Submitted:
22 September 2023
Posted:
25 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
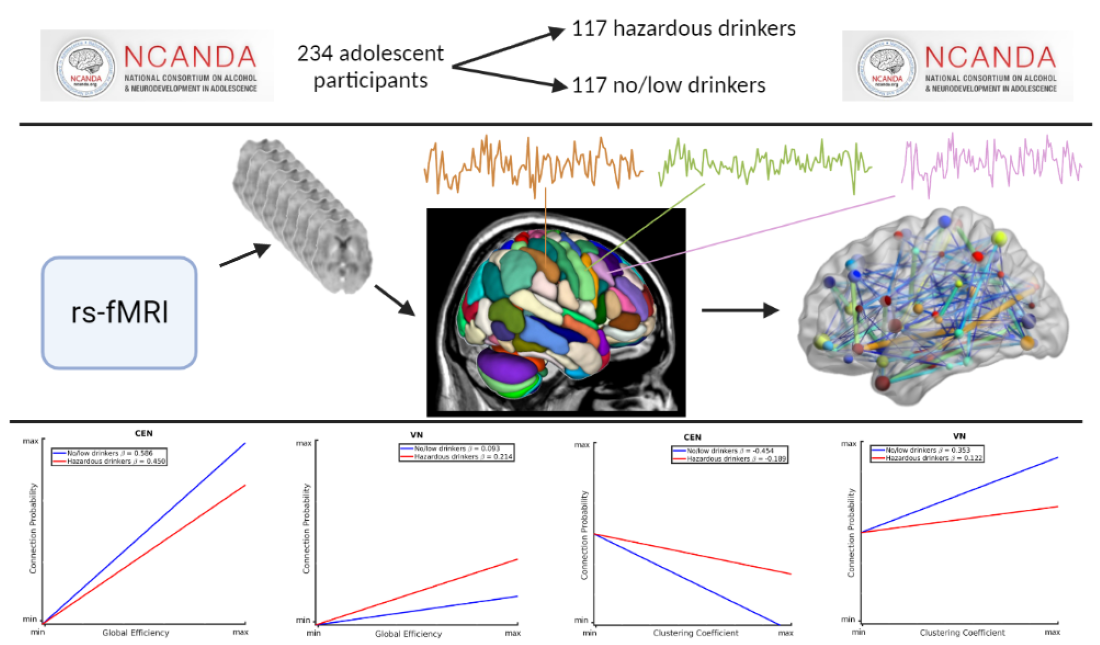
2.1. Participants
2.2. MRI Acquisition and Processing
2.3. Statistical Analysis
3. Results
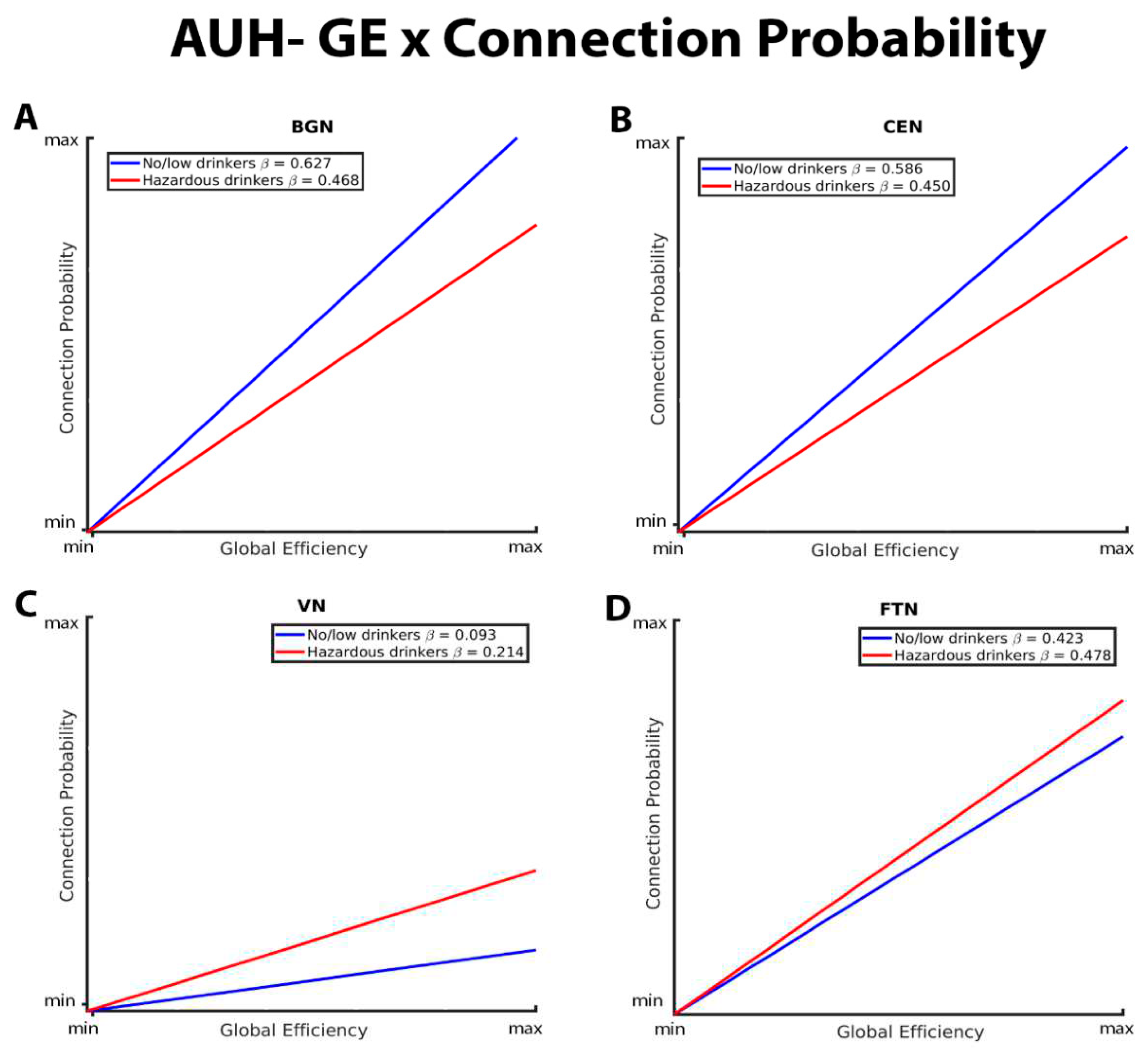
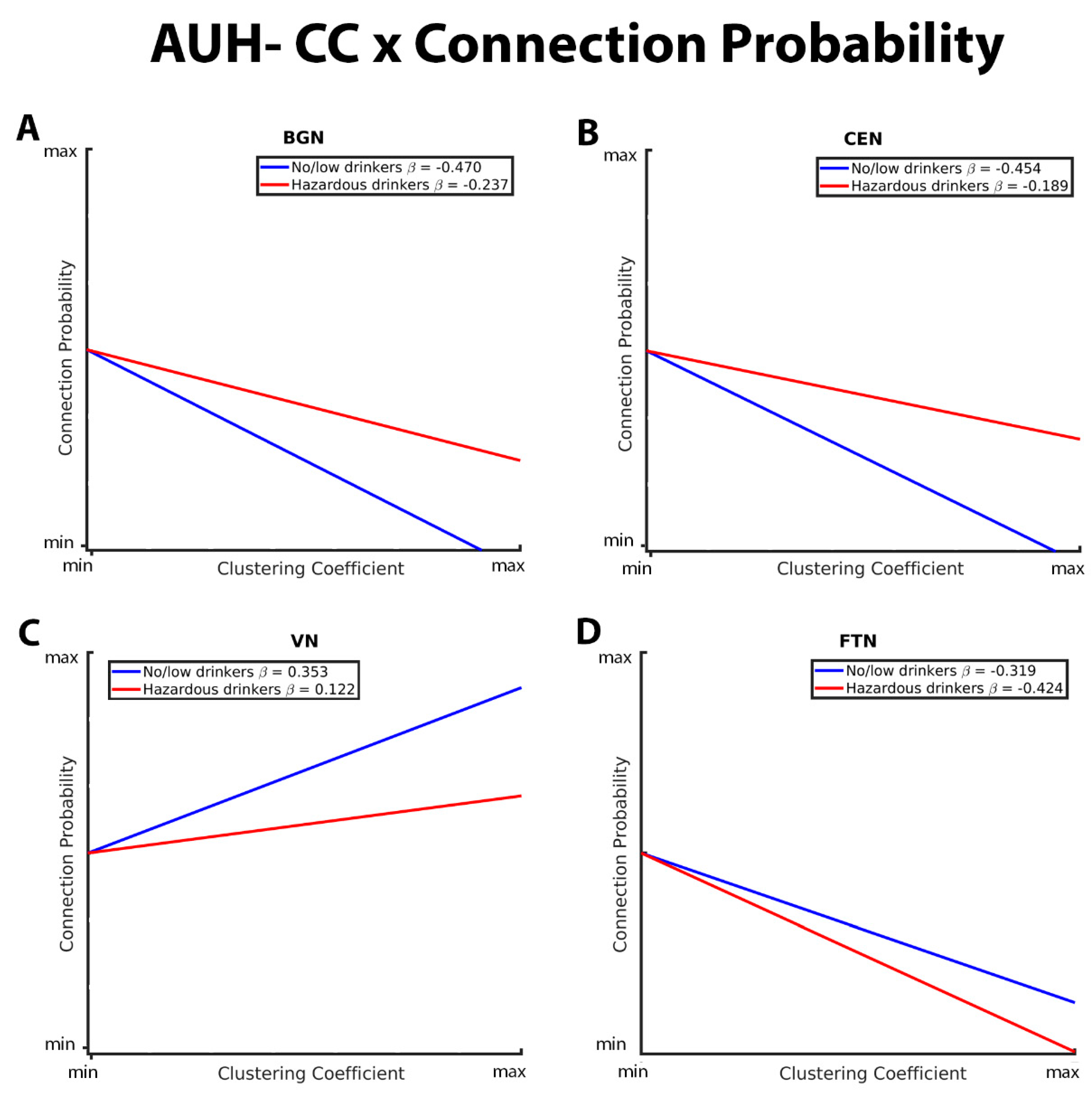
3.1. Mixed-Effects Results from Connection Probability Models
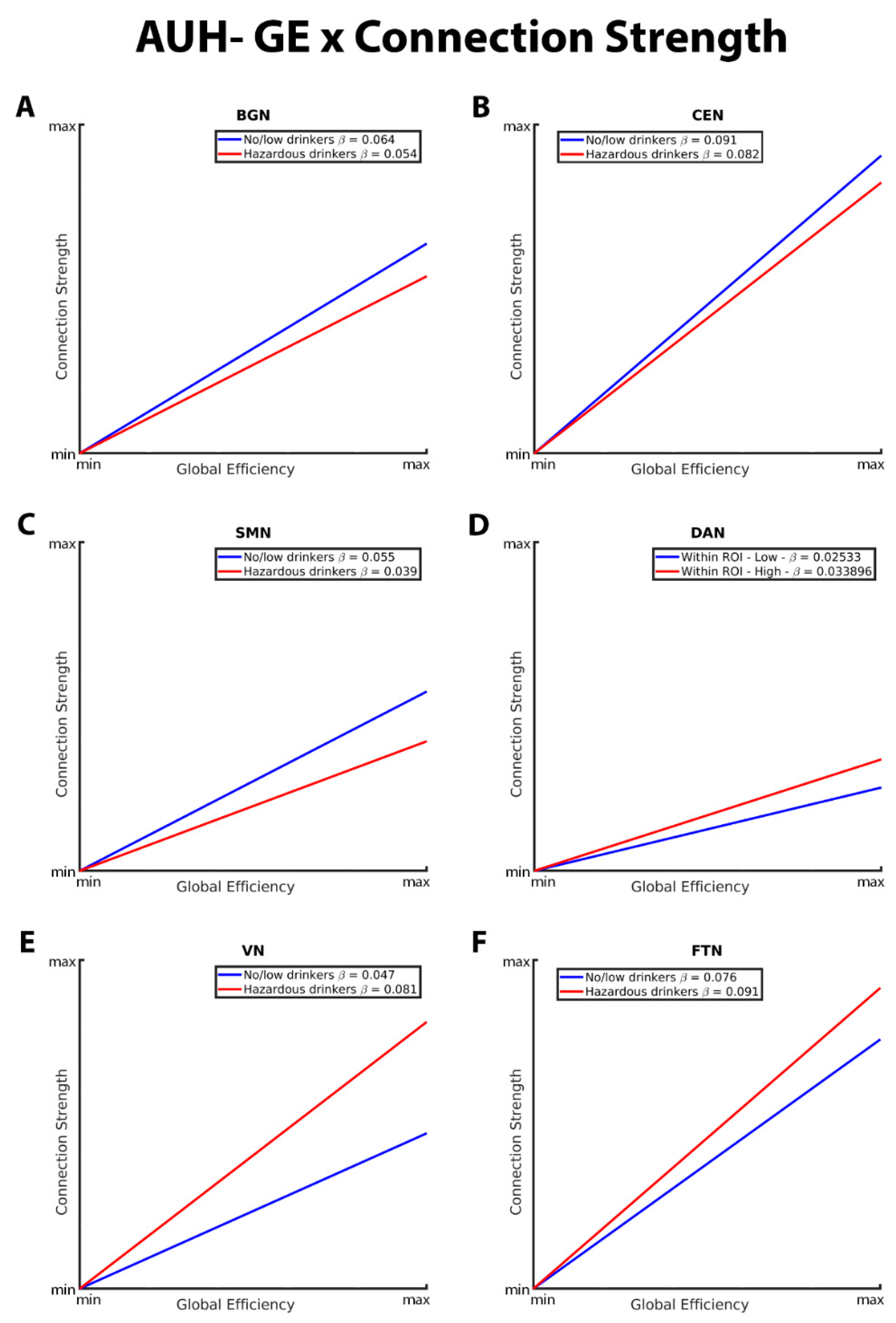
3.2. Mixed-Effects Results from Connection Strength Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giedd, J.N. Structural Magnetic Resonance Imaging of the Adolescent Brain. Ann. New York Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Tessner, K.D.; Toga, A.W. Mapping Continued Brain Growth and Gray Matter Density Reduction in Dorsal Frontal Cortex: Inverse Relationships during Postadolescent Brain Maturation. J. Neurosci. 2001, 21, 8819–8829. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Tallal, P. LATE CHILDHOOD CHANGES IN BRAIN MORPHOLOGY OBSERVABLE WITH MRI. Dev. Med. Child Neurol. 1990, 32, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lenroot, R.K.; Giedd, J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006, 30, 718–729. [Google Scholar] [CrossRef]
- Moorman, D.E. The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2018, 87 Pt A, 85–107. [Google Scholar] [CrossRef]
- Blakemore, S.-J. Imaging brain development: The adolescent brain. NeuroImage 2012, 61, 397–406. [Google Scholar] [CrossRef]
- Barnea-Goraly, N.; Menon, V.; Eckert, M.; Tamm, L.; Bammer, R.; Karchemskiy, A.; Dant, C.C.; Reiss, A.L. White Matter Development During Childhood and Adolescence: A Cross-sectional Diffusion Tensor Imaging Study. Cereb. Cortex 2005, 15, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P.; Bourgeois, J.P.; Goldman-Rakic, P.S. Synaptic development of the cerebral cortex: implications for learning, memory, and mental illness. Prog Brain Res 1994, 102, 227–243. [Google Scholar]
- Bick, J.; Nelson, C.A. Early Adverse Experiences and the Developing Brain. Neuropsychopharmacology 2016, 41, 177–196. [Google Scholar] [CrossRef]
- Hart, H.; Rubia, K. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci. 2012, 6, 52. [Google Scholar] [CrossRef]
- Casey, B.J. , Jones, R. M.; Hare, T.A. The adolescent brain. Ann N Y Acad Sci 2008, 1124, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Winters, K.C.; Arria, A. Adolescent Brain Development and Drugs. Prev Res 2011, 18, 21–24. [Google Scholar]
- DeWitt, S.J.; Aslan, S.; Filbey, F.M. Adolescent risk-taking and resting state functional connectivity. Psychiatry Res. 2014, 222, 157–164. [Google Scholar] [CrossRef]
- NIAAA. Underage Drinking. October 2022 [cited 2022; Available from: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
