Submitted:
26 July 2023
Posted:
04 August 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and Microorganisms Culture Medium
2.2. Plant Material
2.3. Preliminary Phytochemical Profiling
2.4. Preparation of Plants Extracts
2.5. Total Polyphenol Content Determination
2.6. Total Flavonoid Content Determination
2.7. Condensed Tannins Content Determination
2.8. Hydrolyzable Tannins Content Determination
2.9. LC-DAD-MS-ESI + Analysis
2.10. Antimicrobial Activity of J. multifida Leaves Extracts
2.10.1. Microorganisms Strains and Growth Conditions
2.10.2. Antibiogram
2.10.3. Determination of the Minimum Inhibitory Concentration (MIC)
2.10.4. Determination of the Minimum Bactericidal/Fungicidal Concentration (MBC/MFC)
2.11. Microplate Determination of Plant Extracts Antioxydant Activity
2,2-Diphenyl-1-Picrylhydrazyl Assay
The 2,2-Azinobis (3-Ethylbenzthiazoline)-6-Sulfonic Acid Assay
Ferric Reducing Antioxydant Power (FRAP)
Lipid Peroxidation Inhibitory Test (LPO)
2.12. Anti-Inflammatory Capacity of J. multifida Extracts
2.13. Statistical Analysis
3. Results
3.1. Preliminary Phytochemical Profiling
3.2. Total Phenolic and Flavonoid, Condensed and Hydrolyzable Tanins Content
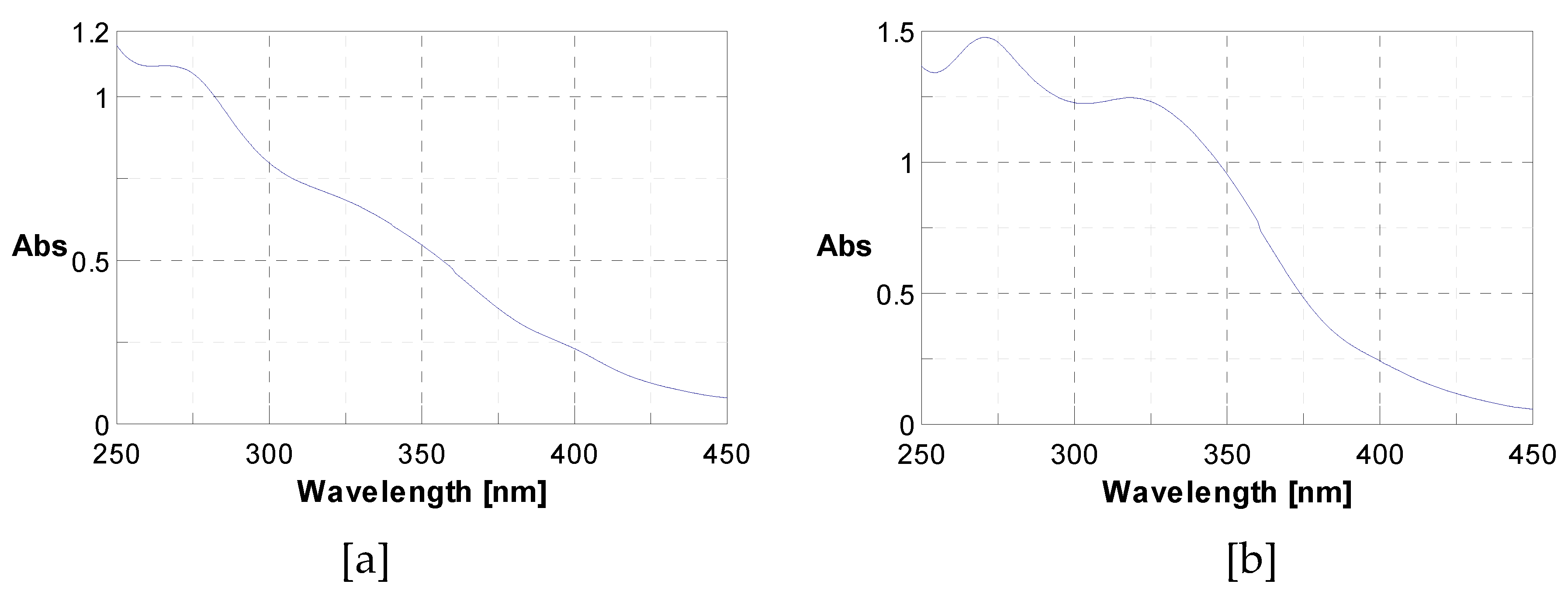
