Submitted:
24 August 2023
Posted:
25 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
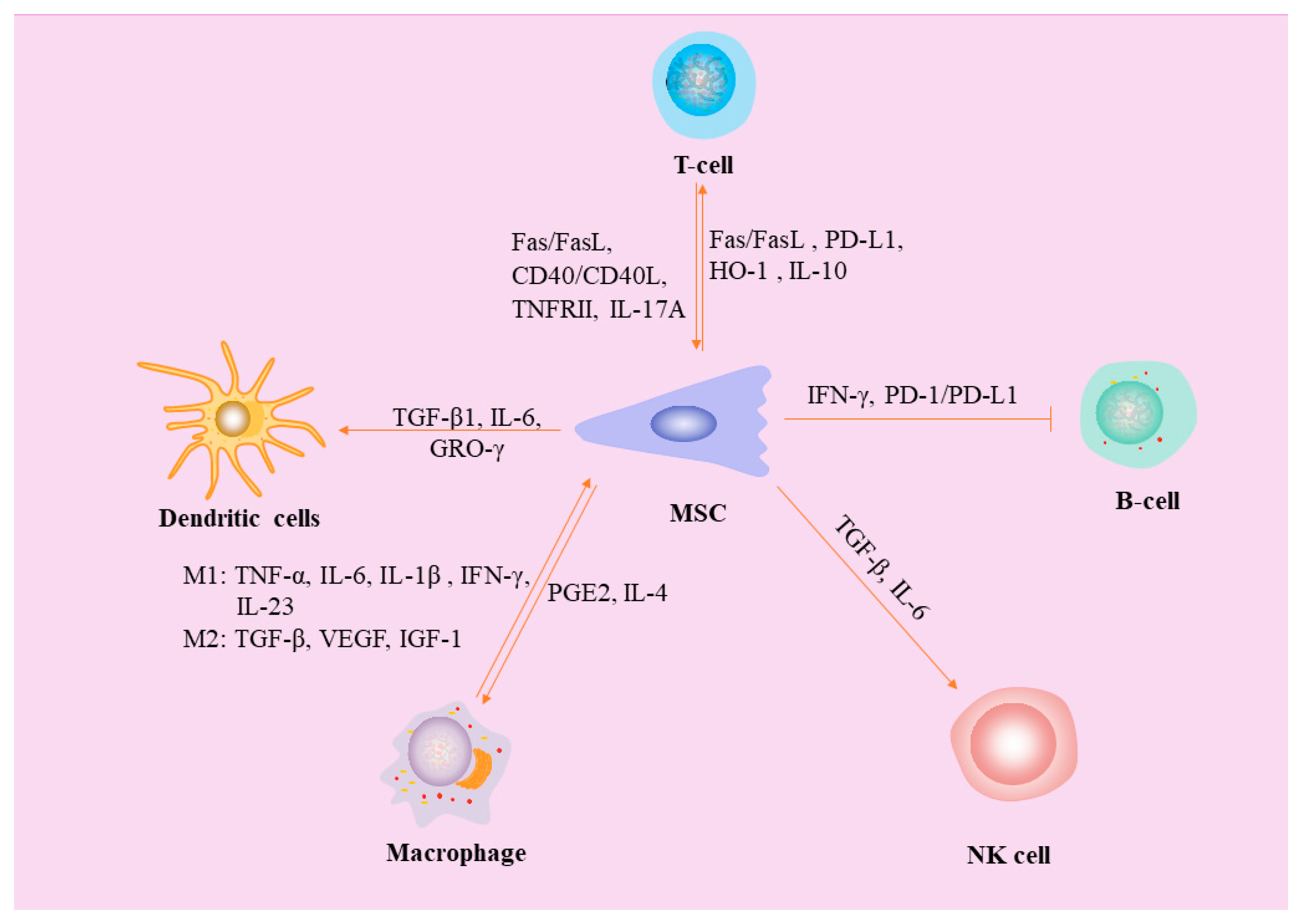
2. Interaction between MSCs and Macrophages
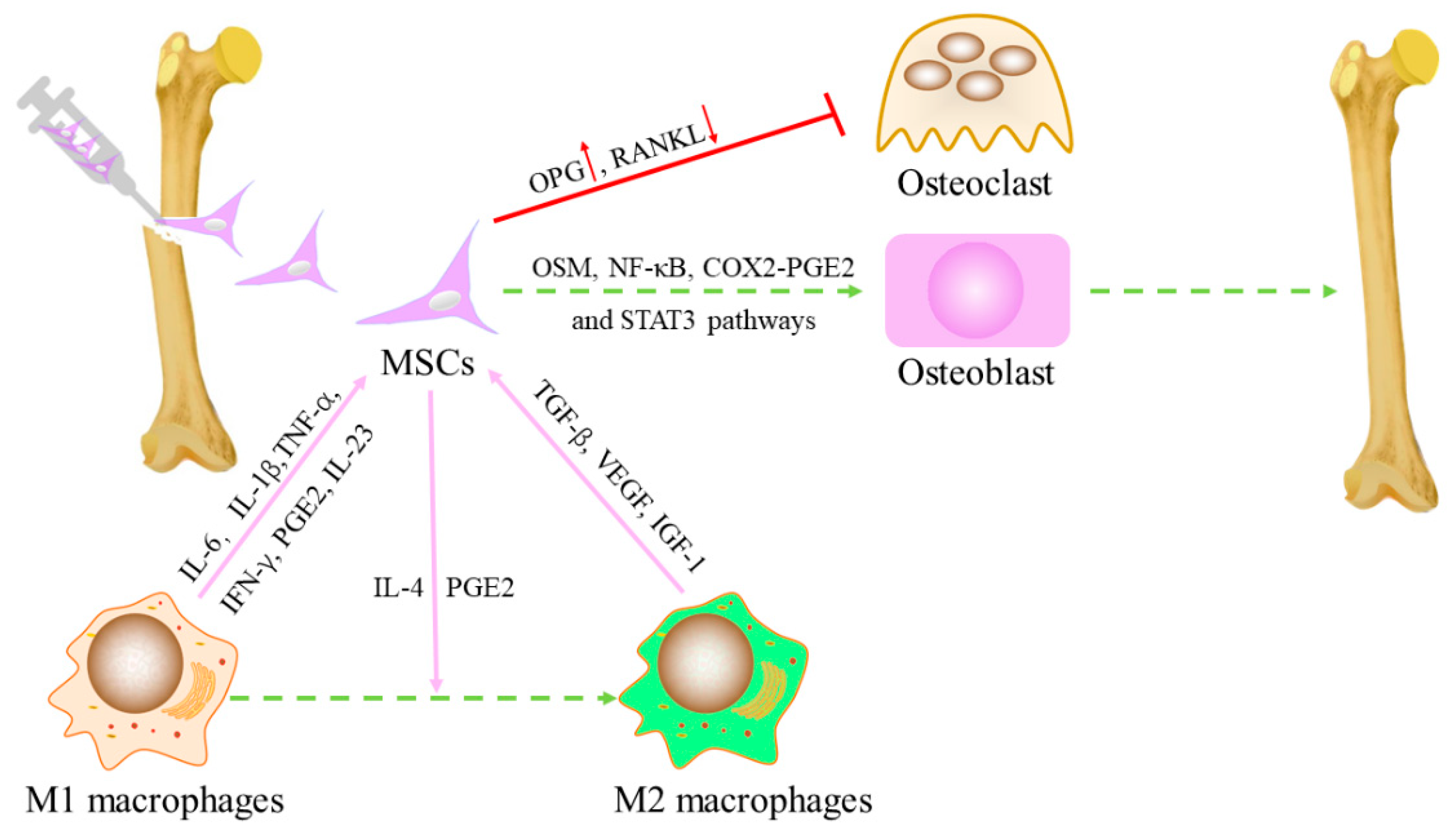
2.1. Effects of macrophages on MSC
2.2. Effects of MSCs on polarization of macrophages
3. MSCs inhibit Dendritic cells (DCs)
4. Interaction between MSCs and Natural killer (NK) cells
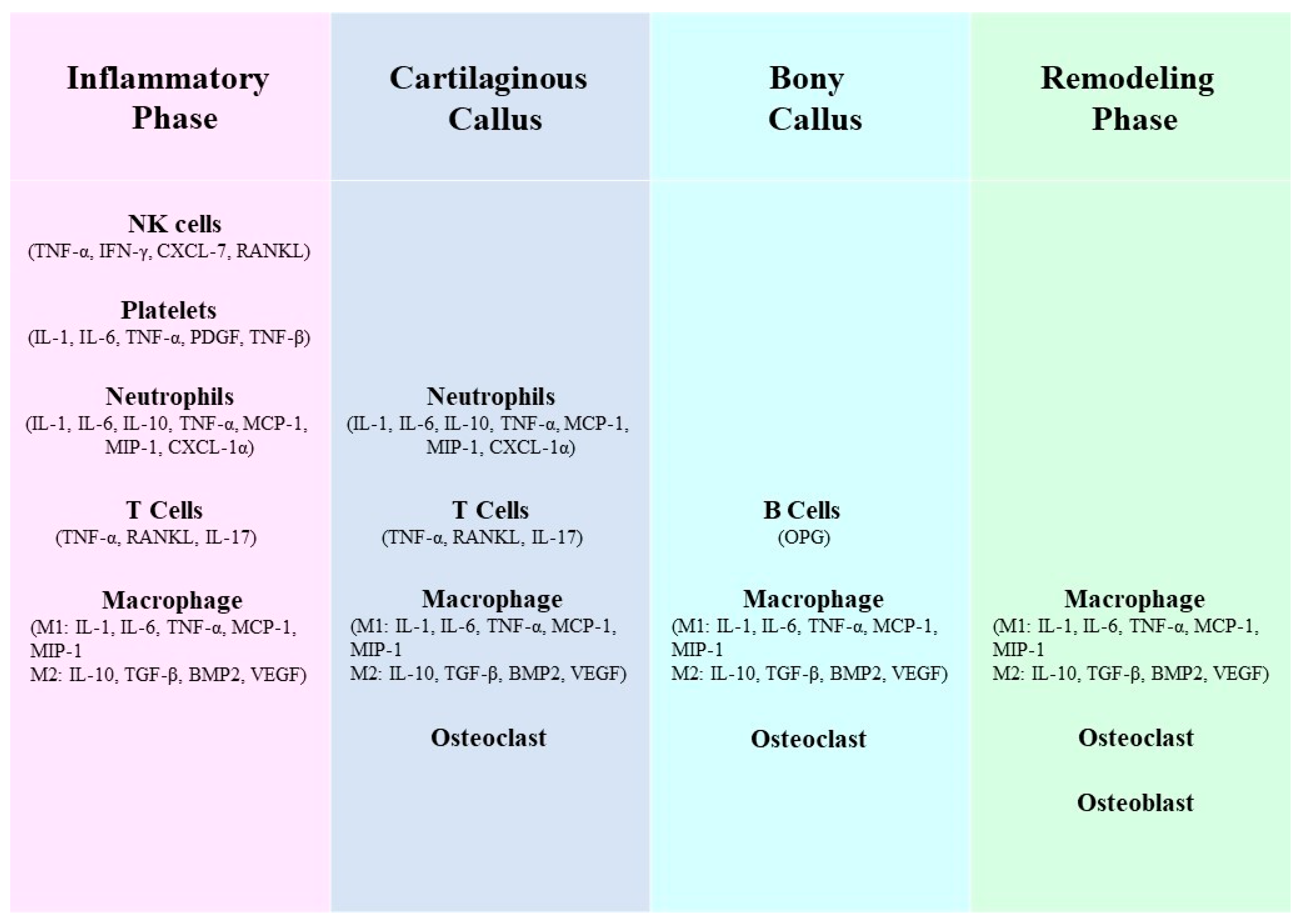
5. Interaction between MSCs and Neutrophils
6. Interactions between MSCs and mast cells (MC)
7. MSCs inhibit B lymphocytes
8. MSCs inhibit T Lymphocytes
9. Effects of B lymphocytes and T lymphocytes on MSCs
10. Effects of pH
11. Effects of exosomes on fractures
12. The effect of immune cells on angiogenesis
13. Application of single cell analysis in fracture repair
14. Main limitations and prospects
15. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Gómez-Barrena, E.; Rosset, P.; Gebhard, F.; Hernigou, P.; Baldini, N.; Rouard, H.; Sensebé, L.; Gonzalo-Daganzo, R.M.; Giordano, R.; Padilla-Eguiluz, N.; et al. Feasibility and safety of treating non-unions in tibia, femur and humerus with autologous, expanded, bone marrow-derived mesenchymal stromal cells associated with biphasic calcium phosphate biomaterials in a multicentric, non-comparative trial. Biomaterials 2019, 196, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chu, W.; Zhuang, Y.; Shi, D.; Tao, H.; Jin, C.; Dai, K.; Zhao, J.; Gan, Y. Bone Mesenchymal Stem Cell-Enriched β-Tricalcium Phosphate Scaffold Processed by the Screen-Enrich-Combine Circulating System Promotes Regeneration of Diaphyseal Bone Non-Union. Cell Transplant 2019, 28, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev 2019, 46, 1–9. [Google Scholar] [CrossRef]
- McGonagle, D.; Baboolal, T.G.; Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol 2017, 13, 719–730. [Google Scholar] [CrossRef]
- Bunpetch, V.; Zhang, Z.Y.; Zhang, X.; Han, S.; Zongyou, P.; Wu, H.; Hong-Wei, O. Strategies for MSC expansion and MSC-based microtissue for bone regeneration. Biomaterials 2019, 196, 67–79. [Google Scholar] [CrossRef]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res Ther 2019, 10, 377. [Google Scholar] [CrossRef]
- Huang, S.; Xu, L.; Zhang, Y.; Sun, Y.; Li, G. Systemic and Local Administration of Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells Promotes Fracture Healing in Rats. Cell Transplant 2015, 24, 2643–2655. [Google Scholar] [CrossRef]
- Zimmermann, G.; Wagner, C.; Schmeckenbecher, K.; Wentzensen, A.; Moghaddam, A. Treatment of tibial shaft non-unions: bone morphogenetic proteins versus autologous bone graft. Injury 2009, 40 Suppl 3, S50–53. [Google Scholar] [CrossRef]
- Calori, G.M.; Colombo, M.; Ripamonti, C.; Bucci, M.; Fadigati, P.; Mazza, E.; Mulas, S.; Tagliabue, L. Polytherapy in bone regeneration: clinical applications and preliminary considerations. Int J Immunopathol Pharmacol 2011, 24, 85–90. [Google Scholar] [CrossRef]
- Chu, W.; Wang, X.; Gan, Y.; Zhuang, Y.; Shi, D.; Liu, F.; Sun, Y.; Zhao, J.; Tang, T.; Dai, K. Screen-enrich-combine circulating system to prepare MSC/β-TCP for bone repair in fractures with depressed tibial plateau. Regen Med 2019, 14, 555–569. [Google Scholar] [CrossRef]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr Osteoporos Rep 2018, 16, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Al-Sebaei, M.O.; Daukss, D.M.; Belkina, A.C.; Kakar, S.; Wigner, N.A.; Cusher, D.; Graves, D.; Einhorn, T.; Morgan, E.; Gerstenfeld, L.C. Role of Fas and Treg cells in fracture healing as characterized in the fas-deficient (lpr) mouse model of lupus. J Bone Miner Res 2014, 29, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ricciardi, B.F.; Hernandez-Soria, A.; Shi, Y.; Pleshko Camacho, N.; Bostrom, M.P. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 2007, 41, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.; Cooney, T.E.; Englund, R.; Lubahn, J.D. Effects of interleukin-6 ablation on fracture healing in mice. J Orthop Res 2011, 29, 1437–1442. [Google Scholar] [CrossRef]
