1. Introduction
Coffee cultivation holds significant socioeconomic importance in Brazil, and its productivity has been jeopardized by adverse climatic factors such as rising temperatures and irregular rainfall patterns [
1,
2]. Severe drought periods can lead to plant mortality, while moderate droughts also prove detrimental, impacting flowering, grain development, and consequentially, coffee yield. Furthermore, fluctuations in precipitation and temperature augment grain defects, alter biochemical composition, and influence the final beverage quality [
3].
The scarcity of water promptly affects leaves, which play a pivotal role in plants [
4]. To curb excessive water loss, stomata close, inducing a reduction in CO
2 absorption and photosynthesis [
5]. This may lead to an imbalance between light capture and utilization, resulting in the overproduction of reactive oxygen species (EROs). These EROs can inflict cellular damage, potentially leading to plant mortality [
6,
7]. However, plants exhibit the capacity to adapt to adverse conditions, including water deficit, by activating physiological, biochemical, and anatomical mechanisms to sustain vital functions [
8]. Responses to water stress may vary among species and genotypes of the same species, implying varying aptitudes for dealing with this condition [
9].
Phenotypic plasticity is a crucial mechanism by which organisms, including plants, adapt to environmental changes, contributing to water stress tolerance. This plasticity manifests through modifications in growth, morphology, physiology, and behavior of plants [
10]. It enables plants to adapt to diverse environmental conditions such as water availability, nutrients, light, and temperature, proving valuable in predicting plant responses to climate changes [
11]. Phenotypic plasticity also serves as an essential tool for exploring plant adaptation to climate change [
12] and can be employed to assess combined effects of various stresses [
13]. However, information regarding phenotypic plasticity in response to water stress in coffee remains limited.
Considering that climate changes significantly impact global climate, comprehending coffee's phenotypic plasticity in response to water stress and identifying genotypes with heightened adaptative capacity assumes paramount importance. This aids in developing management and genetic improvement strategies to maximize productivity and sustainability of coffee cultivation under water restriction [
14].
Traditionally, genotype evaluation and selection in breeding programs involves meticulous analysis of each genotype's phenotypic response to individual parameters. This aim to identify genotypes exhibiting remarkable capacity to elucidate variations in the target variable often linked to productivity. However, the increase of phenotyping capacity through high-throughput methods, coupled with the increasing number of genotypes requiring evaluation, pose challenges, potentially leading to intricate and less efficient processes [
15].
In this context, the possibility of employing a multivariate phenotypic plasticity index emerges as an alternative for genotype evaluation in genetic improvement programs. This index not only considers isolated phenotypic responses of each parameter but also how these responses harmonize under diverse environmental conditions. The multivariate phenotypic plasticity index (MVPi) proposed by Pennacchi et al. [
16] consolidates multiple phenotypic characteristics into a single plasticity marker, based on parameter variance and Euclidean distances in a multidimensional space, enhancing efficiency in genotype evaluation.
Building upon our earlier work, when our research group identified Hybrid Timor accessions from the Epamig Germplasm Collection that displayed varying responses to water stress [
17], our recent investigation aimed to delve deeper. This time, we focused on exploring the phenotypic plasticity present within the germplasm of Hybrid Timor coffee. Additionally, we assessed the utility of the Phenotypic Plasticity Index (MVPi) as a promising tool to predict genotype performance across diverse climatic conditions. The potential to develop coffee plants adapted to low water availability renders this investigation highly pertinent.
2. Materials and Methods
Seven accessions of
Coffea arabica L. from the Germplasm Collection (GC-MG) of the Minas Gerais Agricultural Research Agency (Empresa de Pesquisa Agropecuária de Minas Gerais- EPAMIG), chosen based on productivity parameters, beverage quality, and disease resistance (Supplementary Figure S1), along with two control cultivars, the drought-tolerant (IPR 100) [
18] and drought-sensitive (Rubi MG 1192) [
19], were subjected to physiological, biochemical, and growth evaluations under controlled greenhouse conditions. Additionally, field productivity evaluations were conducted.
Greenhouse Experiment
For seedling formation, seeds of selected Coffea arabica L. accessions were germinated in sand until reaching the stage of cotyledon leaf emergence. Subsequently, they were transplanted into 120 ml polyethylene tubes containing a substrate for plants based on pine bark, peat, expanded vermiculite, enriched with macro and micronutrients from the brand Tropstrato HT. The seedlings were then kept in a nursery until they developed four pairs of true leaves and were acclimated.
After this period, the seedlings were transferred to 20-liter polyethylene pots containing a substrate mixture of 3 parts topsoil, 1 part sand, and 1 part bovine manure (3:1:1). They were grown in a greenhouse for a period of eleven months. Fertilization was carried out according to substrate analysis and regional crop recommendations.
The plants were irrigated to maintain the soil at 100% available water for eleven months, with the hydraulic treatment initiated in April 2019. In the first hydraulic treatment, the plants were maintained with the soil at 100% available water from April 2019 until the end of the experimental period (Irrigated - I). In the second treatment, irrigation was completely suspended (Non-irrigated - NI) until the majority of non-irrigated plants reached a predawn water potential of -3MPa (BRUM et al., 2013). Once this water potential was reached, which occurred 33 days after the stress imposition, irrigation was resumed, again maintaining the soil at 100% available water.
For the assessments of gas exchange and biochemical analyses, three distinct periods were considered: 1 - prior to the imposition of water deficit, 2 at 25 days after the imposition of water deficit (when the majority of plants reached a predawn water potential around -2MPa), and 3 - at 17 days after the resumption of irrigation.
Gas exchange assessments were conducted between 8 and 11 AM under artificial light (1000 µmol m-2 s-1) using a portable infrared gas analyzer (IRGA LICOR – 6400XT). Measurements included net photosynthetic rate (A - µmol CO2 m-2 s-1), stomatal conductance (gs - mol H2O m-1 s-1), transpiration rate (E – mmol H2O m-2 s-1), and instantaneous water use efficiency (WUE - µmol CO2/ mmol H2O m-2 s-1) (A/E). Water potential was determined using a pressure chamber (PMS Instruments Plant Moisture - Model 1000) before sunrise.
