Submitted:
11 August 2023
Posted:
13 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CT Technique
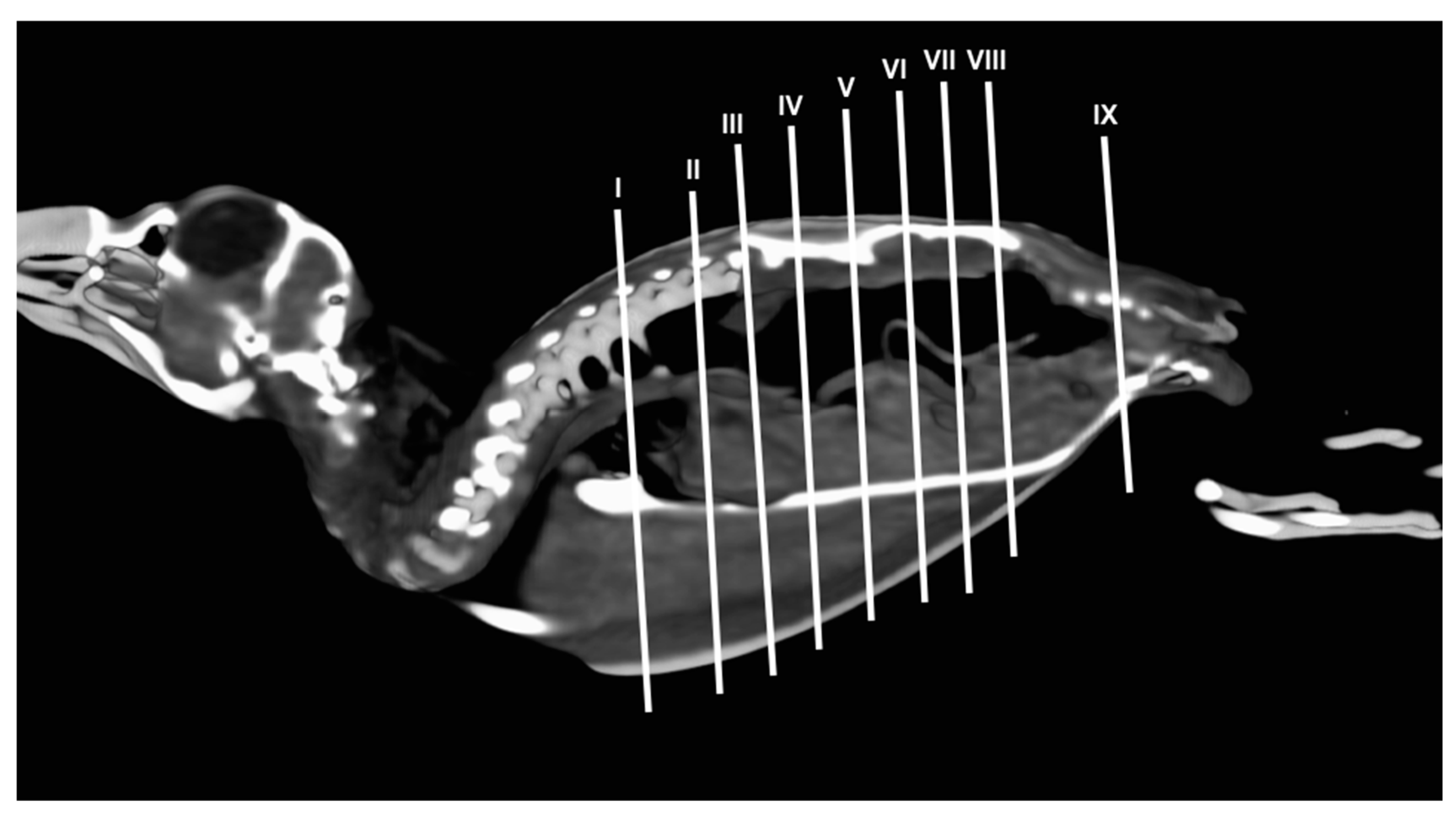
2.3. Anatomical sections
2.4. Anatomic identification
3. Results
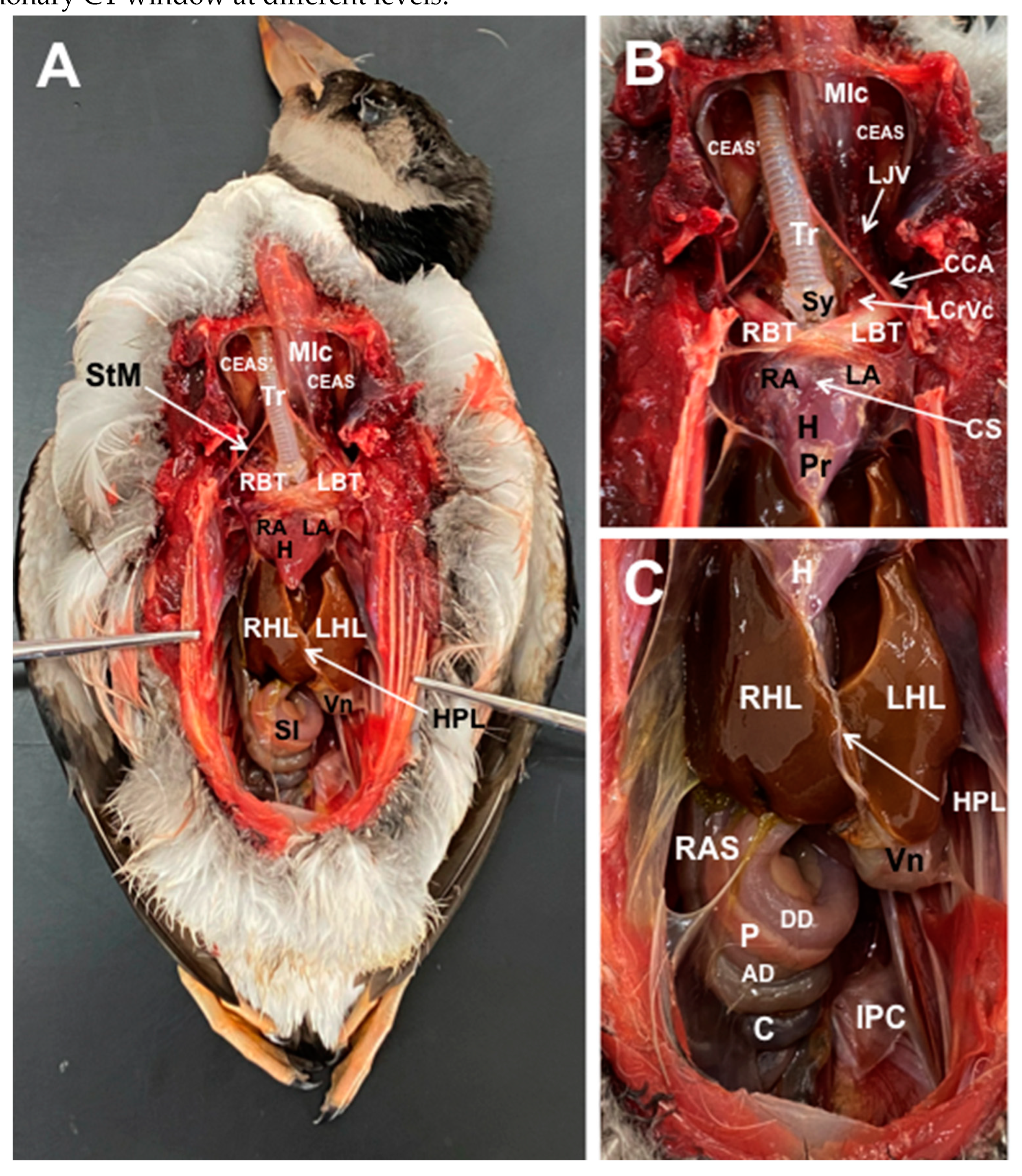
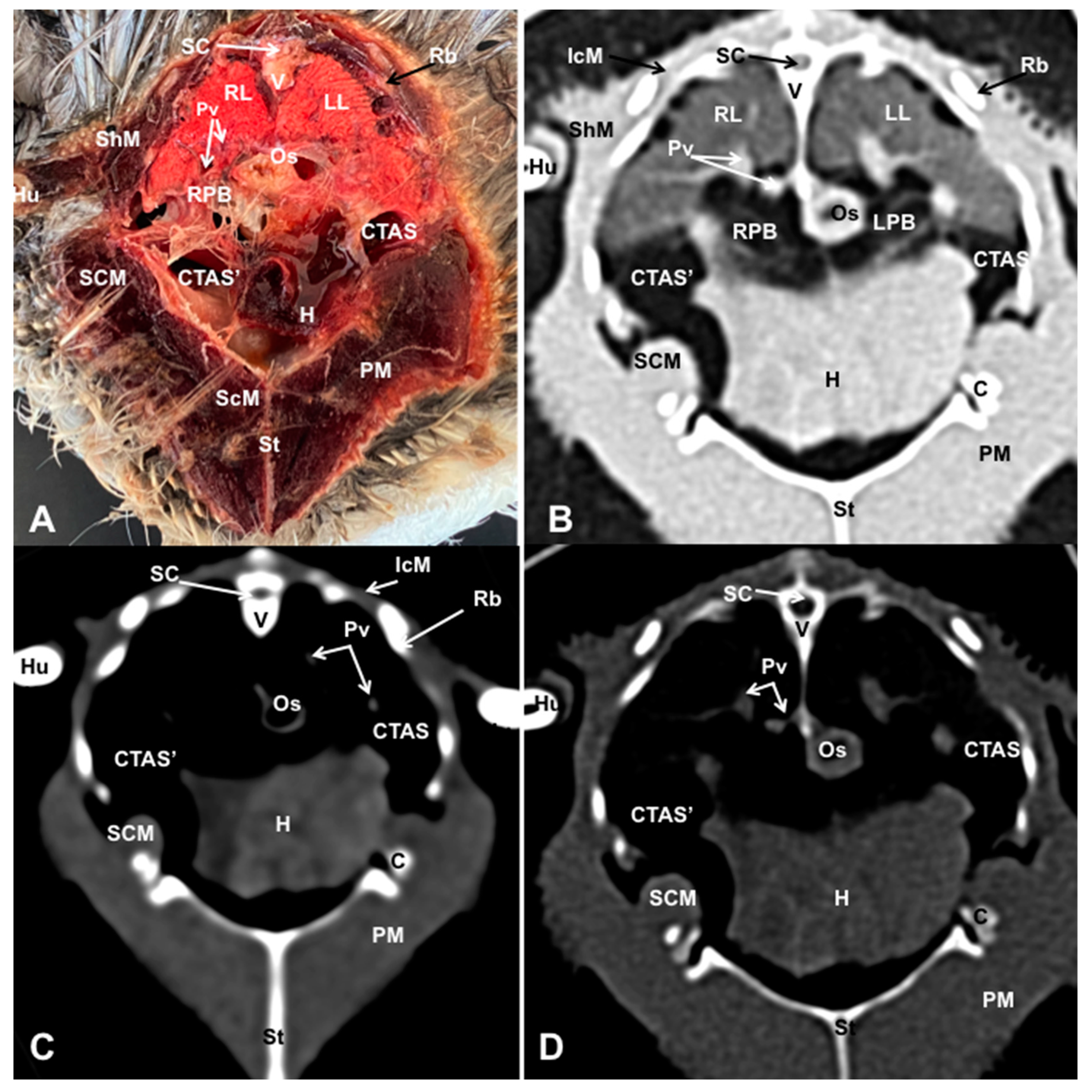