- Nam, D.; Mau, E.; Wang, Y.; Wright, D.; Silkstone, D.; Whetstone, H.; Whyne, C.; Alman, B. T-lymphocytes enable osteoblast maturation via IL-17F during the early phase of fracture repair. PLoS One 2012, 7, e40044. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cho, T.J.; Kon, T.; Aizawa, T.; Tsay, A.; Fitch, J.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 2003, 18, 1584–1592. [Google Scholar] [CrossRef]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A 2011, 108, 1585–1590. [Google Scholar] [CrossRef]
- Könnecke, I.; Serra, A.; El Khassawna, T.; Schlundt, C.; Schell, H.; Hauser, A.; Ellinghaus, A.; Volk, H.D.; Radbruch, A.; Duda, G.N.; et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014, 64, 155–165. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Zhang, M.F.; Wan, S.C.; Chen, W.B.; Yang, D.H.; Liu, W.Q.; Li, B.L.; Aierken, A.; Du, X.M.; Li, Y.X.; Wu, W.P.; et al. Transcription factor Dmrt1 triggers the SPRY1-NF-κB pathway to maintain testicular immune homeostasis and male fertility. Zool Res 2023, 44, 505–521. [Google Scholar] [CrossRef]
- Wei, Y.D.; Du, X.M.; Yang, D.H.; Ma, F.L.; Yu, X.W.; Zhang, M.F.; Li, N.; Peng, S.; Liao, M.Z.; Li, G.P.; et al. Dmrt1 regulates the immune response by repressing the TLR4 signaling pathway in goat male germline stem cells. Zool Res 2021, 42, 14–27. [Google Scholar] [CrossRef] [PubMed]
- O'Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: from discovery to the clinic--an overview. Tissue Eng Part B Rev 2011, 17, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Bellin, G.; Sbricoli, L.; Sivolella, S.; Brunello, G.; Schwartz-Arad, D.; Mijiritsky, E.; Penarrocha, M.; et al. Metal Nanoparticles Released from Dental Implant Surfaces: Potential Contribution to Chronic Inflammation and Peri-Implant Bone Loss. Materials (Basel) 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop Relat Res 1989, 294–309. [Google Scholar]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev 2010, 16, 427–434. [Google Scholar] [CrossRef]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 2000, 106, 1481–1488. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front Immunol 2014, 5, 511. [Google Scholar] [CrossRef]
- Li, N.; Hua, J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci 2017, 74, 2345–2360. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.M.; Kameishi, S.; Grainger, D.W.; Okano, T. Strategies to address mesenchymal stem/stromal cell heterogeneity in immunomodulatory profiles to improve cell-based therapies. Acta Biomater 2021, 133, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Dighe, A.S.; Yang, S.; Madhu, V.; Balian, G.; Cui, Q. Interferon gamma and T cells inhibit osteogenesis induced by allogeneic mesenchymal stromal cells. J Orthop Res 2013, 31, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med 2011, 17, 1594–1601. [Google Scholar] [CrossRef]
- Reinke, S.; Geissler, S.; Taylor, W.R.; Schmidt-Bleek, K.; Juelke, K.; Schwachmeyer, V.; Dahne, M.; Hartwig, T.; Akyüz, L.; Meisel, C.; et al. Terminally differentiated CD8⁺ T cells negatively affect bone regeneration in humans. Sci Transl Med 2013, 5, 177ra136. [Google Scholar] [CrossRef]
- Xue, D.; Chen, E.; Zhong, H.; Zhang, W.; Wang, S.; Joomun, M.U.; Yao, T.; Tan, Y.; Lin, S.; Zheng, Q.; et al. Immunomodulatory properties of graphene oxide for osteogenesis and angiogenesis. Int J Nanomedicine 2018, 13, 5799–5810. [Google Scholar] [CrossRef]
- Hirata, E.; Miyako, E.; Hanagata, N.; Ushijima, N.; Sakaguchi, N.; Russier, J.; Yudasaka, M.; Iijima, S.; Bianco, A.; Yokoyama, A. Carbon nanohorns allow acceleration of osteoblast differentiation via macrophage activation. Nanoscale 2016, 8, 14514–14522. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Z.; Farnaghi, S.; Friis, T.; Mao, X.; Xiao, Y.; Wu, C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater 2016, 30, 334–344. [Google Scholar] [CrossRef]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jämsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res 2017, 35, 2378–2385. [Google Scholar] [CrossRef]