For biochemical analyses, leaves were collected in the afternoon (between 12 and 1 PM), immediately immersed in liquid nitrogen, and stored in an ultra freezer (-80 °C). Subsequent maceration and extraction were performed to quantify hydrogen peroxide (H2O2), lipid peroxidation, antioxidant metabolism, and ascorbate content.
For H
2O
2 quantification, 100 mg of plant material was macerated in liquid nitrogen and polyvinylpolypyrrolidone (PVPP), followed by homogenization in 0.1% (w/v) trichloroacetic acid (TCA). Samples were centrifuged at 12000g for 15 minutes at 4 °C, and the supernatant was mixed with a reaction solution containing 10 mM potassium phosphate buffer (pH 7.0) and 1M potassium iodide. H
2O
2 concentration was determined by measuring absorbance at 390 nm, using a standard curve of known H2O2 concentrations [
20], with modifications. Lipid peroxidation was assessed by quantifying thiobarbituric acid reactive species (TBA) as described by Buege and Aust [
21]. The aliquots were added to the reaction medium composed of 0.5% (w/v) thiobarbituric acid (TBA) and 10% (w/v) trichloroacetic acid (TCA). Subsequently, the medium was incubated at 95°C for 30 minutes. The reaction was halted by rapid cooling on ice, and readings were taken at 535 nm and 600 nm. TBA forms reddish-colored complexes, such as malondialdehyde (MDA), a secondary product of the peroxidation process. The concentration of MDA was calculated using the following equation: [MDA]= (A535 – A600) / (ξ x b), where ξ (molar extinction coefficient = 1,56 x 10
-5); b (optical path = 1). Peroxidation was expressed in mmol of MDA.g
-1 (FW - fresh weight).
For the determination of antioxidant enzyme activity, 100 mg of plant material was macerated in liquid nitrogen and polyvinylpolypyrrolidone (PVPP), followed by homogenization with 3.5 mL of the following extraction buffer: 100 mM potassium phosphate (pH 7.8), 0.1 mM EDTA, and 10 mM ascorbic acid. The extract was centrifuged at 13,000 g for 10 minutes at 4°C. The supernatants were collected and used for the analysis of the enzymes: catalase (CAT), superoxide dismutase (SOD), and ascorbate peroxidase (APX) [
22].
For the determination of catalase (CAT) activity, the protocol of Mengutay et al. [
23] with modifications was employed. Aliquots of the samples were added to the incubation medium, composed of phosphate buffer (45 mM, pH 7.6), Na2EDTA (0.1 mM) (dissolved in the phosphate buffer), and hydrogen peroxide (10 mM). Enzyme activity was determined by the decrease in absorbance at 240 nm every 15 seconds for 3 minutes, monitored by the consumption of hydrogen peroxide. The molar extinction coefficient used was 36 mM
-1cm
-1.
The ascorbate peroxidase (APX) activity was determined by monitoring the oxidation rate of ascorbate at 290 nm over 3 minutes, following Nakano and Asada's methodology (1981), with modifications. Ascorbate quantification was performed using 50 mg of plant material, which was macerated in liquid nitrogen and PVPP, and then homogenized with 5% trichloroacetic acid (m/v). After centrifugation, samples were added to a reaction mixture containing 5% trichloroacetic acid, 99.8% ethanol, ascorbic acid, phosphoric acid (0.4% in ethanol), bathophenanthroline (0.5% in ethanol), and ferric chloride (III) (0.03% in ethanol). The mixture was incubated, and readings were taken at 534 nm using a standard curve with known concentrations [
24].
All biochemical analyses were conducted using 96-well microtitration plates, and readings were performed using a Synergy TM HTX multimode microplate reader.
At the end of the experimental period, dry weights of the aboveground plant parts (MSPA – g), root dry weight (MSR – g), and total dry weight (MST g) were obtained. Roots were washed, and aboveground parts were separated from the root system. The samples were then dried in a forced-air oven at 70 °C for 72 hours, and dry weight was measured using a precision balance.
Field Experiment
The GC-MG was established in 2005 at the EPAMIG Experimental Farm in Patrocínio, MG, located in the Alto Paranaíba region, positioned at approximately 18°59'26" latitude South, 48°58'95" longitude West, and an altitude of about one thousand meters. The adopted spacing was 3.5 x 1.0 m between rows and between plants, respectively. The soil type is Red-Yellow Latosol, and the topography is flat with a slight slope [
25].
Harvesting was conducted in individual plots in 2020 and 2021. The field coffee volume (coffee fruits of mixed maturity) per plot was converted into the number of 60-kg bags of husked coffee produced per hectare (bags ha-1) (PROD). The experimental design followed randomized blocks with two replicates and ten plants per plot. Plant spacing was 4.0 × 1.0 m. Sowing and crop management were carried out according to technical recommendations for the species cultivation.
Statistical Analyses
Calculation of the MVPi
To assess the phenotypic plasticity of the evaluated genotypes, data obtained from physiological and biochemical assessments were subjected to a multivariate approach using an index that considers multiple evaluated traits. The multivariate plasticity index (MVPi), proposed by Pennacchi et al. [
15], was calculated based on the absolute deviation, measured as Euclidean distances in the multidimensional Cartesian plane, between different phenotypic states of the plants, specifically with and without water deficit. The scores for each individual were defined through Principal Componente Analysis (PCA).
The MVPi values were calculated at three different stages, corresponding to the same time points as other physiological and biochemical parameters: before the application of water deficit (WW), during its application (WD), and after irrigation resumed (RW). Using the MVPi values for each point, differences in plasticity values were calculated as the slopes of lines (I) between periods: I1 between the initial measurement and the post-imposition of stress point, and I2 between the post-imposition of stress point and the return of irrigation. The I1 and I2 values were then correlated with differences in total biomass accumulation in both above and belowground parts between control and water deficit treatments, as well as with conventional field management productivity. These correlations were measured through linear regressions using R software [
26].