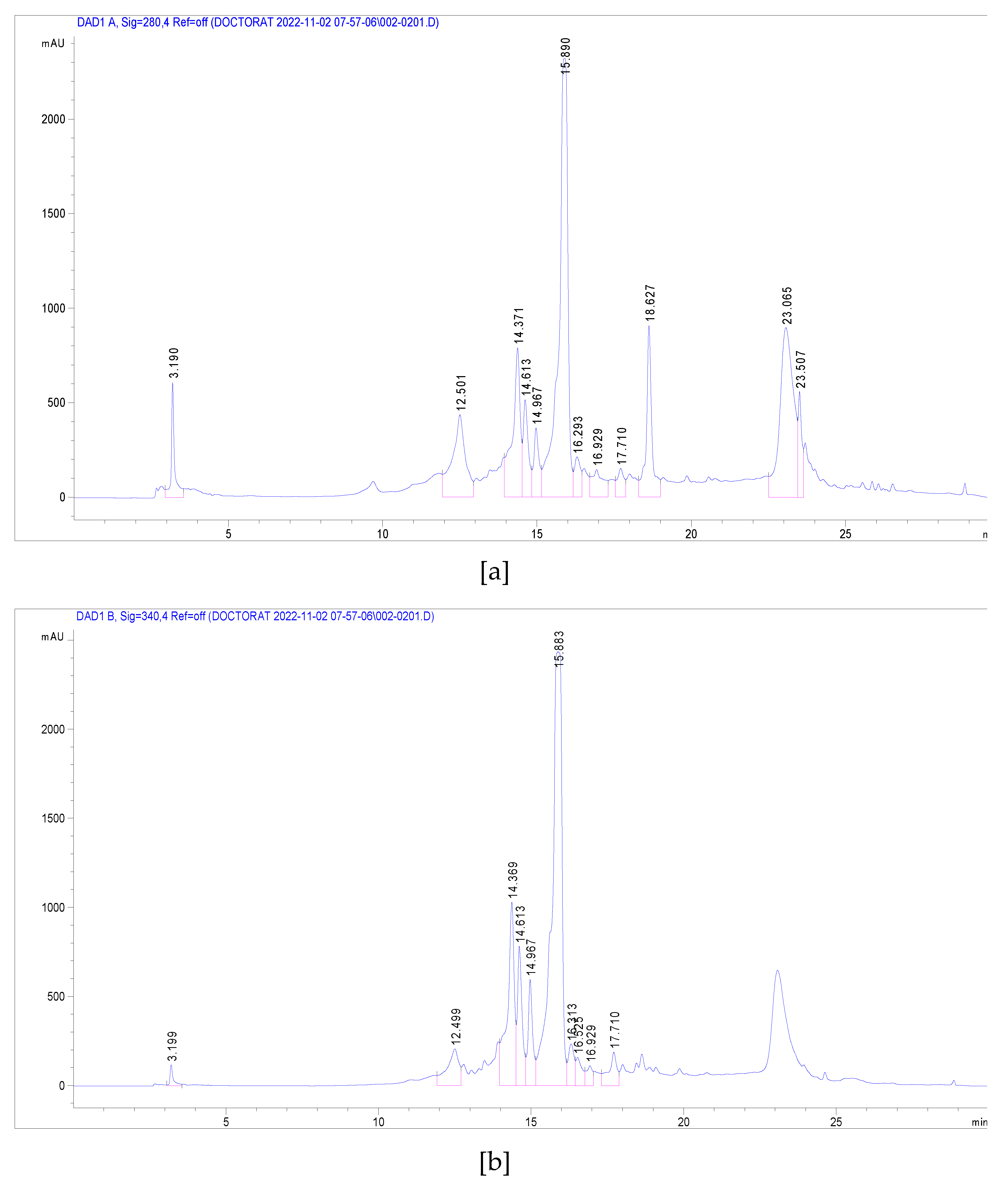
3.3. Identification of Phenolic Compounds in J. multifida Extract by LC-DAD-MS-ESI+ Analysis
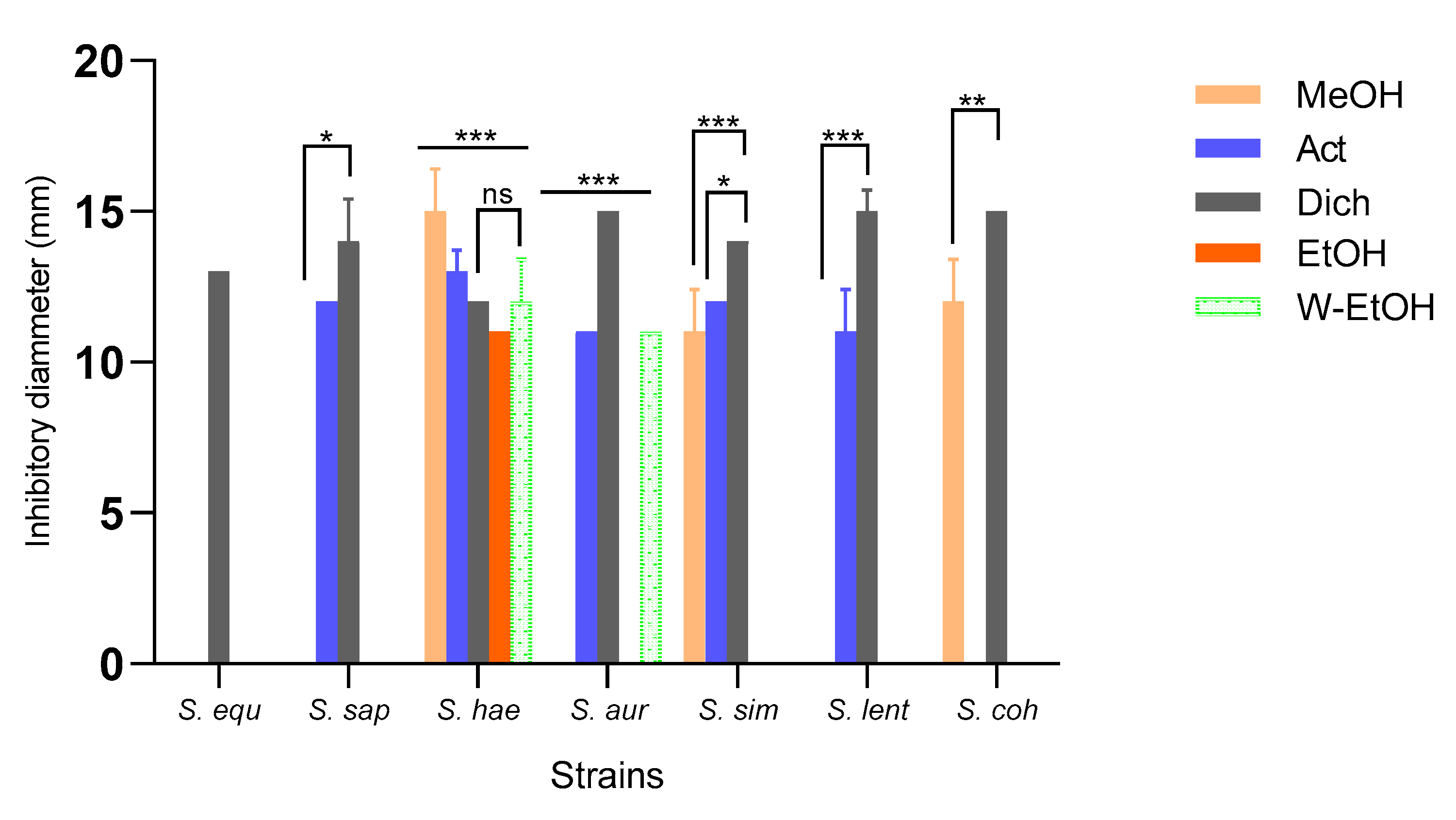
3.4. Antimicrobial Activity of J. multifida Leaves Extracts
3.4.1. Susceptibility of Reference Strains to J. multifida Leaves Extracts
3.4.2. Susceptibility of Maet Isolated Strains to J. multifida Leaves Extracts
