S.K.; Agarwal, N. CYP17 inhibitors in prostate cancer: latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2016, 8, 267–275. [Google Scholar] [CrossRef]
- Rampurwala, M.; Wisinski, K.B.; Burkard, M.E.; Ehsani, S.; O’regan, R.M.; Carmichael, L.; Kim, K.; Kolesar, J.; Tevaarwerk, A.J. Phase 1b study of orteronel in postmenopausal women with hormone-receptor positive (HR+) metastatic breast cancer. Investig. New Drugs 2017, 35, 87–94. [Google Scholar] [CrossRef]
- Peer, C.J.; Schmidt, K.T.; Kindrick, J.D.; Eisner, J.R.; Brown, V.V.; Baskin-Bey, E.; et al. A population pharmacokinetic analysis of the oral CYP17 lyase and androgen receptor inhibitor seviteronel in patients with advanced/metastatic castration-resistant prostate cancer or breast cancer. Cancer Chemother Pharmacol. 2019, 84, 759–70. [Google Scholar] [CrossRef]
- Kono, M.; Fujii, T.; Lim, B.; Karuturi, M.S.; Tripathy, D.; Ueno, N.T. Androgen Receptor Function and Androgen Receptor-Targeted Therapies in Breast Cancer: A Review. JAMA Oncol. 2017, 3, 1266–73. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Abramson, V.G.; Sanders, M.E.; Mayer, E.L.; Haddad, T.C.; Nanda, R.; et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR(+) Metastatic Triple-Negative Breast Cancer. Clin Cancer Res. 2020, 26, 2111–23. [Google Scholar] [CrossRef]
- Eroles, P.; Bosch, A.; Pérez-Fidalgo, J.A.; Lluch, A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat. Rev. 2012, 38, 698–707. [Google Scholar] [CrossRef]
- Bosch, A.; Eroles, P.; Zaragoza, R.; Viña, J.R.; Lluch, A. Triple-negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treat. Rev. 2010, 36, 206–215. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Santonja, A.; Sánchez-Muñoz, A.; Lluch, A.; Chica-Parrado, M.R.; Albanell, J.; Chacón, J.I.; Antolín, S.; Jerez, J.M.; de la Haba, J.; de Luque, V.; et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 2018, 9, 26406–26416. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Colaprico, A.; Silva, T.C.; Chen, J.; An, H.; Ban, Y.; Huang, H.; Wang, L.; James, J.L.; Balko, J.M.; et al. Multi-omics analysis identifies therapeutic vulnerabilities in triple-negative breast cancer subtypes. Nat. Commun. 2021, 12, 6276. [Google Scholar] [CrossRef] [PubMed]
- Gerratana, L.; Basile, D.; Buono, G.; De Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; De Santo, I.; Del Mastro, L.; et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat. Rev. 2018, 68, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Dawood, S.; Holmes, M.D.; Collins, L.C.; Schnitt, S.J.; Cole, K.; et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011, 17, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- You, C.-P.; Leung, M.-H.; Tsang, W.-C.; Khoo, U.-S.; Tsoi, H. Androgen Receptor as an Emerging Feasible Biomarker for Breast Cancer. Biomolecules 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011, 19, 575–86. [Google Scholar] [CrossRef] [PubMed]
- Anestis, A.; Zoi, I.; Papavassiliou, A.G.; Karamouzis, M.V. Androgen Receptor in Breast Cancer—Clinical and Preclinical Research Insights. Molecules 2020, 25, 358. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Selth, L.A.; Chia, K.M.; Laven-Law, G.; Milioli, H.H.; Roden, D.; et al. The androgen receptor is a tumor suppressor in estrogen receptor-positive breast cancer. Nat Med. 2021, 27, 310–20. [Google Scholar] [CrossRef] [PubMed]
- Michmerhuizen, A.R.; Spratt, D.E.; Pierce, L.J.; Speers, C.W. ARe we there yet? Understanding androgen receptor signaling in breast cancer. npj Breast Cancer 2020, 6, 47. [Google Scholar] [CrossRef]
- Parylo, S.; Vennepureddy, A.; Dhar, V.; Patibandla, P.; Sokoloff, A. Role of cyclin-dependent kinase 4/6 inhibitors in the current and future eras of cancer treatment. J Oncol Pharm Pract. 2019, 25, 110–29. [Google Scholar] [CrossRef]