- Tu, B.; Liu, S.; Liu, G.; Yan, W.; Wang, Y.; Li, Z.; Fan, C. Macrophages derived from THP-1 promote the osteogenic differentiation of mesenchymal stem cells through the IL-23/IL-23R/β-catenin pathway. Exp Cell Res 2015, 339, 81–89. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, Y.; Zhang, Y.; Ruan, Z. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro. Ann Clin Lab Sci 2016, 46, 65–71. [Google Scholar] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J Investig Dermatol Symp Proc 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Tasso, R.; Ulivi, V.; Reverberi, D.; Lo Sicco, C.; Descalzi, F.; Cancedda, R. In vivo implanted bone marrow-derived mesenchymal stem cells trigger a cascade of cellular events leading to the formation of an ectopic bone regenerative niche. Stem Cells Dev 2013, 22, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Z.L.; Xiao, M.; Yang, Z.Z.; Peng, M.Z.; Li, C.D.; Zhou, X.J.; Wang, J.W. Impact of bone marrow mesenchymal stem cell immunomodulation on the osteogenic effects of laponite. Stem Cell Res Ther 2018, 9, 100. [Google Scholar] [CrossRef]
- Seebach, E.; Freischmidt, H.; Holschbach, J.; Fellenberg, J.; Richter, W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater 2014, 10, 4730–4741. [Google Scholar] [CrossRef]
- Tour, G.; Wendel, M.; Tcacencu, I. Bone marrow stromal cells enhance the osteogenic properties of hydroxyapatite scaffolds by modulating the foreign body reaction. J Tissue Eng Regen Med 2014, 8, 841–849. [Google Scholar] [CrossRef]
- Lin, T.; Pajarinen, J.; Nabeshima, A.; Lu, L.; Nathan, K.; Jämsen, E.; Yao, Z.; Goodman, S.B. Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodulation and osteogenesis. Stem Cell Res Ther 2017, 8, 277. [Google Scholar] [CrossRef]
- Wei, F.; Li, Z.; Crawford, R.; Xiao, Y.; Zhou, Y. Immunoregulatory role of exosomes derived from differentiating mesenchymal stromal cells on inflammation and osteogenesis. J Tissue Eng Regen Med 2019, 13, 1978–1991. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials (Basel) 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Shin, R.L.; Lee, C.W.; Shen, O.Y.; Xu, H.; Lee, O.K. The Crosstalk between Mesenchymal Stem Cells and Macrophages in Bone Regeneration: A Systematic Review. Stem Cells Int 2021, 2021, 8835156. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Moretta, L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol 2013, 91, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Moretta, L. Interactions between mesenchymal stem cells and dendritic cells. Adv Biochem Eng Biotechnol 2013, 130, 199–208. [Google Scholar] [CrossRef]
- Wehner, R.; Taubert, C.; Mende, T.; Gaebler, C.; de Andrade, A.V.; Bornhäuser, M.; Werner, C.; Tonn, T.; Schäkel, K.; Bachmann, M.; et al. Engineered extracellular matrix components do not alter the immunomodulatory properties of mesenchymal stromal cells in vitro. J Tissue Eng Regen Med 2013, 7, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Liu, J.J.; Wu, J.; Zhang, L.L.; Liu, F.; Yin, L.; Zhang, M.M.; Yu, B. Novel mechanism of inhibition of dendritic cells maturation by mesenchymal stem cells via interleukin-10 and the JAK1/STAT3 signaling pathway. PLoS One 2013, 8, e55487. [Google Scholar] [CrossRef] [PubMed]
- Aldinucci, A.; Rizzetto, L.; Pieri, L.; Nosi, D.; Romagnoli, P.; Biagioli, T.; Mazzanti, B.; Saccardi, R.; Beltrame, L.; Massacesi, L.; et al. Inhibition of immune synapse by altered dendritic cell actin distribution: a new pathway of mesenchymal stem cell immune regulation. J Immunol 2010, 185, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.G.; Xu, W.; Sun, L.; Li, W.M.; Li, Q.B.; Zou, P. The characteristics and immunoregulatory functions of regulatory dendritic cells induced by mesenchymal stem cells derived from bone marrow of patient with chronic myeloid leukaemia. Eur J Cancer 2012, 48, 1884–1895. [Google Scholar] [CrossRef]
- Choi, Y.S.; Jeong, J.A.; Lim, D.S. Mesenchymal stem cell-mediated immature dendritic cells induce regulatory T cell-based immunosuppressive effect. Immunol Invest 2012, 41, 214–229. [Google Scholar] [CrossRef]
- Chen, H.W.; Chen, H.Y.; Wang, L.T.; Wang, F.H.; Fang, L.W.; Lai, H.Y.; Chen, H.H.; Lu, J.; Hung, M.S.; Cheng, Y.; et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol 2013, 190, 5065–5077. [Google Scholar] [CrossRef]
- Deng, Y.; Yi, S.; Wang, G.; Cheng, J.; Zhang, Y.; Chen, W.; Tai, Y.; Chen, S.; Chen, G.; Liu, W.; et al. Umbilical cord-derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL-6-mediated upregulation of SOCS1. Stem Cells Dev 2014, 23, 2080–2092. [Google Scholar] [CrossRef]
