Submitted:
11 October 2023
Posted:
12 October 2023
You are already at the latest version
Abstract
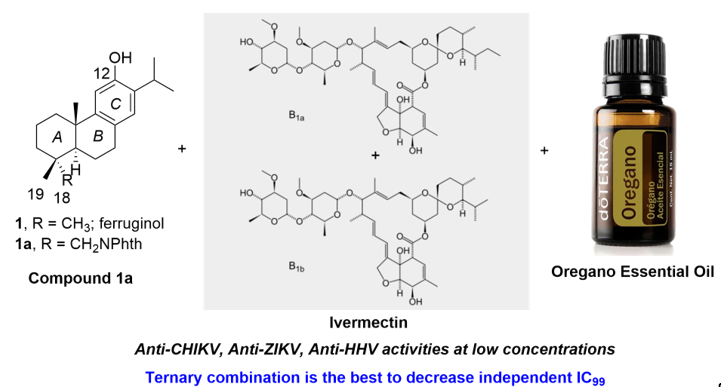
Keywords:
1. Introduction
2. Materials and Methods
2.1. Biological Assays
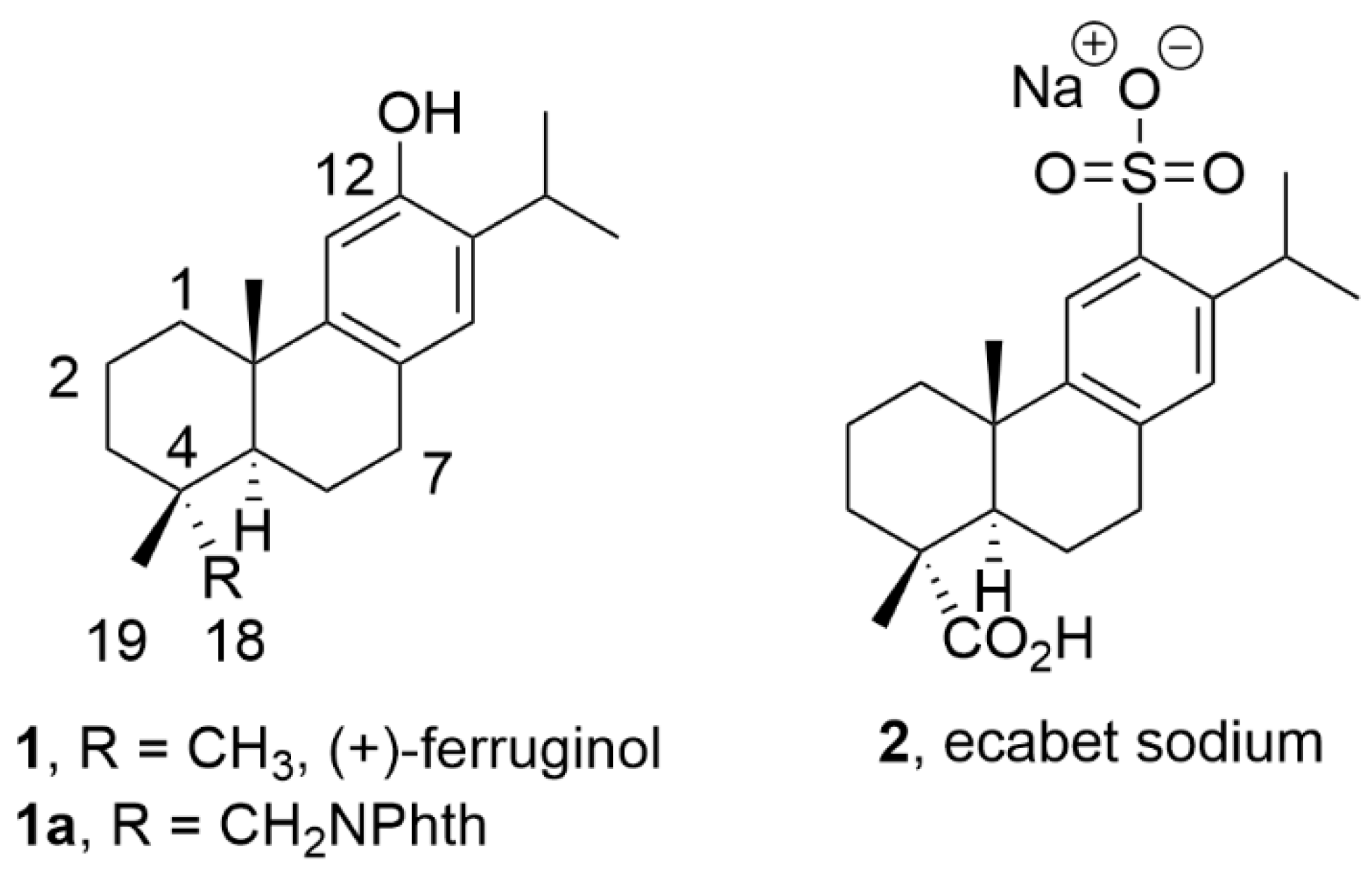
2.1.1. Reagents and compounds
2.1.2. Cell culture and viruses
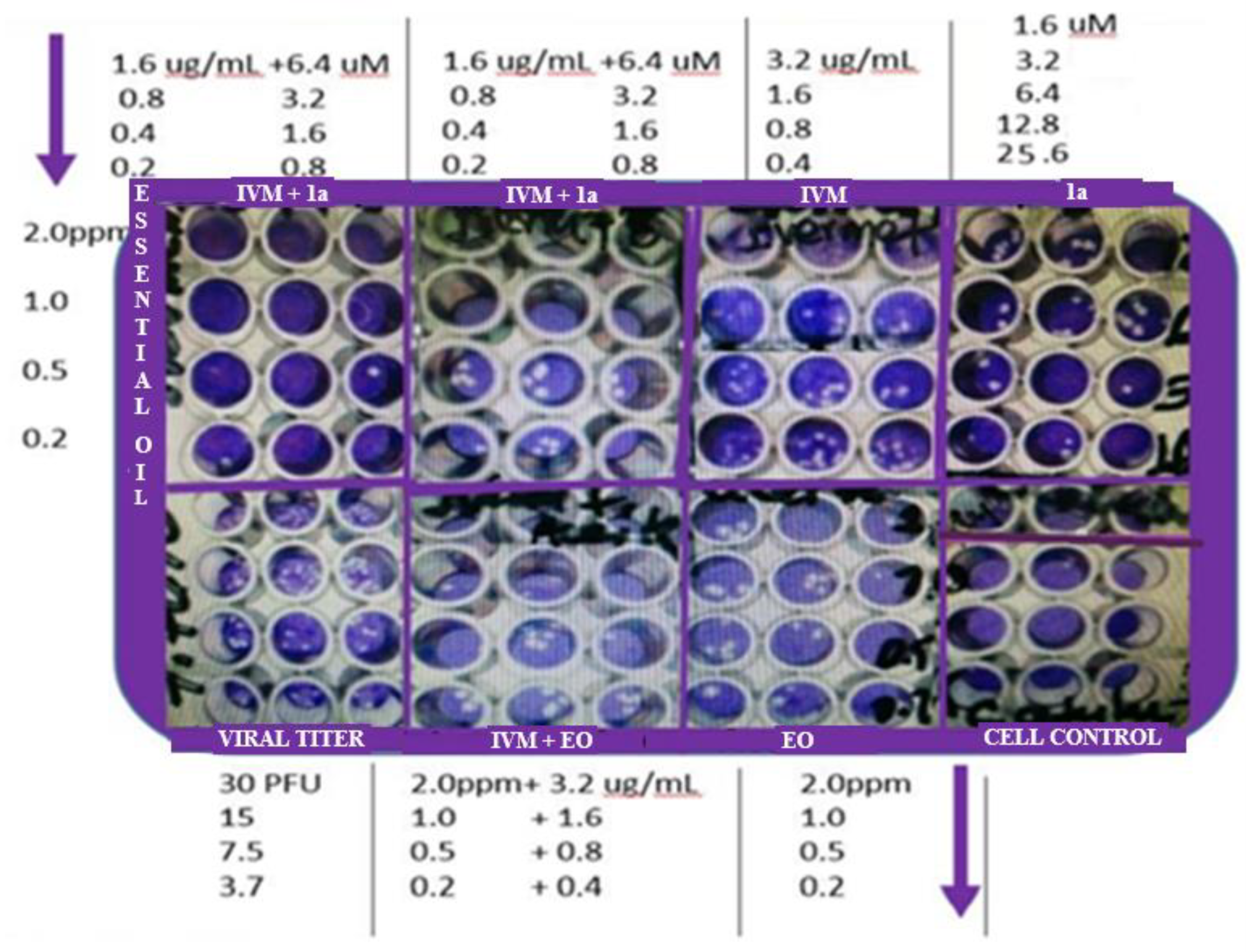
2.1.3. End-point titration technique (EPTT) for evaluation of IC99
2.2. In silico simulations
2.2.1. Calculation of Molecular Properties (Drug-Likeness)
3. Results
3.1. Antiviral evaluation of IVM, EOs, phthFGL 1a against ZIKV, CHIKV and HHV-2
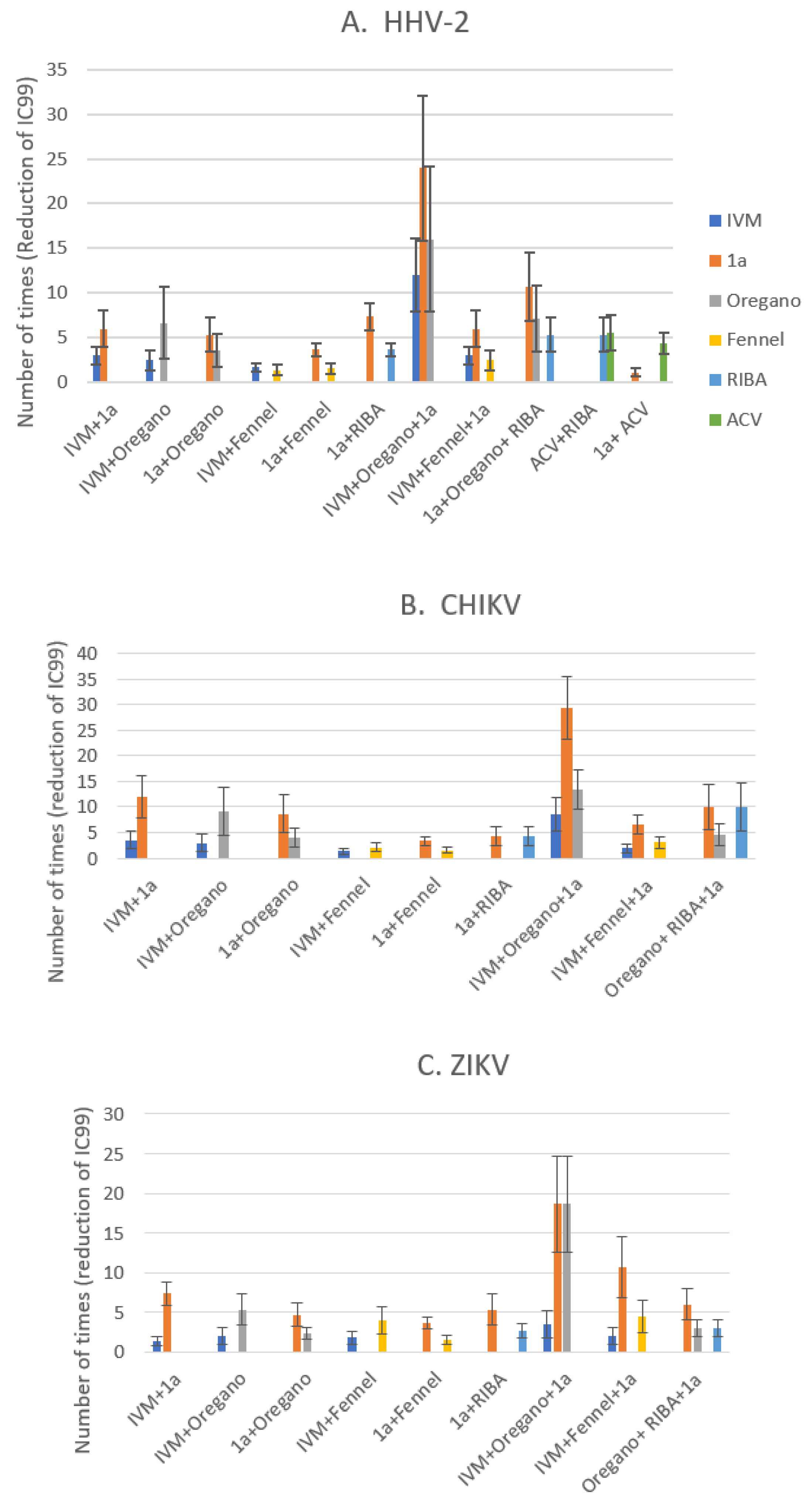
3.2. Antiviral evaluation of binary and ternary combinations of compounds
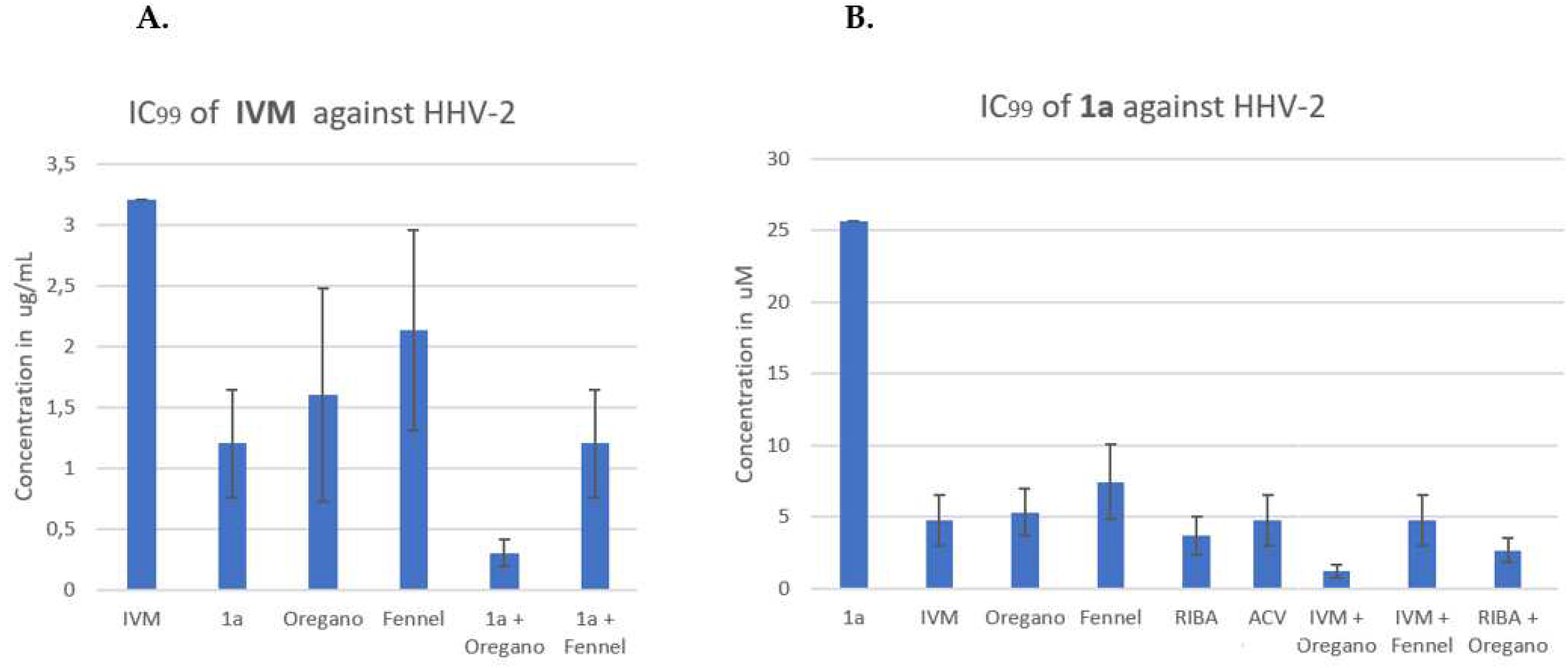
3.2.1. Antiviral activity of IVM or phthFGL (1a) in the context of combinations against HHV-2, CHIKV and ZIKV.
3.2.2. Reduction of IC99 values in comparison with ACV/Ribavirin as synergism control.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Crump, A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020, 178, 104787. [Google Scholar] [CrossRef]
- Heidary, F.; Gharebaghi, R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020, 73, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.N.Y.; Atkinson, S.C.; Wang, C.; Lee, A.; Bogoyevitch, M.A.; Borg, N.A.; Jans, D.A. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020, 177, 104760. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.J.; Jans, D.A. Antivirals that target the host IMPα/β1-virus interface. Biochem. Soc. Tran. 2021, 49, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Chandrakala, V.; Aruna, V.; Angajala, G.; Reddy, P.G. Chemical Composition and Pharmacological Activities of Essential Oils. In Essential oils: extraction methods and applications; Inamuddin, Ed.; Scrivener publishing LLC: Bervely, USA, 2023. [Google Scholar] [CrossRef]
