Submitted:
09 August 2023
Posted:
10 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
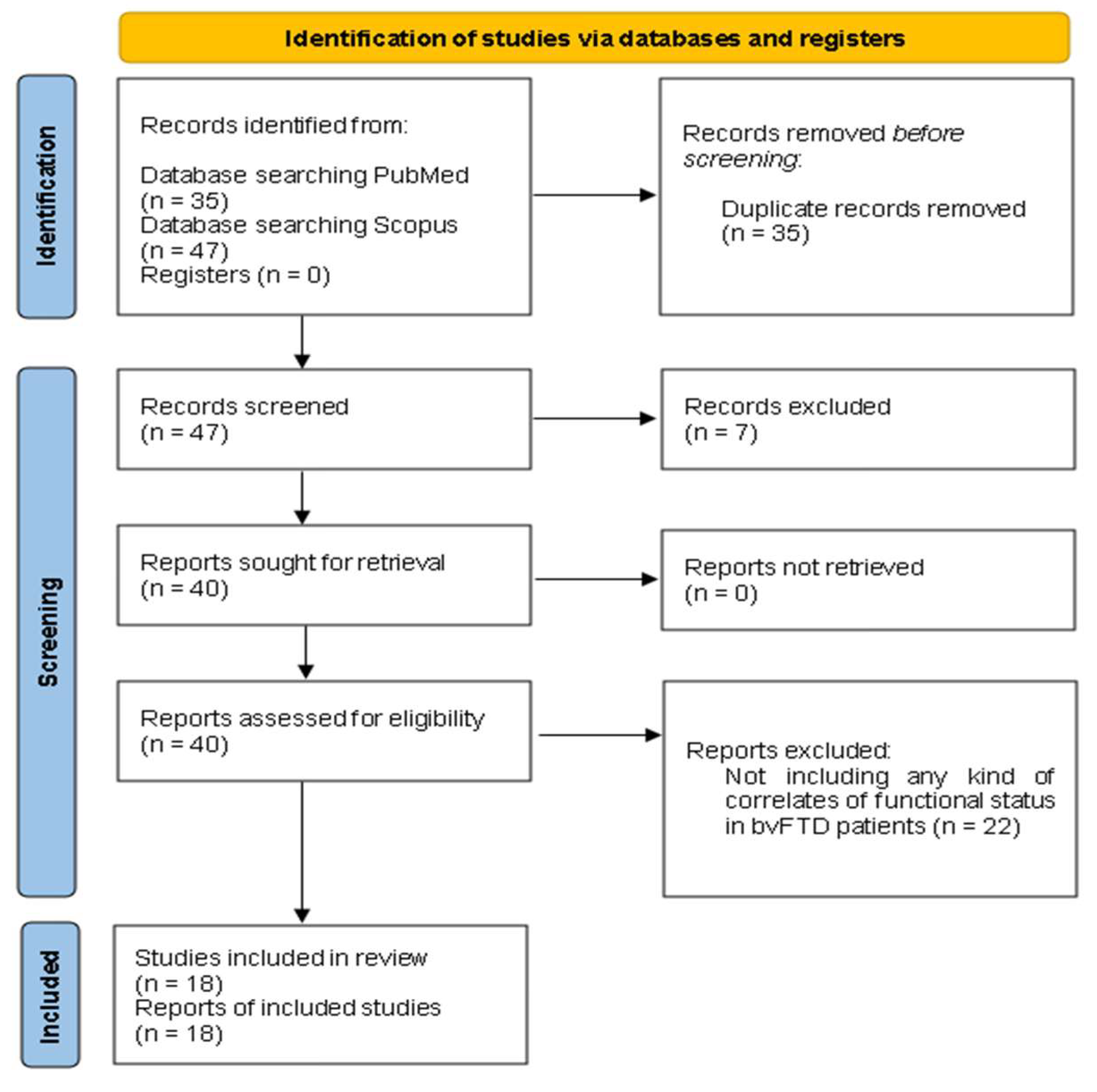
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Synthesis
3. Results
3.1. The Included Papers
| 1st Author, Year | Number of bvFTD patients | Research design | Objective of interest | Functionality measures | Results |
|---|---|---|---|---|---|
| Kipps, 2007 [14] | bvFTD, n = 51 | Cross-sectional | To assess the relationship of focal brain atrophy based on a Magnetic Resonance Imaging (MRI) Visual Rating Scale to clinical data, such as the overall functional disability, in FTD patients | Clinical Dementia Rating (CDR) [15] | BvFTD patients with normal brain scans generally demonstrated milder functional impairment than those with abnormal scans |
| Mioshi, 2007 [9] | bvFTD, n = 15 | Cross-sectional | To investigate the association between functional measures and cognitive tests, age, disease duration, and disease severity in patients with FTD | Disability Assessment for Dementia (DAD) [16] | Functional measures did not correlate with cognitive tests, age, disease duration or disease severity in bvFTD patients |
| Kipps, 2009 [17] | bvFTD, n = 14 | Cross-sectional | To investigate the relationship between perception of emotions, neuropsychiatric symptoms, and ADLs in bvFTD patients | Disability Assessment for Dementia (DAD) [16] | Performance on emotion recognition task did not correlate with ADL ratings, which instead correlated highly with informant-rated apathy levels in bvFTD patients |
| Josephs, 2011 [12] | bvFTD, n = 86 | Longitudinal: multiple serial assessments of functional status per subject (mean 4, range 2–18) over a 15-year period | To determine the baseline (i) demographic, (ii) neuropsychological, (iii) neuropsychiatric, (iv) genetic and (v) anatomic/imaging predictors of the rate of functional decline in bvFTD patients | Clinical Dementia Rating Scale Sum of Boxes (CDR-SOB) [18] | (i) older age at onset, (ii) poorer performance on neuropsychological tests of executive functions, language abilities, and visuospatial function at baseline, (iii) less severe disinhibition, agitation/aggression, and night-time behaviors at presentation, (iv) progranulin (GRN) mutations and (v) predominantly frontal and frontotemporal patterns of atrophy at baseline predicted faster rates of functional decline in bvFTD patients |
| Devenney, 2015 [19] | bvFTD, n = 58 | Longitudinal: patients were assessed at least 2 times over a 6-year period | To identify key predictive features that determine rates of progression in bvFTD | Frontotemporal Dementia Functional Rating Scale (FTDFRS) [20] | The C9orf72 expansion, a positive family history of neurodegeneration, clinical abnormalities at baseline (such as parkinsonism or frontal release signs), episodic memory impairment, deficits on global cognition, and stereotypic/compulsive behaviors were key predictive features of worse prognosis in bvFTD |