- Mota, N.; Parada, M.; Crego, A.; Doallo, S.; Caamaño-Isorna, F.; Holguín, S.R.; Cadaveira, F.; Corral, M. Binge drinking trajectory and neuropsychological functioning among university students: A longitudinal study. Drug Alcohol Depend. 2013, 133, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.L.; Medina, K.L.; Padula, C.B.; Tapert, S.F.; Brown, S.A. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J. Child Adolesc. Subst. Abus. 2011, 20, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.L.; Cummins, K.; Tapert, S.F.; Brown, S.A. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol. Addict. Behav. 2011, 25, 127–142. [Google Scholar] [CrossRef]
- Winward, J.L.; Hanson, K.L.; Tapert, S.F.; Brown, S.A. Heavy Alcohol Use, Marijuana Use, and Concomitant Use by Adolescents Are Associated with Unique and Shared Cognitive Decrements. J. Int. Neuropsychol. Soc. 2014, 20, 784–795. [Google Scholar] [CrossRef]
- Nguyen-Louie, T.T.; Tracas, A.; Squeglia, L.M.; Matt, G.E.; Eberson-Shumate, S.; Tapert, S.F. Learning and Memory in Adolescent Moderate, Binge, and Extreme-Binge Drinkers. Alcohol. Clin. Exp. Res. 2016, 40, 1895–1904. [Google Scholar] [CrossRef]
- Lees, B.; Meredith, L.R.; Kirkland, A.E.; Bryant, B.E.; Squeglia, L.M. Effect of alcohol use on the adolescent brain and behavior. Pharmacol. Biochem. Behav. 2020, 192, 172906. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Spadoni, A.D.; Infante, M.A.; Myers, M.G.; Tapert, S.F. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 2009, 23, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, L.M.; Tapert, S.F.; Sullivan, E.V.; Jacobus, J.; Meloy, M.; Rohlfing, T.; Pfefferbaum, A.; Luby, J.L.; Agrawal, A.; Belden, A.; et al. Brain Development in Heavy-Drinking Adolescents. 2015, 172, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Kwon, D.; Brumback, T.; Thompson, W.K.; Cummins, K.; Tapert, S.F.; Brown, S.A.; Colrain, I.M.; Baker, F.C.; Prouty, D.; et al. Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am. J. Psychiatry 2018, 175, 370–380. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; et al. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry 2000, 157, 737–744. [Google Scholar] [CrossRef]
- De Bellis, M.D.; et al. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res 2005, 29, 1590–1600. [Google Scholar] [CrossRef]
- Jacobus, J.; Tapert, S.F. Neurotoxic Effects of Alcohol in Adolescence. Annu. Rev. Clin. Psychol. 2013, 9, 703–721. [Google Scholar] [CrossRef]
- Tapert, S.F.; Schweinsburg, A.D.; Barlett, V.C.; Brown, S.A.; Frank, L.R.; Brown, G.G.; Meloy, M.J. Blood Oxygen Level Dependent Response and Spatial Working Memory in Adolescents With Alcohol Use Disorders. Alcohol. Clin. Exp. Res. 2004, 28, 1577–1586. [Google Scholar] [CrossRef]
- Tapert, S.F.; Brown, G.G.; Kindermann, S.S.; Cheung, E.H.; Frank, L.R.; A Brown, S. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res 2001, 25, 236–45. [Google Scholar] [CrossRef]
