Submitted:
28 September 2025
Posted:
30 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Method
3. Result
| Characteristic | Patients |
|
HER-2 Status HER-2 Low HER-2 Negative |
297 301 |
|
Median age > 65 years <35 years |
56,5 168 29 |
|
Menopausal status Pre/perimenopausal Postmenopausal |
201 401 |
|
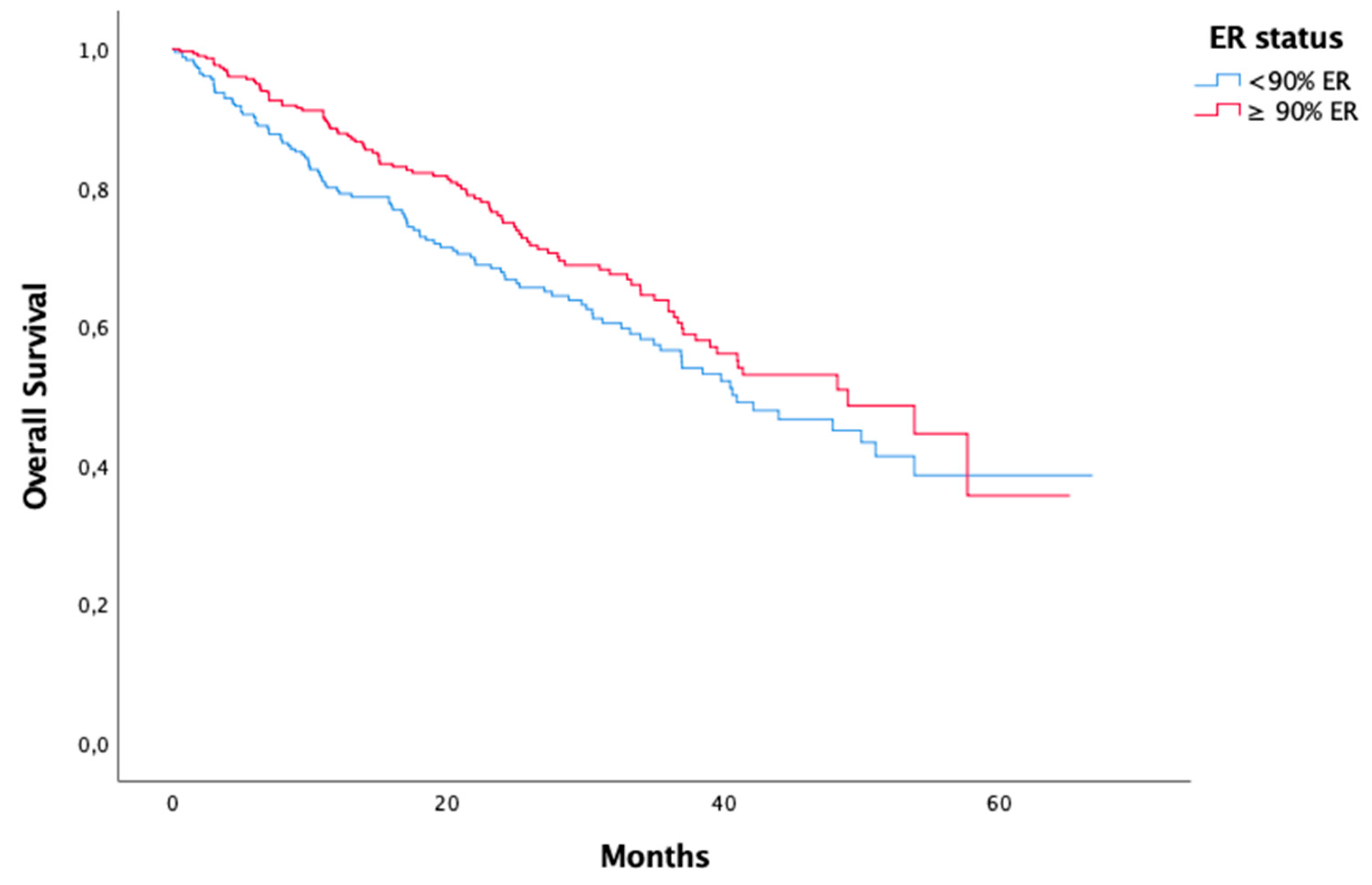
ER status < 90% ≥ 90% Unknown |
259 310 34 |