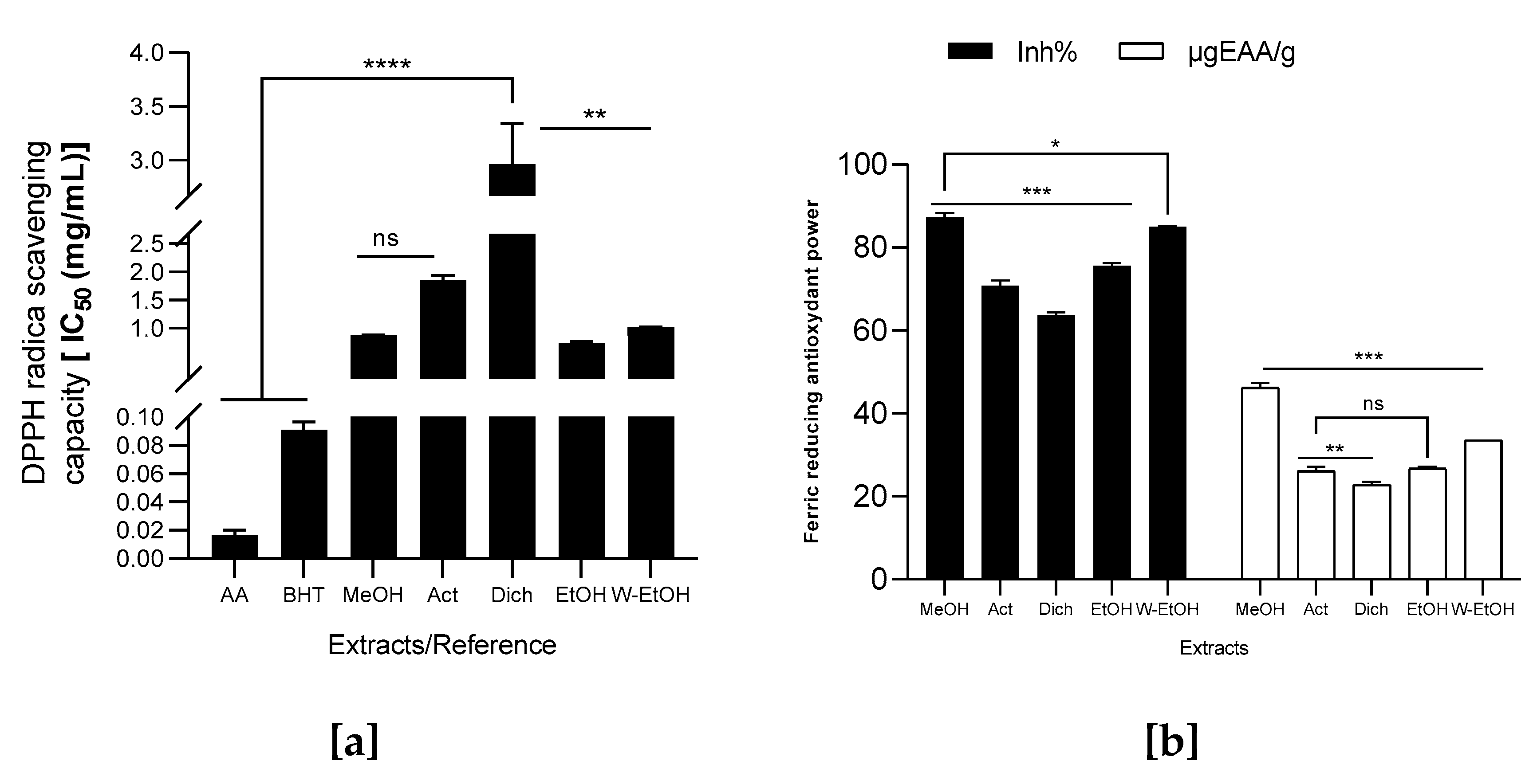
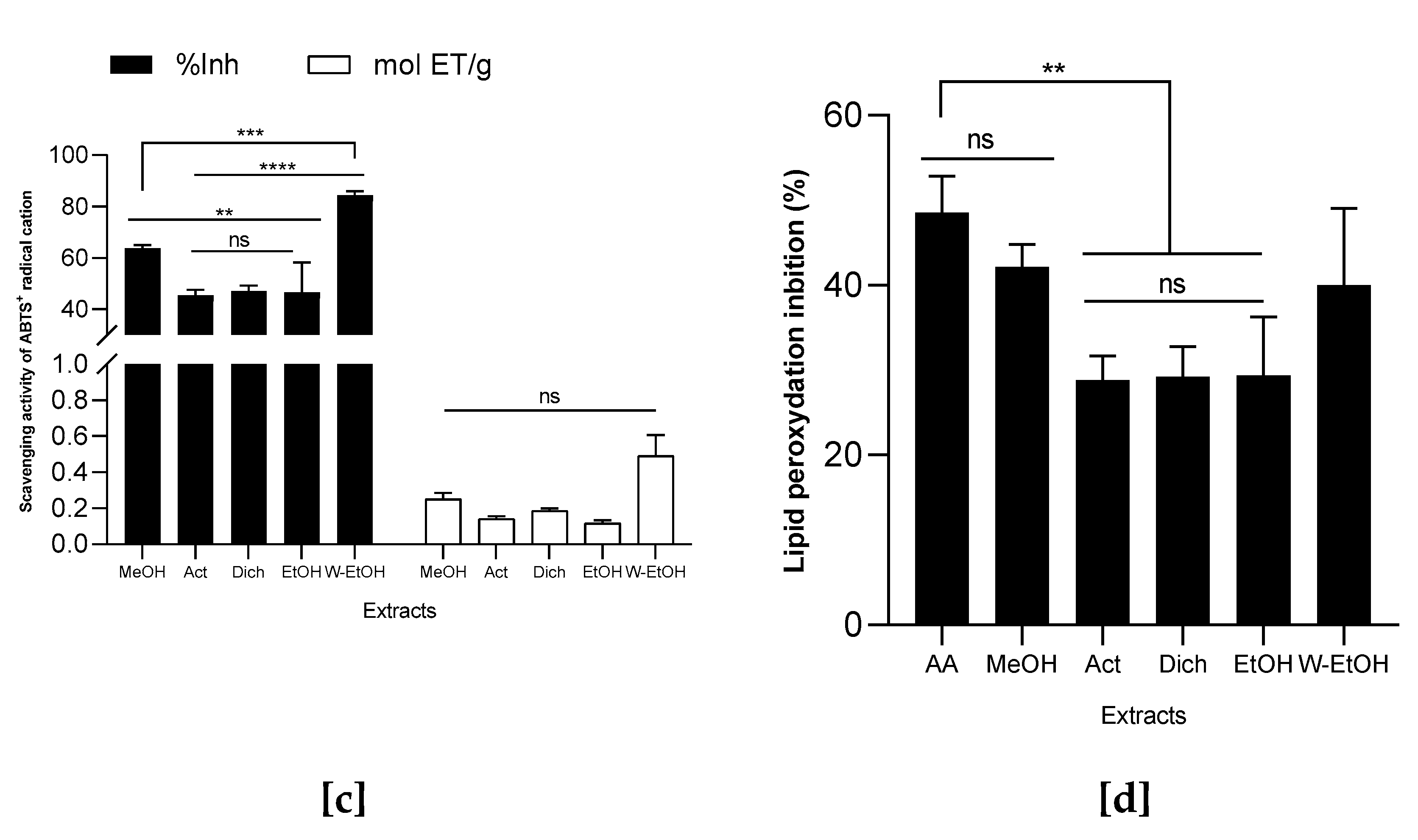
3.5. Antioxydant Activity of J. multifida Leaves Extracts
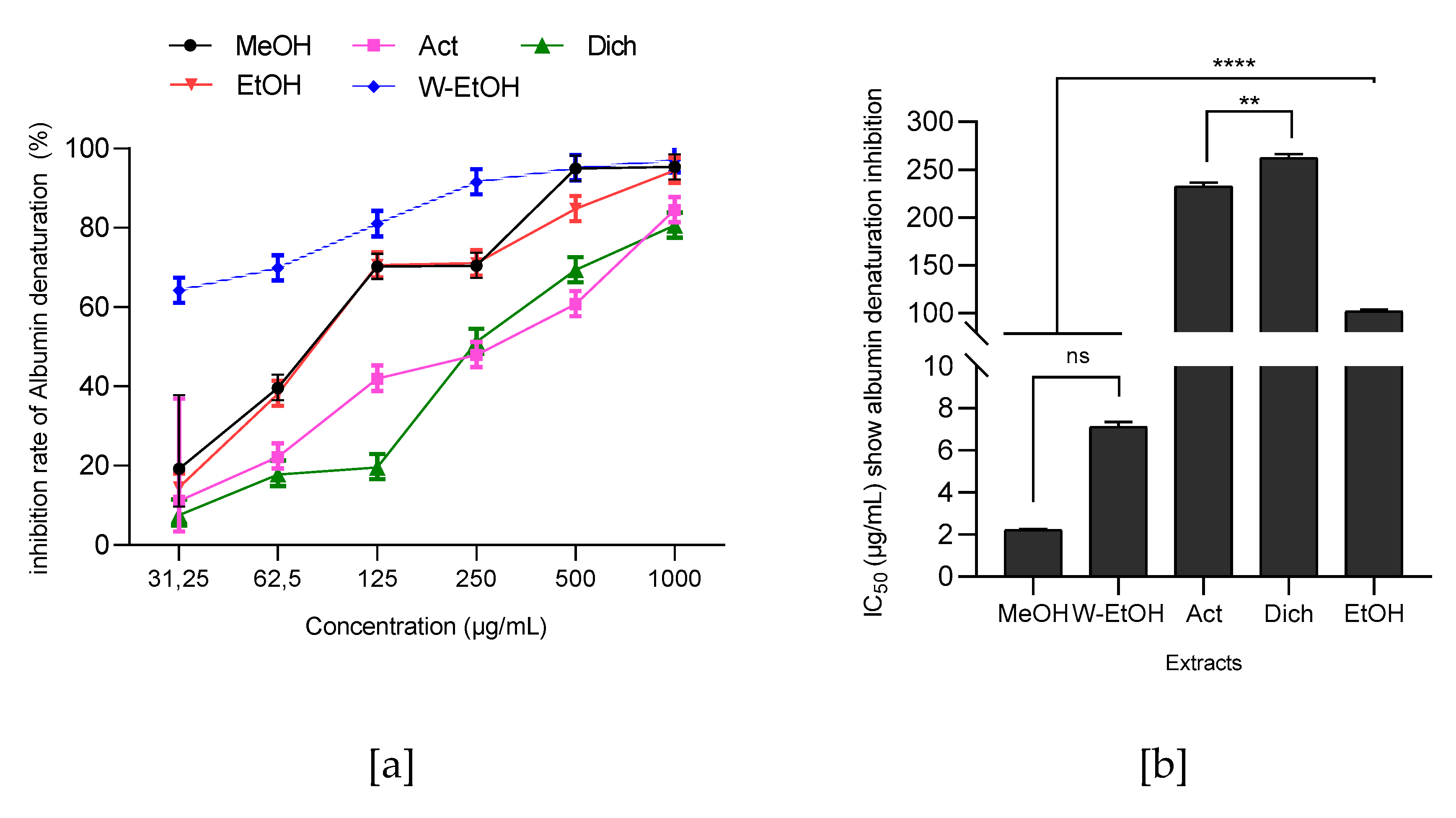
3.6. Antiinflammatory Activity of J. multifida Leaves Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic resistance in foodborne bacteria. Trends Food Sci Technol, 2019, 84, 41–44. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis.