Genetic Parameters
Variance estimation and prediction of random effects were conducted using the restricted maximum likelihood/best linear unbiased prediction (REML/BLUP) procedure with the assistance of the SELEGEN-REML/BLUP software [
27]. For these analysis, the employed model was:
. Where:
is the vector of phenotypic observations;
is the vector of fixed block effects;
is the vector of random genotype effects;
is the vector of errors;
is the block incidence matrix;
is the genotype effect incidence matrix.
Using the estimated variance components, individual heritabilities and other coefficients of determination associated with the random effects of the models were calculated as outlined in Resende [
27]. The variance components were subjected to a likelihood ratio test at a 5% significance level. Subsequently, the selection index was determined based on the Mulamba and Mock [
28] sum of ranks.
3. Results
Microclimatic Data in the Greenhouse
The climatic conditions inside the greenhouse during the experimental period are depicted in Supplementary Figure S2. At the commencement of the experimental period (04/01/2019), the average temperature and relative humidity were 31 °C and 56%, respectively. On the 25th day after the imposition of water deficit (04/26/2019), when the evaluated genotypes exhibited variability in response to water stress, the average temperature was 29 °C, and the relative humidity was 55%. The maximum recorded temperature during the experimental period reached approximately 42 °C. Following the rehydration of the genotypes (05/20/2019), the recorded average temperature and relative humidity were 28 °C and 59%, respectively.
Field Climate Data
The climatic conditions in the field during the experiment were characterized by well-defined dry and rainy seasons. In the year 2019, the average maximum and minimum temperatures were 30.4 °C and 16.1 °C, respectively. The total annual precipitation was 1299 mm (Supplementary Figure S3 A and B). In August 2019, there was an absence of precipitation, with a maximum temperature of 29.6 °C and a minimum of 14.5 °C (Supplementary Figure S3A). In the year 2020, the average maximum and minimum temperatures were 29.4 °C and 15.9 °C, respectively, and the annual precipitation was 1402 mm. In January 2020, there was a precipitation of 282 mm, and the maximum and minimum temperatures were 30.3 °C and 18.7 °C, respectively (Supplementary Figure S3B).
Multivariate Plasticity Index (MVPi) Correlated with Dry Matter and Productivity
The average values of gas exchange and biochemical analyses at three different assessment points are presented in Supplementary Tables S1, S2, S3, and S4, and Supplementary Figures S4, S5, and S6. These values were employed for calculating the MVPi.
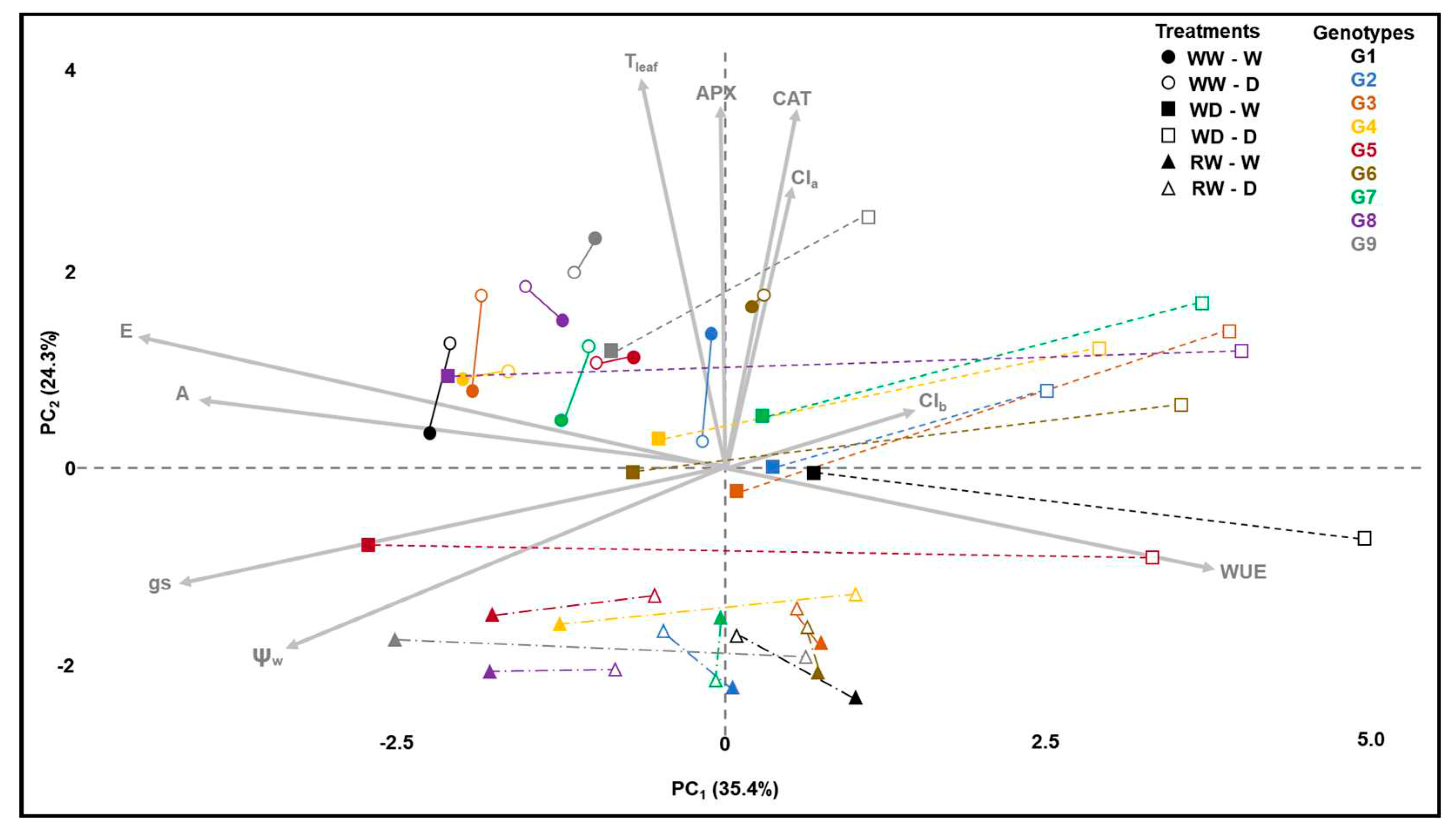
In the principal component analysis (PCA), a grouping of genotypes at the well-watered (WW) stage is observed at the top-left quadrant, in the direction of the arrows representing photosynthesis (A) and transpiration (E). This indicates that among all stages, the WW stage exhibited higher photosynthesis and transpiration. Additionally, stomatal conductance (gs) also trends towards the same left side, albeit closer to leaf water status (ψw) at the bottom-left quadrant (
Figure 1).