|
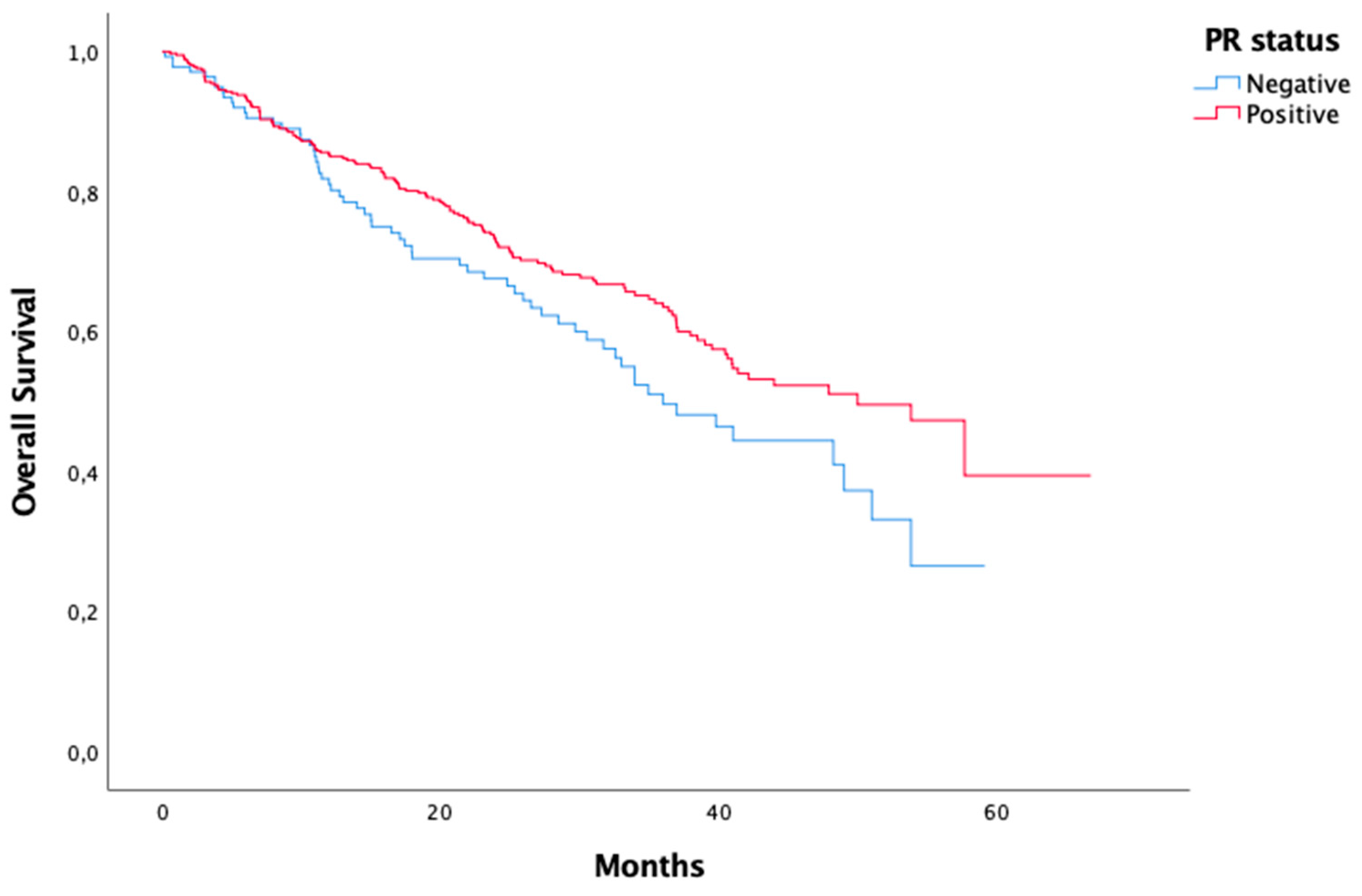
PR status Negative Positive Unknown |
139 430 29 |
|
Pathology Invasive ductal Invasive lobular Other Unknown |
525 51 19 7 |
|
Metastatic status De-novo Recurrent |
308 295 |
|
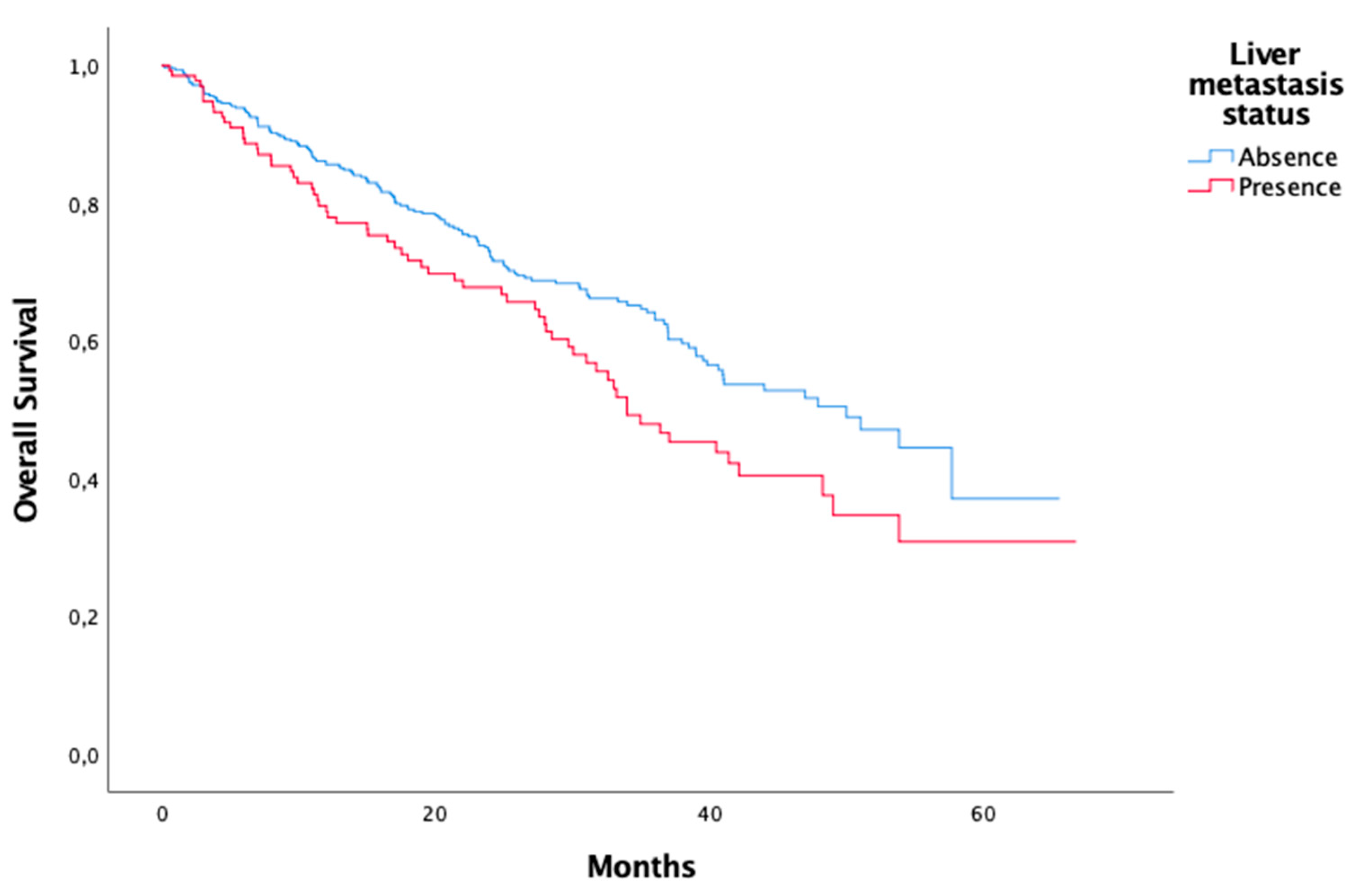
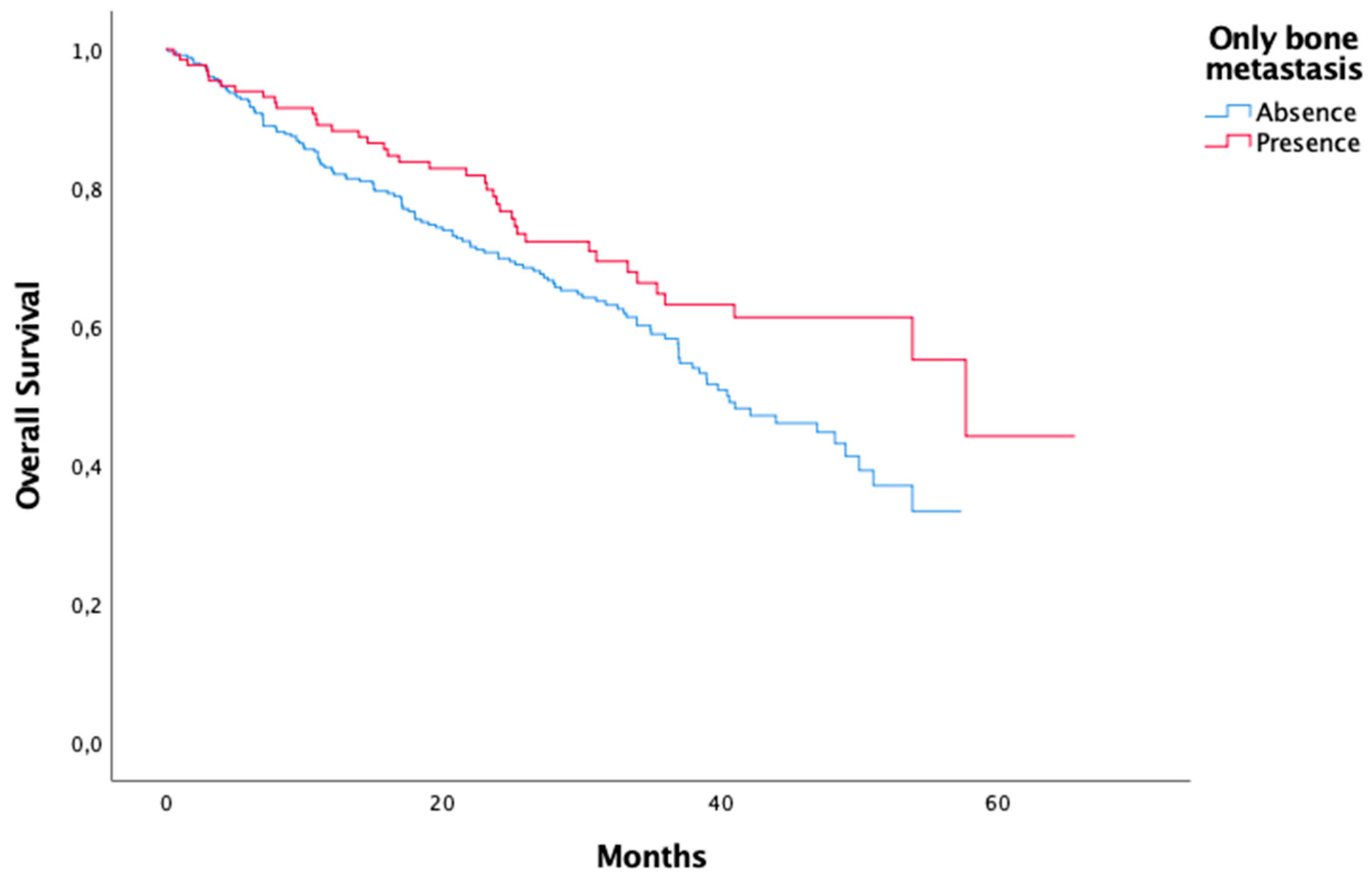
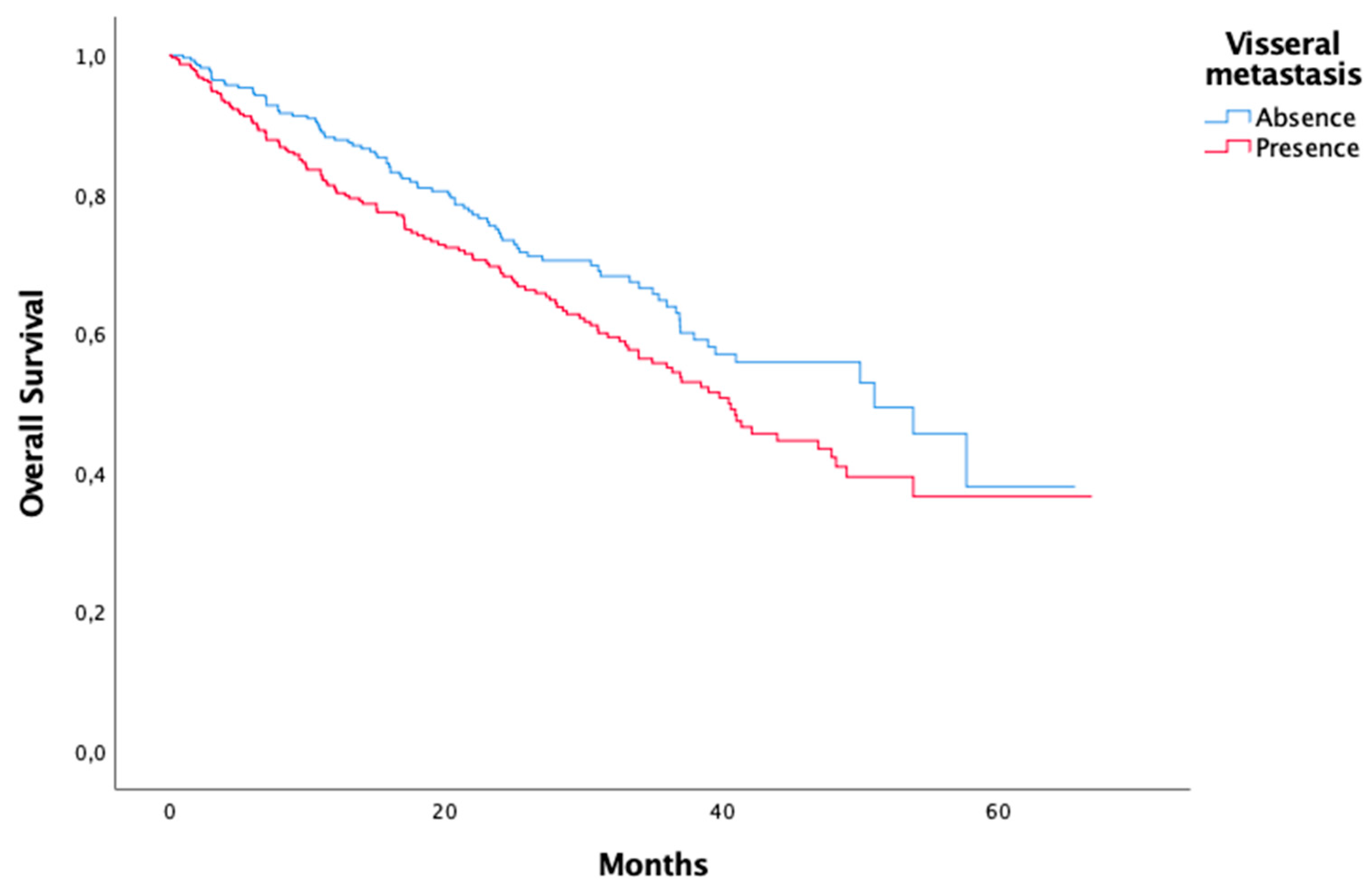
Metastasis Visceral metastasis Only bone metastasis Liver metastasis Unknown |