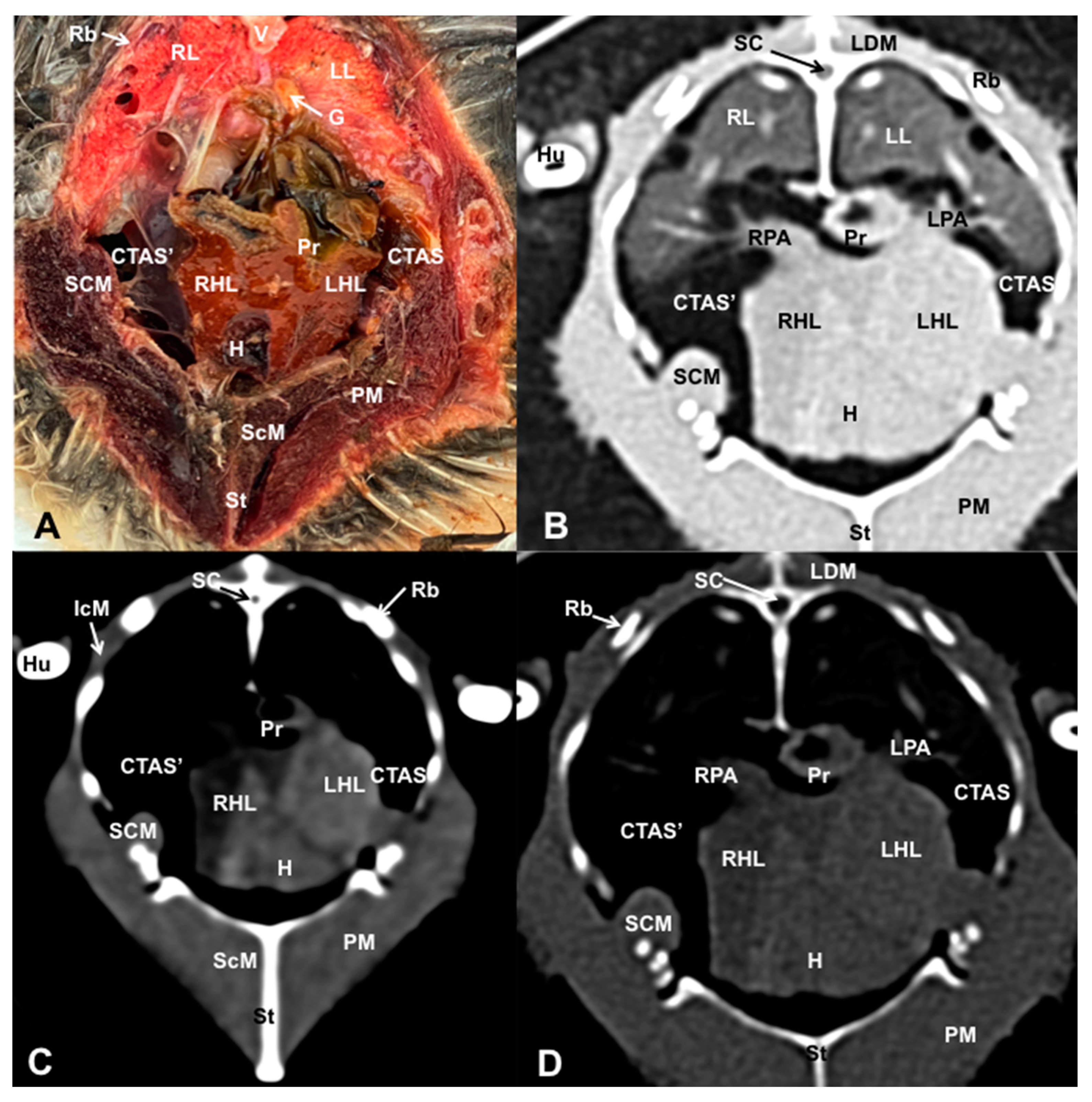
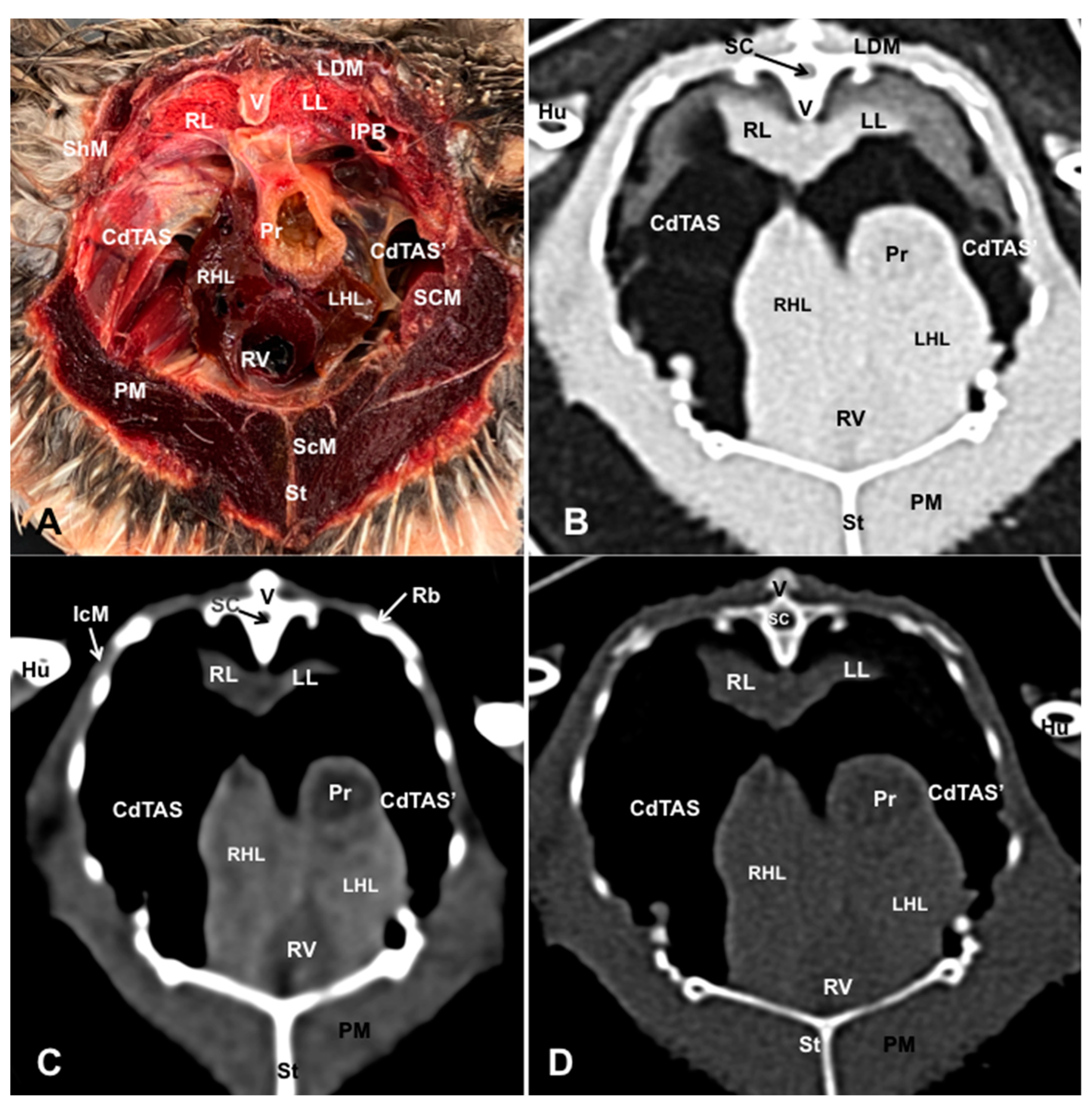
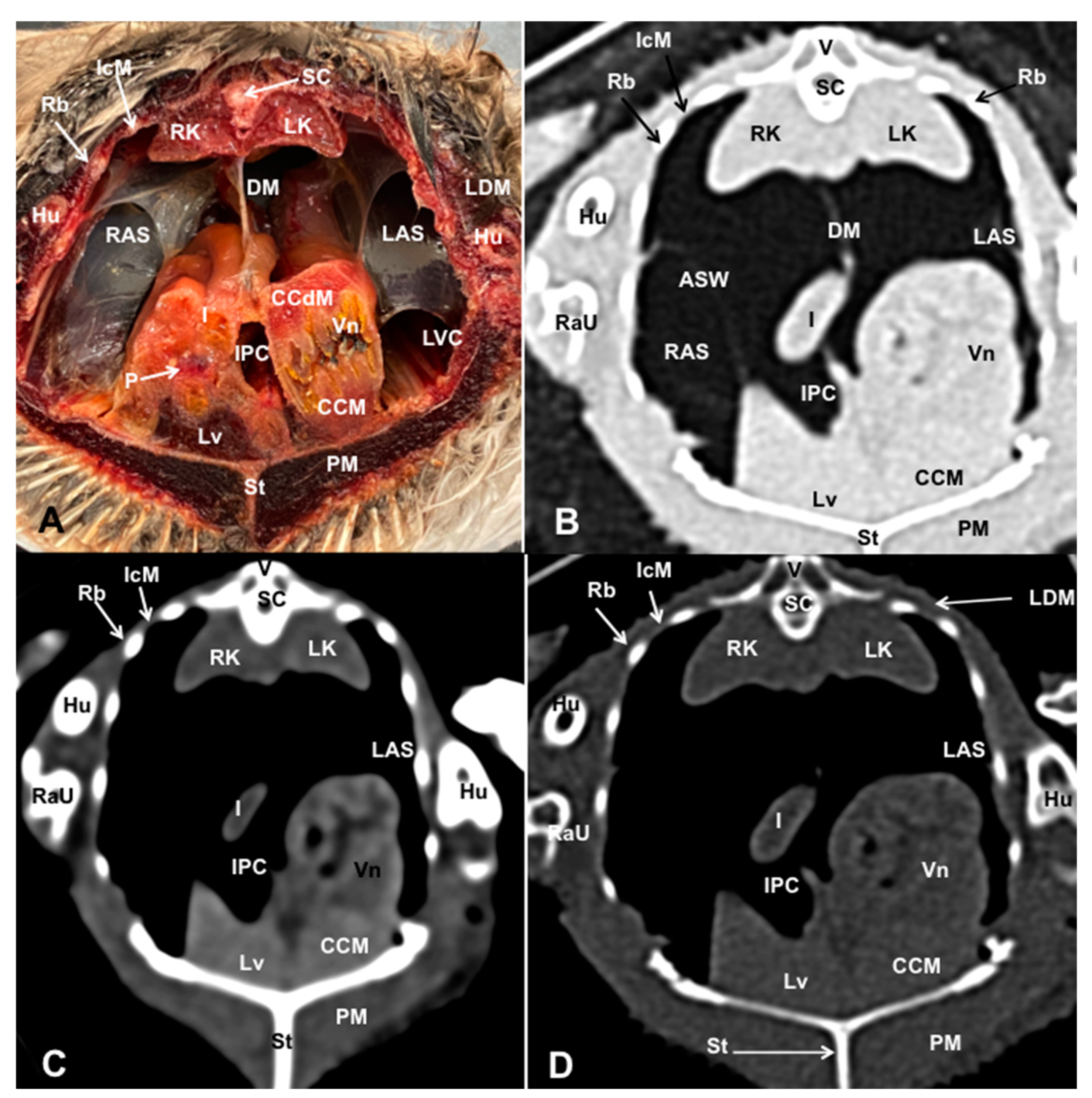
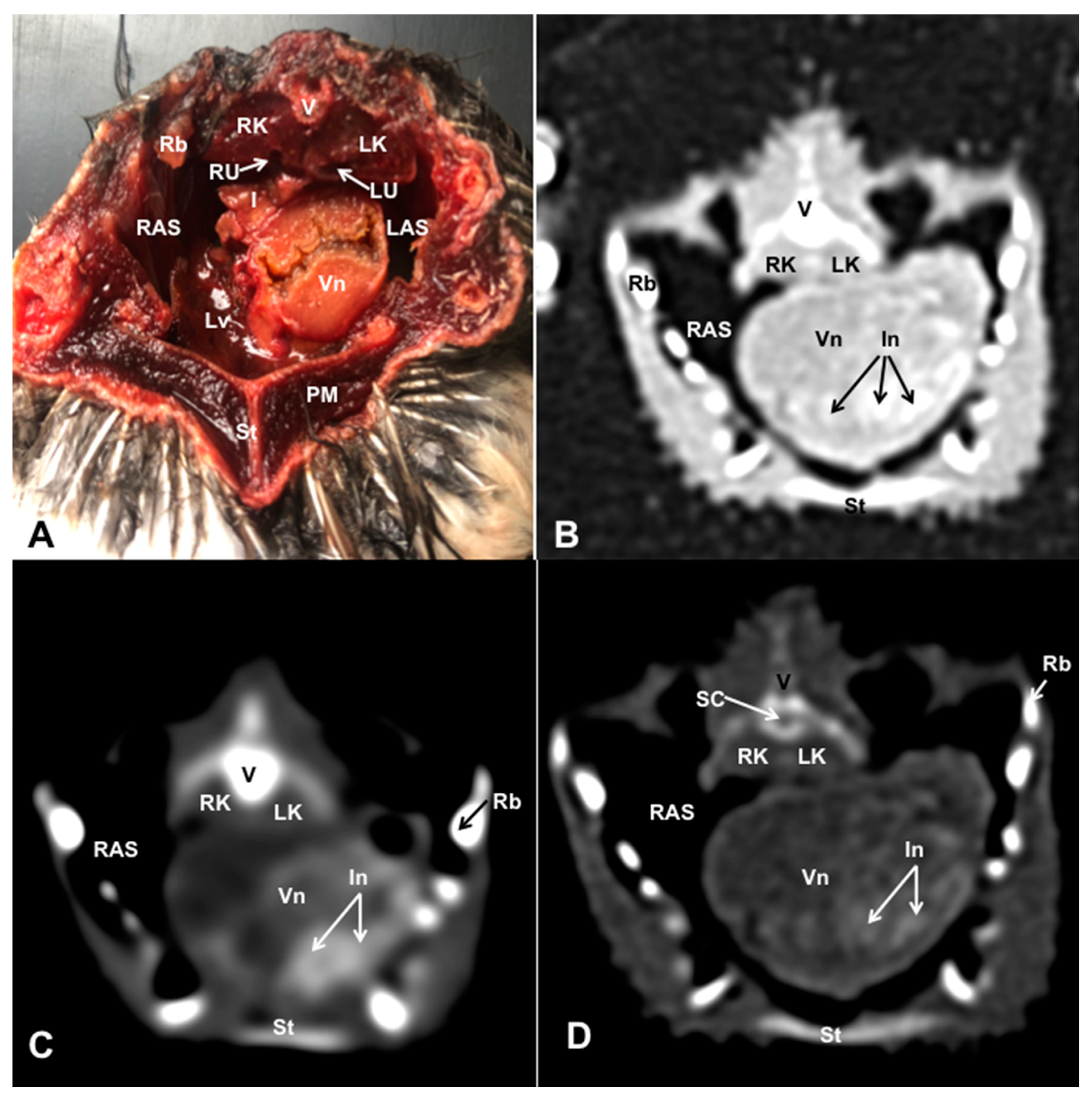
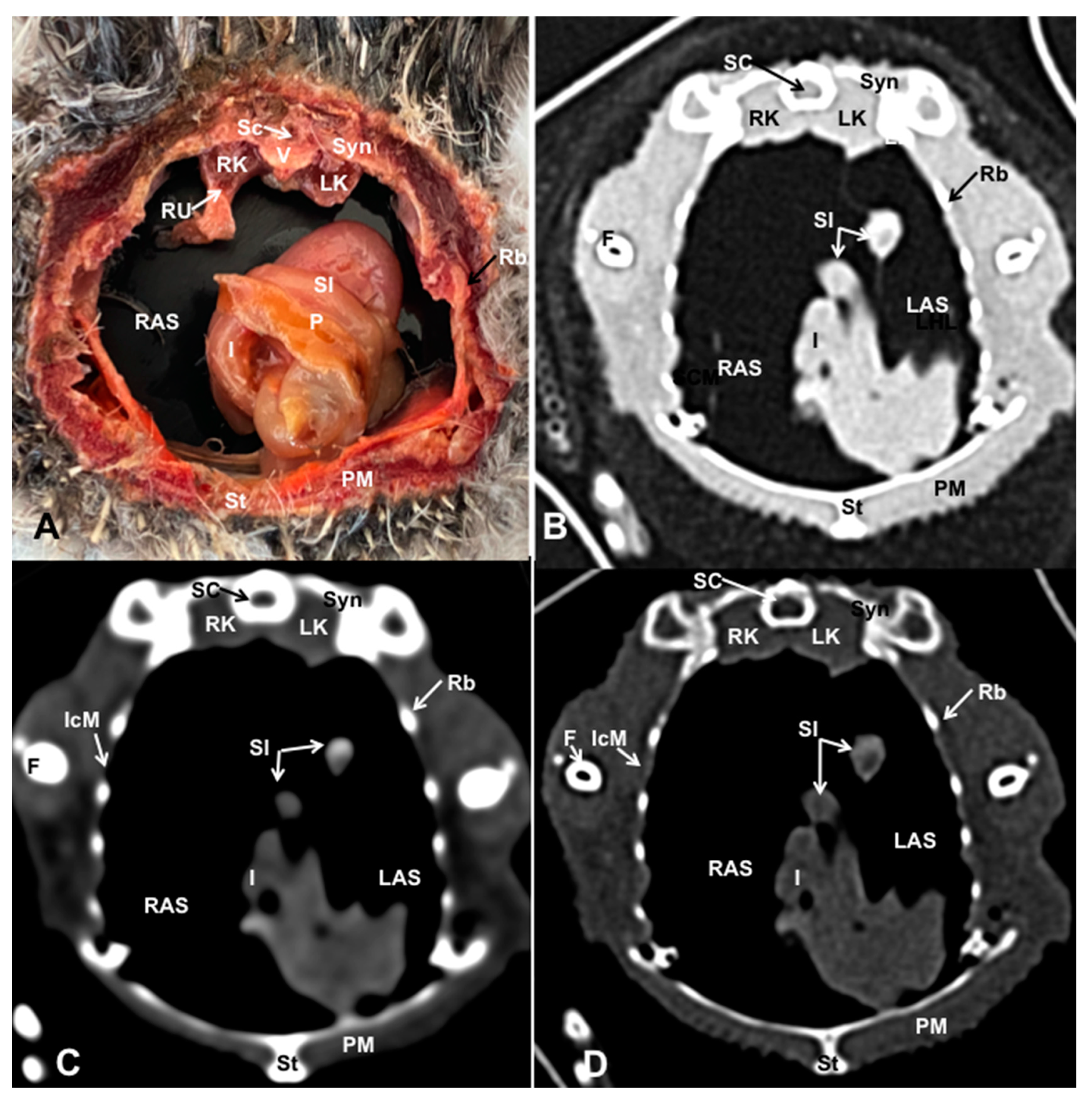
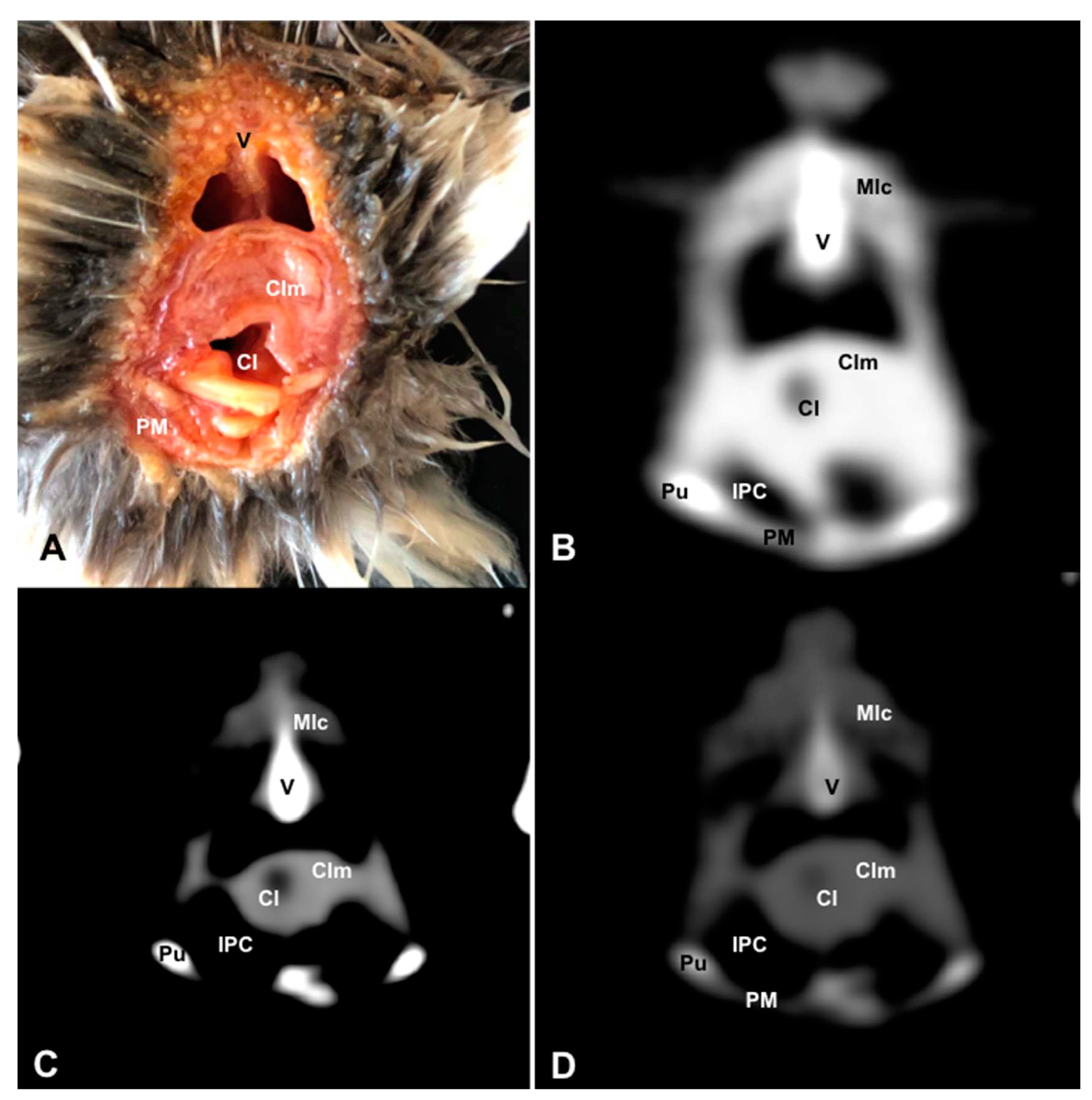
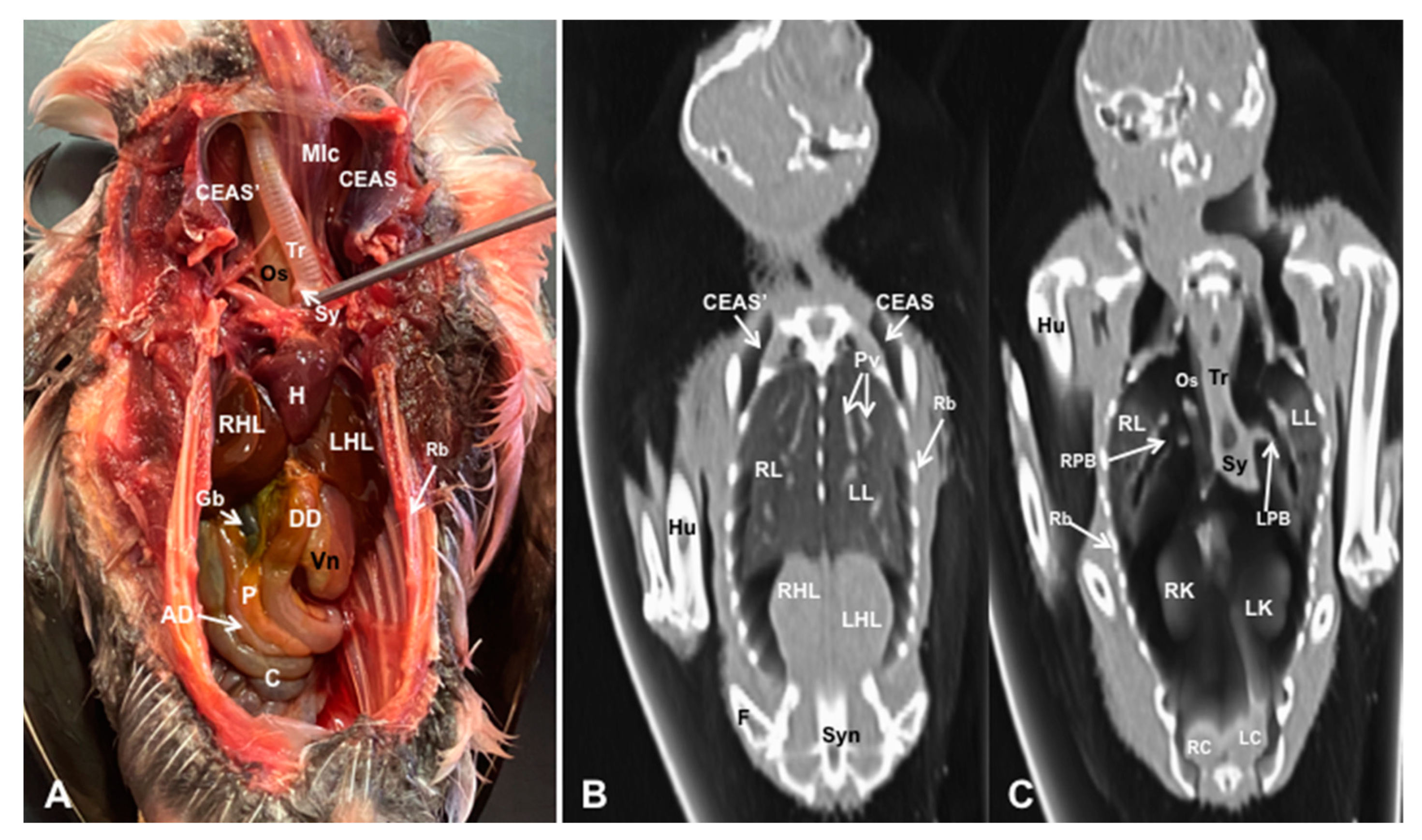