- Xiao, L.; Bechara, A.; Gong, Q.; Huang, X.; Li, X.; Xue, G.; Wong, S.; Lu, Z.-L.; Palmer, P.; Wei, Y.; et al. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol. Addict. Behav. 2013, 27, 443–454. [Google Scholar] [CrossRef]
- Norman, A.L.; Pulido, C.; Squeglia, L.M.; Spadoni, A.D.; Paulus, M.P.; Tapert, S.F. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011, 119, 216–223. [Google Scholar] [CrossRef]
- Gonçalves, S.F.; Turpyn, C.C.; Niehaus, C.E.; Mauro, K.L.; Hinagpis, C.L.; Thompson, J.C.; Chaplin, T.M. Neural activation to loss and reward among alcohol naive adolescents who later initiate alcohol use. Dev. Cogn. Neurosci. 2021, 50, 100978. [Google Scholar] [CrossRef]
- Müller-Oehring, E.M.; Kwon, D.; Nagel, B.J.; Sullivan, E.V.; Chu, W.; Rohlfing, T.; Prouty, D.; Nichols, B.N.; Poline, J.-B.; Tapert, S.F.; et al. Influences of Age, Sex, and Moderate Alcohol Drinking on the Intrinsic Functional Architecture of Adolescent Brains. Cereb. Cortex 2017, 28, 1049–1063. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Schmidt, A.; Denier, N.; Magon, S.; Radue, E.-W.; Huber, C.G.; Riecher-Rossler, A.; A Wiesbeck, G.; E Lang, U.; Borgwardt, S.; Walter, M. Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Transl. Psychiatry 2015, 5, e533–e533. [Google Scholar] [CrossRef] [PubMed]
- Gozdas, E.; Holland, S.K.; Altaye, M. ; CMIND Authorship Consortium Developmental changes in functional brain networks from birth through adolescence. Hum. Brain Mapp. 2019, 40, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Cohen, A.L.; Power, J.D.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Schlaggar, B.L.; Petersen, S.E. Functional Brain Networks Develop from a “Local to Distributed” Organization. PLOS Comput. Biol. 2009, 5, e1000381. [Google Scholar] [CrossRef]
- Lebel, C.; Beaulieu, C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosc 2011, 31, 10937–10947. [Google Scholar] [CrossRef]
- Power, J.D.; Fair, D.A.; Schlaggar, B.L.; Petersen, S.E. The Development of Human Functional Brain Networks. Neuron 2010, 67, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Whelan, R.; Conrod, P.J.; Poline, J.-B.; Lourdusamy, A.; Banaschewski, T.; Barker, G.J.; A Bellgrove, M.; Büchel, C.; Byrne, M.; et al.; the IMAGEN Consortium Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat. Neurosci. 2012, 15, 920–925. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Aoki, Y. Intrinsic Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Science in Development. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2016, 1, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Samea, F.; Soluki, S.; Nejati, V.; Zarei, M.; Cortese, S.; Eickhoff, S.B.; Tahmasian, M.; Eickhoff, C.R. Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neurosci. Biobehav. Rev. 2019, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Hernandez, L.M.; Bookheimer, S.Y.; Dapretto, M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 2019, 12, 53–65. [Google Scholar] [CrossRef]