When water deficit is imposed (WD), the filled squares are positioned closer to the circles, indicating limited metabolic change in well-watered plants despite the change in developmental stages, but not in water treatment. In contrast, empty symbols shift towards the top-right quadrant in the opposite direction of A, E, gs, and ψw. This suggests a decrease in A and E due to lower gs, driven by stomatal control in response to reduced water status. Notably, genotypes 8 (Rubi MG 1192) and 5 (MG 311) exhibit higher distances between filled and empty squares, implying greater systemic phenotypic plasticity as proposed by MVPi (
Figure 1).
Upon re-irrigation (RW), a new phenotypic arrangement is observed at the bottom of the graph. The distance between filled circles, squares, and triangles of the same color represents phenotypic changes in well-watered plants across the crop cycle. The distances between filled and empty symbols, denoted by lines connecting two identical symbols, represent the average Euclidean distance between well-watered (W) and water-restricted (D) treatments. MVPi defines this distance as the integrated phenotypic plasticity of each genotype (
Figure 1).
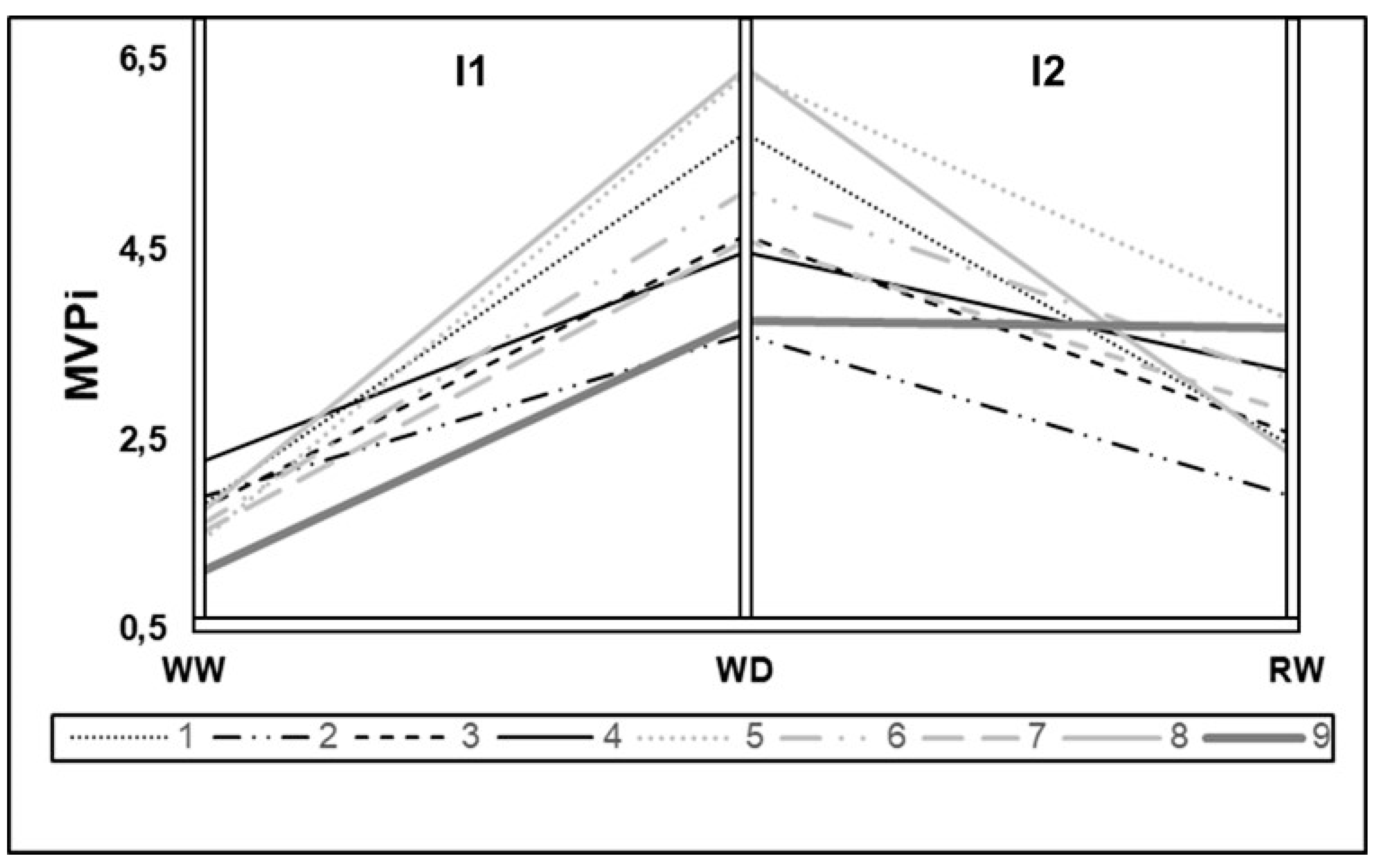
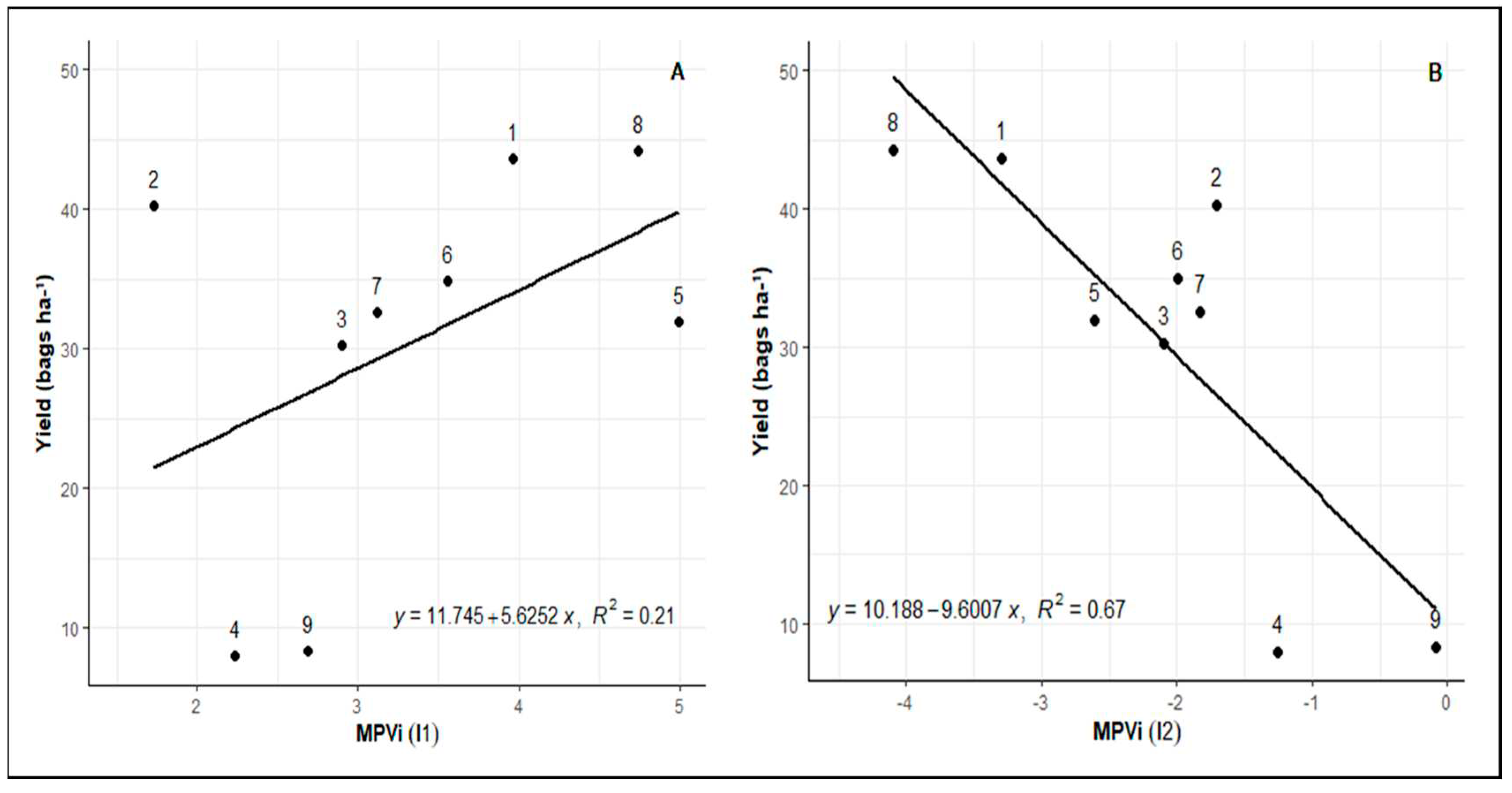
