Submitted:
16 August 2023
Posted:
17 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant material and growth conditions
| Light Intensity (µmol m−2 s−1) | |||
|---|---|---|---|
| Time-point | 100% of sunlight | 50% of sunlight | 30% of sunlight |
| 8 a.m. | 470 | 235 | 141 |
| 12 | 1230 | 615 | 369 |
| 5 p.m. | 650 | 325 | 195 |
2.2. Determination of chlorophyll and carotenoid contents
2.3. Determination of total anthocyanins
2.4. Evaluation of soluble and storage carbohydrate contents
2.5. Chl a fluorescence
2.6. Statistical analysis
3. Results
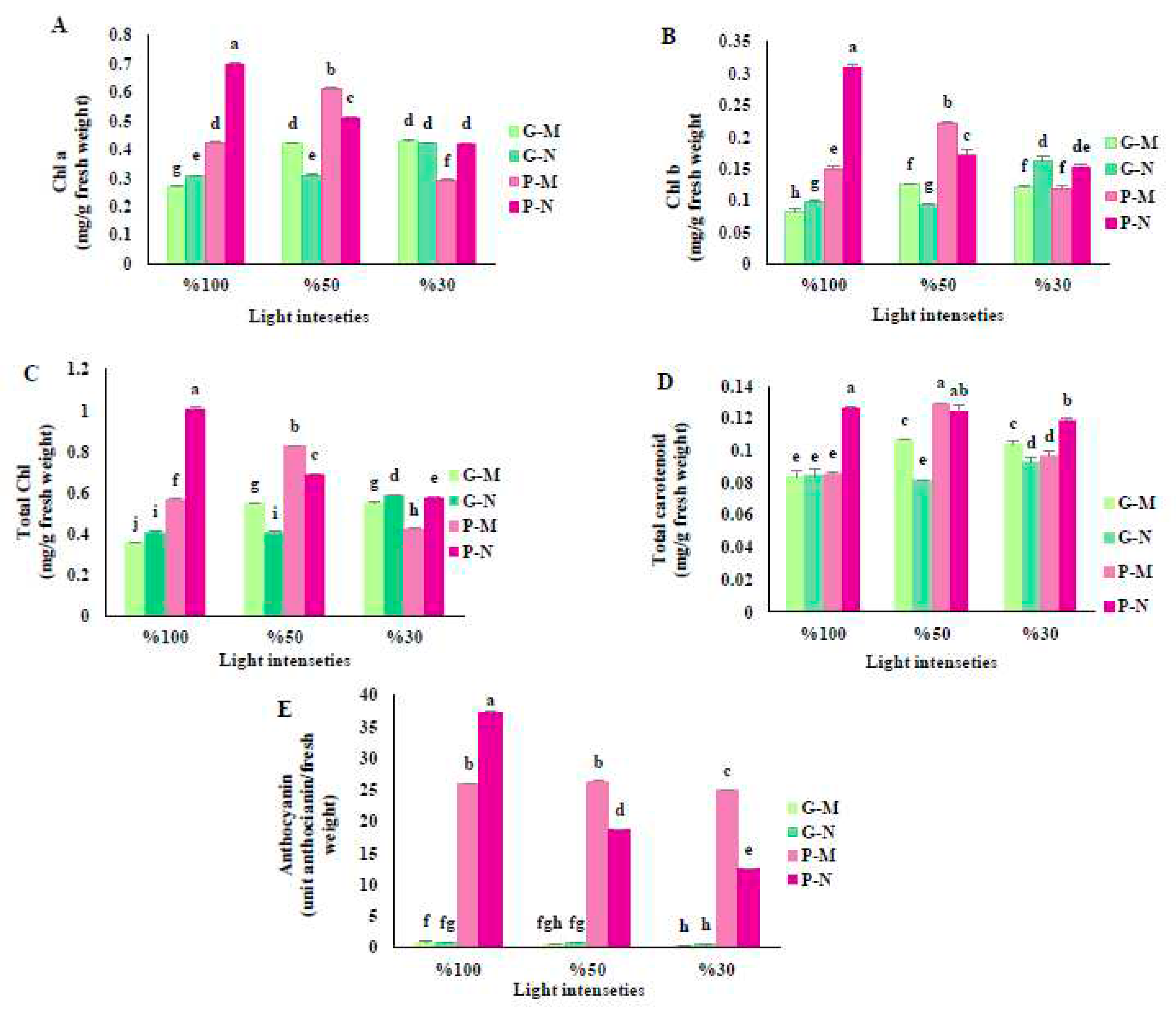
3.1. Effects of shading and harvest time on pigment concentrations of basil plants
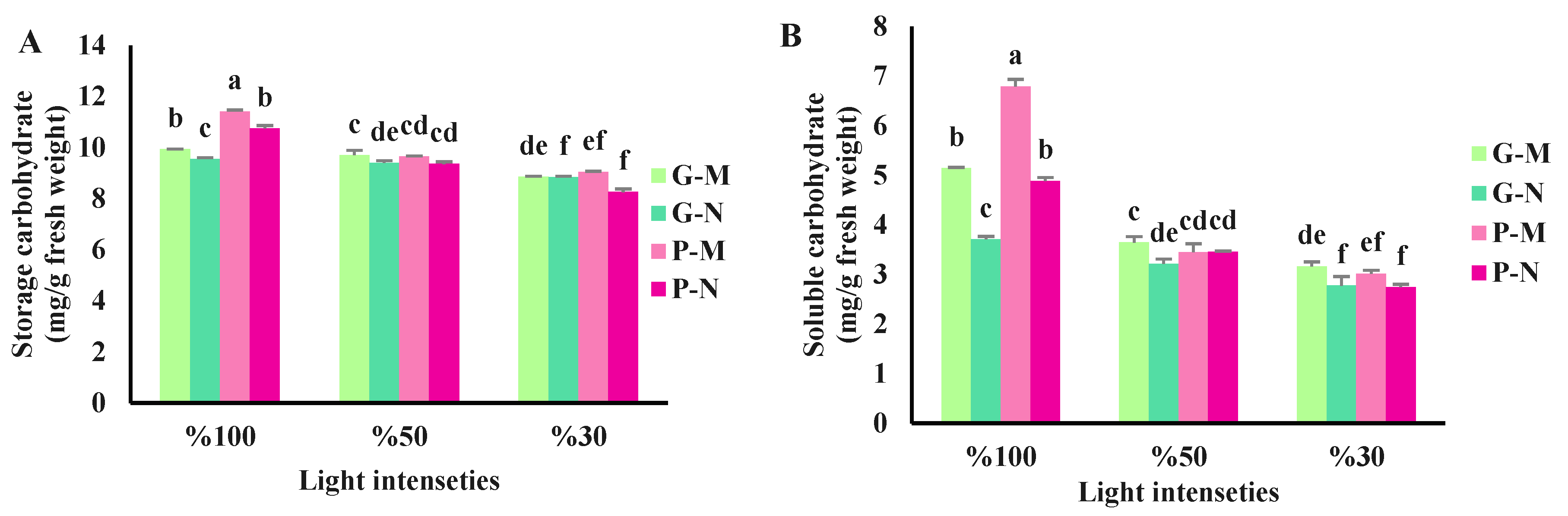
3.2. Effects of shading and harvest time on leaf carbohydrate contents of basil plants
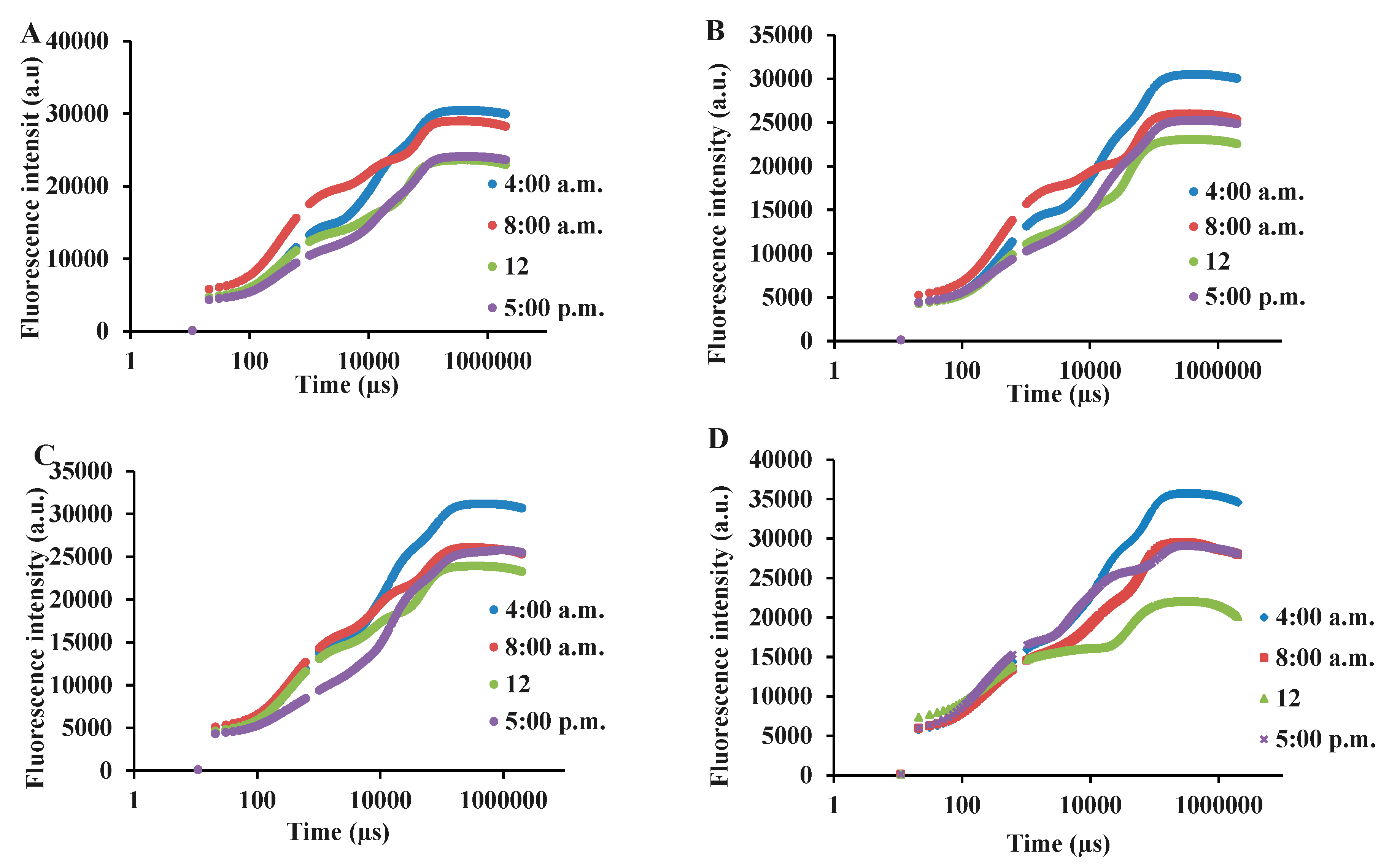
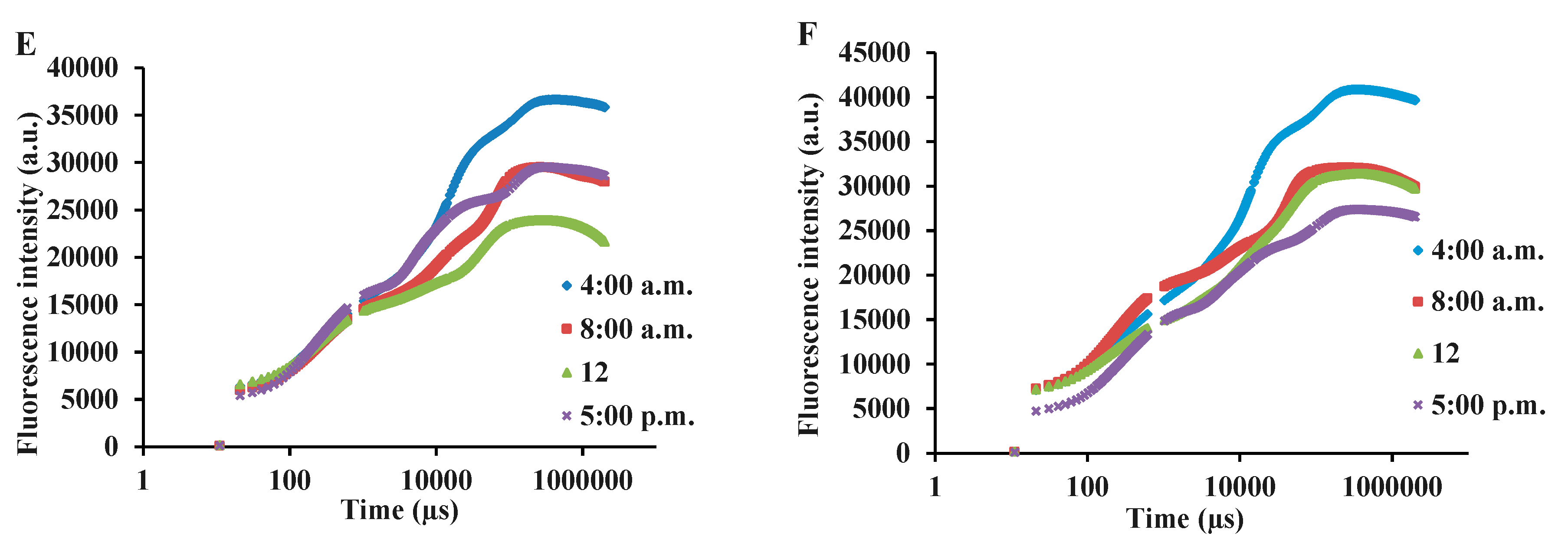
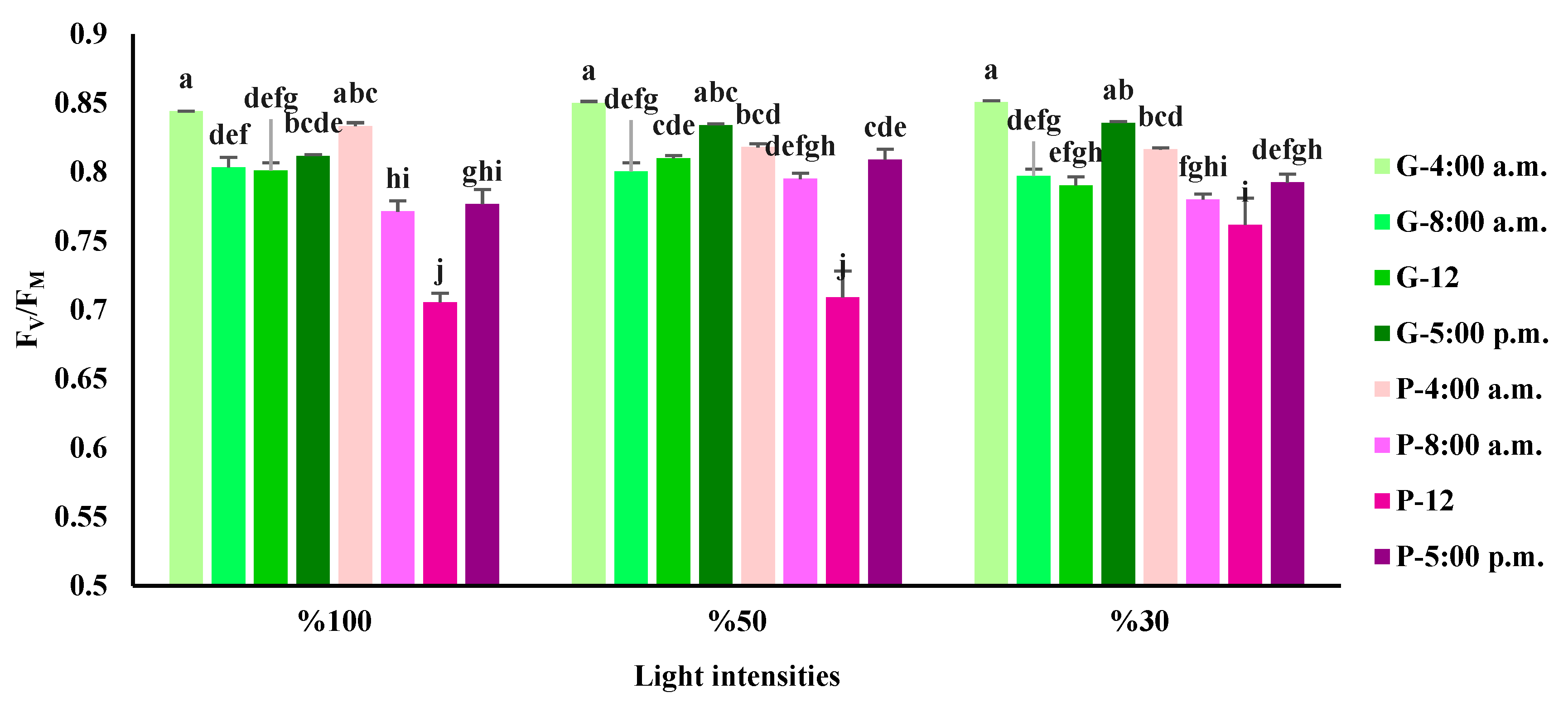
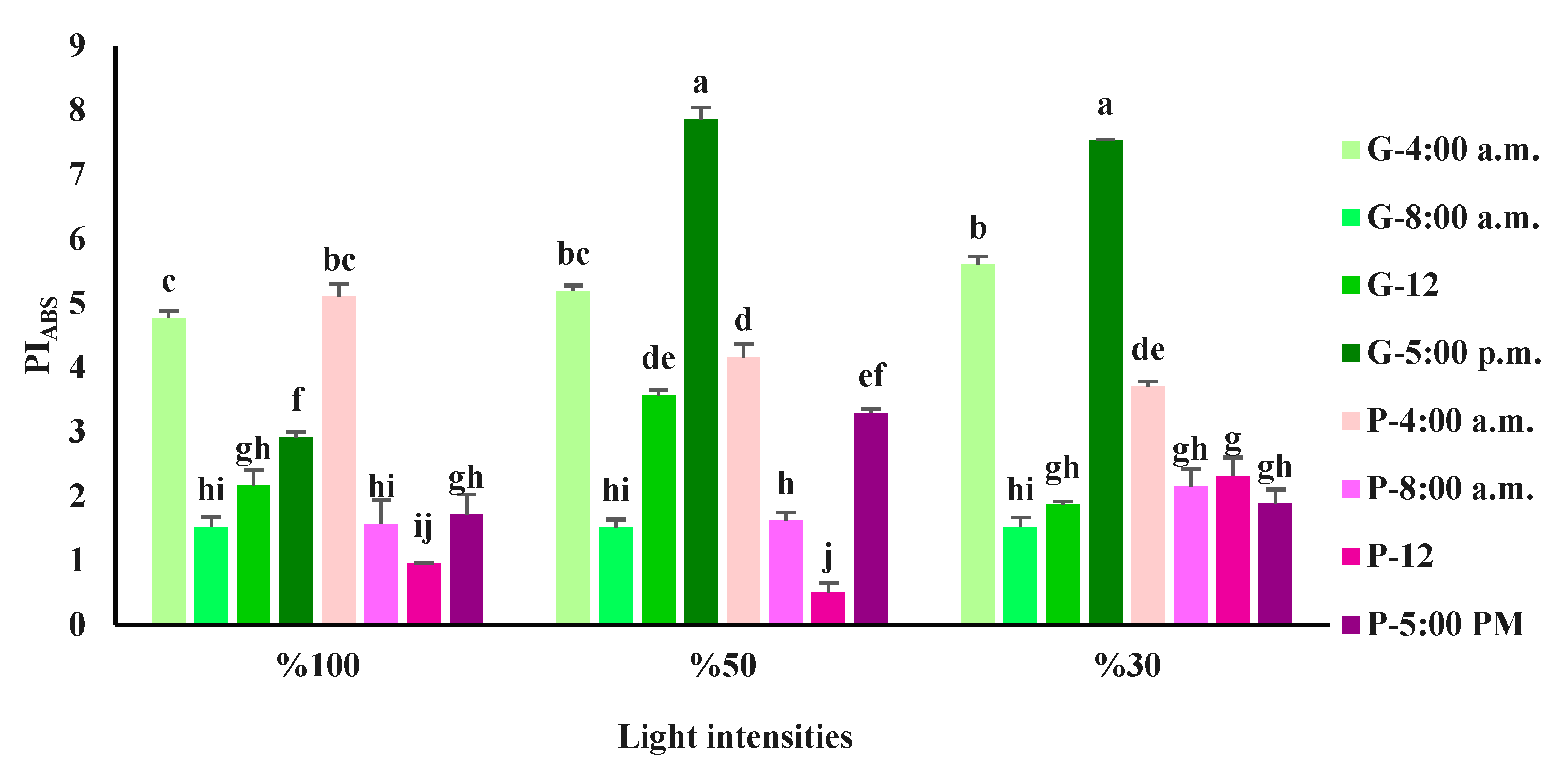
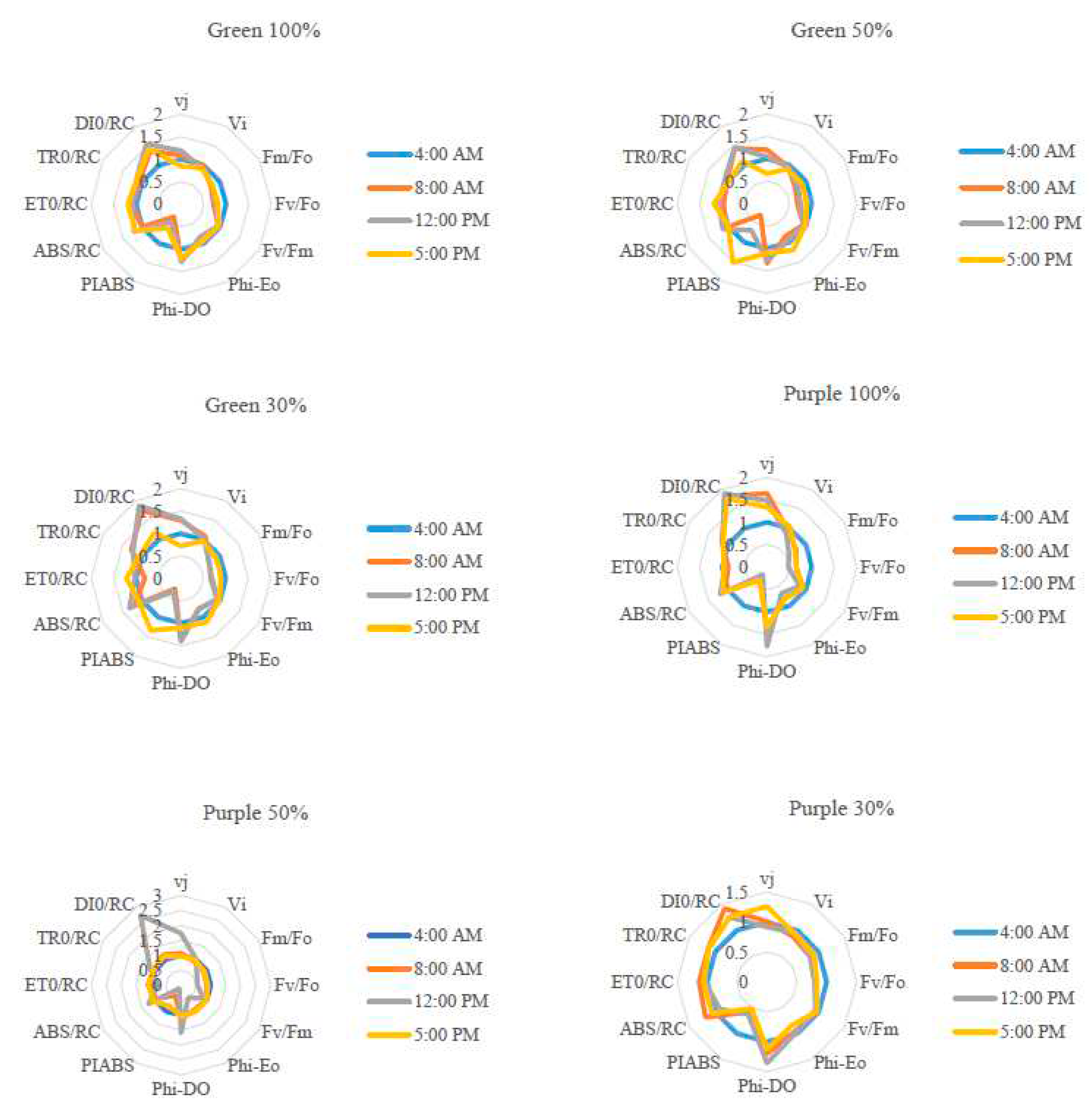
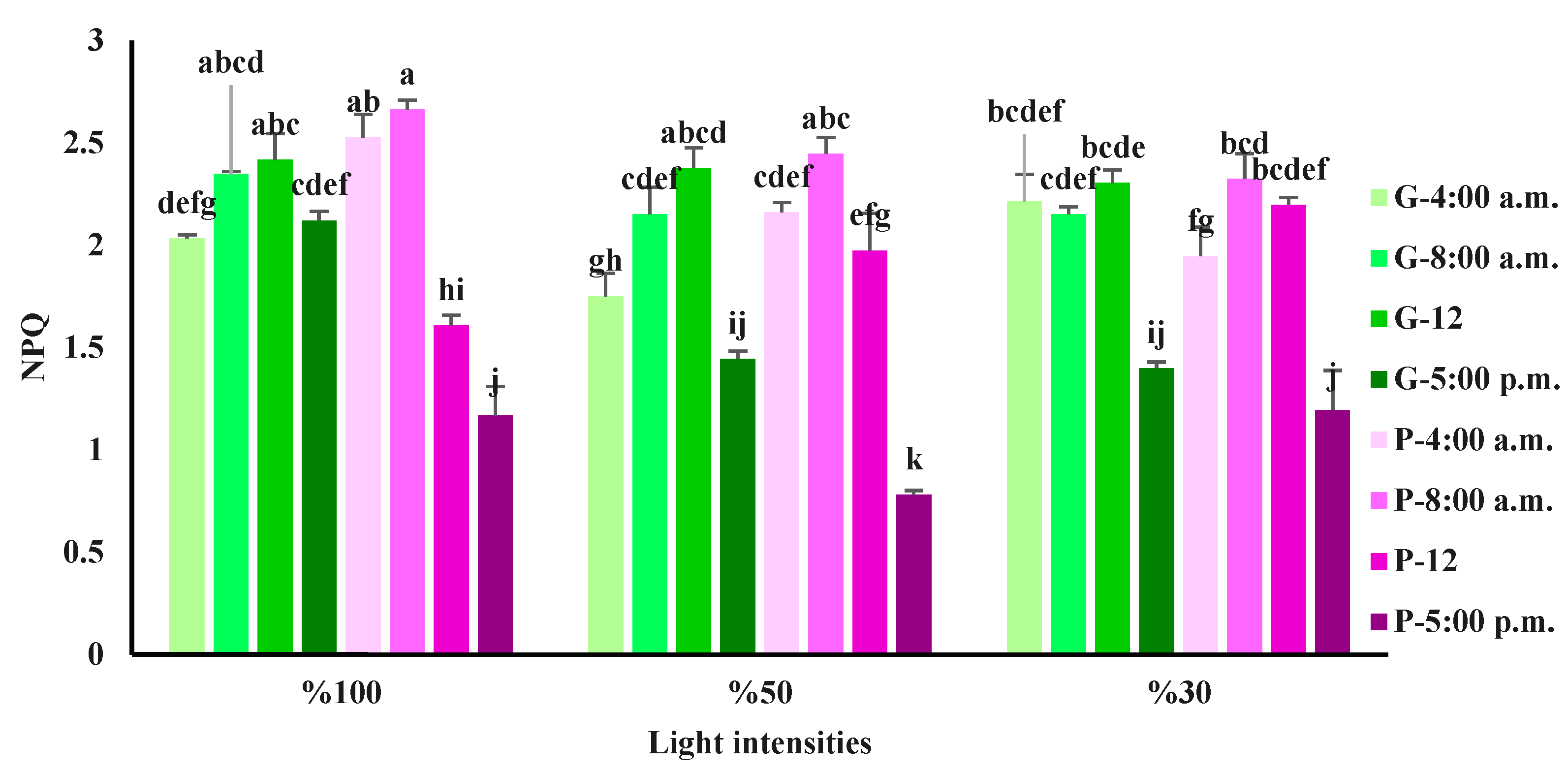