- Belmonte, M.K.; Allen, G.; Beckel-Mitchener, A.; Boulanger, L.M.; Carper, R.A.; Webb, S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004, 24, 9228–9231. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Supekar, K.; Menon, V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013, 7, 458. [Google Scholar] [CrossRef]
- Reese, E.D.; Yi, J.Y.; McKay, K.G.; Stein, E.A.; Ross, T.J.; Daughters, S.B. Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use. J. Clin. Med. 2019, 8, 2135. [Google Scholar] [CrossRef]
- Zhao, Q.; Sullivan, E.V.; Műller-Oehring, E.M.; Honnorat, N.; Adeli, E.; Podhajsky, S.; Baker, F.C.; Colrain, I.M.; Prouty, D.; Tapert, S.F.; et al. Adolescent alcohol use disrupts functional neurodevelopment in sensation seeking girls. Addict. Biol. 2020, 26, e12914. [Google Scholar] [CrossRef]
- Goldfarb, E.V.; Scheinost, D.; Fogelman, N.; Seo, D.; Sinha, R. High-Risk Drinkers Engage Distinct Stress-Predictive Brain Networks. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2022, 7, 805–813. [Google Scholar] [CrossRef]
- Holcomb, L.A.; Huang, S.; Cruz, S.M.; Marinkovic, K. Neural oscillatory dynamics of inhibitory control in young adult binge drinkers. Biol. Psychol. 2019, 146, 107732. [Google Scholar] [CrossRef]
- Bahrami, M.; Laurienti, P.J.; Simpson, S.L. A MATLAB toolbox for multivariate analysis of brain networks. Hum. Brain Mapp. 2019, 40, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, M.; Laurienti, P.J.; Simpson, S.L. Analysis of brain subnetworks within the context of their whole-brain networks. Hum. Brain Mapp. 2019, 40, 5123–5141. [Google Scholar] [CrossRef]
- Simpson, S.L.; Laurienti, P.J. A two-part mixed-effects modeling framework for analyzing whole-brain network data. NeuroImage 2015, 113, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Brumback, T.; Tomlinson, K.; Cummins, K.; Thompson, W.K.; Nagel, B.J.; De Bellis, M.D.; Hooper, S.R.; Clark, D.B.; Chung, T.; et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J. Stud. Alcohol Drugs 2015, 76, 895–908. [Google Scholar] [CrossRef]
- Friedman, E.J.; Landsberg, A.S.; Owen, J.P.; Li, Y.-O.; Mukherjee, P. Stochastic geometric network models for groups of functional and structural connectomes. NeuroImage 2014, 101, 473–484. [Google Scholar] [CrossRef]