Figure 2 presents the MVPi of the nine evaluated genotypes at three time points. MVPi serves as an indicator of integrated phenotypic distance between the control treatment and water deficit treatment. A higher value indicates greater phenotypic distance between irrigated control and water deficit treatments. In the period between initial measurement and post-imposition of stress (I1) on the graph, the phenotypic alteration caused by water deficit imposition is shown in relation to the control.
The inclination between WD and RW points represents the phenotypic alteration caused by re-irrigation after a period of water deficit (I2). Variation in genotype plasticity is observed, with higher plasticity seen in genotypes 8 (Rubi MG 1192), 5 (MG 311), and 1 (MG 270B1), followed by genotypes 6 (MG 279), 3 (MG 364), 7 (MG 308), and 4 (MG 534) exhibiting intermediate values. Conversely, lower plasticity is observed in genotypes 9 (IPR 100) and 2 (MG 270B2). There is a common pattern of reduced phenotypic distance after re-irrigation, indicating similarity between the metabolism of plants subjected to water deficit and those consistently irrigated, except for genotype 9 (IPR 100). Among other genotypes, some show a greater ability to return to metabolic levels similar to the control, such as genotypes 1 (MG 270B1), 2 (MG 270B2), 3 (MG 364), and 8 (Rubi MG 1192).