3.1. Anatomical Dissections and Cross-Sections
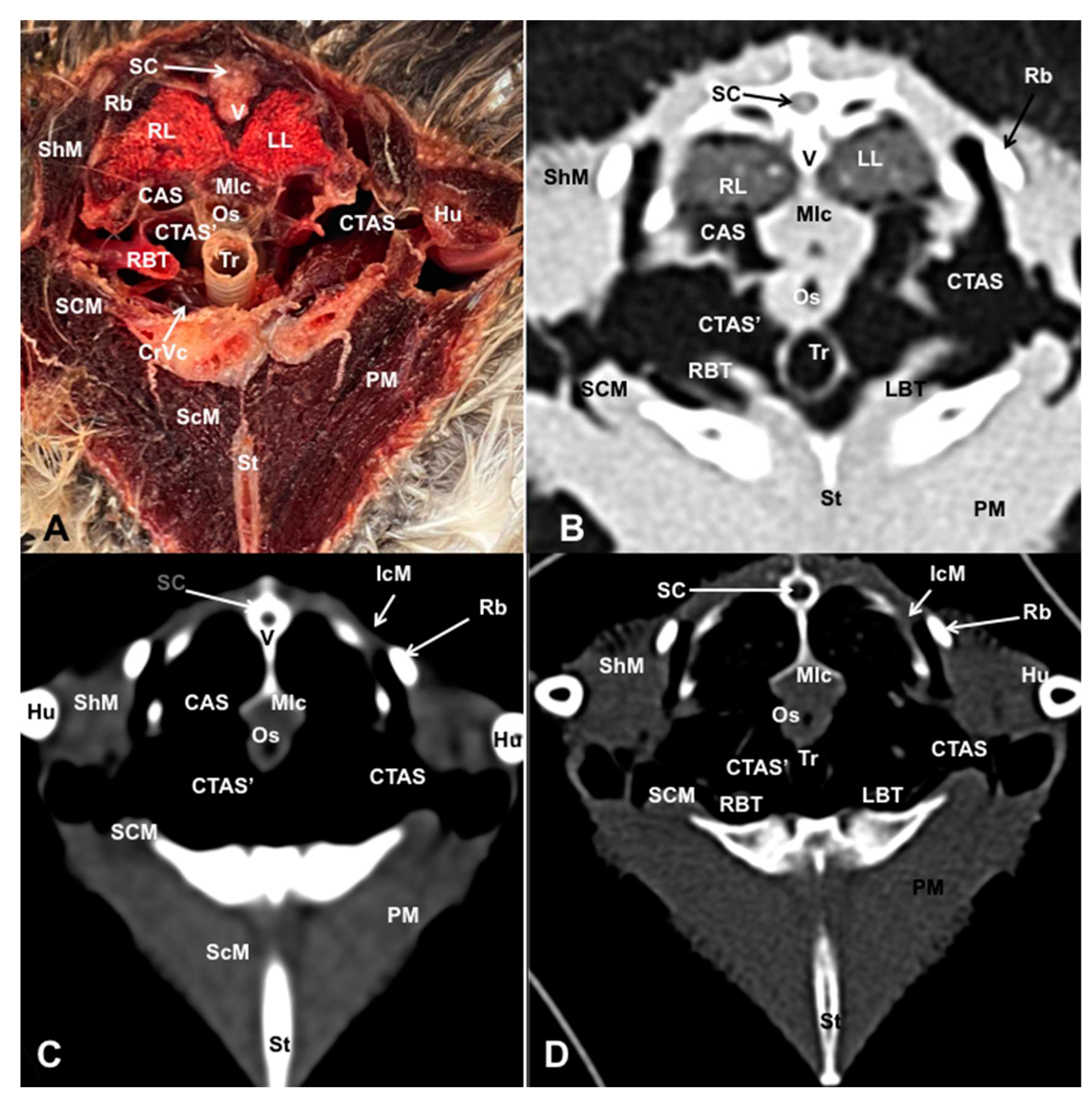
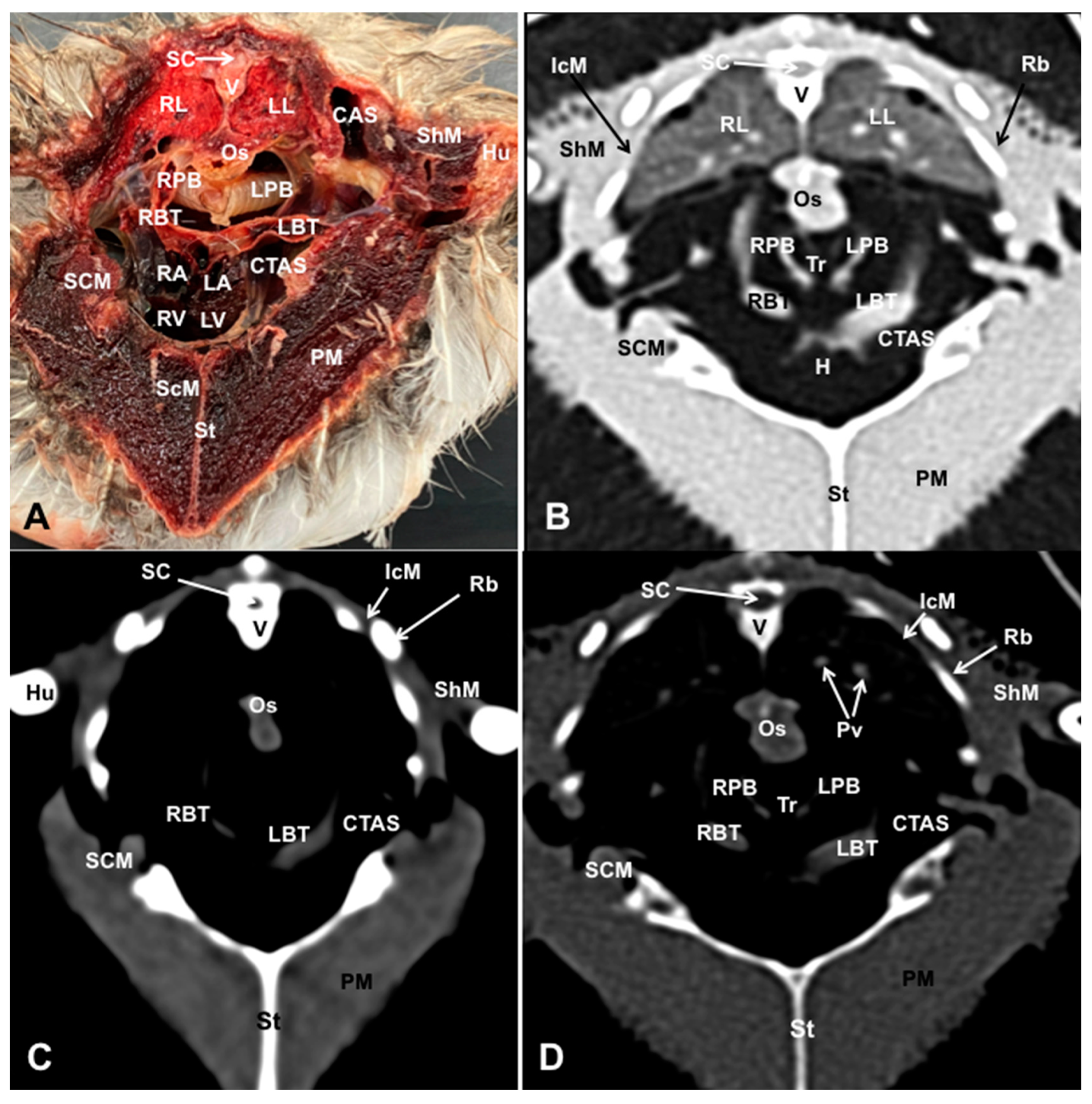
3.2. Computed Tomography Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrowclough, G.F.; Cracraft, J.; Klicka, J.; Zink, R.M. How