- Bahrami, M.; Simpson, S.L.; Burdette, J.H.; Lyday, R.G.; Quandt, S.A.; Chen, H.; Arcury, T.A.; Laurienti, P.J. Altered default mode network associated with pesticide exposure in Latinx children from rural farmworker families. NeuroImage 2022, 256, 119179–119179. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Fair, D.A.; Dosenbach, N.U.F.; Church, J.A.; Cohen, A.L.; Brahmbhatt, S.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. USA 2007, 104, 13507–13512. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press, 2006. [Google Scholar]
- Muller, A.M.; Meyerhoff, D.J. Maladaptive brain organization at 1 month into abstinence as an indicator for future relapse in patients with alcohol use disorder. Eur. J. Neurosci. 2021, 53, 2923–2938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, N.; Liu, H.; Zheng, H.; Zheng, W. Dynamic connectivity patterns of resting-state brain functional networks in healthy individuals after acute alcohol intake. Front. Neurosci. 2022, 16, 974778. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Cservenka, A. Adolescence and drug use vulnerability: findings from neuroimaging. Curr. Opin. Behav. Sci. 2017, 13, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Sawyer, K.S.; Levy, S.; Harris, G.J.; Oscar-Berman, M. Intrinsic brain functional connectivity patterns in alcohol use disorder. Brain Commun. 2022, 4, fcac290. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621–a009621. [Google Scholar] [CrossRef]
- Renteria, R.; Baltz, E.T.; Gremel, C.M. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat. Commun. 2018, 9, 211. [Google Scholar] [CrossRef]
- Sitzia, G.; Lovinger, D.M. Circuit dysfunctions of associative and sensorimotor basal ganglia loops in alcohol use disorder: insights from animal models. Addict. Neurosci. 2023, 5. [Google Scholar] [CrossRef]
- Rzepecki-Smith, C.I.; Meda, S.A.; Calhoun, V.D.; Stevens, M.C.; Jafri, M.J.; Astur, R.S.; Pearlson, G.D. Disruptions in Functional Network Connectivity During Alcohol Intoxicated Driving. Alcohol. Clin. Exp. Res. 2010, 34, 479–487. [Google Scholar] [CrossRef]
- Ersche, K.D.; Meng, C.; Ziauddeen, H.; Stochl, J.; Williams, G.B.; Bullmore, E.T.; Robbins, T.W. Brain networks underlying vulnerability and resilience to drug addiction. Proc. Natl. Acad. Sci. USA 2020, 117, 15253–15261. [Google Scholar] [CrossRef]
- Chenji, S.; Jha, S.; Lee, D.; Brown, M.; Seres, P.; Mah, D.; Kalra, S. Investigating Default Mode and Sensorimotor Network Connectivity in Amyotrophic Lateral Sclerosis. PLOS ONE 2016, 11, e0157443. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.; Shah, R.; Nooner, K.B.; Nagel, B.J.; Tapert, S.F.; de Bellis, M.D.; Mishra, J. Impact of Childhood Trauma on Executive Function in Adolescence—Mediating Functional Brain Networks and Prediction of High-Risk Drinking. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2020, 5, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Bracht, T.; Soravia, L.; Moggi, F.; Stein, M.; Grieder, M.; Federspiel, A.; Tschümperlin, R.; Batschelet, H.M.; Wiest, R.; Denier, N. The role of the orbitofrontal cortex and the nucleus accumbens for craving in alcohol use disorder. Transl. Psychiatry 2021, 11, 267. [Google Scholar] [CrossRef]