- Tang, W.Y.; Liu, J.H.; Peng, C.J.; Liao, Y.; Luo, J.S.; Sun, X.; Tang, Y.L.; Luo, X.Q. Functional Characteristics and Application of Mesenchymal Stem Cells in Systemic Lupus Erythematosus. Arch Immunol Ther Exp (Warsz) 2021, 69, 7. [Google Scholar] [CrossRef]
- Petri, R.M.; Hackel, A.; Hahnel, K.; Dumitru, C.A.; Bruderek, K.; Flohe, S.B.; Paschen, A.; Lang, S.; Brandau, S. Activated Tissue-Resident Mesenchymal Stromal Cells Regulate Natural Killer Cell Immune and Tissue-Regenerative Function. Stem Cell Reports 2017, 9, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Rekasi, H.; Hepner-Schefczyk, M.; Fessmann, K.; Petri, R.M.; Bruderek, K.; Brandau, S.; Jäger, M.; Flohé, S.B. Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther 2016, 7, 88. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J Allergy Clin Immunol 2010, 125, S3–23. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Crop, M.J.; Korevaar, S.S.; de Kuiper, R.; JN, I.J.; van Besouw, N.M.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Human mesenchymal stem cells are susceptible to lysis by CD8(+) T cells and NK cells. Cell Transplant 2011, 20, 1547–1559. [Google Scholar] [CrossRef]
- Giuliani, M.; Bennaceur-Griscelli, A.; Nanbakhsh, A.; Oudrhiri, N.; Chouaib, S.; Azzarone, B.; Durrbach, A.; Lataillade, J.J. TLR ligands stimulation protects MSC from NK killing. Stem Cells 2014, 32, 290–300. [Google Scholar] [CrossRef]
- Chen, R.; Hao, Z.; Wang, Y.; Zhu, H.; Hu, Y.; Chen, T.; Zhang, P.; Li, J. Mesenchymal Stem Cell-Immune Cell Interaction and Related Modulations for Bone Tissue Engineering. Stem Cells Int 2022, 2022, 7153584. [Google Scholar] [CrossRef]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef]
- Brandau, S.; Jakob, M.; Hemeda, H.; Bruderek, K.; Janeschik, S.; Bootz, F.; Lang, S. Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J Leukoc Biol 2010, 88, 1005–1015. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; François, M.; Boivin, M.N.; Bouchentouf, M.; Spaner, D.E.; Galipeau, J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol 2009, 182, 7963–7973. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Mosna, F.; Micheletti, A.; Lisi, V.; Tamassia, N.; Cont, C.; Calzetti, F.; Pelletier, M.; Pizzolo, G.; Krampera, M. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells 2011, 29, 1001–1011. [Google Scholar] [CrossRef]
- Brandau, S.; Jakob, M.; Bruderek, K.; Bootz, F.; Giebel, B.; Radtke, S.; Mauel, K.; Jäger, M.; Flohé, S.B.; Lang, S. Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS One 2014, 9, e106903. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, R.H.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood 2011, 118, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Roddy, G.W.; Oh, J.Y.; Lee, R.H.; Bartosh, T.J.; Ylostalo, J.; Coble, K.; Rosa, R.H., Jr.; Prockop, D.J. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cells 2011, 29, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Muschhammer, J.; Qi, Y.; Kügler, A.; de Vries, J.C.; Saffarzadeh, M.; Sindrilaru, A.; Beken, S.V.; Wlaschek, M.; Kluth, M.A.; et al. Suppression of Neutrophil-Mediated Tissue Damage-A Novel Skill of Mesenchymal Stem Cells. Stem Cells 2016, 34, 2393–2406. [Google Scholar] [CrossRef]
- Ragipoglu, D.; Dudeck, A.; Haffner-Luntzer, M.; Voss, M.; Kroner, J.; Ignatius, A.; Fischer, V. The Role of Mast Cells in Bone Metabolism and Bone Disorders. Front Immunol 2020, 11, 163. [Google Scholar] [CrossRef]
- Docheva, D.; Popov, C.; Mutschler, W.; Schieker, M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med 2007, 11, 21–38. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front Immunol 2018, 9, 2837. [Google Scholar] [CrossRef]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 2018, 282, 121–150. [Google Scholar] [CrossRef]
- Ehnert, S.; Relja, B.; Schmidt-Bleek, K.; Fischer, V.; Ignatius, A.; Linnemann, C.; Rinderknecht, H.; Huber-Lang, M.; Kalbitz, M.; Histing, T.; et al. Effects of immune cells on mesenchymal stem cells during fracture healing. World J Stem Cells 2021, 13, 1667–1695. [Google Scholar] [CrossRef]