3.3. Chlorophyll a fluorescence influenced by light intensity and day-time in basil varieties
4. Discussion
5. Conclusions
References
- Ioannidis, D., L. Bonner, and C.B. Johnson, UV-B is required for normal development of oil glands in Ocimum basilicum L.(Sweet Basil). Annals of Botany, 2002, 90, 453–460. [CrossRef] [PubMed]
- Labra, M., et al., Morphological characterization, essential oil composition and DNA genotyping of Ocimum basilicum L. cultivars. Plant Science 2004, 167, 725–731. [CrossRef]
- Sajjadi, S.E. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. DARU Journal of Pharmaceutical Sciences, 2006, 14, 128–130. [Google Scholar]
- Paez, A., et al., Growth, soluble carbohydrates, and aloin concentration of Aloevera plants exposed to three irradiance levels. Environmental and Experimental Botany, 2000, 44, 133–139. [CrossRef]
- Ashrostaghi, T., et al., Light intensity: The role player in cucumber response to cold stress. Agronomy, 2022, 12, 201. [CrossRef]
- Esmaeili, S., et al., Elevated light intensity compensates for nitrogen deficiency during chrysanthemum growth by improving water and nitrogen use efficiency. Scientific Reports, 2022, 12, 10002. [CrossRef]
- Long, S.P., S. Humphries, and P.G. Falkowski, Photoinhibition of photosynthesis in nature. Annual review of plant biology, 1994, 45, 633–662. [CrossRef]
- Ghorbanzadeh, P., et al., Dependency of growth, water use efficiency, chlorophyll fluorescence, and stomatal characteristics of lettuce plants to light intensity. Journal of Plant Growth Regulation, 2021, 40, 2191–2207. [CrossRef]
- Vatankhah, A., et al., Plants exposed to titanium dioxide nanoparticles acquired contrasting photosynthetic and morphological strategies depending on the growing light intensity: a case study in radish. Scientific Reports, 2023, 13, 5873. [CrossRef]
- Zhang, S., K. Ma, and L. Chen, Response of photosynthetic plasticity of Paeonia suffruticosa to changed light environments. Environmental and Experimental Botany, 2003, 49, 121–133. [CrossRef]
- Whitelam, G.C. and K.J. Halliday, Light and plant development. 2007: Blackwell Pub.