- Schnitzler, P. Essential oils for the treatment of herpes simplex virus infections. Chemotherapy 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Lai, W.-L.; Chuang, H.-S.; Lee, M.-H.; Wei, C.-L.; Lin, C.-F.; Tsai, Y.-C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Brand, Y.M.; Roa-Linares, V.C.; Betancur-Galvis, L.A.; Durán-García, D.C.; Stashenko, E. Antiviral activity of colombian Labiatae and Verbenaceae family essential oils and monoterpenes on human herpes viruses. J. Essent. Oil Res. 2016, 28, 130–137. [Google Scholar] [CrossRef]
- Mustafa, A.; El-Kashef, D.H.; Abdelwahab, M.F.; Gomaa, A.A.-R.; Mustafa, M.; Abdel-Wahab, N.M.; Ibrahim, A.H. Investigation of antiviral effects of essential oils. In Essential oils: extraction methods and applications; Inamuddin, Ed.; Scrivener publishing LLC: Bervely, USA, 2023. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential oils as antiviral agents, potential of essential oils to treat SARS-CoV-2 infection: an in-silico investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Serifi, I.; Tzima, E.; Bardouki, H.; Lampri, E.; Papamarcaki, T. Effects of the essential oil from Pistacia lentiscus var. chia on the lateral line system and the gene expression profile of zebrafish (Danio rerio). Molecules 2019, 24, 3919. [Google Scholar] [CrossRef]
- Sawerus, M.G.; Shehata, O.; Ahmed, W.M.S.; Shany, S.; Hassan, K.E.; Mahdi, E.A.; Mohamed, A.H. The modulatory effect of carvacrol on viral shedding titer and acute phase response in broiler chickens experimentally infected with infectious bronchitis virus. Microb. Pathog. 2022, 163, 105410. [Google Scholar] [CrossRef]
- González, M. A. Aromatic abietane diterpenoids: their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Ferruginol and sugiol: a short review of their chemistry, sources, contents, pharmacological properties and patents. Trop. J. Nat. Prod. Res. 2023, 7, 2325–2336. [Google Scholar]