316 135 136 16 |
|
CDK 4/6 Inhibitors Ribosiclib Palbosiclib |
422 181 |
|
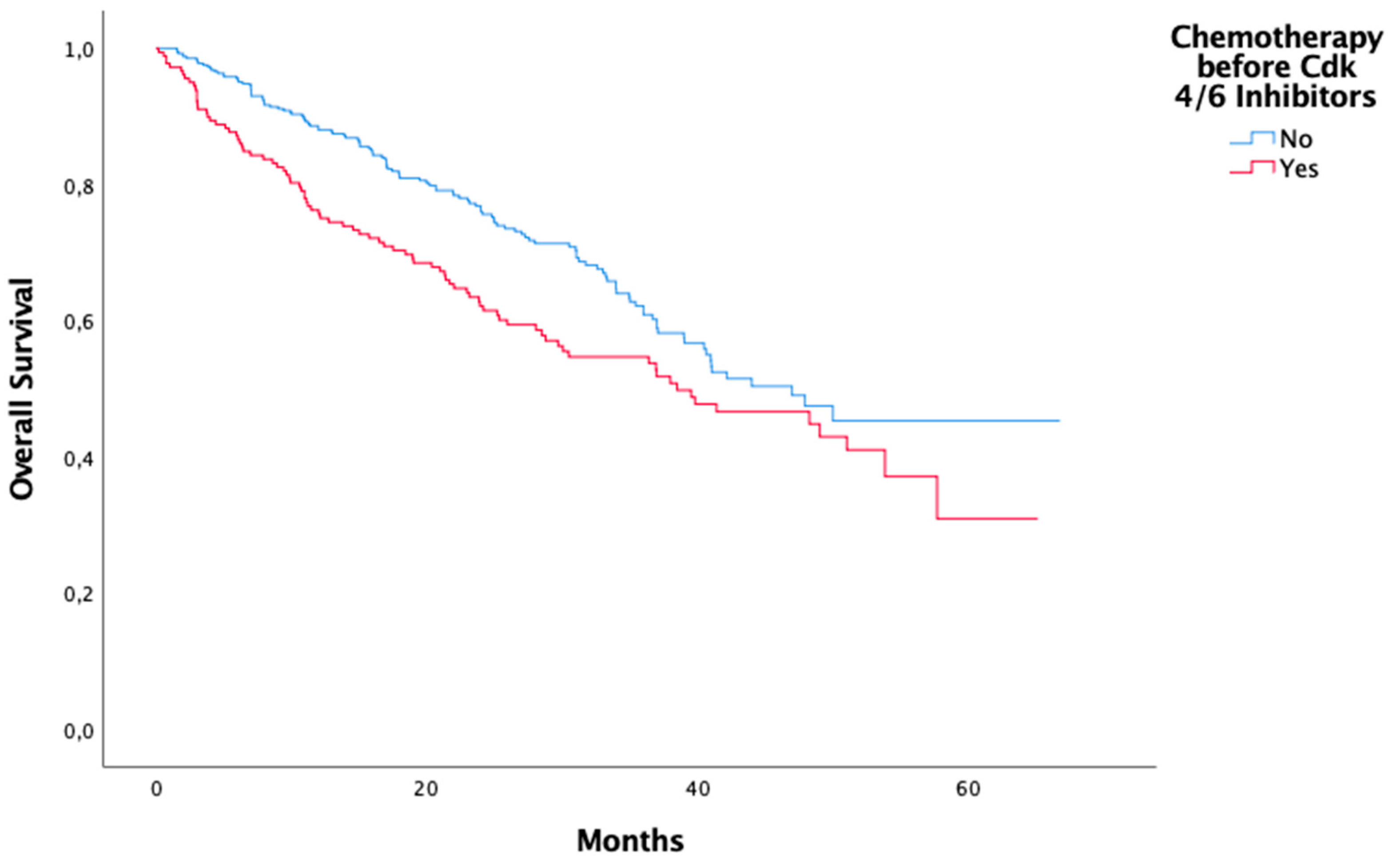
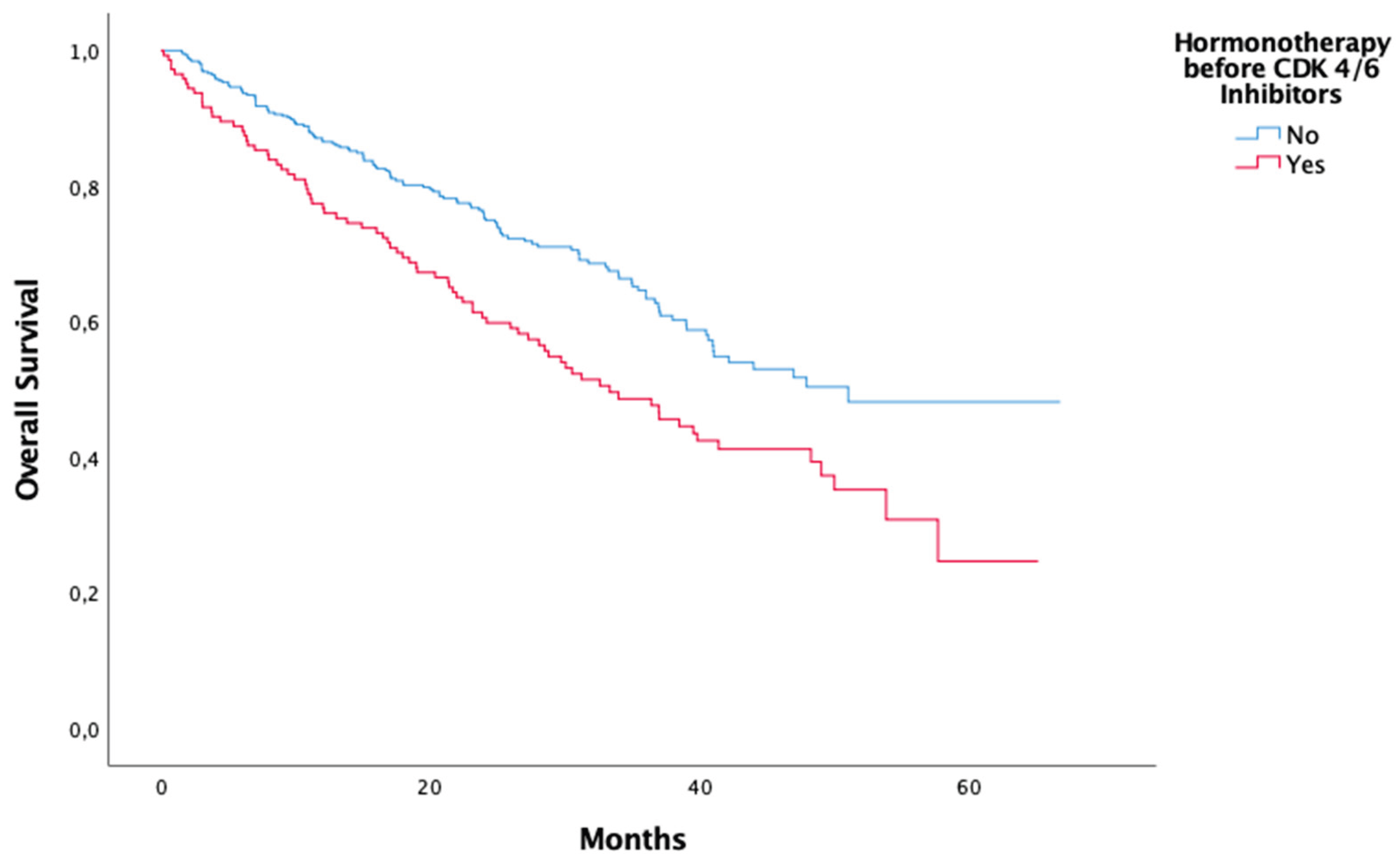
Tretatment before CDK 4/6 Inhibitors Hormonotherapy before CDK 4/6 Inhibitors Yes No Chemotherapy before CDK 4/6 Inhibitors Yes No |
144 458 181 418 |
| Variables | P |
| Her-2 status Her-2 low Her-2 negative |
