1. Introduction
Lymphangiogenesis, the growth of lymphatic vessels, has recently received considerable attention because lymphatic disorders due to trauma, infection, surgery, radiotherapy, or a combination of these are increasing worldwide. Cancer therapy with surgical lymph node dissection and radiotherapy can cause severe lymphatic disorders, including lymphocele, lymphorrhea, and lymphedema. The latest estimates report that 250 million people worldwide experience lymphedema, impaired immunity, and the accumulation of subcutaneous fat tissue [
1]. Therefore, understanding the mechanisms underlying lymphangiogenesis is important for clinicians and can provide new possibilities for treating lymphatic disorders.
Lymphangiogenesis generally occurs in adult tissues during wound healing. The main function of the lymphatic vasculature is to return extravasated fluid to the blood vascular system. In healthy adults, the lymphatic system returns approximately 1–2 L of interstitial fluid with 20–30 g/L protein to the venous circulation daily [
2]. The lymphatic vascular system plays an important role in immune responses, serving as a conduit for extravasated leukocytes, activated antigen-presenting cells, and cancer metastasis. Two concepts explain the mechanism of lymphangiogenesis under inflammatory conditions [
3]. The first is sprouting lymphangiogenesis. Activated lymphatic endothelial cells (LECs) proliferate and extend outside the existing lymphatic vessels. Bone marrow-derived myeloid cells (BMDMs) contribute to sprouting lymphangiogenesis by producing paracrine factors, such as vascular endothelial growth factor (VEGF)-A and VEGF-C, and activating LECs [
4,