- Roa-Linares, V.C.; Brand, Y.M.; Agudelo-Gomez, L.S.; Tangarife-Castaño, V.; Betancur-Galvis, L.A.; Gallego-Gomez, J.C.; González, M.A. Anti-herpetic and anti-dengue activity of abietane ferruginol analogues synthesized from (+)-dehydroabietylamine. Eur. J. Med. Chem. 2016, 108, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.T.G.; Nunes, C.; Romano, C. M.; Sabino, E. C.; González-Cardenete, M. A. Anti-Zika virus activity of several abietane-type ferruginol analogues. Rev. Inst. Med. Trop. São Paulo 2020, 62, e97. [Google Scholar] [CrossRef] [PubMed]
- González-Cardenete, M.A.; Hamulić, D.; Miquel-leal, F.J.; González-Zapata, N.; Jimenez-Jarava, O.J.; Brand, Y.M.; Restrepo-Mendez, L.C.; Martinez-Gutierrez, M.; Betancur-Galvis, L.A.; Marín, M.L. Antiviral profiling of C18- or C19-functionalized semisynthetic abietane diterpenoids. J. Nat. Prod. 2022, 85, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, M.; Philippot, S.; González-Cardenete, M.A. Anticoronavirus evaluation of antimicrobial diterpenoids: application of new ferruginol analogues. Viruses 2023, 15, 1342. [Google Scholar] [CrossRef]
- Roa-Linares, V.C.; Escudero-Flórez, M.; Vicente-Manzanares, M.; Gallego-Gómez, J.C. Host cell targets for unconventional antivirals against RNA viruses. Viruses 2023, 15, 776. [Google Scholar] [CrossRef]
- González, M. A. Synthetic derivatives of aromatic abietane diterpenoids and their biological activities. Eur. J. Med. Chem. 2014, 87, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Yarovaya, O.I.; Laev, S.S.; Salakhutdinov, N.F. Antiviral properties of diterpenes and their derivatives. Russ. Chem. Rev. 2023, 92, RCR5056. [Google Scholar] [CrossRef]
- Lakshmanan, M. Drugs used in acid peptic disorders. In Introduction to Basics of Pharmacology and Toxicology: Essentials of Systemic Pharmacology: From Principles to Practice; Paul, A., Anandabaskar, N., Mathaiyan, J., Raj, G.M., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Shyr, Z.A.; Cheng, Y.-S.; Lo, D.C.; Zheng, W. Drug combination therapy for emerging viral diseases. Drug Discov. Today 2021, 26, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, J.; Herring, S.; Hsiang, T.