| Lima-Silva, 2015 [21] | bvFTD, n = 20 | Cross-sectional | To contrast a direct and an indirect measure of functional status as to their degree of association with cognitive measures in bvFTD patients |
Direct Assessment of Functional Status (DAFS) [22] and Disability Assessment for Dementia (DAD) [16] |
Both direct and indirect measures of functional status correlated with the bvFTD patients’ performance on cognitive screening tools, such as the Mini-Mental State Examination (MMSE) |
| Torralva, 2015 [23] | bvFTD, n = 391 | Cross-sectional | To investigate the role of vascular changes on the functional status of bvFTD patients | Clinical Dementia Rating (CDR) [15] | The presence of vascular changes was not associated with greater functional disability in bvFTD cases |
| Amanzio, 2016 [24] | bvFTD, n = 23 | Cross-sectional | To investigate the neuroanatomic correlates of IADLs deficits in bvFTD patients | Basic Activities of Daily Living (BADL) [25] and Instrumental Activities of Daily Living (IALD) [26] |
There was a positive association between IADLs and left insula volume, indicating greater grey matter in more independent bvFTD patients |
| De Silva, 2016 [27] | bvFTD, n = 14 | Longitudinal: assessment at baseline and at 9-17 months follow-up | To examine the relationship between motor impairment and functional decline in ALS-FTD spectrum | Frontotemporal Dementia Functional Rating Scale (FTDFRS) [20] | There was no correlation between motor impairment and functional decline either at baseline or at follow-up assessment in bvFTD patients |
| O'Connor, 2016 [28] | bvFTD, n = 21 | Longitudinal: patients were assessed on 2-4 separate occasions over a 4-year period | To investigate the longitudinal relationship between behavioral changes and functional decline in bvFTD |
Disability Assessment for Dementia (DAD) [16] | Apathy and stereotypical behavior made longitudinal contributions to functional disability in bvFTD patients, whereas disinhibition did not play a major role in patients’ functional status |
| Premi, 2016 [29] | bvFTD, n = 64 | Cross-sectional | To evaluate the correlation between brain volume (by means of voxel-based morphometry) and clinical scales of functional impairment in FTD | Basic Activities of Daily Living (BADL) [25] and Instrumental Activities of Daily Living (IALD) [26] |
Lower grey matter volume in frontotemporal regions, especially on the right side, correlated with poorer performance on daily activities in bvFTD patients |
| Moheb, 2017 [30] | bvFTD, n = 607 | Cross-sectional | To determine the cognitive and behavioral correlates of IADLs deficits in FTD patients | Functional Activities Questionnaire (FAQ) [31] | Poorer performance on measures of executive functions, processing speed and memory, as well as more severe behavioral disturbances, especially hallucinations and anxiety, predicted decreased IADL performance in bvFTD patients |