5]. Another concept is the fusion of LEC progenitor cells with existing or sprouting lymphatic vessels. In inflammatory conditions, BMDMs often express LEC markers such as VEGF receptor-3, lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1), or podoplanin [
6,
7,
8,
9,
10]. Thus, these BMDM are called LEC progenitor cells.
In a corneal transplantation mouse model, CD11b+ macrophages contributed to lymphangiogenesis in the corneal stroma. The depletion of these cells by clodronate liposomes suppressed CD11b+ macrophage infiltration and lymphangiogenesis in the cornea [
6]. Additionally, CD11b+ macrophages integrate into lymphatic vessels in lipopolysaccharide (LPS)-induced inflamed diaphragm [
11]. Ligands of Toll-like receptor 4 (LPS, high mobility group box 1 protein, paclitaxel) and Th2 cytokines, such as interleukin (IL)-4, IL-10, and IL-13, have been reported to promote myeloid-lymphatic transition [
3,
12]. However, studies on the mechanism of lymphatic regeneration using three-dimensional images of the lymphatic structures are limited [
13]. In this study, we used scanning electron microscopy (SEM) to observe the three-dimensional structure of lymphatic vessels and integrated spherical cells, presumed to be LEC progenitor cells, in a secondary lymphedema mouse model.
2. Results
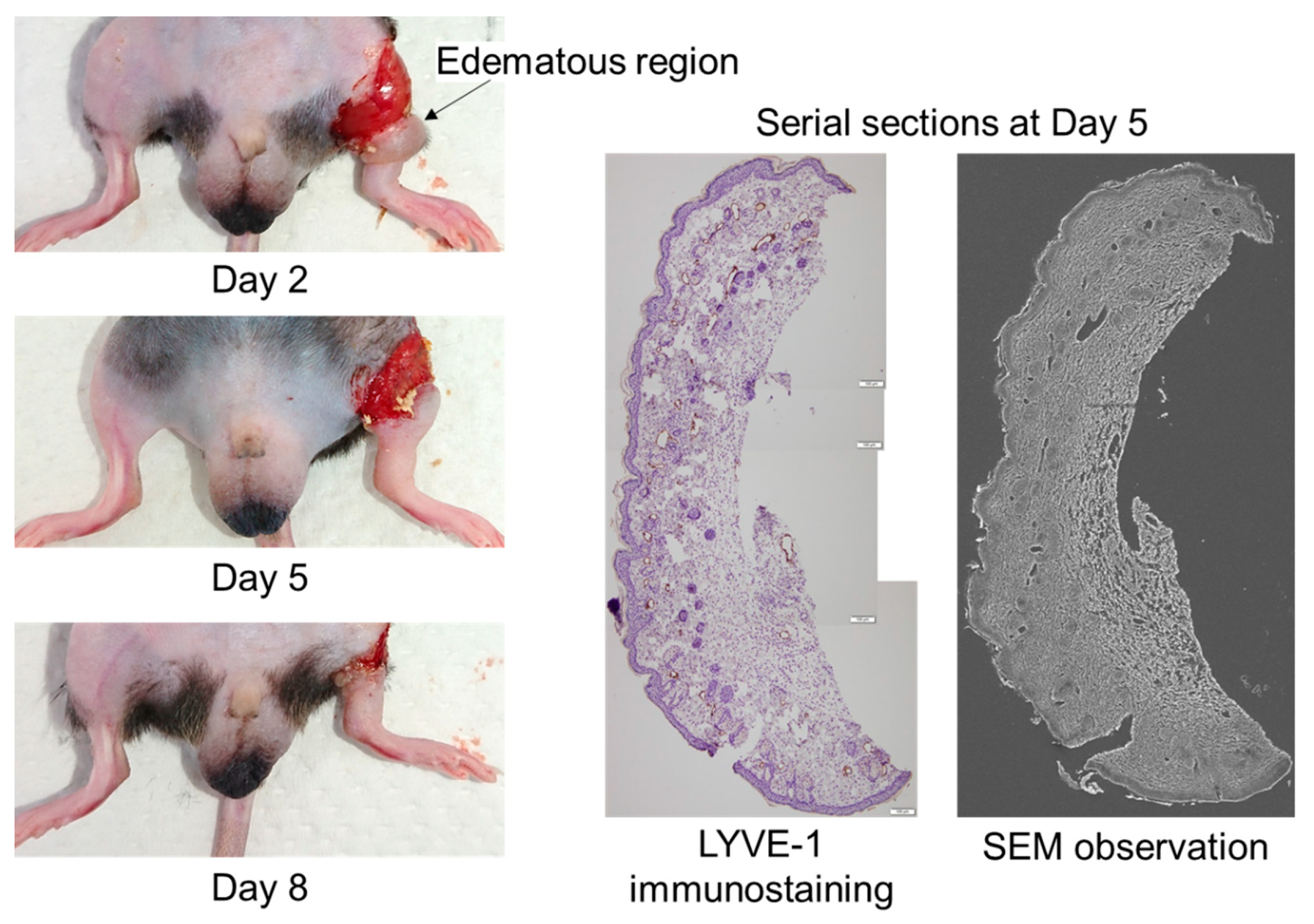
To obtain three-dimensional structures of lymphatic regeneration in edematous regions, we prepared serial sections from the left hind limbs of the mice following a surgical lymphatic vessel incision. Although the edema prepared from only surgical incision was spontaneously resolved in approximately 3–4 weeks after surgery, obvious hind limb edema occurred during days 2–8 (
Figure 1). The lymphatic vessel architecture was observed using SEM overlaying images of the same serial sections stained with the anti-LYVE-1 antibody (
Figure 1).