-Y.; Ianevski, A.; Biering, S.B.; Xu, S.; Hoffmann, M.; Pöhlmann, S.; Gale Jr., M.; Aittokallio, T.; Schiffer, J.T.; White, J.M.; Polyak, S.J. Combinations of host- and virus-targeting antiviral drugs confer synergistic suppression of SARS-CoV-2. Microbiol. Spectr. 2022, 10, e0333122. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Recommendations for Laboratory Detection and Diagnosis of Arbovirus Infections in the Region of the Americas; Pan American Health Organization: Washington, D.C, 2023. [Google Scholar]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir resistance in herpes simplex viruses: prevalence and therapeutic alternatives. Biochem Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, L.A.; Upadhyay, R.; Greeley, Z.W.; Margulies, B.J. Current drugs to treat infections with herpes simplex viruses-1 and -2. Viruses 2021, 13, 1228. [Google Scholar] [CrossRef]
- Laiton-Donato, K.; Álvarez-Díaz, D.A.; Rengifo, A.C.; Torres-Fernández, O.; Usme-Ciro, J.A.; Rivera, J.A.; Santamaría, G.; Naizaque, J.; Monroy-Gómez, J.; Sarmiento, L.; Gunturiz, M.L.; Muñoz, A.; Vanegas, R.; Rico, A.; Pardo, L.; Peláez-Carvajal, D. Complete genome sequence of Colombian Zika virus strain obtained from BALB/c mouse brain after intraperitoneal inoculation. Microbiol. Resour. Announc. 2019, 8, e01719–18. [Google Scholar] [CrossRef]
- Vlietinck, A.J.; Van Hoof, L.; Totté, J.; Lasure, A.; Vanden Berghe, D.; Rwangabo, P.C.; Mvukiyumwami, J. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J. Ethnopharmacology 1995, 46, 31–47. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pancheva, S.N. Potentiating effect of ribavirin on the anti-herpes activity of acyclovir. Antiviral Res. 1991, 16, 151–161. [Google Scholar] [CrossRef]
- Shishkov, S.; Pancheva, S. The synergistic antiviral effect of acyclovir and ribavirin against the herpes simplex type-1 virus and the pseudorabies virus in vitro. Acta Microbiol. Bulg. 1990, 25, 69–75. [Google Scholar] [PubMed]