- Marusic, A.; Grcevic, D.; Katavic, V.; Kovacic, N.; Lukic, I.K.; Kalajzic, I.; Lorenzo, J.A. Role of B lymphocytes in new bone formation. Lab Invest 2000, 80, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Han, Q.; Liao, L.; You, S.; Deng, H.; Zhao, R.C. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol 2005, 24, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef]
- Tabera, S.; Pérez-Simón, J.A.; Díez-Campelo, M.; Sánchez-Abarca, L.I.; Blanco, B.; López, A.; Benito, A.; Ocio, E.; Sánchez-Guijo, F.M.; Cañizo, C.; et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica 2008, 93, 1301–1309. [Google Scholar] [CrossRef]
- Asari, S.; Itakura, S.; Ferreri, K.; Liu, C.P.; Kuroda, Y.; Kandeel, F.; Mullen, Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol 2009, 37, 604–615. [Google Scholar] [CrossRef]
- Schena, F.; Gambini, C.; Gregorio, A.; Mosconi, M.; Reverberi, D.; Gattorno, M.; Casazza, S.; Uccelli, A.; Moretta, L.; Martini, A.; et al. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum 2010, 62, 2776–2786. [Google Scholar] [CrossRef] [PubMed]
- Traggiai, E.; Volpi, S.; Schena, F.; Gattorno, M.; Ferlito, F.; Moretta, L.; Martini, A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells 2008, 26, 562–569. [Google Scholar] [CrossRef]
- Kon, T.; Cho, T.J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res 2001, 16, 1004–1014. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, R.; Shi, S. Systemic infusion of mesenchymal stem cells improves cell-based bone regeneration via upregulation of regulatory T cells. Tissue Eng Part A 2015, 21, 498–509. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef]
- Najar, M.; Fayyad-Kazan, H.; Faour, W.H.; Merimi, M.; Sokal, E.M.; Lombard, C.A.; Fahmi, H. Immunological modulation following bone marrow-derived mesenchymal stromal cells and Th17 lymphocyte co-cultures. Inflamm Res 2019, 68, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Klyushnenkova, E.; Mosca, J.D.; Zernetkina, V.; Majumdar, M.K.; Beggs, K.J.; Simonetti, D.W.; Deans, R.J.; McIntosh, K.R. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci 2005, 12, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffin, M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther 2011, 2, 34. [Google Scholar] [CrossRef]
- Glennie, S.; Soeiro, I.; Dyson, P.J.; Lam, E.W.; Dazzi, F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood 2005, 105, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef]
- Davies, L.C.; Heldring, N.; Kadri, N.; Le Blanc, K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar] [CrossRef]
- English, K.; Wood, K.J. Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harb Perspect Med 2013, 3, a015560. [Google Scholar] [CrossRef]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells 2013, 31, 1980–1991. [Google Scholar] [CrossRef]
- Yan, Z.; Zhuansun, Y.; Chen, R.; Li, J.; Ran, P. Immunomodulation of mesenchymal stromal cells on regulatory T cells and its possible mechanism. Exp Cell Res 2014, 324, 65–74. [Google Scholar] [CrossRef]
- Meert, K.L.; Ofenstein, J.P.; Sarnaik, A.P. Altered T cell cytokine production following mechanical trauma. Ann Clin Lab Sci 1998, 28, 283–288. [Google Scholar]
- Nauta, A.J.; Fibbe, W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef]
- Noël, D.; Djouad, F.; Bouffi, C.; Mrugala, D.; Jorgensen, C. Multipotent mesenchymal stromal cells and immune tolerance. Leuk Lymphoma 2007, 48, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Campioni, D.; Stignani, M.; Melchiorri, L.; Bagnara, G.P.; Bonsi, L.; Alviano, F.; Lanzoni, G.; Moretti, S.; Cuneo, A.; et al. A functional role for soluble HLA-G antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy 2008, 10, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Raffaghello, L.; Bianchi, G.; Meloni, F.; Salis, A.; Millo, E.; Ferrone, S.; Barnaba, V.; Pistoia, V. Immunogenicity of human mesenchymal stem cells in HLA-class I-restricted T-cell responses against viral or tumor-associated antigens. Stem Cells 2008, 26, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Montespan, F.; Deschaseaux, F.; Sensébé, L.; Carosella, E.D.; Rouas-Freiss, N. Osteodifferentiated mesenchymal stem cells from bone marrow and adipose tissue express HLA-G and display immunomodulatory properties in HLA-mismatched settings: implications in bone repair therapy. J Immunol Res 2014, 2014, 230346. [Google Scholar] [CrossRef] [PubMed]