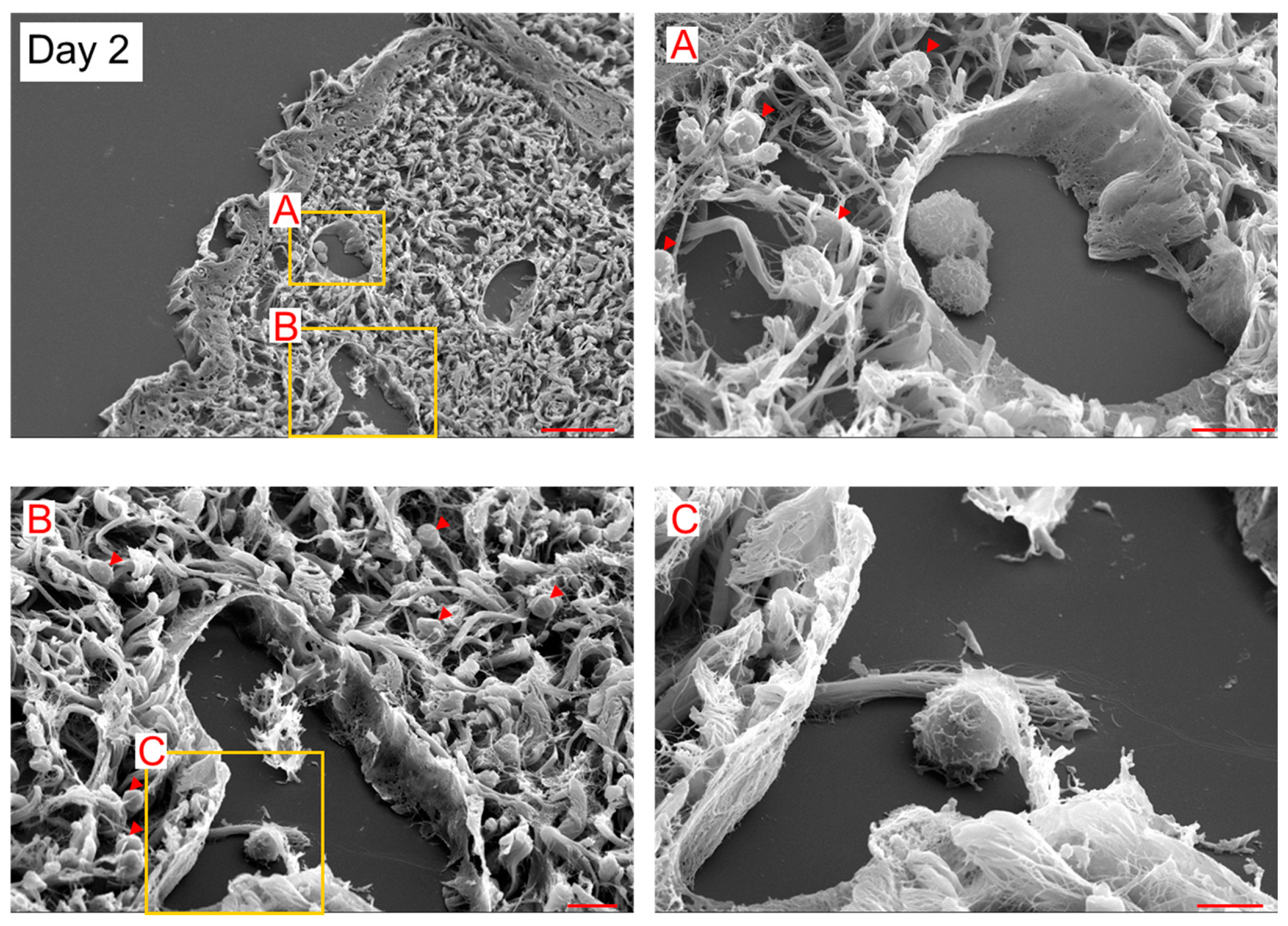
In SEM observation, 5–10 μm spherical cells (probably myeloid cells) existed inside and outside LYVE-1+ lymphatic vessels on day 2 (
Figure 2). The spherical cells were attached to LECs inside the lymphatic vessels on day 2 (
Figure 2). On day 5, the spherical cells were integrated with LEC. The complex of these cells was elongated toward the lumen (
Figure 3). These observational results suggest that the spherical cells were LEC progenitor cells and integrated into existing lymphatic vessels in the lymphedematous region.