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Pharmaceut. Sci. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Hartmann, T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochem. 2007, 68, 2831–2846. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 621–276. [Google Scholar] [CrossRef]
- Akoègninou, A.; van der Burg, W.J.; van der Maesen, L.J.G. Flore analytique du Bénin; Backhuys Publishers: Leiden, Netherlands, 2006; 1034 p. [Google Scholar]
- Shoji, M.; Woo, S.Y.; Masuda, A.; Win, N.N.; Ngwe, H.; Takahashi, E.; Kido, H.; Morita, H.; Ito, T.; Kuzuhara, T. Anti-influenza virus activity of extracts from the stems of Jatropha multifida Linn. collected in Myanmar. BMC Complement Altern Med. 2017, 17, 1–7. [Google Scholar] [CrossRef]
- Houghton, P.J.; Raman, A. Laboratory Hand Book for the Fractionation of Natural Extracts; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Dicko, M.H.; Hilhorst, R.; Gruppen, H.; Traore, A.S.; Laane, C.; van Berkel, W.J.H.; Voragen, A.G.J. Comparison of Content in Phenolic Compounds, Polyphenol Oxidase, and Peroxidase in Grains of Fifty Sorghum Varieties from Burkina Faso. J. Agric. Food Chem. 2002, 50, 3780–3788. [Google Scholar] [CrossRef]
- Cudalbeanu, M.; Ghinea, I.O.; Furdui, B.; Dah-Nouvlessounon, D.; Raclea, R.; Costache, T.; Cucolea, I.E.; Urlan, F.; Dinica, R.M. Exploring New Antioxidant and Mineral Compounds from Nymphaea alba Wild-Grown in Danube Delta Biosphere. Molecules. 2018, 23, 1–16. [Google Scholar] [CrossRef]
- Belem-Kabré, W.L.M.E.; Kaboré, B.; Compaoré-Coulibaly, A.; Traoré, T.K.; Thiombiano, E.A.M.; Nebié-Traoré, M.; Compaoré, M.; Kini, F.B.; Ouédraogo, S.; Kiendrebeogo, M.; Ouédraogo, N. Phytochemical and biological investigations of extracts from the roots of Cocos nucifera L. (Arecaceae) and Carica papaya L. (Caricaceae), two plants used in traditional medicine. Afr. J. Biochem. Res. 2021, 15, 28–35. [Google Scholar]
- Attien, P.; Sina, H.; Moussaoui, W.; Dadié, T.; Chabi, K.; Djéni, T.; Bankole, H.S.; Kotchoni, S.O.; Edoh, V.; Prévost, G.; Djè, M.; Baba-Moussa, L. Prevalence and antibiotic resistance of Staphylococcus strains isolated from meat products sold in Abidjan streets (Ivory Coast),” Afr. J. Microbiol. Res. 2011, 7, 3285–3293. [Google Scholar]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food. Drug. Analys. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, T.C.; Truck, M. Antibiotic susceptibility testing by a standardized single disc method,” Amer. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent. 2020, 23, 105–109. [Google Scholar] [CrossRef]
- Chokki, M.; Cudalbeanu, M.; Zongo, C.; Dah-Nouvlessounon, D.; Otilia Ghinea, I.; Furdui, B.; Raclea, R.; Savadogo, A.; Baba-Moussa, L.; Avamescu, S.M.; Dinica, R.M.; Baba-Moussa, F. Exploring Antioxidant and Enzymes (A-Amylase and B-Glucosidase) Inhibitory Activity of Morinda lucida and Momordica charantia leaves from Benin. Foods 2020, 9, 434. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Rodriguez, J.A.; Theoduloz, C.; Astudillo, S.L.; Feresin, G.E.; Tapia, A. Free-radical scavengers and antioxidants from Peumus boldus Mol. (‘Boldo’). Free. Rad. Res. 2003, 37, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food. Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Cudalbeanu, M.; Furdui, B.; Cârâc, G.; Barbu, V.; Iancu, A.V.; Marques, F.; Leitão, J.H.; Sousa, S.A.; Dinica, R.M. Antifungal, Antitumoral and Antioxidant Potential of the Danube Delta Nymphaea alba Extracts. Antibiotics (Basel). 2019, 9(1), 7. [Google Scholar] [CrossRef]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Bashir, R.; Mahon, P.J. Shalliker R.A. Ferric reducing antioxidant potential (FRAP) of antioxidants using reaction flow chromatography. Anal. Chim. Acta. 2017, 967, 93–101. [Google Scholar] [CrossRef]
- Kabré, W.J.D.; Dah-Nouvlessounon, D.; Hama, F.; Kohonou, N.A.; Sina, H.; Senou, M.; Baba-Moussa, L. Savadogo A. Anti-Inflammatory and Anti-Colon Cancer Activities of Mung Bean Grown in Burkina Faso. Evid Based Complement Alternat Med 2022, 9, 7873572. [Google Scholar] [CrossRef]
- Rampadarath, S.; Puchooa, D.; Ranghoo-Sanmukhiya, V.M. Antimicrobial, phytochemical and larvicidal properties of Jatropha multifida Linn. Asian Pac J Trop Med 2014, 7, S380–S383. [Google Scholar] [CrossRef]
- Hanafi, H.R.; Septilina, M.S.; Lintannisa, R.; Sri, R.S. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Volatile components from Coral “BETADINE” (Jatropha multifida Linn) Leaves. Res. J. Pharm. Technol. 2022, 15, 4633–4636. [Google Scholar] [CrossRef]
- Adamski, Z.; Blythe, L.L.; Milella, L.; Bufo, S.A. Biological Activities of Alkaloids: From Toxicology to Pharmacology. Toxins 2020, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Aiyelaagbe, O. Antibacterial activity of Jatropha multifida roots. Fitoterapia, 2001, 72, 544–546. [Google Scholar] [CrossRef]
- Nwokocha Blessing, A.; Agbagwa, I.O.; Okoli, B.E. Comparative phytochemical screening of Jatropha L. species in the Niger Delta. Res J Phytochem 2011, 5, 107–114. [Google Scholar]
- Chokchaisiri, R.; Srijun, J.; Chaichompoo, W.; Cheenpracha, S.; Ganranoo, L.; Suksamrarn, A. Anti-herpes simplex type-1 (HSV-1) activity from the roots of Jatropha multifida L. Med. Chem. Res. 2019, 29, 328–333. [Google Scholar] [CrossRef]
- Bruneton, J. Phytochimie, plantes médicinales Les tanins. 3th ed. Editions TEC. Et DOC., Editions médicales internationales, Paris 1999.