- Tesfaye, K., S. Walker, and M. Tsubo, Radiation interception and radiation use efficiency of three grain legumes under water deficit conditions in a semi-arid environment. European journal of Agronomy, 2006, 25, 60–70. [CrossRef]
- Zervoudakis, G., et al., Influence of light intensity on growth and physiological characteristics of common sage (Salvia officinalis L.). Brazilian archives of biology and technology, 2012, 55, 89–95. [CrossRef]
- De la Mata, L., et al., Study of the senescence process in primary leaves of sunflower (Helianthus annuus L.) plants under two different light intensities. Photosynthetica, 2013, 51, 85–94. [CrossRef]
- Zhu, H., et al., Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PloS one, 2017, 12, e0179305.
- Santana, A.C., et al., Effect of harvest at different times of day on the physical and chemical characteristics of vegetable-type soybean. Food Science and Technology, 2012, 32, 351–356. [CrossRef]
- Teng, S., et al., Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant physiology, 2005, 139, 1840–1852. [CrossRef]
- Irigoyen, J., D. Einerich, and M. Sánchez-Díaz, Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativd) plants. Physiologia plantarum, 1992, 84, 55–60. [CrossRef]
- McCready, R., et al., Determination of starch and amylose in vegetables. Analytical chemistry, 1950, 22, 1156–1158. [CrossRef]
- Aliniaeifard, S., P. Malcolm Matamoros, and U. van Meeteren, Stomatal malfunctioning under low VPD conditions: induced by alterations in stomatal morphology and leaf anatomy or in the ABA signaling? Physiologia plantarum, 2014, 152, 688–699. [CrossRef]
- Aliniaeifard, S. and U. van Meeteren, Natural variation in stomatal response to closing stimuli among Arabidopsis thaliana accessions after exposure to low VPD as a tool to recognize the mechanism of disturbed stomatal functioning. Journal of Experimental Botany, 2014, 65, 6529–6542. [CrossRef]
- Genty, B., J.-M. Briantais, and N.R. Baker, The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)-General Subjects, 1989, 990, 87–92. [CrossRef]
- Abreu, P.P., et al., Photosynthetic responses of ornamental passion flower hybrids to varying light intensities. Acta physiologiae plantarum, 2014, 36, 1993–2004. [CrossRef]
- Wadhwa, R., N. Kumari, and V. Sharma, Varying light regimes in naturally growing Jatropha curcus: pigment, proline and photosynthetic performance. Journal of Stress Physiology & Biochemistry, 2010, 6.
- Zhao, S., et al., Anthocyanin accumulation provides protection against high light stress while reducing photosynthesis in apple leaves. International Journal of Molecular Sciences, 2022, 23, 12616. [CrossRef]
- Biswal, A.K., et al., Light intensity-dependent modulation of chlorophyll b biosynthesis and photosynthesis by overexpression of chlorophyllide a oxygenase in tobacco. Plant physiology, 2012, 159, 433–449. [CrossRef] [PubMed]
- Muhidin, et al. The effect of shade on chlorophyll and anthocyanin content of upland red rice. in IOP Conference Series: Earth and Environmental Science. 2018. IOP Publishing.
- Zhang, H., et al., Adaptive changes in chlorophyll content and photosynthetic features to low light in Physocarpus amurensis Maxim and Physocarpus opulifolius “Diabolo”. PeerJ, 2016, 4, e2125. [CrossRef]
- He, F., et al., Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules, 2010, 15, 9057–9091. [CrossRef]
- Oren-Shamir, M. and A. Levi-Nissim, UV-light effect on the leaf pigmentation of Cotinus coggygria ‘Royal Purple’. Scientia horticulturae, 1997, 71, 59–66. [CrossRef]
- Merzlyak, M.N. and O.B. Chivkunova, Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. Journal of Photochemistry and Photobiology B: Biology, 2000, 55, 155–163. [CrossRef]
- Bayat, L., et al., Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB Plants, 2018, 10.
- Albert, N.W., et al., Light-induced vegetative anthocyanin pigmentation in Petunia. Journal of experimental botany, 2009, 60, 2191–2202. [CrossRef] [PubMed]
- Hosseini, A., et al., Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiology and Molecular Biology of Plants, 2019, 25, 741–752. [CrossRef] [PubMed]
- Tenore, G.C., et al., Antioxidant and antimicrobial properties of traditional green and purple “Napoletano” basil cultivars (Ocimum basilicum L.) from Campania region (Italy). Natural product research, 2017, 31, 2067–2071. [CrossRef]
- Landi, M., et al., Antioxidant and photosynthetic response of a purple-leaved and a green-leaved cultivar of sweet basil (Ocimum basilicum) to boron excess. Environmental and experimental botany, 2013, 85, 64–75. [CrossRef]
- Chanoca, A., et al., Anthocyanin vacuolar inclusions form by a microautophagy mechanism. The plant cell, 2015, 27, 2545–2559. [CrossRef] [PubMed]
- Gould, K.S., D.A. Dudle, and H.S. Neufeld, Why some stems are red: cauline anthocyanins shield photosystem II against high light stress. Journal of Experimental Botany, 2010, 61, 2707–2717. [CrossRef]
- Hosseini, A., et al., Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiology and Molecular Biology of Plants, 2019, 25, 741–752. [CrossRef] [PubMed]
- Bryant, D.A. and N.-U. Frigaard, Prokaryotic photosynthesis and phototrophy illuminated. Trends in microbiology, 2006, 14, 488–496. [CrossRef]
- Cakmak, I. and V. Römheld, Boron deficiency-induced impairments of cellular functions in plants. Plant and Soil, 1997, 193, 71–83. [CrossRef]
- Chen, M., J. Chory, and C. Fankhauser, Light signal transduction in higher plants. Annu. Rev. Genet., 2004, 38, 87–117. [CrossRef]
- Zlatev, Z.S. and I.T. Yordanov, Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. Bulg. J. Plant Physiol, 2004, 30, 3–18.