- Myrick, H.; Anton, R.F.; Li, X.; Henderson, S.; Drobes, D.; Voronin, K.; George, M.S. Differential Brain Activity in Alcoholics and Social Drinkers to Alcohol Cues: Relationship to Craving. Neuropsychopharmacology 2003, 29, 393–402. [Google Scholar] [CrossRef]
- Maia, T.V.; Cooney, R.E.; Peterson, B.S. The neural bases of obsessive–compulsive disorder in children and adults. Dev. Psychopathol. 2008, 20, 1251–1283. [Google Scholar] [CrossRef]
- Peters, S.; Peper, J.S.; Van Duijvenvoorde, A.C.; Braams, B.R.; Crone, E.A. Amygdala–orbitofrontal connectivity predicts alcohol use two years later: a longitudinal neuroimaging study on alcohol use in adolescence. Dev. Sci. 2017, 20. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Abnormal Functional Connectivity in Children with Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2012, 71, 443–450. [Google Scholar] [CrossRef]
- Sami, M.B.; A McCutcheon, R.; Ettinger, U.; Williams, S.; Lythgoe, D.; McGuire, P.; Bhattacharyya, S. Cannabis Use Linked to Altered Functional Connectivity of the Visual Attentional Connectivity in Patients With Psychosis and Controls. Schizophr. Bull. Open 2020, 1. [Google Scholar] [CrossRef]
- Menon, V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 2013, 17, 627–640. [Google Scholar] [CrossRef]
- Canessa, N.; Basso, G.; Carne, I.; Poggi, P.; Gianelli, C. Increased decision latency in alcohol use disorder reflects altered resting-state synchrony in the anterior salience network. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.-W.; Hwang, S.; Cheong, C. Functional and Structural Alteration of Default Mode, Executive Control, and Salience Networks in Alcohol Use Disorder. Front. Psychiatry 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- (NIAAA), N.I.o.A.A.a.A., Alcohol screening and brief intervention for youth: A practitioner's guide, N.I.o.H. (NIH), Editor. 2011: Bethesda, MD.
- dministration, S.A.a.M.H.S., Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings, NSDUH, Editor. 2012: Rockville, MD.
- Benjamini, Y.; Hochberg, Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing With Independent Statistics. Journal of Educational and Behavioral Statistics 2000, 25, 60–83. [Google Scholar] [CrossRef]
| Age | Maximum drinks per occasion: Female | Maximum drinks per occasion: Male | Total days of drinking in lifetime | |
|---|---|---|---|---|
| 12-13.9 | ≤ 3 | ≤ 3 | ≤ 5 | |
| 14-15.9 | ≤ 3 | ≤ 4 | ≤ 5 | |
| 16-16.9 | ≤ 3 | ≤ 4 | ≤ 11 | |
| 17-17.9 | ≤ 3 | ≤ 4 | ≤ 23 | |
| 18-19.9 | ≤ 3 | ≤ 4 | ≤ 51 | |
| ≥ 20 | ≤ 3 | ≤ 5 | ≤ 51 |
| Matched Groups | |||||
|---|---|---|---|---|---|