- Najar, M.; Raicevic, G.; Fayyad-Kazan, H.; De Bruyn, C.; Bron, D.; Toungouz, M.; Lagneaux, L. Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming. Stem Cell Rev Rep 2012, 8, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Pistoia, V.; Moretta, L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 2007, 28, 219–226. [Google Scholar] [CrossRef]
- Sun, G.; Wang, Y.; Ti, Y.; Wang, J.; Zhao, J.; Qian, H. Regulatory B cell is critical in bone union process through suppressing proinflammatory cytokines and stimulating Foxp3 in Treg cells. Clin Exp Pharmacol Physiol 2017, 44, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Meednu, N.; Rosenberg, A.; Rangel-Moreno, J.; Wang, V.; Glanzman, J.; Owen, T.; Zhou, X.; Zhang, H.; Boyce, B.F.; et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat Commun 2018, 9, 5127. [Google Scholar] [CrossRef]
- van Mierlo, G.J.; Scherer, H.U.; Hameetman, M.; Morgan, M.E.; Flierman, R.; Huizinga, T.W.; Toes, R.E. Cutting edge: TNFR-shedding by CD4+CD25+ regulatory T cells inhibits the induction of inflammatory mediators. J Immunol 2008, 180, 2747–2751. [Google Scholar] [CrossRef]
- Ono, T.; Okamoto, K.; Nakashima, T.; Nitta, T.; Hori, S.; Iwakura, Y.; Takayanagi, H. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun 2016, 7, 10928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, Q.; Gu, B.; Yin, C.; Shen, K.; Tang, H.; Xia, H.; Zhang, X.; Zhao, Y.; Yang, X.; et al. Minimally invasive implantation and decreased inflammation reduce osteoinduction of biomaterial. Theranostics 2020, 10, 3533–3545. [Google Scholar] [CrossRef]
- Spector, J.A.; Mehrara, B.J.; Greenwald, J.A.; Saadeh, P.B.; Steinbrech, D.S.; Bouletreau, P.J.; Smith, L.P.; Longaker, M.T. Osteoblast expression of vascular endothelial growth factor is modulated by the extracellular microenvironment. Am J Physiol Cell Physiol 2001, 280, C72–80. [Google Scholar] [CrossRef] [PubMed]
- Hazehara-Kunitomo, Y.; Hara, E.S.; Ono, M.; Aung, K.T.; Komi, K.; Pham, H.T.; Akiyama, K.; Okada, M.; Oohashi, T.; Matsumoto, T.; et al. Acidic Pre-Conditioning Enhances the Stem Cell Phenotype of Human Bone Marrow Stem/Progenitor Cells. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Ichim, T.E.; Miller, K.; Anneling, A.; Grinstein, V.; Vargas, V.; Silva, F.J. Comparing atmospheric and hypoxic cultured mesenchymal stem cell transcriptome: implication for stem cell therapies targeting intervertebral discs. J Transl Med 2018, 16, 222. [Google Scholar] [CrossRef]
- Jadaun, P.K.; Zhang, S.; Koedam, M.; Demmers, J.; Chatterjee, S.; van Leeuwen, J.P.; van der Eerden, B.C. Inhibition of hypoxia-induced Mucin 1 alters the proteomic composition of human osteoblast-produced extracellular matrix, leading to reduced osteogenic and angiogenic potential. J Cell Physiol 2022, 237, 1440–1454. [Google Scholar] [CrossRef]
- Hu, S.; Qiao, L.; Cheng, K. Generation and Manipulation of Exosomes. Methods Mol Biol 2021, 2158, 295–305. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, B.; Zullo, M.J.; Codispoti, B.; Paduano, F.; Benincasa, C.; Fortunato, F.; Scacco, S.; Zavan, B.; Cocco, T. Exosomes from Human Periapical Cyst-MSCs: Theranostic Application in Parkinson's Disease. Int J Med Sci 2020, 17, 657–663. [Google Scholar] [CrossRef]
- Chachques, J.C.; Gardin, C.; Lila, N.; Ferroni, L.; Migonney, V.; Falentin-Daudre, C.; Zanotti, F.; Trentini, M.; Brunello, G.; Rocca, T.; et al. Elastomeric Cardiowrap Scaffolds Functionalized with Mesenchymal Stem Cells-Derived Exosomes Induce a Positive Modulation in the Inflammatory and Wound Healing Response of Mesenchymal Stem Cell and Macrophage. Biomedicines 2021, 9. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Gardin, C.; Zamparini, F.; Ferroni, L.; Esposti, M.D.; Parchi, G.; Ercan, B.; Manzoli, L.; Fava, F.; Fabbri, P.; et al. Mineral-Doped Poly(L-lactide) Acid Scaffolds Enriched with Exosomes Improve Osteogenic Commitment of Human Adipose-Derived Mesenchymal Stem Cells. Nanomaterials (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Zuo, R.; Liu, M.; Wang, Y.; Li, J.; Wang, W.; Wu, J.; Sun, C.; Li, B.; Wang, Z.; Lan, W.; et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res Ther 2019, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater 2020, 103, 196–212. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, J.; Jing, W. Motivating role of type H vessels in bone regeneration. Cell Prolif 2020, 53, e12874. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Guder, C.; Gravius, S.; Burger, C.; Wirtz, D.C.; Schildberg, F.A. Osteoimmunology: A Current Update of the Interplay Between Bone and the Immune System. Front Immunol 2020, 11, 58. [Google Scholar] [CrossRef]
- Sasse, S.; Skorska, A.; Lux, C.A.; Steinhoff, G.; David, R.; Gaebel, R. Angiogenic Potential of Bone Marrow Derived CD133(+) and CD271(+) Intramyocardial Stem Cell Trans- Plantation Post MI. Cells 2019, 9. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Schell, H.; Lienau, J.; Schulz, N.; Hoff, P.; Pfaff, M.; Schmidt, G.; Martin, C.; Perka, C.; Buttgereit, F.; et al. Initial immune reaction and angiogenesis in bone healing. J Tissue Eng Regen Med 2014, 8, 120–130. [Google Scholar] [CrossRef]
- Chen, X.; He, W.; Sun, M.; Yan, Y.; Pang, Y.; Chai, G. STING inhibition accelerates the bone healing process while enhancing type H vessel formation. Faseb j 2021, 35, e21964. [Google Scholar] [CrossRef]
- Gao, B.; Deng, R.; Chai, Y.; Chen, H.; Hu, B.; Wang, X.; Zhu, S.; Cao, Y.; Ni, S.; Wan, M.; et al. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J Clin Invest 2019, 129, 2578–2594. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, G.; Zhang, B.; Wang, C.; Li, D.; Ding, C.; Wei, Q.; Fan, Z.; Tang, H.; et al. Single-Cell RNA Sequencing Reveals B Cells Are Important Regulators in Fracture Healing. Front Endocrinol (Lausanne) 2021, 12, 666140. [Google Scholar] [CrossRef]
- Avin, K.G.; Dominguez, J.M., 2nd; Chen, N.X.; Hato, T.; Myslinski, J.J.; Gao, H.; Liu, Y.; McKinley, T.O.; Brown, K.M.; Moe, S.M.; et al. Single-cell RNAseq provides insight into altered immune cell populations in human fracture nonunions. J Orthop Res 2023, 41, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Jeong, H.W.; Dharmalingam, B.; Zeuschner, D.; Adams, S.; Potente, M.; Adams, R.H. Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Rep 2021, 36, 109352. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl Med 2017, 6, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.; Santos, S.G.; Gonçalves, R.M.; Teixeira, J.H.; Barbosa, M.A.; Almeida, M.I. Genetically Engineered-MSC Therapies for Non-unions, Delayed Unions and Critical-size Bone Defects. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.H.G.; et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 2020, 239, 119833. [Google Scholar] [CrossRef]
- Humbert, P.; Brennan, M.; Davison, N.; Rosset, P.; Trichet, V.; Blanchard, F.; Layrolle, P. Immune Modulation by Transplanted Calcium Phosphate Biomaterials and Human Mesenchymal Stromal Cells in Bone Regeneration. Front Immunol 2019, 10, 663. [Google Scholar] [CrossRef]
- Hou, Y.; Cai, K.; Li, J.; Chen, X.; Lai, M.; Hu, Y.; Luo, Z.; Ding, X.; Xu, D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int J Nanomedicine 2013, 8, 3619–3630. [Google Scholar] [CrossRef]
- Takebe, J.; Champagne, C.M.; Offenbacher, S.; Ishibashi, K.; Cooper, L.F. Titanium surface topography alters cell shape and modulates bone morphogenetic protein 2 expression in the J774A.1 macrophage cell line. J Biomed Mater Res A 2003, 64, 207–216. [Google Scholar] [CrossRef]
- Refai, A.K.; Textor, M.; Brunette, D.M.; Waterfield, J.D. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res A 2004, 70, 194–205. [Google Scholar] [CrossRef]
- Smith, B.S.; Capellato, P.; Kelley, S.; Gonzalez-Juarrero, M.; Popat, K.C. Reduced in vitro immune response on titania nanotube arrays compared to titanium surface. Biomater Sci 2013, 1, 322–332. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. Advancing dental implant surface technology--from micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Paul, N.E.; Skazik, C.; Harwardt, M.; Bartneck, M.; Denecke, B.; Klee, D.; Salber, J.; Zwadlo-Klarwasser, G. Topographical control of human macrophages by a regularly microstructured polyvinylidene fluoride surface. Biomaterials 2008, 29, 4056–4064. [Google Scholar] [CrossRef]