Many Kinds of Birds Are There and Why Does It Matter?. PLOS ONE 2016, 11 (11).
- American Veterinary Medical Association. Available online: https://www.avma.org/resources-tools/reports-statistics/us-pet-ownership-statistis (accessed on 1 June 2023).
- Raftery, A. BSAVA Manual of Psittacine Birds. 2nd ed.; British small animal veterinary association: Gloucester, U.K, 2005; pp. 35–49.
- Krautwald-Junghanns, M.E.; Pees, M.; Reese, S.; Tully, T. Diagnostic imaging of exotic pets. 1st ed.: Schlütersche: Hannover, Germany, 2011.
- McMillan, M. C. Imaging techniques. Wingers Publishing: Lake Worth, Florida, U.S.A. 1994; pp. 246–326.
- Zeeland, Y. R. A.; Schoemaker, N. J.; Hsu, E. W. Current therapy in avian medicine and surgery; Elsevier: St. Louis, U.S.A. 2016; pp. 531–549.
- Haacke, E.M.; Brown, R.W.; Thompson, M.R.; Vankatesan, R. Magnetic Resonance Imaging. John Wiley & Sons: New York, U.S.A., 1999; pp. 331–380 and 669–702.
- Beuf, O.; Jaillon, F.; Saint-Jalmes, H. Small-animal MRI: signal-to-noise ratio comparison at 7 and 15 T with multiple-animal acquisition strategies. Mag Reson Mater Phy 2006, 19, 202–208.