- Azam, F.; Taban, I.M.; Eid, E.E.M.; Iqbal, M.; Alam, O.; Khan, S.; Mahmood, D.; Anwar, M.J.; Khalilullah, H.; Khan, M.U. An in-silico analysis of ivermectin interaction with potential SARS-CoV-2 targets and host nuclear importin α. J. Biomol. Struct. Dyn. 2022, 40, 2851–2864. [Google Scholar] [CrossRef] [PubMed]
- Jermain, B.; Hanafin, P.O.; Cao, Y.; Lifschitz, A.; Lanusse, C.; Rao, G.G. Development of a minimal physiologically-based pharmacokinetic model to simulate lung exposure in humans following oral administration of ivermectin for COVID-19 drug repurposing. J. Pharm. Sci. 2020, 109, 3574–3578. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Karim, M.M.; Ross, A.G.; Hossain, M.S.; Clemens, J.D.; Sumiya, M.K.; Phru, C.S.; Rahman, M.; Zaman, K.; Somani, J.; Yasmin R, Hasnat, M. A.; Kabir, A.; Aziz, A.B.; Khan, W.A. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 2021, 103, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Suputtamongkol, Y.; Avirutnan, P.; Mairiang, D.; Angkasekwinai, N.; Niwattayakul, K.; Yamasmith, E.; Saleh-Arong, F.A.; Songjaeng, A.; Prommool, T.; Tangthawornchaikul, N.; Puttikhunt, C.; Hunnangkul, S.; Komoltri, C.; Thammapalo, S.; Malasit, P. Ivermectin accelerates circulating nonstructural protein 1 (NS1) clearance in adult dengue patients: a combined phase 2/3 randomized double-blinded placebo controlled trial. Clin. Infect. Dis. 2021, 72, e586–e593. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Y.; Fraser, J.E.; Chan, W.K.; Moreland, N.J.; Rathore, A.P.; Wang, C.; Vasudevan, S.G.; Jans, D.A. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013, 99, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Jitobaom, K.; Boonarkart, C.; Manopwisedjaroen, S.; Punyadee, N.; Borwornpinyo, S.; Thitithanyanont, A.; Avirutnan, P.; Auewarakul, P. Synergistic anti-SARS-CoV-2 activity of repurposed anti-parasitic drug combinations. BMC Pharmacol. Toxicol. 2022, 23, 41. [Google Scholar] [CrossRef]