- Qiu, P.; Li, M.; Chen, K.; Fang, B.; Chen, P.; Tang, Z.; Lin, X.; Fan, S. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials 2020, 227, 119552. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 2012, 21, 2724–2752. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Locatelli, F.; Fibbe, W.E. Mesenchymal stromal cells. Ann N Y Acad Sci 2009, 1176, 101–117. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 2011, 9, 12. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med 2013, 2, 455–463. [Google Scholar] [CrossRef]
- Ding, H.; Gao, Y.S.; Wang, Y.; Hu, C.; Sun, Y.; Zhang, C. Dimethyloxaloylglycine increases the bone healing capacity of adipose-derived stem cells by promoting osteogenic differentiation and angiogenic potential. Stem Cells Dev 2014, 23, 990–1000. [Google Scholar] [CrossRef]
- Zimmerlin, L.; Donnenberg, V.S.; Pfeifer, M.E.; Meyer, E.M.; Péault, B.; Rubin, J.P.; Donnenberg, A.D. Stromal vascular progenitors in adult human adipose tissue. Cytometry A 2010, 77, 22–30. [Google Scholar] [CrossRef]
- Storti, G.; Scioli, M.G.; Kim, B.S.; Orlandi, A.; Cervelli, V. Adipose-Derived Stem Cells in Bone Tissue Engineering: Useful Tools with New Applications. Stem Cells Int 2019, 2019, 3673857. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Jimi, E.; Hirata, S.; Shin, M.; Yamazaki, M.; Fukushima, H. Molecular mechanisms of BMP-induced bone formation: Cross-talk between BMP and NF-κB signaling pathways in osteoblastogenesis. Japanese Dental ence Review 2010, 46, 33–42. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 2011, 13, 27–38. [Google Scholar] [CrossRef]
- Shafaei, H.; Kalarestaghi, H. Adipose-derived stem cells: An appropriate selection for osteogenic differentiation. J Cell Physiol 2020, 235, 8371–8386. [Google Scholar] [CrossRef]
- Yang, J.; Andre, P.; Ye, L.; Yang, Y.Z. The Hedgehog signalling pathway in bone formation. Int J Oral Sci 2015, 7, 73–79. [Google Scholar] [CrossRef]
- Senarath-Yapa, K.; McArdle, A.; Renda, A.; Longaker, M.T.; Quarto, N. Adipose-derived stem cells: a review of signaling networks governing cell fate and regenerative potential in the context of craniofacial and long bone skeletal repair. Int J Mol Sci 2014, 15, 9314–9330. [Google Scholar] [CrossRef]
- Malladi, P.; Xu, Y.; Yang, G.P.; Longaker, M.T. Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Eng 2006, 12, 2031–2040. [Google Scholar] [CrossRef]
- Wang, C.Z.; Chen, S.M.; Chen, C.H.; Wang, C.K.; Wang, G.J.; Chang, J.K.; Ho, M.L. The effect of the local delivery of alendronate on human adipose-derived stem cell-based bone regeneration. Biomaterials 2010, 31, 8674–8683. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, J.; Liu, Y.; Yu, Q.; Liu, Y.; Deng, N.; Liu, J. Functional Selenium Nanoparticles Enhanced Stem Cell Osteoblastic Differentiation through BMP Signaling Pathways. John Wiley & Sons, Ltd 2014.
- Scioli, M.G.; Bielli, A.; Gentile, P.; Cervelli, V.; Orlandi, A. Combined treatment with platelet-rich plasma and insulin favours chondrogenic and osteogenic differentiation of human adipose-derived stem cells in three-dimensional collagen scaffolds. J Tissue Eng Regen Med 2017, 11, 2398–2410. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.C.; Young, D.W.; Golenbock, D.T.; Christ, W.J.; Gusovsky, F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 1999, 274, 10689–10692. [Google Scholar] [CrossRef] [PubMed]
- Herzmann, N.; Salamon, A.; Fiedler, T.; Peters, K. Lipopolysaccharide induces proliferation and osteogenic differentiation of adipose-derived mesenchymal stromal cells in vitro via TLR4 activation. Exp Cell Res 2017, 350, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.; Ushmorov, A.; Fiedler, J.; Brenner, R.E.; Wirth, T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone 2009, 45, 367–376. [Google Scholar] [CrossRef]
- Fiedler, T.; Salamon, A.; Adam, S.; Herzmann, N.; Taubenheim, J.; Peters, K. Impact of bacteria and bacterial components on osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Exp Cell Res 2013, 319, 2883–2892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