- Lauridsen, H.; Hansen, K.; Wang, T.; Agger, P.; Andersen, J.L.; Knudsen, P.S.; Rasmussen, A.S.; Uhrenholt, L.; Pedersen, M. Inside Out: Modern Imaging Techniques to Reveal Animal Anatomy. PLOS ONE 2011, 6(3).
- Dinley, J.; Hawkins, L.; Paterson, G.; Ball, A.D.; Sinclair, I.; Sinnett-Jones, P.; Lanham, S. Micro-computed X-ray tomography: a new non-destructive method of assessing sectional, fly-through and 3D imaging of a soft-bodied marine worm. J Microsc 2010, 238, 123–133.
- Ziegler, A.; Faber, A.; Mueller, S.; Bartolomaeus, T. Systematic comparison and reconstruction of sea urchin (Echinoidea) internal anatomy: a novel approach using magnetic resonance imaging. BMC Biol 2008, 6, 1–15.
- Rieppel, O.; Gauthier, J.; Maisano, J. Comparative morphology of the dermal palate in squamate reptiles, with comments on phylogenetic implications. Zool J Linn Soc 2008, 152, 131–152.
- Digital Fish Library. A collaborative project at the University of California, San Diego funded by the National Science Foundation's (NSF) Biological Infrastructure program. Available online: www.digitalfishlibrary.org (accessed on 8 June 2023).
- Webb, E.; Yuan, M.; Lemoine, N.; Wang, Y. Imaging in animal models. Integr Cancer Sci Ther 2016, 3, 428–431.
- Schambach, S.J.; Bag, S.; Schilling, L.; Groden, C.; Brockmann, M.A. Application of micro-CT in small animal imaging. Methods 2010, 50, 2–13.
- Farrag, M.; Pukale, D.D.; Leipzig, N.D. Micro-computed tomography utility for estimation of intraparenchymal spinal cord cystic lesions in small animals. Neural Regen Res 2021, 16(11), 2293-2298.
- Delk, K.W.; Mejia-Fava, J.; Jiménez, D.A.; Kent, M.; Myrna, K.; Mayer, J.; Divers, S. Diagnostic imaging of peripheral vestibular disease in a Chinese goose (Anser cygnoides). J Avian Med Surg 2014, 28, 31–37.
- Fleming, G.J.; Lester, N.V.; Stevenson, R.; Silver, X.S. High field strength (4.7 T) magnetic resonance imaging of hydrocephalus in an African Grey parrot (Psittacus erithacus). Vet Radiol Ultrasound 2003, 44, 542– 545.
- Jirak, D.; Janacek, J.; Kear, B. P. A combined MR and CT study for precise quantitative analysis of the avian brain. Sci. Rep 2015, 5(1), 1-7.
- Romagnano, A.; Shiroma, J.T.; Heard, D.J.; Johnson, R.D.; Schiering, M.R.; Mladinich, M.S. Magnetic resonance imaging of the brain and coelomic cavity of the domestic pigeon (Columba livia domestica). Vet Radiol Ultrasound 1996, 37, 431–440.
- Fumero-Hernández, M.; Encinoso, M.; Ramírez, A.S.; Morales, I.; Suárez Pérez, A.; Jaber, J.R. A Cadaveric Study Using Computed Tomography for Measuring the Ocular Bulb and Scleral Skeleton of the Atlantic Puffin (Aves, Alcidae, Fratercula arctica) Animals 2023, 13 (15): 2418.
- Hall, M.I. The anatomical relationships between the avian eye, orbit and sclerotic ring: Implications for inferring activity patterns in extinct birds. J. Anat 2008, 212, 781–794.
- Andrade, S.B.D.; Araujo, N.L.L.C.D.; Raposo, A.C.S.; Muramoto, C.; Oriá, A.P. Morphometric descriptive report of scleral ossicle rings, by ultrasound and computed tomography, in three Testudines specimens. Ciência Rural 2023, 53, 1–10.
- Orosz, S. E.; Toal, R.L. Tomographic anatomy of the golden eagle (Aquila chrysaetos). J. Zoo Wildl. Med 1992, 23, 39-46.
- Veladiano, I.A.; Banzato, T.; Bellini, L.; Montani, A.; Catania, S.; Zotti, A. Normal computed tomographic features and reference values for the coelomic cavity in pet parrots. BMC Vet.Res 2016, 12 (1),1-9.
- da Silva, .J.P.; Rahal, S.C.; Castiglioni, M.C.R.; Baldissera Gonçalves, R.A.; Doiche, D.P.; Moresco, A.; Mamprim, M.J.; Vulcano, L.C. Radiography and computed tomography of the heart and lower respiratory tract in toco toucans (Ramphastos toco). Anat Histol Embryol 2020, 49(4), 541-549.
- Pepperberg, I.M.; Howell, K.S.; Banta, P.A.; Patterson, D.K.; Meisser, M. Measurement of grey parrot (Psittacus erithacus) trachea via magnetic resonance imaging, dissection, and electron beam computed tomography. J Morphol 1998, 238(1), 81–91.
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 2 June 2023).
- Painter, J. Fratercula arctica. Available online: https://animaldiversity.org/accounts/Fratercula_arctica/ (accessed on 30 May 2023).
- Sisson, S.; Getty, R.; Grossman, J.D. Anatomía de los animales domésticos, 5th ed.; Elsevier Masson: Issy les Moulineaux , France, 1982.
- MacLelland, J. Atlas en color de anatomía de las aves. McGraw-Hill Interamericana: Madrid, Spain, 1992.
- König, E.H.; Liebich, H.G.; Bragulla, H. Anatomie und Propädeutik des Geflügels. Schattauer: Stuttgart, German, 2001.
- Isaac, I.; Richardson, J.; Liuti, T.; Longo, M. Safety of intravenous iodinated contrast medium injection in rabbits undergoing conscious computed tomography. Veterinary Record Open 2022, 9(1), e31.
- Seamon, A.B.; Hofmeister, E.H.; Divers, S.J. Outcome following inhalation anesthesia in birds at a veterinary referral hospital: 352 cases (2004–2014). J Am Vet Med Assoc 2017, 251(7), 814-817.
- Arencibia, A.; Corbera JA.; Ramírez, G.; Díaz-Bertrana, ML.; Pitti, L.; Morales, M.; Jaber, JR. Anatomical Assessment of the Thorax in the Neonatal Foal Using Computed Tomography Angiography, Sectional Anatomy, and Gross Dissections. Animals 2020, 10(6):1045.
- Banzato, T.; Selleri, P.; Veladiano, IA.; Zotti, A. Comparative evaluation of the cadaveric and computed tomographic features of the coelomic cavity in the green iguana (Iguana iguana), black and white tegu (Tupinambis merianae) and bearded dragon (Pogona vitticeps). Anat Histol Embryol 2013, 42(6):453-60.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