- Tan, Y.L.; Tan, K.S.W.; Chu, J.J.H.; Chow, V.T. Combination treatment with remdesivir and ivermectin exerts highly synergistic and potent antiviral activity against murine coronavirus infection. Front. Cell Infect. Microbiol. 2021, 11, 700502. [Google Scholar] [CrossRef]
- Errecalde, J.; Lifschitz, A.; Vecchioli, G.; Ceballos, L.; Errecalde, F.; Ballent, M.; Marín, G.; Daniele, M.; Turic, E.; Spitzer, E.; Toneguzzo, F.; Gold, S.; Krolewiecki, A.; Alvarez, L.; Lanusse, C. Safety and pharmacokinetic assessments of a novel ivermectin nasal spray formulation in a pig model. J. Pharm. Sci. 2021, 110, 2501–2507. [Google Scholar] [CrossRef]
- Varghese, F.S.; Kaukinen, P.; Gläsker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kümmerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Slońska, A.; Cymerys, J.; Skwarska, J.; Golke, A.; Bańbura, M.W. Influence of importin alpha/beta and exportin 1 on equine herpesvirus type 1 (EHV-1) replication in primary murine neurons. Pol. J. Vet. Sci. 2013, 16, 749–751. [Google Scholar] [CrossRef]
- Lv, C.; Liu, W.; Wang, B.; Dang, R.; Qiu, L.; Ren, J.; Yan, C.; Yang, Z.; Wang, X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 2018, 159, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Döhner, K.; Ramos-Nascimento, A.; Bialy, D.; Anderson, F.; Hickford-Martinez, A.; Rother, F.; Koithan, T.; Rudolph, K.; Buch, A.; Prank, U.; Binz, A.; Hügel, S.; Lebbink, R.J.; Hoeben, R.C.; Hartmann, E.; Bader, M.; Bauerfeind, R.; Sodeik, B. Importin α1 is required for nuclear import of herpes simplex virus proteins and capsid assembly in fibroblasts and neurons. PLoS Pathog. 2018, 14, e1006823. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, D.H.; Min, K.A.; Maeng, H.-J.; Jang, D.-J.; Cho, K.H. Preparation, characterization, and in vitro release of chitosan-ecabet electrolyte complex for the mucosal delivery. J. Nanosci. Nanotechnol. 2019, 19, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, X.; Liu, M.; Li, H.; Jiang, Y.; Zhao, L.; Gu, J. Determination of ecabet in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2008, 863, 223–228. [Google Scholar] [CrossRef]