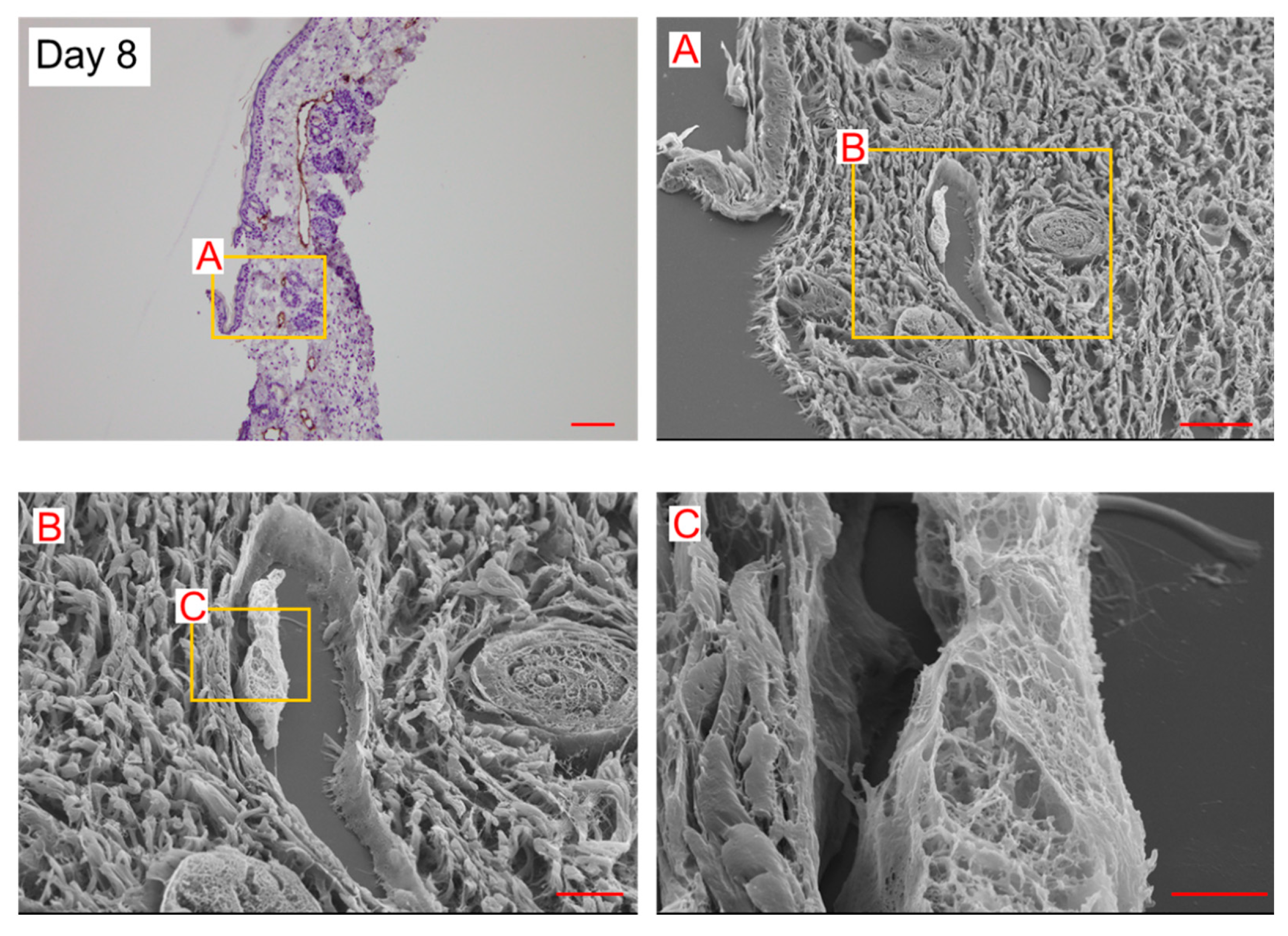
On day 8, a few spherical cells were observed in the lymphatic vessels. The internal wall consisted of a string-shaped structure (
Figure 4). In our previous study, using the secondary lymphedema model made with X-ray irradiation and surgery [
13], we reported almost the same structure in the histological analysis of the lymphedematous region (Figure 5). The internal wall comprised a string-shaped structure and spherical cells (Figure 5). These results suggest that spherical cells are converted into string-shaped cells, forming a new lymphatic vessel wall.
Dilated lymphatic vessels and LECs protruding toward the lumen (
Figure 4 and Figure 5) were considered structures analogous to an intraluminal pillar, which is the hallmark of intussusceptive angiogenesis. We anticipated that intussusceptive lymphangiogenesis would occur in lymphedema lesions.
3. Discussion
The SEM results suggested that spherical cells were integrated into the existing lymphatic vessel walls and transformed into string-shaped cells to form a new lymphatic vessel wall in a lymphedema lesion. We expected that surgical incision would cause BMDM infiltration into the lymphedema region and induce lymphangiogenesis in BMDM-derived LEC progenitor cells.