- Koryakina, Y.; E Knudsen, K.; Gioeli, D. Cell-cycle-dependent regulation of androgen receptor function. Endocr. -Relat. Cancer 2015, 22, 249–264. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; et al. CDK inhibitors in cancer therapy, an overview of recent development. Am J Cancer Res. 2021, 11, 1913–35. [Google Scholar]
- Martin, J.M.; Goldstein, L.J. Profile of abemaciclib and its potential in the treatment of breast cancer. OncoTargets Ther. 2018, 11, 5253–5259. [Google Scholar] [CrossRef]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016, 6, 740–53. [Google Scholar] [CrossRef] [PubMed]
- Sharifi MW, K.B.; Burkard, M.E.; Tevaarwerk, A.J.; Tamkus, D.; Chan, N.; Truica, C.; Danciu, O.; Hoskins, K.; O’Regan, R.M. Abstract OT1-02-01: Phase I trial of bicalutamide and ribociclib in androgen receptor-positive triple negative breast cancer. Cancer Research. 2019, 79. [Google Scholar] [CrossRef]
- Penson, D.F.; Armstrong, A.J.; Concepcion, R.S.; Agarwal, N.; Olsson, C.A.; Karsh, L.I.; Dunshee, C.J.; Duggan, W.; Shen, Q.; Sugg, J.; et al. Correction: Enzalutamide versus bicalutamide in patients with nonmetastatic castration-resistant prostate cancer: a prespecified subgroup analysis of the STRIVE trial. Prostate Cancer Prostatic Dis. 2022, 25, 597–597. [Google Scholar] [CrossRef]
- Choupani, E.; Madjd, Z.; Saraygord-Afshari, N.; Kiani, J.; Hosseini, A. Combination of androgen receptor inhibitor enzalutamide with the CDK4/6 inhibitor ribociclib in triple negative breast cancer cells. PLoS One. 2022, 17, e0279522. [Google Scholar] [CrossRef]
- Gucalp AB, L.A.; Alano, T.; Arumov, A.; Gounder, M.M.; Patil, S.; Feigin, K.; Edelweiss, M.; D'Andrea, G.; Bromberg, J.; Goldfarb, S.B.; Ligresti, L.; Tsan-Lai Wong, S.; Traina, T.A. Phase II trial of bicalutamide in combination with palbociclib for the treatment of androgen receptor (+) metastatic breast cancer. J Clin Oncol. 2020, 38, 1017. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Lau, K.-Y.; Hsu, C.-C.; Chen, J.-L.; Lee, C.-H.; Huang, T.-T.; Chen, Y.-T.; Huang, C.-T.; Lin, P.-H.; Tseng, L.-M. Combination of palbociclib with enzalutamide shows in vitro activity in RB proficient and androgen receptor positive triple negative breast cancer cells. PLOS ONE 2017, 12, e0189007. [Google Scholar] [CrossRef]
- Gao, S.; Gao, Y.; He, H.H.; Han, D.; Han, W.; Avery, A.; Macoska, J.A.; Liu, X.; Chen, S.; Ma, F.; et al. Androgen Receptor Tumor Suppressor Function Is Mediated by Recruitment of Retinoblastoma Protein. Cell Rep. 2016, 17, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.; Brufsky, A.; Rugo, H.S.; Vogel, C.L.; O'Shaughnessy, J.; Getzenberg, R.H.; Barnette, K.G.; Rodriguez, D.; Bird, G.; Steiner, M.S.; et al. Phase 3 ENABLAR-2 study to evaluate enobosarm and abemaciclib combination compared to estrogen-blocking agent for the second-line treatment of AR+, ER+, HER2- metastatic breast cancer in patients who previously received palbociclib and estrogen-blocking agent combination therapy. J. Clin. Oncol. 2022, 40, TPS1121. [Google Scholar] [CrossRef]
- Yoshimoto, F.K.; Auchus, R.J. The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1). J. Steroid Biochem. Mol. Biol. 2015, 151, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.D.; Ellison, S.J.; Baker, J.G.; Stagg, D.B.; Wardell, S.E.; Park, S.; Alley, H.M.; Baldi, R.M.; Yllanes, A.; Andreano, K.J.; et al. Androgen receptor antagonism drives cytochrome P450 17A1 inhibitor efficacy in prostate cancer. J. Clin. Investig. 2017, 127, 2326–2338. [Google Scholar] [CrossRef] [PubMed]
- Reese JMB, B.L.; Christenson, J.L.; Spoelstra, N.S.; Elias, A.; Eisner, J.R.; Baskin-Bey, E.S.; Gertz, J.; Richer, J.K. Abstract P5-05-05: Targeting the Androgen Receptor with Seviteronel, a CYP17 Lyase and AR Inhibitor, in Triple Negative Breast Cancer. Cancer Research. 2019, 79. [Google Scholar] [CrossRef]