- Shomali, A., et al., Artificial neural network (ANN)-based algorithms for high light stress phenotyping of tomato genotypes using chlorophyll fluorescence features. Plant Physiology and Biochemistry, 2023, 2023, 107893.
- Strasser, R.J., A. Srivastava, and M. Tsimilli-Michael, The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing photosynthesis: mechanisms, regulation and adaptation, 2000, 25, 445–483.
- Mehta, P., et al., Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiology and biochemistry, 2010, 48, 16–20. [CrossRef] [PubMed]
- Chaves, M.M., J. Flexas, and C. Pinheiro, Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of botany, 2009, 103, 551–560. [CrossRef]
- Kocheva, K., et al., Evaluation of chlorophyll fluorescence and membrane injury in the leaves of barley cultivars under osmotic stress. Bioelectrochemistry, 2004, 63, 121–124. [CrossRef]
- Shomali, A., et al., Photoinhibition in horticultural crops: An overview of the effect of light quality and signaling in the underlying photoprotection mechanisms. International Journal of Horticultural Science and Technology, 2023, 10, 39–50.
- Willadino, L., et al., Estresse salino em duas variedades de cana-de-açúcar: enzimas do sistema antioxidativo e fluorescência da clorofila. Revista Ciência Agronômica, 2011, 42, 417–422. [CrossRef]
- Li, J., et al., Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. Journal of The Saudi Society of Agricultural Sciences, 2017, 16, 82–88. [CrossRef]
- Broetto, F., H.M. Duarte, and U. Lüttge, Responses of chlorophyll fluorescence parameters of the facultative halophyte and C3–CAM intermediate species Mesembryanthemum crystallinum to salinity and high irradiance stress. Journal of plant physiology, 2007, 164, 904–912. [CrossRef]
- Hazrati, S., et al., Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L. Plant Physiology and Biochemistry, 2016, 106, 141–148. [CrossRef]
- Shomali, A., et al., Synergistic effects of melatonin and gamma-aminobutyric acid on protection of photosynthesis system in response to multiple abiotic stressors. Cells, 2021, 10, 1631.
- Kalhor, M.S., et al., Enhanced salt tolerance and photosynthetic performance: Implication of ɤ-amino butyric acid application in salt-exposed lettuce (Lactuca sativa L.) plants. Plant physiology and biochemistry, 2018, 130, 157–172. [CrossRef] [PubMed]
- Martinazzo, E.G., A. Ramm, and M.A. Bacarin, The chlorophyll a fluorescence as an indicator of the temperature stress in the leaves of Prunus persica. Brazilian journal of plant physiology, 2012, 24, 237–246.
- Mathur, S., P. Mehta, and A. Jajoo, Effects of dual stress (high salt and high temperature) on the photochemical efficiency of wheat leaves (Triticum aestivum). Physiology and Molecular Biology of Plants, 2013, 19, 179–188. [CrossRef] [PubMed]
- Sousaraei, N., et al., Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Horticulture, environment, and biotechnology, 2021, 62, 521–535. [CrossRef]
- Kalaji, H.M., et al., Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant physiology and biochemistry, 2014, 81, 16–25. [CrossRef]
- Liang, Y., et al., Responses of Jatropha curcas seedlings to cold stress: photosynthesis-related proteins and chlorophyll fluorescence characteristics. Physiologia Plantarum, 2007, 131, 508–517. [CrossRef]
- Zivcak, M., et al., Measurements of chlorophyll fluorescence in different leaf positions may detect nitrogen deficiency in wheat. 2014.
- Porcar-Castell, A., et al., Linking chlorophyll a fluorescence to photosynthesis for remote sensing applications: mechanisms and challenges. Journal of experimental botany, 2014, 65, 4065–4095. [CrossRef]
- Shomali, A., et al., Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants, 2022, 11, 3158. [CrossRef]
- Zhu, H., et al., Anthocyanins function as a light attenuator to compensate for insufficient photoprotection mediated by nonphotochemical quenching in young leaves of Acmena acuminatissima in winter. Photosynthetica 2018, 56, 445–454. [CrossRef]
- Agati, G., et al., Anthocyanins in photoprotection: knowing the actors in play to solve this complex ecophysiological issue. The New Phytologist, 2021, 232, 2228. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