- Cavalcante, N.B.; Diego da Conceição Santos, A.; Guedes da Silva Almeida, J.R. The genus Jatropha (Euphorbiaceae): A review on secondary chemical metabolites and biological aspects. Chem-Biologic. Int. 2020, 108976. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-S.; Zhang, Y.; Li, S.; Ahmed, A.; Tang, G-H.; Yin, S. Cytotoxic macrocyclic diterpenoids from Jatropha multifida. Bioorganic Chem. 2018, 80, 511–518. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food. Chem. X. 2022, 13, 100217. [Google Scholar] [CrossRef]
- Alvarez-Martínez, F.J.; Barrajon-Catalan, E.; Herranz-Lopez, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine, 2021, 90, 153626. [Google Scholar] [CrossRef]
- Zhao, D.-D.; Jiang, L.L.; Li, H.Y.; Yan, P.F.; Zhang, Y.L. Chemical components and pharmacological activities of terpene natural products from the genus paeonia. Molecules 2016, 21, 1362. [Google Scholar] [CrossRef]
- Anani, K.; Adjrah, Y.; Ameyapoh, Y.; Karou, S.D.; Agbonon, A.; de Souza, C.; et al. Antimicrobial, Anti-inflammatory and antioxidant activities of Jatropha multifida L. (Euphorbiaceae). Phcog Res 2016, 8, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Banso, A.; Adeyemo, S.O. Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr J Biotechnol 2007, 6, 1785–1787. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Ooi, L.S.M.; Wang, H.; He, Z.; Ooi, V.E.C. Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). J. Ethnopharmacol. 2006, 106, 187–191. [Google Scholar] [CrossRef]
- Mijangos-Ramos, I.F.; Zapata-Estrella, H.E.; Ruiz-Vargas, J.A.; Escalante-Erosa, F.; Gómez-Ojeda, N.; García-Sosa, K.; Peña-Rodríguez, L.M. Bioactive dicaffeoylquinic acid derivatives from the root extract of Calea urticifolia. Rev. Bra. Farmaco. 2018, 28, 339–343. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.-Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.-J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Andersen, O.; Markham, R. Flavonoids, Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005; Volume 1, p. 1256. [Google Scholar]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef]
- De Stefano, A.; Caporali, S.; Di Daniele, N.; Rovella, V.; Cardillo, C.; Schinzari, F.; Minieri, M.; Pieri, M.; Candi, E.; Bernardini, S.; et al. Anti-Inflammatory and Proliferative Properties of Luteolin-7-O-Glucoside. Int. J. Mol. Sci 2021, 22, 1321. [Google Scholar] [CrossRef]
- Huang, W.C.; Liou, C.J.; Shen, S.C.; Hu, S.; Hsiao, C.Y.; Wu, S.J. Luteolin Attenuates IL-1beta-Induced THP-1 Adhesion to ARPE-19 Cells via Suppression of NF-kappaB and MAPK Pathways. Mediat. Inflamm 2020, 2020, 9421340. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cheng, C.; Li, X.; Zhou, S.; Hua, J.; Huang, J.; Li, Y.; Yang, K.; Zhang, P.; Zhang, Y.; Tian, J. Luteolin alleviates ochratoxin A induced oxidative stress by regulating Nrf2 and HIF-1alpha pathways in NRK-52E rat kidney cells. Food Chem. Toxicol. 2020, 141, 111436. [Google Scholar] [CrossRef] [PubMed]
- Alekhya Sita, G.J.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Rama Sireesha, K.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A.; SreeHarsha, N. Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/ HO-1 pathway. IUBMB Life. 2019, 71, 1041–1047. [Google Scholar] [CrossRef]
- Palombo, R.; Caporali, S.; Falconi, M.; Iacovelli, F.; Morozzo Della Rocca, B.; Lo Surdo, A.; Campione, E.; Candi, E.; Melino, G.; Bernardini, S.; Terrinoni, A. Luteolin-7-O-beta-d-Glucoside Inhibits Cellular Energy Production Interacting with HEK2 in Keratinocytes. Int. J. Mol. Sci. 2019, 20, 2689. [Google Scholar] [CrossRef]