Previously, we expected intussusceptive lymphangiogenesis to occur in lymphedematous regions because the newly formed lymphatic vessel wall protruded toward the lumen, likely an intraluminal pillar, which is a hallmark of intussusceptive angiogenesis [
13]. Intussusceptive lymphangiogenesis is a process like intussusceptive angiogenesis occurring by the division of the lumen of a preexisting vessel through the insertion of an intraluminal pillar [
14,
15,
16]. Díaz-Flores et al. mentioned that intussusceptive angiogenesis and lymphangiogenesis are mechanisms by which blood and lymphatic vessels split, expand, and remodel through transluminal pillar formation (hallmarks of intussusception) [
16]. According to Burri et al., when the formation of intraluminal pillars is localized at the free ends of vessels, intussusceptive arborization occurs, i.e., vascular net expansion [
17]. However, when it occurs in vessel bifurcation, it induces the remodeling or pruning of vascular sprouts. The destiny and effect of these pillars formed in the bifurcation of small vessels depend on their size, shape, and localization. These pillars can optimize hemodynamic conditions and adapt the mechanisms of increased blood flow (lymph flow) and pressure. They can induce complete occlusion, followed by regression, retraction, and atrophy of the affected sprout. Therefore, intussusceptive lymphangiogenesis is not only responsible for the increase in the vascular bed but is also directly involved in structural remodeling to optimize the shape and function of the lymphatic vessel.
To overlay serial section images with LYVE-1 immunostaining, we identified lymphatic vessels and analyzed their internal structure using SEM. However, this method is unsuitable for analyzing the outside of the vessels because the region is filled with collagen and elastic fibers. Therefore, sprouting lymphangiogenesis, which elongates to new lymphatic vessels toward the outside of existing lymphatic vessels, is difficult to observe using this method. If this method is performed in a tissue such as the cornea, which is transparent and has almost no other structures, analyzing sprouting lymphangiogenesis using SEM may be possible.
In a previous study, we showed that ADSC transplantation accelerated LEC proliferation, increased the number of lymphatic vessels, and mitigated fibrosis in the surrounding interstitial tissue [
13,
18,
19]. However, the exact mechanism and function of lymphangiogenesis in lymphedema and wound healing remain unknown. Additionally, the characteristics of the spherical cells have not yet been validated in this study. To identify the origin of these cells, double staining for myeloid cell markers and LEC markers are required. Additionally, SEM observation with double-stained slides is needed to observe the three-dimensional structure. However, performing double staining and SEM sample preparation on the same slide is difficult because 5-μm sections are easily disrupted during lyophilization. The role of LEC progenitor cells in intussusceptive lymphangiogenesis is an emerging research phase. We need to continue analyzing the lymphatic function in irradiated tissues and/or the wound-healing process.
4. Materials and Methods
This study was approved by the Animal Research Committee of Shimane University (Approval No. IZ30-127).
2.1. Surgical Procedure
C57BL/6J male mice, 8-week-old, were intraperitoneally anesthetized with medetomidine, midazolam, and butorphanol at doses of 0.15 mg/kg, 2.0 mg/kg, and 2.5 mg/kg, respectively. They were then subjected to a circumferential incision in the inguinal region of the muscle layer. A 5-mm wide gap was left open. Adipose-derived mesenchymal stem cells (ADSCs) were purchased from Cyagen (MUBMD-01001, Santa Clara, CA, USA) and cultured as previously described [
13]. ADSCs (1.0×10
5 cells/mice) were injected into the incised site 24 h after surgery. Tissues from the distal skin of the incision at 2, 5, and 8 days after surgery were used for histological analysis.
2.2. Observation of Lymphangiogenesis Using a Combination Method of SEM and LYVE-1 Immunoreactivity
Tissue obtained from the distal side of the incision was washed with 0.1 M phosphate buffer, fixed immediately with 10% formalin/70% methanol solution for 24 h, and embedded in paraffin. For immunohistochemistry, embedded specimens were serially sectioned in 5 µm. We performed antigen retrieval treatment using Tris-EDTA buffer (pH 9.0) at 85°C for 20 min. Antigen-retrieved specimens were immersed in 3% H2O2 for 15 min, washed with Tris-buffered saline (TBS), and incubated with Blocking One Histo (Nacalai Tesque, Inc., Kyoto, Japan) for 10 min. After washing with TBS-0.1% Tween-20 (TBS-T), the cells were incubated for 1 h at 20°C with rabbit polyclonal anti-mouse LYVE-1 antibody (2 μg/mL; DP3513P, OriGene Technologies Inc., Rockville, MD, USA). The specimens were then washed with TBS-T, incubated with Donkey Anti-Rabbit IgG H&L (HRP), preadsorbed (1:500 dilution, ab7083, Abcam, Cambridge, UK) for 1 h, and visualized using a Peroxidase Stain DAB Kit (Nacalai Tesque) and hematoxylin.