| No/low drinkers | Hazardous drinkers | No/low drinkers | |||
| Total | 581 | 117 | 117 | ||
| Girls/Boys | 306/275 | 62/55 | 62/55 | ||
| Age | Girls | 15.9 ± 2.4 | 18.6 ± 2 | 18.4 ± 1.9 | |
| Boys | 15.9 ± 2.3 | 18.7 ± 1.9 | 18.4 ± 1.7 | ||
| GE/Siemens | 385/196 | 80/37 | 72/45 | ||
| Pubertal Development Scale | Girls | 3.4 ± 0.6 | 3.8 ± 0.2 | 3.8 ± 0.3 | |
| Boys | 2.9 ± 0.7 | 3.5 ± 0.5 | 3.5 ± 0.4 | ||
| Alcohol use | # days lifetime | 1.3 ± 4.1 | 50.6 ± 75.5 | 3.1 ± 7.2 | |
| # days past year | 0.7 ± 2.9 | 23.2 ± 31.8 | 1.8 ± 4.8 | ||
| Nicotine use | # cigarettes lifetime | 0.3 ± 2.4 | 11.4 ± 45.3 | 0.7 ± 4.7 | |
| # cigarettes past year | 0.1 ± 1.3 | 6 ± 28.1 | 0.3 ± 2.3 | ||
| Marijuana use | # days lifetime | 0.6 ± 2.5 | 10.8 ± 17.7 | 1 ± 3.9 | |
| # days past year | 0.3 ± 1.6 | 7.5 ± 16 | 0.6 ± 2.5 | ||
| Parental education (years) | 16.9 ± 2.4 | 17.4 ± 2 | 17 ± 2 | ||
| Estimate | SE | t Value | P-Value | |||
|---|---|---|---|---|---|---|
| Basal Ganglia Network (BGN) | ||||||
| GE*AUH | -0.02109 | 0.01815 | -1.16 | 0.2453 | ||
| CC*AUH | 0.01752 | 0.02166 | 0.81 | 0.4187 | ||
| GE*AUH*BGN | -0.1386 | 0.01973 | -7.03 | <.0001 | ||
| CC*AUH*BGN | 0.2160 | 0.02256 | 9.57 | <.0001 | ||
| Central Executive Network (CEN) | ||||||
| GE*AUH | -0.02511 | 0.01873 | -1.34 | 0.1802 | ||
| CC*AUH | 0.02172 | 0.02240 | 0.97 | 0.3323 | ||
| GE*AUH*CEN | -0.1113 | 0.02654 | -4.19 | <.0001 | ||
| CC*AUH*CEN | 0.2436 | 0.02663 | 9.15 | <.0001 | ||
| Visual Network (VN) | ||||||
| GE*AUH | -0.02992 | 0.01942 | -1.54 | 0.1234 | ||
| CC*AUH | 0.02984 | 0.02357 | 1.27 | 0.2055 | ||
| GE*AUH*VN | 0.1504 | 0.05072 | 2.97 | 0.0030 | ||
| CC*AUH*VN | -0.2611 | 0.04617 | -5.65 | <.0001 | ||
| Fronto-Temporal Network (FTN) | ||||||
| GE*AUH | -0.02687 | 0.01839 | -1.46 | 0.1440 | ||
| CC*AUH | 0.02617 | 0.02215 | 1.18 | 0.2374 | ||
| GE*AUH*FTN | 0.08199 | 0.03451 | 2.38 | 0.0175 | ||
| CC*AUH*FTN | -0.1313 | 0.03949 | -3.33 | 0.0009 | ||
| Sensorimotor Network (SMN) | ||||||
| GE*AUH | -0.02580 | 0.01811 | -1.42 | 0.1543 | ||
| CC*AUH | 0.02294 | 0.02158 | 1.06 | 0.2878 | ||
| GE*AUH*SMN | 0.07347 | 0.02388 | 3.08 | 0.0021 | ||
| CC*AUH*SMN | -0.07970 | 0.02145 | -3.72 | 0.0002 | ||
| Default Mode Network (DMN) | ||||||
| GE*AUH | -0.02346 | 0.01925 | -1.22 | 0.2229 | ||
| CC*AUH | 0.02431 | 0.02364 | 1.03 | 0.3037 | ||
| GE*AUH*DMN | 0.06644 | 0.02115 | 3.14 | 0.0017 | ||
| CC*AUH*DMN | -0.04831 | 0.02071 | -2.33 | 0.0196 | ||
| Estimate | SE | t Value | P-Value | |
|---|---|---|---|---|
| GE*AUH within BGN | -0.1597 | 0.02648 | -6.03 | <.0001 |
| CC*AUH within BGN | 0.2335 | 0.03098 | 7.54 | <.0001 |
| GE*AUH within CEN | -0.1364 | 0.03221 | -4.24 | <.0001 |
| CC*AUH within CEN | 0.2653 | 0.03452 | 7.69 | <.0001 |
| GE*AUH within VN | 0.1205 | 0.05416 | 2.23 | 0.0261 |