- Gucalp AD, M.A.; Elias, A.D.; Bardia, A.; Ali, H.Y.; Potter, D.; Gabrail, N.Y.; Haley, B.B.; Khong, H.T.; Riley, E.C.; Ervin, L.; Eisner, J.R.; Baskin-Bey, E.; Moore, W.R.; Traina, T.A. Phase (Ph) 2 stage 1 clinical activity of seviteronel, a selective CYP17-lyase and androgen receptor (AR) inhibitor, in women with advanced AR+ triple-negative breast cancer (TNBC) or estrogen receptor (ER)+ BC: CLARITY-01. J Clin Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Yardley, D.A.; Young, R.R.; Adelson, K.B.; Silber, A.L.; Najera, J.E.; Daniel, D.B.; Peacock, N.; Finney, L.; Hoekstra, S.J.; Shastry, M.; et al. A Phase II Study Evaluating Orteronel, an Inhibitor of Androgen Biosynthesis, in Patients With Androgen Receptor (AR)-Expressing Metastatic Breast Cancer (MBC). Clin. Breast Cancer 2022, 22, 269–278. [Google Scholar] [CrossRef]
- Michmerhuizen, A.R.; Chandler, B.; Olsen, E.; Wilder-Romans, K.; Moubadder, L.; Liu, M.; Pesch, A.M.; Zhang, A.; Ritter, C.; Ward, S.T.; et al. Seviteronel, a Novel CYP17 Lyase Inhibitor and Androgen Receptor Antagonist, Radiosensitizes AR-Positive Triple Negative Breast Cancer Cells. Front. Endocrinol. 2020, 11, 35. [Google Scholar] [CrossRef]
- Paplomata, E.; O’regan, R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef]
- Lehmann, B.D.; A Bauer, J.; Schafer, J.M.; Pendleton, C.S.; Tang, L.; Johnson, K.C.; Chen, X.; Balko, J.M.; Gómez, H.; Arteaga, C.L.; et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014, 16, 406. [Google Scholar] [CrossRef]
- Qi, W.; Morales, C.; Cooke, L.S.; Johnson, B.; Somer, B.; Mahadevan, D. Reciprocal feedback inhibition of the androgen receptor and PI3K as a novel therapy for castrate-sensitive and -resistant prostate cancer. Oncotarget 2015, 6, 41976–41987. [Google Scholar] [CrossRef]
- Won, K.; Spruck, C. Triple-negative breast cancer therapy: Current and future perspectives (Review). Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. New Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Park, H.; Murthy, R.K.; Iwata, H.; Tamura, K.; Tsurutani, J.; Moreno-Aspitia, A.; Doi, T.; Sagara, Y.; Redfern, C.; et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low–Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J. Clin. Oncol. 2020, 38, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Li, Y.; Deng, Z.; Zhang, Z.; Xie, Q.; Zhang, H.; Zhong, W.; Pan, B. IGHG1 Regulates Prostate Cancer Growth via the MEK/ERK/c-Myc Pathway. BioMed Res. Int. 2019, 2019, 7201562–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Q.; Li, N.; Xu, M.; Miyamoto, T.; Liu, J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. npj Breast Cancer 2022, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Regua, A.; Papp, C.; Grageda, A.; Porter, B.A.; Caza, T.; Bichindaritz, I.; Krendel, M.; Sivapiragasam, A.; Bratslavsky, G.; Kuznetsov, V.A.; et al. ABI1-based expression signature predicts breast cancer metastasis and survival. Mol. Oncol. 2022, 16, 2632–2657. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.A.; Li, X.; Arya, N.; Zhang, F.; Kung, S.H.Y.; Fazli, L.; et al. ABI1 regulates transcriptional activity of Androgen Receptor by novel DNA and AR binding mechanism. bioRxiv 2023. [Google Scholar]
| TRIAL NUMBER | TRIAL NAME | DRUG NAME(S) | PURPOSE | OUTCOMES | PHASE |
|---|---|---|---|---|---|
| NCT05095207 | Abemaciclib in combination with bicalutamide for AR+, HER2-metastatic breast cancer | Abemaciclib and Bicalutamide | Determine the dose-limiting toxicity and efficacy of this combination therapy | Accrual Status: Active Hypothesized that the two drugs together will improve CBR based on preclinical data and the drug properties |