0,479 |
| Metastasis Visceral metastasis Only bone metastasis Liver metastasis |
0,025 0,022 0,019 |
| ER status < %90 ≥ %90 |
0,072 |
| PR status Negative Positive |
0,037 |
| Menopausal status Pre/perimenopausal Postmenopausal |
0,775 |
| Pathology Invasive ductal Invasive lobular Other |
0,723 |
| Metastatic status De-novo Recurrent |
0,757 |
| CDK 4/6 Inhibitors Ribosiclib Palbosiclib |
0,267 |
| Treatment before CDK 4/6 Inhibitors Hormonotherapy before CDK 4/6 Inhibitors Chemotheray before CDK 4/6 Inhibitors |
<0,01 0,01 |
| Age < 35 years >65 years |
0,848 0,695 |
| Variables | P |
| Metastasis Visceral metastasis Only bone metastasis Liver metastasis |
0,237 0,586 0,332 |
| ER status < %90 ≥ %90 |
0,031 |
| PR status Negative Positive |
0,231 |
| Metastatic status De-novo Recurrent |
0,813 |
| CDK 4/6 Inhibitors Ribosiclib Palbosiclib |
0,127 |
| Treatment before CDK 4/6 Inhibitors Hormonotherapy before CDK 4/6 Inhibitors Chemotherapy before CDK 4/6 Inhibitors |
0,045 0,530 |
4. Discussion
5. Conclusions
References
- Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends: an update. Cancer Epidemiol Biomarkers Prev 2016;25(1):16–27.