- Kluska, M.; Juszczak, M.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Effect of Kaempferol and Its Glycoside Derivatives on Antioxidant Status of HL-60 Cells Treated with Etoposide. Molecules 2022, 27, 333. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; Tamokou, J.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res Notes 2012, 5, 158. [Google Scholar] [CrossRef]
| Group of compounds | Class | J. multifida |
|---|---|---|
| Nitrogenous compound | Alkaloids | + |
| Poly-phenolics compound | Tanin catéchique | - |
| Tanin gallique | + | |
| Flavonoids | + | |
| Anthocyans | + | |
| Leuco-anthocyane | + | |
| Coumarin | - | |
| Quinonics derivate | + | |
| Terpenic compound | Triterpenoids | + |
| Steroids | - | |
| Cardenolids | - | |
| Heterosides | Saponosids (IM) | + (112) |
| Cyanogenics derivate | - | |
| Reducing compounds | - | |
| Free anthracénics | - | |
| Mucilags | + |
| Solvents | Polyphenols (mgEqGA/g) | Flavonoids (mgEqQ/g) | Condensed Tanins (mgEqC/g) | Hydrolysable Tanins (mgEqGA/g) |
|---|---|---|---|---|
| Water | 11.25±1.37 | 6.58±1.36 | 2.05±0.28 | 2.18±0.15 |
| Ethanol | 23.03±6.9 | 7.18±2.85 | 5.49±0.35 | 3.03±0.38 |
| Water-Ethanol | 21.57±2.20 | 7.43±0.12 | 4.78±0.42 | 2.11±0.12 |
| Methanol | 45.01±11.87 | 3.65±0.09 | 6.49±0.46 | 3.31±0.52 |
| Acetone | 26.83±0.27 | 0.90±0.03 | 8.58±0.45 | 3.19±0.11 |
| Dichloromethane | 12.86±0.44 | 0.32±0.01 | 6.79±0.34 | 4.12±0.29 |
| PeakNo. | Rt (min) | UVλmax (nm) | [M+H]+(m/z) | Phenolic Compound | Subclass | Content(mg/g) |
|---|---|---|---|---|---|---|
| 1 | 3.19 | 270 | 139 | 2-Hydroxybenzoic acid | Hydroxybenzoic acid | 1.250 |
| 2 | 12.50 | 333 | 339 | o-Coumaroylquinic acid | Hydroxycinnamic acid | 2.291 |
| 3 | 14.37 | 330, 280 | 565, 271 | Apigenin-apiosyl-glucoside | Flavone | 5.587 |
| 4 | 14.61 | 350, 260 | 449, 287 | Luteolin-galactoside | Flavone | 2.890 |
| 5 | 14.96 | 350, 260 | 449, 287 | Luteolin-glucoside | Flavone | 2.173 |
| 6 | 15.88 | 350, 260 | 433, 287 | Luteolin-rhamnoside | Flavone | 19.732 |
| 7 | 16.31 | 360, 260 | 465, 303 | Quercetin-glucoside | Flavonol | 1.092 |
| 8 | 16.52 | 360, 260 | 434, 303 | Quercetin-arabinoside | Flavonol | 0.779 |
| 9 | 16.93 | 332 | 517 | Dicaffeoyquinic acid | Hydroxycinnamic acid | 0.636 |
| 10 | 17.71 | 350, 250 | 433, 287 | Kaempferol-rhamnoside | Flavonol | 1.223 |
| Total phenolics | 37.652 |
| Extracts | Parameters (mg/mL) | Staphylococcus aureus ATCC 6538P | Escherichia coli ATCC 25922 | Salmonella enteritidis ATCC 13076 | Listeria monocytogenes ATCC 19114. |
Candida albicans ATCC 10231 |
|---|---|---|---|---|---|---|
| Water | MIC | 22.67 | 47.61 | 47.61 | 47.61 | 47.61 |
| MBC | 47.61 | >47.61 | >47.61 | >47.61 | >47.61 | |
| MBC/MIC | 2.10 | nd | nd | nd | nd | |
| Ethanol | MIC | 5.14 | 34.54 | 22.67 | 22.67 | 22.67 |
| MBC | 22.67 | 47.61 | 22.67 | 22.67 | 22.67 | |
| MBC/MIC | 4.41 | 1.37* | 1* | 1* | 1* |
| Extracts | Parameters (mg/mL) | S. equ | S. sap | S. hae | S. aur | S. sim | S. len | S. coh |
|---|---|---|---|---|---|---|---|---|
| MeOH | MIC | - | - | 20 | - | 20 | - | 10 |
| MBC | - | - | >20 | - | >20 | - | 20 | |
| MBC/MIC | - | - | - | - | - | - | 2* | |
| Act | MIC | - | 10 | 20 | 20 | 20 | 20 | - |
| MBC | - | >20 | >20 | >20 | >20 | >20 | - | |
| MBC/MIC | - | - | - | - | - | - | - | |
| Dich | MIC | 2.5 | 2.5 | 5 | 2.5 | 2.5 | 2.5 | 5 |
| MBC | 5 | 5 | 5 | 20 | 10 | 5 | 20 | |
| MBC/MIC | 2* | 2* | 1* | 8 | 4 | 2* | 4 | |
| EtOH | MIC | - | - | 10 | - | - | - | - |
| MBC | - | - | 20 | - | - | - | - | |
| MBC/MIC | - | - | 2* | - | - | - | - | |
| W-EtOH | MIC | - | - | 20 | 20 | - | - | - |
| MBC | - | - | >20 | >20 | - | - | - | |
| MBC/MIC | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