| O'Connor, 2017 [32] | bvFTD, n = 88 | Cross-sectional | To identify the contribution of different behavioral phenotypes to functional disability in bvFTD patients | Disability Assessment for Dementia (DAD) [16] | Patients with severely apathetic behavioral profiles had more extensive brain atrophy and were more functionally impaired than those with mild apathy or severe disinhibition alone |
| Steinacker, 2018 [33] | bvFTD, n = 74 | Longitudinal: assessment at baseline and at 1-year follow-up | To determine the association of serum neurofilament light chain (NfL) levels with functional deterioration in bvFTD | Clinical Dementia Rating Scale Sum of Boxes (CDR-SOB) [18] and Frontotemporal Lobar Degeneration (FTLD)-specific CDR-SOB [34] |
Serum NfL levels are positively correlated with functional impairment at different disease stages in bvFTD |
| Yassuda, 2018 [35] | bvFTD, n = 109 | Cross-sectional | To investigate the contribution of cognitive and neuropsychiatric factors to functional disability in bvFTD patients | Disability Assessment for Dementia (DAD) [16] | Cognitive deficits and apathy are key contributors to functional disability in bvFTD patients |
| Benussi, 2020 [36] | bvFTD, n = 122 | Longitudinal: assessment at baseline and at 12-month follow-up | To examine if transcranial magnetic stimulation (TMS) measures predict functional decline in FTD patients | Basic Activities of Daily Living (BADL) [25] and Instrumental Activities of Daily Living (IALD) [26] |
The dysfunction of inhibitory and facilitatory intracortical circuits, evaluated with TMS, accurately predicted functional decline at 12 months in bvFTD patients, beyond any other investigated variable |
| Marin, 2021 [37] | bvFTD, n = 30 | Cross-sectional | To correlate the swallowing problems with functionality in bvFTD patients | The Index of Independence in Activities of Daily Living (ADL) [38] | Swallowing problems in bvFTD correlated with impaired functionality |
| Musa Salech, 2022 [39] | bvFTD, n = 27 | Cross-sectional | To investigate the cognitive and neuropsychiatric correlates of functional impairment in patients with bvFTD | Technology-Activities of Daily Living Questionnaire (T-ADLQ) [40] | The factors associated with functional impairment in bvFTD varied across the different ADL domains: Apathy and disinhibition contributed significantly to BADL impairment Apathy, impaired emotion recognition and deficits in executive functions contributed significantly to IADL impairment Only apathy contributed significantly to advanced ADL (a-ADL) impairment Apathy was the strongest correlate of functional decline throughout all the ADL domains in patients with bvFTD |
3.2. Characteristics of the Included Papers
3.3. Summary of Findings
3.3.1. Demographic Correlates
3.3.2. Clinical Correlates
3.3.3. Genetic Correlates
3.3.4. Neural Correlates
- Findings based on MRI measures
- 2.
- Findings based on TMS measures
- 3.