- Reinert, T., de Paula, B., Shafaee, M. N., Souza, P. H., Ellis, M. J., & Bines, J. (2018). Endocrine therapy for ER-positive/HER2-negative metastatic breast cancer. Chinese Clinical Oncology, 7(3), 25–25. [CrossRef]
- Cardoso F, Costa A, Senkus E, et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann Oncol 2017;28:3111.
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12.
- Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1and S phases. Nat Rev Mol Cell Biol. 2013;14:518–28.
- Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A. Cyclin-dependentkinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past,present, and future. Lancet. 2020;395:817–27.
- Ding L, Cao J, Lin W, Chen H, Xiong X, Ao H, et al. The roles of cyclin-dependentkinases in cell-cycle progression and therapeutic strategies in human breastcancer. Int J Mol Sci. 2020;21:1960.
- Roskoski R Jr. Cyclin-dependent protein serine/threonine kinase inhibitors asanticancer drugs. Pharm Res. 2019;139:471–88.
- Pernas S, Tolaney SM, Winer EP, Goel S. CDK4/6 inhibition in breast cancer:current practice and future directions. Ther Adv Me Oncol.2018;10:1758835918786451.
- Alves CL, Ehmsen S, Terp MG, Portman N, Tuttolomondo M, Gammelgaard OL,et al. Co-targeting CDK4/6 and AKT with endocrine therapy prevents progres-sion in CDK4/6 inhibitor and endocrine therapy-resistant breast cancer. NatCommun. 2021;12:5112.
- Im SA, Lu YS, Bardia A, et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med 2019; 381:307.
- Slamon DJ, Diéras V, Rugo HS, et al. Overall Survival With Palbociclib Plus Letrozole in Advanced Breast Cancer. J Clin Oncol 2024; 42:994.
- Rugo H, et al. Overall survival with first-line palbociclib plus an aromatase inhibitor(AI) vs AI in metastatic breast cancer: A large real-world database analysis. Ann Oncol 2022; 33 (S3): S194-S223.
- Finn RS, Martin M, Rugo HS, et al: Palbociclib and letrozole in advanced breast cancer. N Engl J Med375:1925-1936, 2016.
- Cristofanilli M, Turner NC, Bondarenko I, et al: Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17:425-439, 2016.
- Hortobagyi GN, Stemmer SM, Burris HA, et al: Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738-1748, 2016.
- llison, K. H., Hammond, M. E. H., Dowsett, M., McKernin, S. E., Carey, L. A., Fitzgibbons, P. L., Hayes, D. F., Lakhani, S. R., Chavez-MacGregor, M., Perlmutter, J., Perou, C. M., Regan, M. M., Rimm, D. L., Symmans, W. F., Torlakovic, E. E., Varella, L., Viale, G., Weisberg, T. F., McShane, L. M., & Wolff, A. C. (2020). Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 38(12), 1346–1366. [CrossRef]
- Finn, R., Jiang, Y., Rugo, H., Moulder, S. L., Im, S.-A., Gelmon, K. A., Dieras, V., Martin, M., Joy, A. A., Toi, M., Gauthier, E., Lu, D. R., Bartlett, C. H., & Slamon, D. (2016). late-breaking and deferred publication abstracts LBA15 Biomarker analyses from the phase 3 PALOMA-2 trial of palbociclib (P) with letrozole (L) compared with placebo (PLB) plus L in postmenopausal women with ER + /HER2-advanced breast cancer (ABC). Abstract Book of the 41st ESMO Congress (ESMO 2016), 7-11 October 2016, Copenhagen, Denmark, 27, vi554. [CrossRef]
- Fang H, Huang D, Yang F, Guan X. Potential biomarkers of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer. Breast Cancer Res Treat. 2018;168(2):287-297. [CrossRef]
- Pandey K, An HJ, Kim SK, et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: a review. Int J Cancer. 2019;145(5): 1179-1188. [CrossRef]
- Öner, İ., İnci, K., Tolunay, K., Karabuğa, B., Türkel, A., Ateş, Ö., & Karaçin, C. (2025). ANATOLIAN CURRENT MEDICAL Predictive value of progesterone receptor in advanced-stage breast cancer patients treated with CDK 4/6 inhibitors. Anatolian Curr Med J, 7(3), 375–383. [CrossRef]
- Toss A, Venturelli M, Sperduti I, et al. First-line treatment for endocrine-sensitive bone-only metastatic breast cancer: systematic review and meta-analysis. Clin Breast Cancer. 2019;19(6):e701-e716.
- Niikura N, Liu J, Hayashi N, et al. Treatment outcome and prognostic factors for patients with bone-only metastasesof breast cancer: a single-institution retrospective analysis.Oncologist. 2011;16(2):155-164.