- Wei, Y.; He, J.; Qin, H.; Wu, X.; Yao, X. Determination of ferruginol in rat plasma via high-performance liquid chromatography and its application in pharmacokinetics study. Biomed. Chromatogr. 2009, 23, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- De Forni, D.; Poddesu, B.; Cugia, G.; Chafouleas, J.; Lisziewicz, J.; Lori, F. Synergistic drug combinations designed to fully suppress SARS-CoV-2 in the lung of COVID-19 patients. PLoS One 2022, 17, e0276751. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille, C.; Lisco, A.; Introini, A.; Grivel, J.C.; Munawwar, A.; Merbah, M.; Schinazi, R.F.; Derudas, M.; McGuigan, C.; Balzarini, J.; Margolis, L. Exploiting the anti-HIV-1 activity of acyclovir: suppression of primary and drug-resistant HIV isolates and potentiation of the activity by ribavirin. Antimicrob. Agents Chemother. 2012, 56, 2604–2611. [Google Scholar] [CrossRef] [PubMed]
- Mediouni, S.; Jablonski, J.A.; Tsuda, S.; Barsamian, A.; Kessing, C.; Richard, A.; Biswas, A.; Toledo, F.; Andrade, V.M.; Even, Y.; Stevenson, M.; Tellinghuisen, T.; Choe, H.; Cameron, M.; Bannister, T. D.; Valente, S. T. Oregano oil and its principal component, carvacrol, inhibit HIV-1 fusion into target cells. J. Virol. 2020, 94, e00147–20. [Google Scholar] [CrossRef]
- Lai, W.-L.; Chuang, H.-S.; Lee, M.-H.; Wei, C.-L.; Lin, C.-F.; Tsai, Y.-C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Shah, M.; Kumar, S. Role of cholesterol in anatid herpesvirus 1 infections in vitro. Virus Res. 2020, 290, 198174. [Google Scholar] [CrossRef]
- Glitscher, M.; Hildt, E. Endosomal Cholesterol in Viral Infections - A Common Denominator? Front Physiol. 2021, 12, 750544. [Google Scholar] [CrossRef]