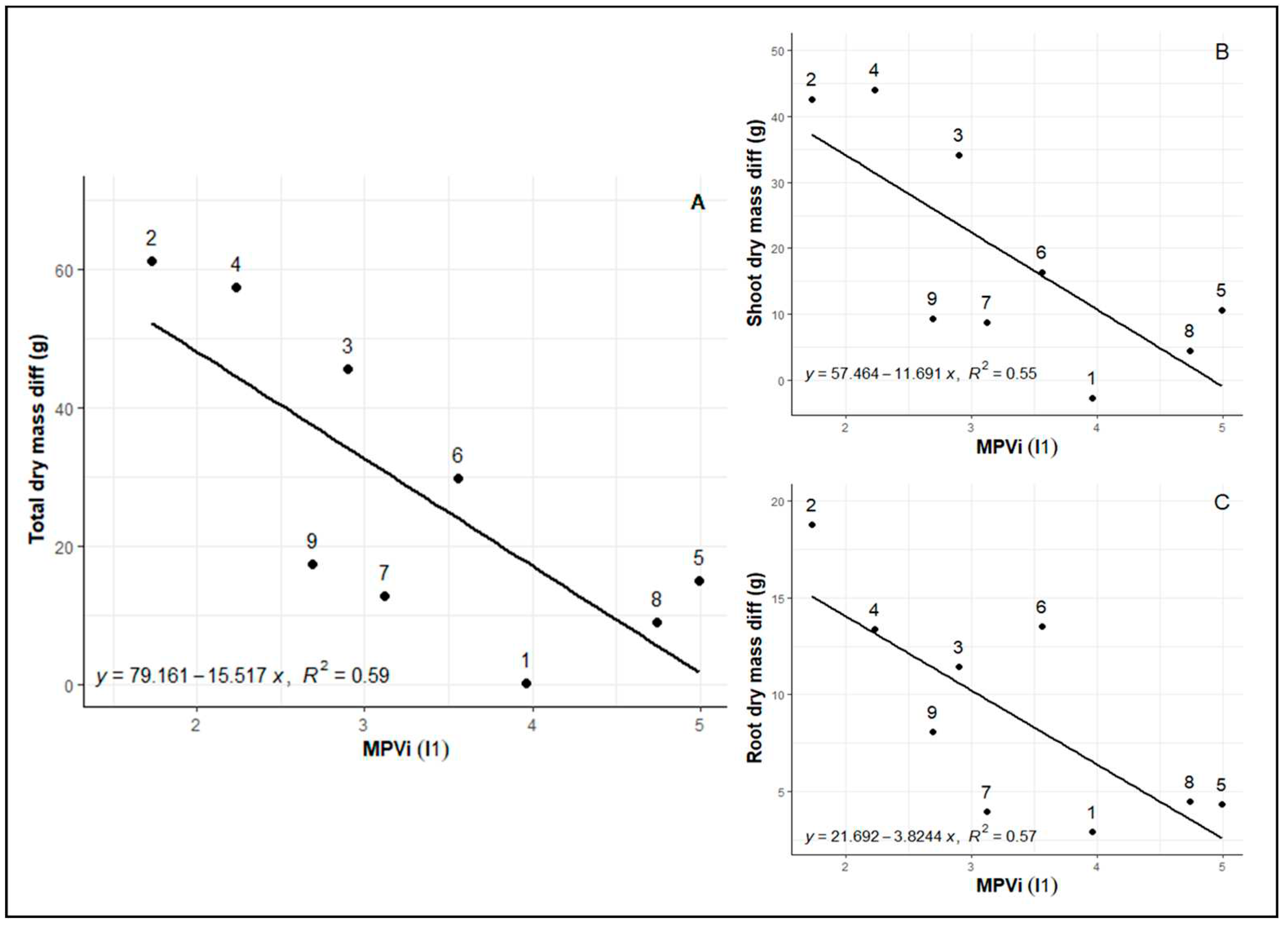
When evaluating integrated phenotypic plasticity, a correlation is observed between MVPi values and patterns of total biomass loss in the shoot and root parts (
Figure 3A, B, C), between control and water deficit plants. This pattern suggests that genotypes exhibiting greater plasticity in response to water deficit also experienced lower biomass losses compared to irrigated control.
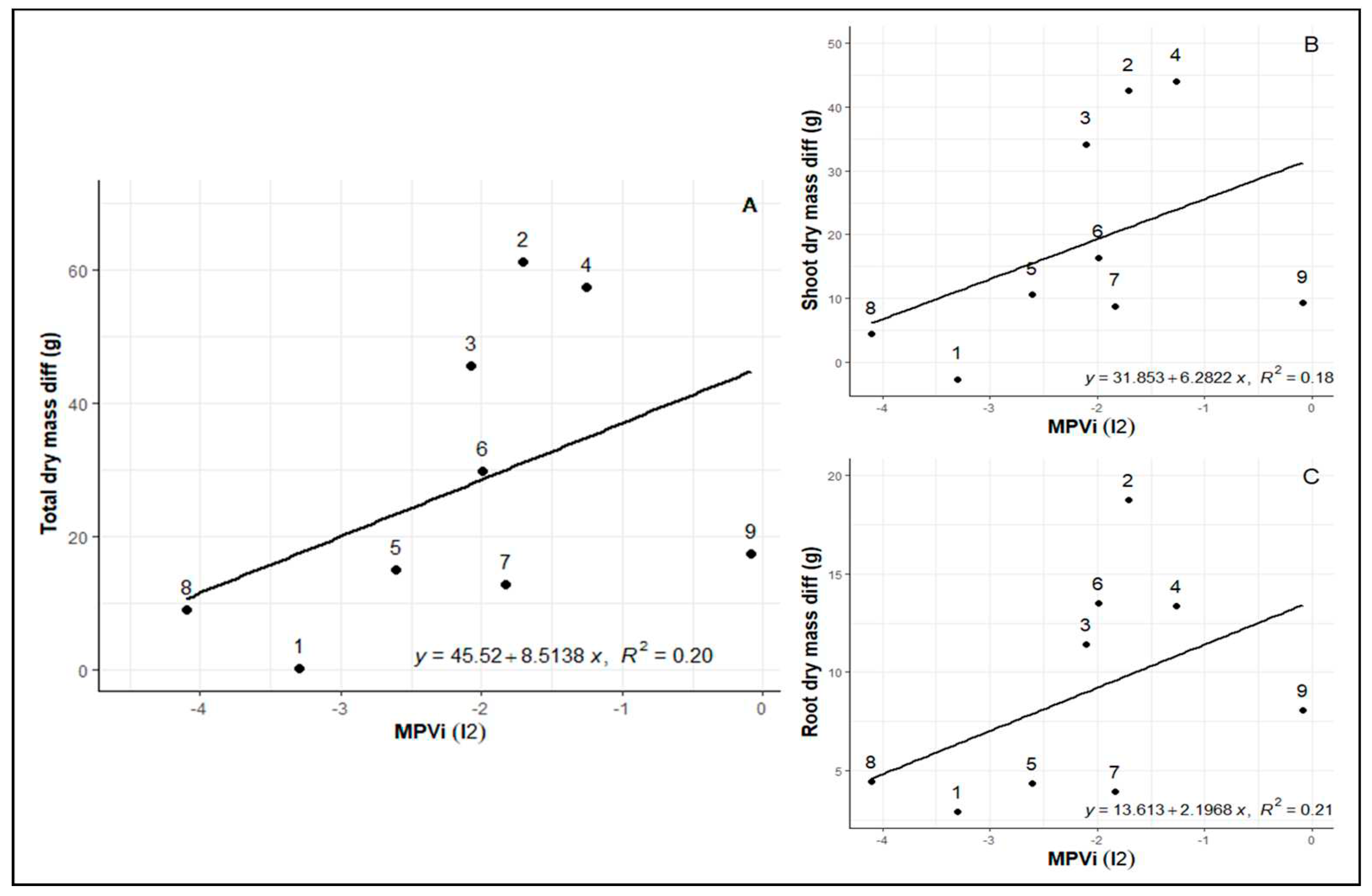
Likewise, after re-irrigation, a very similar pattern between plasticity and biomass loss is evident. In this case, more negative values indicate higher plasticity, showing that genotypes 8 (Rubi MG 1192), 1 (MG 270B1), and 5 (MG 311), exhibiting higher plasticity, had lower biomass losses at the end of the cycle (
Figure 4A, B, C). Despite genotypes 2 (MG 270B2) and 3 (MG 364) returning to a phenotypic state very close to the control after re-irrigation, there is substantial biomass loss due to the water limitation cycle, indicating reduced plasticity and low adaptation capacity during stress.