- Cummings MC et al. (2014) Metastatic progression of breast cancer: insights from 50 years of autopsies. J Pathol 232(1):23–31. 10.1002/path.4288.
- Zhao HY, Gong Y, Ye FG, Ling H, Hu X (2018) Incidence and prognostic factors of patients with synchronous liver metastases upon initial diagnosis of breast cancer: a population-based study. Cancer Manag Res 10:5937–5950. [CrossRef]
- Dawood S, Broglio K, Ensor J, et al: Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 21:2169-2174, 2010.
- Lobbezoo D, Van Kampen R, Voogd A, et al: Prognosis of metastatic breast cancer: Are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer112:1445, 2015.
- Den Brok WD, Speers CH, Gondara L, et al: Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat 161:549-556, 2017.
- Yardley DA, Kaufman PA, Brufsky A, et al: Treatment patterns and clinical outcomes for patients with de novo versus recurrent HER2-positive metastatic breast cancer. Breast Cancer Res Treat 145:725-734, 2014.
- Barcenas, C. H., Song, J., Murthy, R. K., Raghavendra, A. S., Li, Y., Hsu, L., Carlson, R. W., Tripathy, D., & Hortobagyi, G. N. (2021). Prognostic Model for De Novo and Recurrent Metastatic Breast Cancer. JCO Clinical Cancer Informatics, 5, 789–804. https://doi.org/10.1200/CCI.21.00020/SUPPL_FILE/DS_CCI.21.00020.PDF.
- Modi, S., Jacot, W., Yamashita, T., Sohn, J., Vidal, M., Tokunaga, E., Tsurutani, J., Ueno, N. T., Prat, A., Chae, Y. S., Lee, K. S., Niikura, N., Park, Y. H., Xu, B., Wang, X., Gil-Gil, M., Li,W., Pierga, J.-Y., Im, S.-A., … Cameron, D. A. (2022). Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. The New England Journal of Medicine, 387(1),9. [CrossRef]
- Kahraman, S., Hızal, M., Gümüşay, Ö., Başaran, G., Seyyar, M., Sahin, E., Çabuk, D., Yaşar, A., Bayoğlu, V., Bayram, E., Paydaş, S., Gülbağcı, B., Hacıbekiroğlu, İ., Demirel, B. Ç., Yaren, A., Özçelik, M., Yılmaz, F., Doğan, M., Paksoy, N., … Şendur, M. A. (2025). HER2-low expression in patients with hormone receptor positive and HER2 negative advanced breast cancer treated with ribociclib or palbociclib in combination with endocrine therapy. Turkish Journal of Clinics and Laboratory. [CrossRef]
- Önder, T., Ateş, Öner, & Karaçin, C. (2024). Relationship between HER2-low status and efficacy of CDK4/6 inhibitors in advanced breast cancer: a real-world study. International Journal of Clinical Oncology, 29(7), 972–984. [CrossRef]
- Thill, M., Zahn, M. O., Welt, A., Nusch, A., Zaiss, M., Engelken, K., Kaltenecker, G., Ringwald, K., Gratzke, K., Dille, S., Kruggel, L., Jänicke, M., Schulz, H., Hagen, V., Fricker, R., Stickeler, E., Harbeck, N., Wöckel, A., & Decker, T. (2025). Head-to-head comparison of palbociclib and ribociclib in first-line treatment of HR-positive/HER2-negative metastatic breast cancer with real-world data from the OPAL registry. International Journal of Cancer, 156(9), 1770–1782. [CrossRef]
- Schettini F, Giudici F, Giuliano M, Cristofanilli M, Arpino G, Del Mastro L, Puglisi F, De Placido S, Paris I, De Placido P, Venturini S, De Laurentis M, Conte P, Juric D, Llombart-Cussac A, Pusztai L, Prat A, Jerusalem G, Di Leo A, Generali D. Overall Survival of CDK4/6-Inhibitor-Based Treatments in Clinically Relevant Subgroups of Metastatic Breast Cancer: Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2020 Nov 1;112(11):1089-1097. [CrossRef] [PubMed] [PubMed Central]
- Tripathy, D., Im, S. A., Colleoni, M., Franke, F., Bardia, A., Harbeck, N., Hurvitz, S. A., Chow, L., Sohn, J., Lee, K. S., Campos-Gomez, S., Villanueva Vazquez, R., Jung, K. H., Babu, K. G., Wheatley-Price, P., de Laurentiis, M., Im, Y. H., Kuemmel, S., El-Saghir, N., … Lu, Y. S. (2018). Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. The Lancet Oncology, 19(7), 904–915. [CrossRef]
- Toda, E., Hoshino, M., Shimoi, T., Yamanaka, T., Kitadai, R., Saito, A., Kita, S., Kawachi, A., Maejima, A., Kojima, Y., Noguchi, E., Fujiwara, Y., Sudo, K., Koyama, T., & Yonemori, K. (2025). 66P Genetic insights into CDK4/6 inhibitor efficacy in invasive lobular carcinoma and invasive ductal carcinoma. ESMO Open, 10, 104620. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