- Findings based on blood-related measures
3.3.5. Motor correlates
3.3.6. Cognitive Correlates
3.3.7. Correlates Related to Social Cognition
3.3.8. Behavioral Correlates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bang, J.; Spina, S.; Miller, B. L. Frontotemporal dementia. The Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Pressman, P.; Miller, B. L. Diagnosis and management of behavioral variant frontotemporal dementia. Biological Psychiatry 2014, 75, 574–581. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J. R.; Kipps, C.; Johnson, J. K.; Seeley, W. W.; Mendez, M. F.; Knopman, D. S.; Kertesz, A.; Mesulam, M.; Salmon, D. P.; Galasko, D.; Chow, T. W.; DeCarli, C.; Hillis, A. E.; Josephs, K. A.; Kramer, J. H.; Weintraub, S.; Grossman, M.; Gorno-Tempini, M. L.; Miller, B. Diagnostic criteria for the behavioral variant of frontotemporal dementia (BVFTD): current limitations and future directions. Alzheimer Disease & Associated Disorders 2007, 21, S14–S18. [Google Scholar] [CrossRef]
- Onyike, C. U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. International Review of Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Johnson, J. K.; Diehl, J.; Mendez, M. F.; Neuhaus, J.; Shapira, J.; Forman, M. S.; Chute, D. J.; Roberson, E. D.; Pace-Savitsky, C.; Neumann, M.; Chow, T. W.; Rosen, H. J.; Förstl, H.; Kurz, A.; Miller, B. L. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Archives of Neurology 2005, 62. [Google Scholar] [CrossRef]
- Nunnemann, S.; Schuster, T.; Förstl, H.; Kurz, A.; Diehl-Schmid, J. Survival in a German population with frontotemporal lobar degeneration. Neuroepidemiology 2011, 37, (3–4). [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, E.; Ioannidis, P.; Moraitou, D.; Konstantinopoulou, E.; Aretouli, E. The cognitive and behavioral correlates of functional status in patients with frontotemporal dementia: a pilot study. Frontiers in Human Neuroscience 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Lima-Silva, T. B.; Bahia, V. S.; Nitrini, R.; Yassuda, M. S. Functional status in behavioral variant frontotemporal dementia: a systematic review. BioMed Research International 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Mioshi, E.; Kipps, C.; Dawson, K.; Mitchell, J. C.; Graham, A.; Hodges, J. R. Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology 2007, 68, 2077–2084. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J. R.; Knopman, D. S.; Mendez, M. F.; Kramer, J. H.; Neuhaus, J.; Van Swieten, J. C.; Seelaar, H.; Dopper, E. G. P.; Onyike, C. U.; Hillis, A. E.; Josephs, K. A.; Boeve, B. F.; Kertesz, A.; Seeley, W. W.; Rankin, K. P.; Johnson, J. K.; Gorno-Tempini, M. L.; Rosen, H. J.; Prioleau-Latham, C. E.; Lee, A.; Kipps, C.; Lillo, P.; Piguet, O.; Rohrer, J. D.; Rossor, M. N.; Warren, J. D.; Fox, N. C.; Galasko, D.; Salmon, D. P.; Black, S. E.; Mesulam, M.; Weintraub, S.; Dickerson, B. C.; Diehl-Schmid, J.; Pasquier, F.; Deramecourt, V.; Lebert, F.; Pijnenburg, Y. A. L.; Chow, T. W.; Manes, F.; Grafman, J.; Cappa, S. F.; Freedman, M.; Grossman, M.; Miller, B. L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Hodges, J. R. Rate of change of functional abilities in frontotemporal dementia. Dementia and Geriatric Cognitive Disorders 2009, 28, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K. A.; Whitwell, J. L.; Weigand, S. D.; Senjem, M. L.; Boeve, B. F.; Knopman, D. S.; Smith, G. E.; Ivnik, R. J.; Jack, C. R.; Petersen, R. C. Predicting functional decline in behavioural variant frontotemporal dementia. Brain 2011, 134, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Page, M. J.; McKenzie, J. E.; Bossuyt, P. M.; Boutron, I.; Hoffmann, T.; Mulrow, C. D.; Shamseer, L.; Tetzlaff, J.; Akl, E. A.; Brennan, S.; Chou, R.; Glanville, J.; Grimshaw, J.; Hróbjartsson, A.; Lalu, M. M.; Li, T.; Loder, E.; Mayo-Wilson, E.; McDonald, S.; McGuinness, L. A.; Stewart, L.; Thomas, J.; Tricco, A. C.; Welch, V.; Whiting, P.; Moher, D. The