When genotypes are compared in relation to field productivity (
Figure 5), a consistent pattern emerges wherein those demonstrating higher plasticity in response to water deficit and re-irrigation in greenhouse experiments also exhibit greater field productivities. Based on the I1 of the study, genotypes 8 (Rubi MG 1192), 5 (MG 311), 1 (MG 270 B1), and 6 (MG 279) stood out in terms of plasticity and productivity. In the context of I2, where plasticity reflects the capacity to return to the physiological state after the water deficit period, notably, genotypes 8 (Rubi MG 1192) and 1 (MG 270 B1) excelled.
However, it is important to note that genotype 2 (MG 270 B2) presents an anomaly concerning this linear pattern, as it displays high field productivity despite its low plasticity in the greenhouse.
Genetic Parameters and Genotype Selection
To examine whether the MVPi indices studied at the two inclinations (I1 and I2) could be employed in the selection process, we estimated genetic parameters (
Table 1). The likelihood ratio test revealed genetic variability for the indices in both studied inclinations. The average heritability of genotypes indicated the potential for selection with high magnitudes, suggesting that the studied genotypes tend to maintain stable expression of phenotypic plasticity traits (I1 and I2) in response to environmental changes. The coefficients of relative variation showed values of 0.97 and 0.82 for I1 and I2, indicating a high level of variability in characters due to genetic and environmental causes. Moreover, the selection accuracy among genotypes demonstrated high precision in inferring genotypic values (0.94 to 0.93), indicating that the experimental setup was appropriate and the evaluation based on these traits can characterize plastic genotypes.
Inclinations I1 and I2 can be selected as traits for greenhouse selection of progenies with greater plasticity, as these traits exhibited satisfactory genetic parameters (
Table 1). According to the Mulamba and Mock index [
28] (
Table 2), the summed ranks indicated that genotypes 8 (Rubi MG 1192), 5 (MG 311), 1 (MG 270), and 6 (MG 279) were superior in plasticity compared to the other genotypes, corroborating the results presented in
Figure 5. In the selection of improved progenies, the predicted genotypic values favored genetic gains relative to the genotypic mean, with an increase of 57.09% in I1 and 36.67% in I2 for the selection of progenies with greater phenotypic plasticity.
4. Discussion
Water stress is one of the most significant environmental factors affecting agricultural production, leading to growth reduction, development problems, and decreased yield. Responses to water stress vary among species and within genotypes of the same species, with some being more adapted due to genetic, environmental, and management factors [
11,
16]. Tolerance to stress is determined by these complex elements.
Given the increasing demand for drought-tolerant coffee genotypes and the emergence of new automated phenotyping techniques, the need for a more efficient genotypic selection process has significantly intensified. Analyzing these multiple parameters individually and their intercorrelations complicates decision-making in the breeding process. To address this challenge, our research group has been seeking integrated parameters for assessing plasticity that align with breeding selection criteria. Phenotypic plasticity, the ability of a specific genotype to display multiple phenotypes in response to the environment, can be used to predict plant behavior under stress conditions. This phenomenon has been extensively studied in the context of plant adaptation to adverse environmental conditions [
3]. The MVPi is applied to quantify plant phenotypic plasticity based on multiple characteristics in response to varying growth conditions [
16].
In this study, we investigated the relationship between MVPi, biomass accumulation, and production of genotypes subjected to water limitation. We found that the MVPi fulfilled three main desired characteristics to be considered a potential marker for genetic improvement. Firstly, we observed a clear correlation between MVPi and productivity and productive resilience in different growth environments. Additionally, we noted wide genetic variation among the evaluated genotypes in relation to MVPi, highlighting its relevance as a parameter of interest for selection. Lastly, our results demonstrated that MVPi is heritable, meaning it is genetically transmitted to subsequent generations, solidifying its utility as a valuable tool in agricultural crop improvement programs.
When analyzing the I1 of MVPi, related to the period of irrigation suspension, we noticed that genotypes 8 (Rubi MG 1192), 5 (MG 311 Hybrid Timor UFV 428-02), 1 (MG 270 B1 Hybrid Timor UFV 377-21), and 6 (MG 279 Hybrid Timor UFV 376-31) exhibited the highest degree of phenotypic plasticity. Indicating significant shifts from the irrigated control, these genotypes showcased pronounced deviations. Such adjustments facilitated the preservation of metabolic activities, illustrating an adaptive approach for confronting water stress while sustaining dry matter content and overall productivity.