IB/II |
| NCT03090165 | Ribociclib and bicalutamide in AR+ TNBC | Ribociclib and Bicalutamide | Determine the safety and efficacy of this combination therapy | Accrual Status: Active Primary outcome to be measured by MTD for combination of drugs without dose-limiting toxicity, and CBR |
I/II |
| NCT02605486 | Palbociclib in combination with bicalutamide for the treatment of AR+ metastatic breast cancer (MBC) | Palbociclib and Bicalutamide | Determine the safety and efficacy of this combination therapy as well as determining effective dosage | Accrual Status: Active Primary outcomes to be measured by determination of the RP2D (phase I) and PFS (phase II) |
I/II |
| NCT05065411 | Efficacy and safety evaluation of enobosarm in combo with abemaciclib in treatment of ER+HER2- metastatic breast cancer | Enobosarm and Abemaciclib Combo | Determine the safety of enobosarm used with abemaciclib as compared to the estrogen blocking control group, exemestane and fulvestrant | Accrual Status: Active Primary outcome to be measured by PFS in the combination drug group as compared to the control group |
III |
| NCT01808040 | A Phase IB Study of TAK-700 in postmenopausal women with HER2+ metastatic breast cancer | TAK700 (CYP17 Lyase Inhibitor) | Determine dose escalation and dose expansion of TAK700 in female metastatic breast cancer patients, test TAK700’s ability to decrease estrogen levels | Accrual Status: Completed Results not yet submitted, primary outcomes to be measured by number of patients with adverse effects to determine RP2D, decrease in serum estradiol levels |
I |
| NCT02130700 | Oral VT-464 in patients with castration-resistant prostate cancer (CRPC) previously treated with enzalutamide, AR+ TNBC patients and men with ER+ breast cancer | VT-464 (lyase-selective inhibitor of CYP17) | Determine the efficacy and safety of VT-464 in CRPC patients treated with enzalutamide, AR+ TNBC patients and male AR+ BC patients | Accrual Status: Completed Results not yet posted, primary outcomes to be measured by serum PSA decrease, PFS, and CBR |
II |
| NCT02580448 | CYP17 Lyase and AR inhibitor treatment with seviteronel trial | Seviteronel | Determine the RP2D of seviteronel for TNBC or ER+ BC patients in phase I. as well as safety, pharmacokinetics, and pharmacodynamics in phase II | Accrual Status: Completed Results not yet submitted, primary outcome to be measured by CBR at 16 weeks for TNBC female patients and all male patients, CBR at 24 weeks for ER+ BC female patients |
I/II |
| NCT02457910 | Taselisib and enzalutamide in treating patients with AR+ triple-negative metastatic breast cancer | Taselisib and Enzalutamide | Determine the side effects and most effective dose of this combination therapy in AR+ TNBC | Accrual Status: Active 0.357 CBR in combination therapy patients, dose limiting toxicity of 0 measured in doses ranging 2-8 mg of taselisib with 160 mg enzalutamide |
IB/II |
| NCT03207529 | Alpelisib and enzalutamide in treating patients with AR+, PTEN+ metastatic breast cancer | Alpelisib and Enzalutamide | Determine the maximum tolerated dose (MTD) of this combination therapy | Accrual Status: Active Primary outcome will be measured using MTD |
I |
| NCT03840200 | A study evaluating safety, pharmacokinetics and efficacy of ipatasertib administered in combination with rucaparib in participants with advanced breast, ovarian, and prostate cancer | Ipatasertib and Rucaparib | Determine safety and pharmacokinetics of this combination therapy as well as dose escalation and expansion | Accrual Status: Completed Results not yet submitted, primary outcome to be measured by percentage of patients with adverse effects and dose-limiting toxicities |
I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