PRISMA 2020 Statement: An updated guideline for reporting systematic reviews. International Journal of Surgery 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Kipps, C.; Davies, R.; Mitchell, J. C.; Kril, J. J.; Halliday, G. M.; Hodges, J. R. Clinical Significance of lobar atrophy in frontotemporal dementia: application of an MRI visual rating scale. Dementia and Geriatric Cognitive Disorders 2007, 23, 334–342. [Google Scholar] [CrossRef]
- Morris, J. C. The Clinical Dementia Rating (CDR). Neurology 1993, 43, 2412.2–a. [Google Scholar] [CrossRef]
- Gélinas, I.; Gauthier, L.; McIntyre, M.; Gauthier, S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. American Journal of Occupational Therapy 1999, 53, 471–481. [Google Scholar] [CrossRef]
- Kipps, C.; Mioshi, E.; Hodges, J. R. Emotion, social functioning and activities of daily living in frontotemporal dementia. Neurocase 2009, 15, 182–189. [Google Scholar] [CrossRef]
- Hughes, C. P.; Berg, L.; Danziger, W. L.; Coben, L. A.; Martin, R. L. A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Devenney, E.; Bartley, L.; Hoon, C.; O’Callaghan, C.; Kumfor, F.; Hornberger, M.; Kwok, J. B.; Halliday, G. M.; Kiernan, M. C.; Piguet, O.; Hodges, J. R. Progression in behavioral variant frontotemporal dementia. JAMA Neurology 2015, 72. [Google Scholar] [CrossRef]
- Mioshi, E.; Hsieh, S.; Savage, S. A.; Hornberger, M.; Hodges, J. R. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010, 74, 1591–1597. [Google Scholar] [CrossRef]
- Lima-Silva, T. B.; Bahia, V. S.; Carvalho, V. A.; Guimarães, H. C.; Caramelli, P.; Balthazar, M. L. F.; Damasceno, B.; De Campos Bottino, C. M.; Brucki, S. M. D.; Yassuda, M. S. Direct and indirect assessments of activities of daily living in behavioral variant frontotemporal dementia and Alzheimer disease. Journal of Geriatric Psychiatry and Neurology 2015, 28, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, D.; Amigo, E.; Duara, R.; Guterman, A.; Hurwitz, D.; Berkowitz, N.; Wilkie, F. L.; Weinberg, G.; Black, B.; Gittelman, B.; Eisdorfer, C. A new scale for the assessment of functional status in Alzheimer’s disease and related disorders. Journal of Gerontology 1989, 44, P114–P121. [Google Scholar] [CrossRef] [PubMed]
- Torralva, T.; Sposato, L. A.; Riccio, P. M.; Gleichgerrcht, E.; Roca, M.; Toledo, J. B.; Trojanowski, J. Q.; Kukull, W. A.; Manes, F.; Hachinski, V. Role of brain infarcts in behavioral variant frontotemporal dementia. Neurobiology of Aging 2015, 36, 2861–2868. [Google Scholar] [CrossRef] [PubMed]
- Amanzio, M.; D’Agata, F.; Palermo, S.; Rubino, E.; Zucca, M.; Galati, A.; Pinessi, L.; Castellano, G.; Rainero, I. Neural correlates of reduced awareness in instrumental activities of daily living in frontotemporal dementia. Experimental Gerontology 2016, 83, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Katz, S. Studies of illness in the aged. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M. P.; Brody, E. M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- De Silva, D.; Hsieh, S.; Caga, J.; Leslie, F.; Kiernan, M. C.; Hodges, J. R.; Mioshi, E.; Burrell, J. R. Motor function and behaviour across the ALS-FTD spectrum. Acta Neurologica Scandinavica 2016, 133, 367–372. [Google Scholar] [CrossRef]
- O’Connor, C. M.; Clemson, L.; Hornberger, M.; Leyton, C. E.; Hodges, J. R.; Piguet, O.; Mioshi, E. Longitudinal change in everyday function and behavioral symptoms in frontotemporal dementia. Neurology 2016, 6, 419–428. [Google Scholar] [CrossRef]