The sensitivity to water deficit of the 'Rubi MG 1192' cultivar (genotype 8), described by other authors [
19], was not observed in this study. The observed tolerance behavior may be related to greater phenotypic plasticity, indicated by the larger Euclidean distance between the control and water deficit treatments (
Figure 1, G8, distance between filled and empty squares). It is evident that the control treatment in the WD stage has a phenotypic state similar to the same treatment in the previous stage (WW), shifted in the direction of the vectors A, E, gs, and ψw. On the other hand, the plants under water deficit are shifted to the far-right side of the Cartesian plane, indicating stomatal closure and increased water-use efficiency. The tolerance mechanism may be related to increased carbon fixation during periods of higher water availability and enhanced control of water loss during water deficit periods. This mechanism differs from that studied by Freire et al. [
18].who evaluated the expression of the CaM6FR gene related to mannitol sy nthesis.
Among the most plastic genotypes, accessions 5 (MG 311 Hybrid Timor UFV 428-02), 1 (MG 270 B1 Hybrid Timor UFV 377-21), and 6 (MG 279 Hybrid Timor UFV 376-31) belong to the Hybrid of Timor germplasm (Supplementary Table S1), commonly used in breeding programs as a source of resistance to major coffee diseases [
28]. In another study, these genotypes were found to have leaf anatomical structures that optimize water transport and prevent excessive transpiration, as well as physiological mechanisms that minimize impacts during dry periods [
17]. This adaptive capacity is crucial in future scenarios of climate change, where extreme events such as prolonged droughts may become more frequent.
In plants subjected to recurring water stress, such as coffee, the ability to restore their pre-stress behavior is crucial for resuming growth and productivity, as demonstrated by Beacham et al. [
30]. Both recovery ability and drought tolerance are essential, as indicated by Hassan et al. [
8]. This phenomenon was observed in genotypes 1 (MG 270 B1 Hybrid Timor UFV 377-21), 2 (MG 270 B2 Hybrid Timor UFV 377-21), 3 (MG 364 Hybrid Timor UFV 442-42), and control 8 (Rubi MG 1192), which exhibited a greater ability to return to metabolic levels similar to the irrigated control.
Phenotypic plasticity ranges from stable to highly plastic in genotypes [
13]. The 'IPR 100' cultivar demonstrated lower plasticity, possibly related to different drought tolerance mechanisms [
18]. Its distinction may stem from hybridization origin, "Catuaí" x coffee ("Catuaí" x coffee genotype from 'BA-10' series) bearing
C. liberica genes, diverging from other materials in the Hybrid of Timor germplasm (Supplementary
Table 1). Future studies can explore these mechanisms for sustainable agronomic approaches in the face of climate change.
The concept of "Physiological Breeding" [
31] proposes the use of parameters related to plant metabolism, primarily those of physiological and biochemical nature, as indicators for selecting genotypes in plant breeding. The growing capacity to measure these parameters, driven by high-throughput phenotyping methods, is remarkable. However, this progress demands the development of new statistical approaches that translate phenotyping advancements into substantial genetic gains [
32]. In this study, genetic parameters were estimated, indicating promising prospects for selecting individuals with greater phenotypic plasticity. Subsequently, the application of the Mulamba and Mock selection index [
28] was proposed, using plasticity index during the interval between the initial measurement and the subsequent point after stress imposition (I1), as well as during the period between post-stress imposition and irrigation return (I2), when the plants were under water stress conditions. By amalgamating the genotype rankings evaluated by Mulamba and Mock [
28] we selected genotypes 8 (Rubi MG 1192), 5 (MG 311 Hybrid Timor UFV 428-02), 1 (MG 270 B1 Hybrid Timor UFV 377-21), and 6 (MG 279 Hybrid Timor UFV 376-31). These selections exhibited significant enhancements in phenotypic plasticity, with increments of 57.09% in I1 and 36.67% in I2. This reaffirms the consistency of prior findings across diverse assessments (
Figure 1,
Figure 2 and
Figure 3).
The outcomes of this investigation align cohesively with antecedent studies, which also accentuated the pivotal role of phenotypic plasticity as a valuable tool in prognosticating plant responses under the aegis of climate change [
12,
13,
21] The prognostication of genotypic responses to diverse environmental stress scenarios stands as a pivotal imperative in the realm of devising strategies for the management and selection of more resilient and productive cultivars.
Our findings underscore the fundamental significance of phenotypic plasticity as an intrinsic adaptive mechanism for heightening plant productivity in water-stress environments. It is evident from our observations that the more plastic genotypes—those endowed with the capability to meaningfully modulate their physiological and biochemical parameters in response to environmental stimuli—exhibited diminished biomass losses in both aerial and root components, thus engendering a favorable impact on their overall productivity. The MVPi emerged as a valuable instrument for assessing the adaptive capacity of genotypes and forecasting their performance across varying climatic scenarios. Heightened plasticity was discernible within the GC-MG accessions: MG 311 Hybrid Timor UFV 428-02, MG 270 Hybrid Timor UFV 377-21, and MG 279 Hybrid Timor UFV 376-31, as well as the Rubi MG 1192 cultivar.
It is imperative to underscore that while phenotypic plasticity has demonstrated benefits in the context of biomass preservation and productivity under water stress, substantial uncharted realms remain within this sphere of inquiry. Prospective studies could delve into the molecular underpinnings of phenotypic plasticity and the adaptive response of genotypes to an array of environmental stimuli. A comprehensive comprehension of these processes is paramount in formulating sustainable agronomic paradigms in the face of global climatic transformations.
Author Contributions
CSS, AFF, GHBS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. JPP, MAFC, MOS,: Conceptualization, Data curation, Formal analysis, Investigation, Methodology. TSJM,, JCRA, AAP, GRC,: Investigation, Methodology, Validation,Visualization, Writing – original draft, Writing – review & editing. VAS: Conceptualization, Investigation, Methodology, Resources, Supervision,Validation, Visualization, Writing – original draft, Writing – review & editing.