| CC*AUH within VN | -0.2312 | 0.05165 | -4.48 | <.0001 |
| GE*AUH within FTN | 0.05511 | 0.03888 | 1.42 | 0.1564 |
| CC*AUH within FTN | -0.1051 | 0.04506 | -2.33 | 0.0196 |
| GE*AUH within SMN | 0.04766 | 0.02970 | 1.60 | 0.1085 |
| CC*AUH within SMN | -0.05676 | 0.03014 | -1.88 | 0.0597 |
| GE*AUH within DMN | 0.04298 | 0.02827 | 1.52 | 0.1285 |
| CC*AUH within DMN | -0.02400 | 0.03110 | -0.77 | 0.4403 |
| Estimate | SE | t Value | P-Value | |
|---|---|---|---|---|
| Basal Ganglia Network (BGN) | ||||
| GE*AUH | 0.001081 | 0.001052 | 1.03 | 0.3040 |
| CC*AUH | 0.000708 | 0.001478 | 0.48 | 0.6319 |
| GE*AUH*BGN | -0.01102 | 0.001897 | -5.81 | <.0001 |
| CC*AUH*BGN | 0.005730 | 0.002092 | 2.74 | 0.0062 |
| Central Executive Network (CEN) | ||||
| GE*AUH | 0.000684 | 0.001050 | 0.65 | 0.5151 |
| CC*AUH | 0.000676 | 0.001487 | 0.45 | 0.6496 |
| GE*AUH*CEN | -0.00889 | 0.002535 | -3.51 | 0.0005 |
| CC*AUH*CEN | 0.01810 | 0.002437 | 7.43 | <.0001 |
| Sensorimotor Network (SMN) | ||||
| GE*AUH | 0.001498 | 0.001131 | 1.33 | 0.1850 |
| CC*AUH | -0.00025 | 0.001599 | -0.15 | 0.8776 |
| GE*AUH*SMN | -0.01663 | 0.001929 | -8.62 | <.0001 |
| CC*AUH*SMN | 0.01991 | 0.001550 | 12.85 | <.0001 |
| Dorsal Attention Network (DAN) | ||||
| GE*AUH | 0.000106 | 0.001001 | 0.11 | 0.9159 |
| CC*AUH | 0.001683 | 0.001420 | 1.19 | 0.2359 |
| GE*AUH*DAN | 0.008460 | 0.003021 | 2.80 | 0.0051 |
| CC*AUH*DAN | -0.00750 | 0.002712 | -2.77 | 0.0056 |
| Visual Network (VN) | ||||
| GE*AUH | -0.00104 | 0.000961 | -1.08 | 0.2784 |
| CC*AUH | 0.002881 | 0.001370 | 2.10 | 0.0355 |
| GE*AUH*VN | 0.03491 | 0.003501 | 9.97 | <.0001 |
| CC*AUH*VN | -0.00537 | 0.002965 | -1.81 | 0.0700 |
| Fronto-Temporal Network (FTN) | ||||
| GE*AUH | 0.000445 | 0.001019 | 0.44 | 0.6620 |
| CC*AUH | 0.001431 | 0.001422 | 1.01 | 0.3142 |
| GE*AUH*FTN | 0.01523 | 0.003358 | 4.53 | <.0001 |
| CC*AUH*FTN | -0.02452 | 0.003820 | -6.42 | <.0001 |
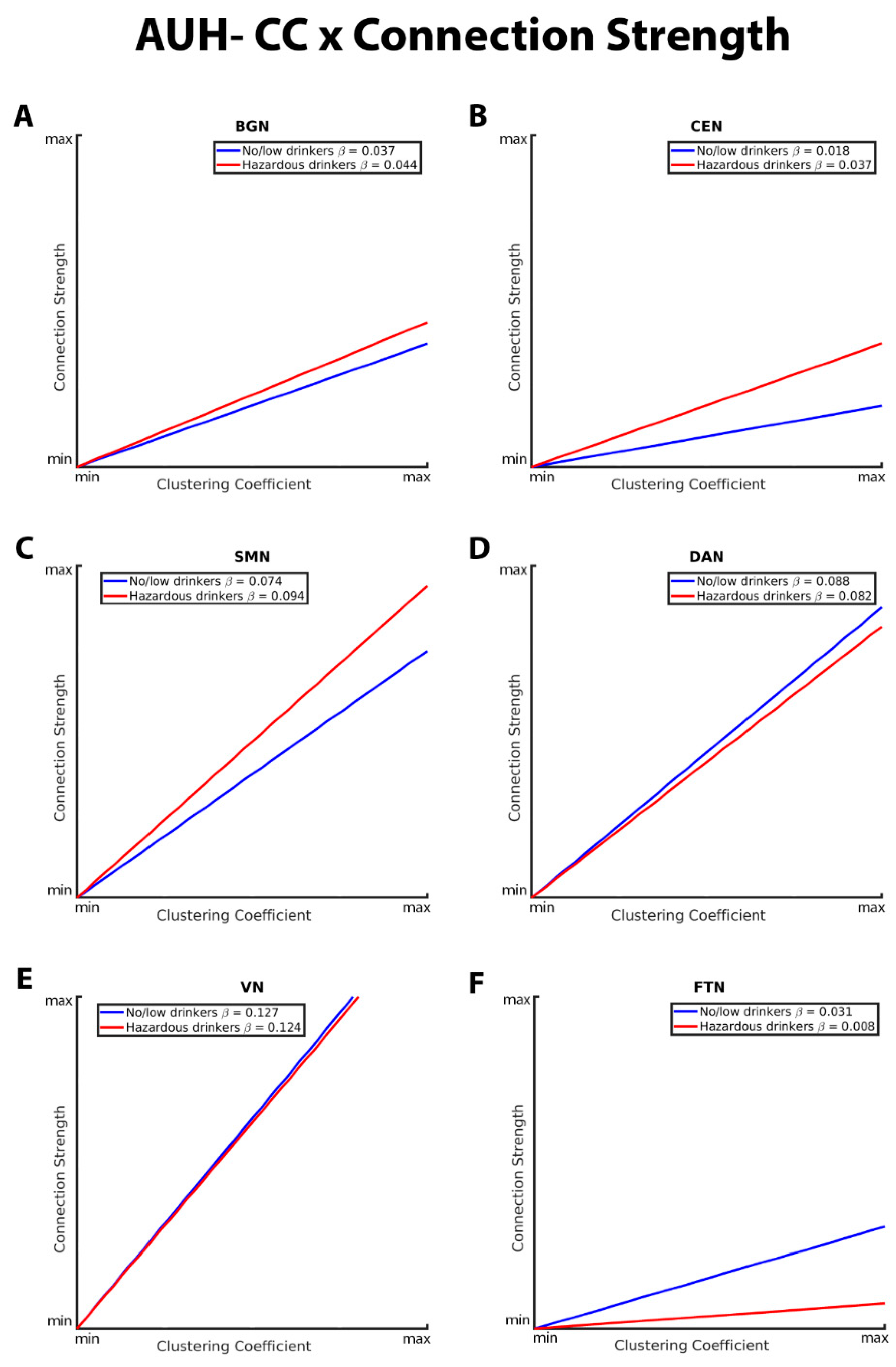
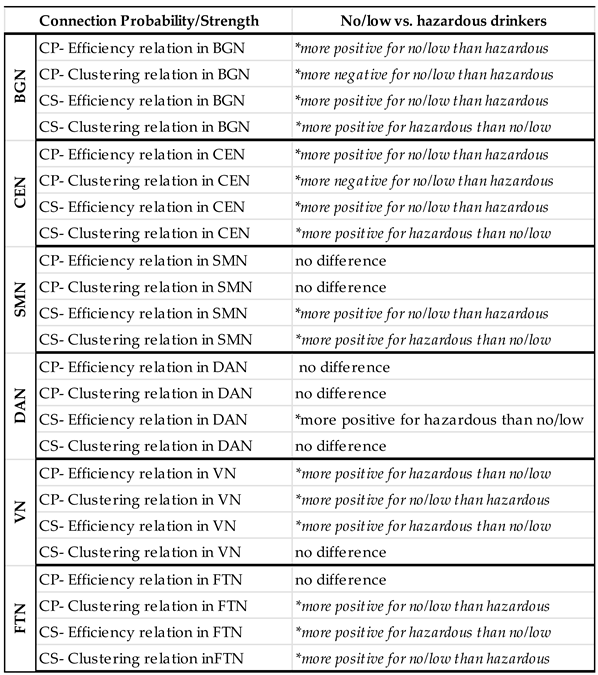
| Estimate | SE | t Value | P-Value | |
|---|---|---|---|---|
| GE*AUH within BGN | -0.00994 | 0.002110 | -4.71 | <.0001 |
| CC*AUH within BGN | 0.006438 | 0.002511 | 2.56 | 0.0104 |
| GE*AUH within CEN | -0.00821 | 0.002697 | -3.04 | 0.0023 |
| CC*AUH within CEN | 0.01878 | 0.002807 | 6.69 | <.0001 |
| GE*AUH within SMN | -0.01514 | 0.002187 | -6.92 | <.0001 |
| CC*AUH within SMN | 0.01967 | 0.002180 | 9.02 | <.0001 |
| GE*AUH within DAN | 0.008566 | 0.003145 | 2.72 | 0.0065 |
| CC*AUH within DAN | -0.00582 | 0.003017 | -1.93 | 0.0537 |
| GE*AUH within VN | 0.03387 | 0.003597 | 9.42 | <.0001 |
| CC*AUH within VN | -0.00249 | 0.003226 | -0.77 | 0.4400 |
| GE*AUH within FTN | 0.01567 | 0.003474 | 4.51 | <.0001 |
| CC*AUH within FTN | -0.02309 | 0.004042 | -5.71 | <.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