- Premi, E.; Gualeni, V.; Costa, P.; Cosseddu, M.; Gasparotti, R.; Padovani, A.; Borroni, B. Looking for measures of disease severity in the frontotemporal dementia continuum. Journal of Alzheimer’s Disease 2016, 52, 1227–1235. [Google Scholar] [CrossRef]
- Moheb, N.; Mendez, M. F.; Kremen, S.; Teng, E. Executive dysfunction and behavioral symptoms are associated with deficits in instrumental activities of daily living in frontotemporal dementia. Dementia and Geriatric Cognitive Disorders 2017, 43, (1–2). [Google Scholar] [CrossRef]
- Pfeffer, R. I.; Kurosaki, T.; Harrah, C. H.; Chance, J. M.; Filos, S. Measurement of functional activities in older adults in the community. Journal of Gerontology 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C. M.; Landin-Romero, R.; Clemson, L.; Kaizik, C.; Daveson, N.; Hodges, J. R.; Hsieh, S.; Piguet, O.; Mioshi, E. Behavioral-variant frontotemporal dementia: distinct phenotypes with unique functional profiles. Neurology 2017, 89, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Anderl-Straub, S.; Diehl-Schmid, J.; Semler, E.; Uttner, I.; Von Arnim, C. a. F.; Barthel, H.; Danek, A.; Fassbender, K.; Fliessbach, K.; Foerstl, H.; Grimmer, T.; Huppertz, H.-J.; Jahn, H.; Kassubek, J.; Kornhuber, J.; Landwehrmeyer, G. B.; Lauer, M.; Maler, J. M.; Mayer, B.; Oeckl, P.; Prudlo, J.; Schneider, A.; Volk, A. E.; Wiltfang, J.; Schroeter, M. L.; Ludolph, A. C.; Otto, M. Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 2018, 91, e1390–e1401. [Google Scholar] [CrossRef]
- Borroni, B.; Agosti, C.; Premi, E.; Cerini, C.; Cosseddu, M.; Paghera, B.; Bellelli, G.; Padovani, A. The FTLD-modified Clinical Dementia Rating scale is a reliable tool for defining disease severity in frontotemporal lobar degeneration: evidence from a brain SPECT study. European Journal of Neurology 2010, 17, 703–707. [Google Scholar] [CrossRef]
- Yassuda, M. S.; Da Silva, T. B. L.; O’Connor, C. M.; Mekala, S.; Alladi, S.; Bahia, V. S.; Almaral-Carvalho, V.; Guimarães, H. C.; Caramelli, P.; Balthazar, M. L. F.; Damasceno, B.; Brucki, S. M. D.; Nitrini, R.; Hodges, J. R.; Piguet, O.; Mioshi, E. Apathy and functional disability in behavioral variant frontotemporal dementia. Neurology 2018, 8, 120–128. [Google Scholar] [CrossRef]
- Benussi, A.; Dell’Era, V.; Cantoni, V.; Cotelli, M. S.; Cosseddu, M.; Spallazzi, M.; Micheli, A.; Turrone, R.; Alberici, A.; Borroni, B. TMS for staging and predicting functional decline in frontotemporal dementia. Brain Stimulation 2020, 13, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Marin, S. D. M.; Mansur, L. L.; Oliveira, F. F. D.; Marin, L. F.; Wajman, J. R.; Bahia, V. S.; Bertolucci, P. H. F. Swallowing in behavioral variant frontotemporal dementia. Arquivos De Neuro-Psiquiatria 2021, 79, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, P. The hierarchy of the activities of daily living in the Katz index in residents of skilled nursing facilities. Journal of Geriatric Physical Therapy 2013, 36, 87–91. [Google Scholar] [CrossRef]
- Musa Salech, G.; Lillo, P.; Van Der Hiele, K.; Méndez-Orellana, C.; Ibáñez, A.; Slachevsky, A. Apathy, executive function, and emotion recognition are the main drivers of functional impairment in behavioral variant of frontotemporal dementia. Frontiers in Neurology 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Barion, A.; Rademaker, A.; Rehkemper, G.; Weintraub, S. The Activities of Daily Living Questionnaire: a validation study in patients with dementia. Alzheimer Disease & Associated Disorders 2004, 18, 223–230. [Google Scholar] [CrossRef]
- Rohrer, J. D.; Ridgway, G. R.; Modat, M.; Ourselin, S.; Mead, S.; Fox, N. C.; Rossor, M. N.; Warren, J. D. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. NeuroImage 2010, 53, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Whitwell, J. L.; Weigand, S. D.; Gunter, J. L.; Boeve, B. F.; Rademakers, R.; Baker, M.; Knopman, D. S.; Wszolek, Z. K.; Petersen, R. C.; Jack, C. R.; Josephs, K. A. Trajectories of brain and hippocampal atrophy in FTD with mutations in MAPT or GRN. Neurology 2011, 77, 393–398. [Google Scholar] [CrossRef]