- Martínez-Gutierrez, M.; Castellanos, J.E.; Gallego-Gómez, J.C. Statins reduce dengue virus production via decreased virion assembly. Intervirology 2011, 54, 202–216. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.A.; Jimenez-Jarava, O.J. Actividad anti-chikungunya de los aceites esenciales de plantas pertenecientes a las familias verbenaceae, piperaceae, poaceae, lamiaceae, lauraceae y myrtaceae: estudios de docking molecular. In Entre Ciencia e Ingenieria 4; Pereira da Silva, A.F., Ed.; Atena Editora: Ponta Grossa, Brazil, 2022. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, Q.; Hu, P.; Huang, X.; Yang, M.; Ren, G.; Du, Q.; Luo, J.; Zhang, K.; Li, J.; Wu, H.; Guo, Y.; Liu, S. Sedative and hypnotic effects of compound Anshen essential oil inhalation for insomnia. BMC Complement. Altern. Med. 2019, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Tran, M.; González, M.A.; Gautam, L.N.; Connelly, M.; Wood, R.K.; Fatima, I.; Miranda-Carboni, G.; Rivas, F. (+)-Dehydroabietylamine derivatives target triple-negative breast cancer. Eur. J. Med. Chem. 2015, 102, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S. Novel antiviral agents: synthesis, molecular modelling studies and biological investigation. Viruses 2023, 15, 2042. [Google Scholar] [CrossRef] [PubMed]
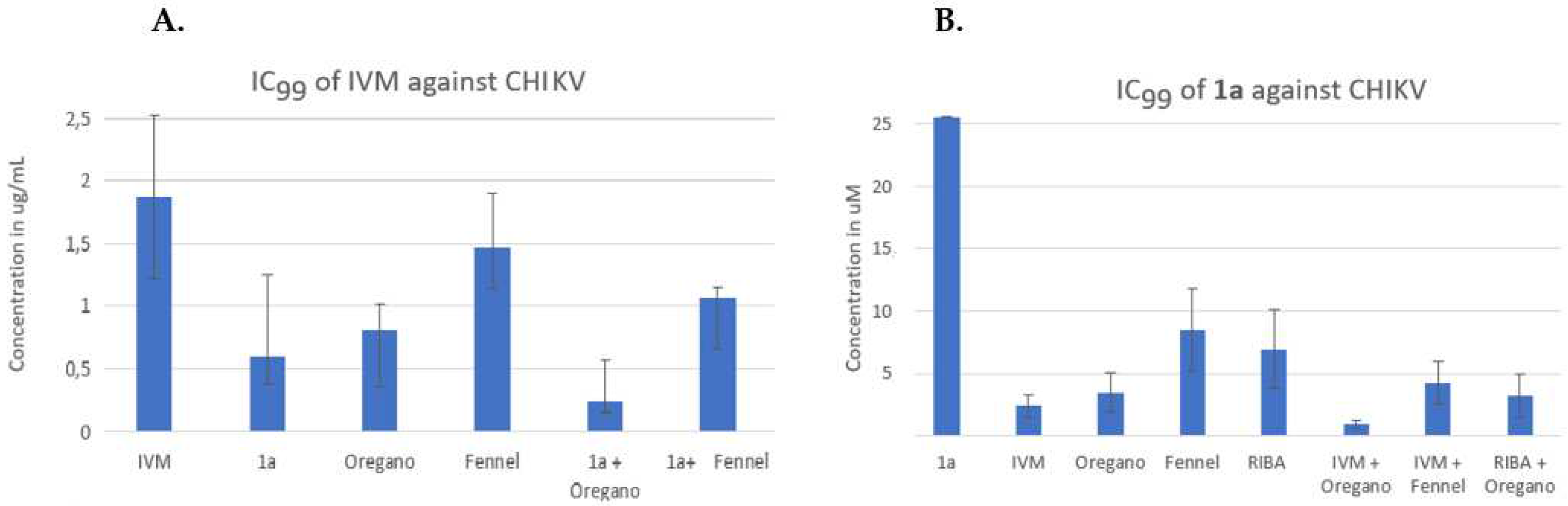
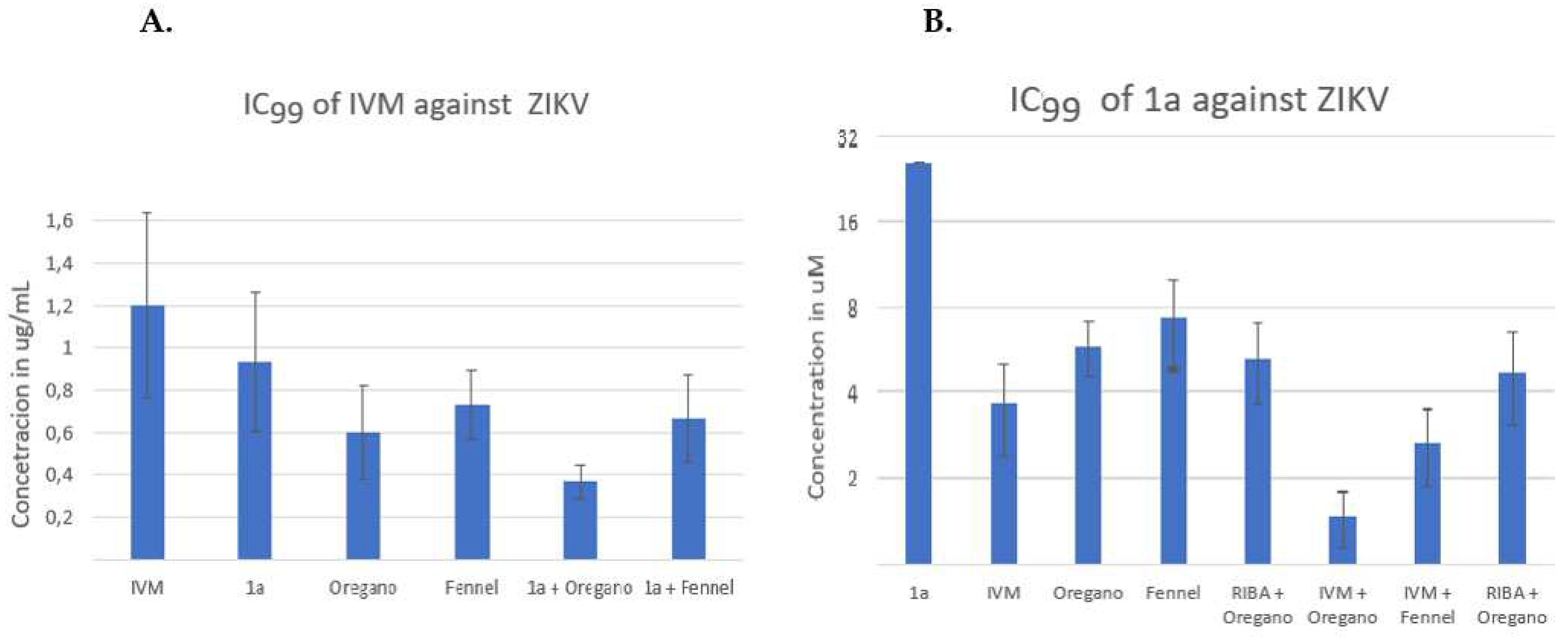
| ZIKV | CHIKV | HHV-2 | |||||
|---|---|---|---|---|---|---|---|
| Compounds | CC100 | IC99 | SI | IC99 | SI | IC99 | SI |
| 1a, µM | 410 | 25,6 | 16 | 25,6 | 16 | 25,6 | 16 |
| IVM, µg/mL | 6.4 | 1,2 ± 0,4 | 5,3 | 1,8 ± 0,6 | 3,5 | 3,2* | 2 |
| Oregano, ppm | 18,6 ± 0,6 | 8 | 2,3 | 3,6 ± 0,7 | 5,1 | 5,3 ± 1,8 | 3,5 |
| Fennel, ppm | 64 | 12 ± 4 | 5,3 | 14,6 ± 2,9 | 4,4 | 12 ± 4 | 5,3 |
| RIBA, µM | >700 | 176 | >4 | 44 | >16 | 176 | >4 |
| ACV, µM | >660 | NT | -- | NT | -- | 6,6 | >100 |
| Heparin, U.I/mL | 640 | NT | -- | NT | -- | 10 | 64 |
| RIBA/ACV 1.3 µM | NT | NT | -- | NT | -- | 36,6 ± 10 | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