- Devenney, E.; Hornberger, M.; Irish, M.; Mioshi, E.; Burrell, J. R.; Tan, R.; Kiernan, M. C.; Hodges, J. R. Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurology 2014, 71, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Irish, M.; Devenney, E.; Wong, S.; Dobson-Stone, C.; Kwok, J. B.; Piguet, O.; Hodges, J. R.; Hornberger, M. Neural substrates of episodic memory dysfunction in behavioural variant frontotemporal dementia with and without C9ORF72 expansions. NeuroImage: Clinical 2013, 2, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, C.; Beck, J.; Rohrer, J. D.; Lashley, T.; Mok, K. Y.; Shakespeare, T. J.; Yeatman, T.; Warrington, E. K.; Schott, J. M.; Fox, N. C.; Rossor, M. N.; Hardy, J.; Collinge, J.; Revesz, T.; Mead, S.; Warren, J. D. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain 2012, 135, 736–750. [Google Scholar] [CrossRef]
- Miller, B. L.; Cummings, J. L. (Eds.) . The Human Frontal Lobes: Functions and Disorders, 3rd ed.; Guilford Press: New York, NY, 2017. [Google Scholar]
- Whitwell, J. L.; Przybelski, S. A.; Weigand, S. D.; Ivnik, R. J.; Vemuri, P.; Gunter, J. L.; Senjem, M. L.; Shiung, M. M.; Boeve, B. F.; Knopman, D. S.; Parisi, J. E.; Dickson, D. W.; Petersen, R. C.; Jack, C. R.; Josephs, K. A. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain 2009, 132, 2932–2946. [Google Scholar] [CrossRef]
- Rascovsky, K.; Salmon, D. P.; Lipton, A. M.; Leverenz, J. B.; DeCarli, C.; Jagust, W. J.; Clark, C. M.; Mendez, M. F.; Tang-Wai, D. F.; Graff-Radford, N. R.; Galasko, D. Rate of progression differs in frontotemporal dementia and Alzheimer disease. Neurology 2005, 65, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, J. D.; Woollacott, I. O. C.; Dick, K. M.; Brotherhood, E. V.; Gordon, E.; Fellows, A. D.; Toombs, J.; Druyeh, R.; Cardoso, M. J.; Ourselin, S.; Nicholas, J. M.; Norgren, N.; Mead, S.; Andreasson, U.; Blennow, K.; Schott, J. M.; Fox, N. C.; Warren, J. D.; Zetterberg, H. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 2016, 87, 1329–1336. [Google Scholar] [CrossRef]
- Lezak, M. D. Neuropsychological assessment, 3rd ed.; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- McCabe, D. P.; Roediger, H. L.; McDaniel, M. A.; Balota, D. A.; Hambrick, D. Z. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychology 2010, 24, 222–243. [Google Scholar] [CrossRef]
- Bertoux, M.; De Souza, L. C.; O’Callaghan, C.; Greve, A.; Sarazin, M.; Dubois, B.; Hornberger, M. Social cognition deficits: The key to discriminate behavioral variant frontotemporal dementia from Alzheimer’s disease regardless of amnesia? Journal of Alzheimer’s Disease 2016, 49, 1065–1074. [Google Scholar] [CrossRef]
- Maresca, G.; Maggio, M. G.; Latella, D.; Naro, A.; Portaro, S.; Calabrò, R. S. Understanding the role of social cognition in neurodegenerative Disease: A scoping review on an overlooked problem. Journal of Clinical Neuroscience 2020, 77, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D. E.; Van Reekum, R.; Simard, M.; Streiner, D. L.; Conn, D.; Cohen, T.; Freedman, M. Apathy in dementia: clinical and sociodemographic correlates. Journal of Neuropsychiatry and Clinical Neurosciences 2008, 20, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.; Perani, D.; Herholz, K.; Holthoff, V.; Beuthien-Baumann, B.; Sorbi, S.; Pupi, A.; Degueldre, C.; Lemaire, C.; Collette, F.; Salmon, E. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dementia and Geriatric Cognitive Disorders 2006, 21, (5–6). [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