For SEM analysis, the specimens were processed on glass slides as previously described [
13]. Briefly, 15-µm-thick serial sections were mounted on a MAS-GP type A-coated glass slide (Matsunami Glass Ind., Ltd., Osaka, Japan). After deparaffinization with xylene and 99% ethanol, slides were transferred to 100% ethanol and tert-butyl alcohol. Slides in frozen tert-butyl alcohol were lyophilized with JFD-320 (JEOL Ltd., Tokyo, Japan). Then, gold ions were coated with VX-10A (EIKO ENGINEERING, LTD., Ibaraki, Japan), mounted on an aluminum sample stage using double-sided tape, and observed using a JSM-6510 (JEOL Ltd.) in the secondary electron image mode. In parallel, 5-µm-thick serial sections were prepared for LYVE-1 staining to identify the lymphatic vessel position using SEM.
5. Conclusions
In this study, we observed that the three-dimensional structures of spherical cells fused to existing lymphatic vessels, transformed into string-shaped cells, and formed a new lymphatic vessel wall in a mouse model of secondary lymphedema. Furthermore, the newly formed lymphatic vessel wall extended into the lumen, suggesting intussusceptive lymphangiogenesis. These limited but definitive observational results may contribute to elucidating the mechanisms underlying lymphangiogenesis in adult tissues.
Author Contributions
Conceptualization, K. H. and R. O.; methodology, R. O.; investigation, R. O.; resources, K. H.; data curation, R. O.; writing—original draft preparation, K. H. and R. O.; writing—review and editing, K. H., R. O., S. S., and S. Y.; visualization, R. O.; supervision, K. H.; project administration, K. H.; funding acquisition, K. H. All authors have read and agreed to the published version of the manuscript.”.
Funding
This research was funded by the Japan Society for the Promotion of Science (JSPS), KAKENHI (grant number 18K16062).
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank Editage (www. editage.com) for English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahmadzadeh, N.; Robering, J.W.; Kengelbach-Weigand, A.; Al-Abboodi, M.; Beier, J.P.; Horch, R.E.; Boos, A.M. Human adipose-derived stem cells support lymphangiogenesis in vitro by secretion of lymphangiogenic factors. Exp Cell Res. 2020, 388, 111816. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Volk-Draper, L.D.; Hall, K.L.; Wilber, A.C.; Ran, S. Lymphatic endothelial progenitors originate from plastic myeloid cells activated by toll-like receptor-4. PLoS One. 2017, 12, e0179257. [Google Scholar] [CrossRef]
- Ji, R.C. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol Life Sci. 2012, 69, 897–914. [Google Scholar] [CrossRef]
- Ding, M.; Fu, X.; Tan, H.; Wang, R.; Chen, Z.; Ding, S. The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer. Mol Med Rep. 2012, 6, 1023–1029. [Google Scholar] [CrossRef]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D'Amore, P.A.; Stein-Streilein, J.; Losordo, D.W.; Streilein, J.W. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, A.; Baeriswyl, V.; Imaizumi, N.; Schwendener, R.; Rüegg, C.; Christofori, G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One. 2009, 4, e7067. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, C.; Cho, Y.P.; Lee, E.; Kim, H.; Kim, P.; Yun, S.H.; Yoon, Y.S. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010, 122, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Kröber, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; Wick, N.; Mazal, P.R. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006, 12, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Religa, P.; Cao, R.; Bjorndahl, M.; Zhou, Z.; Zhu, Z.; Cao, Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005, 106, 4184–4190. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.L.; Volk-Draper, L.D.; Flister, M.J.; Ran, S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One. 2012, 7, e31794. [Google Scholar] [CrossRef] [PubMed]
- Espinosa Gonzalez, M.; Volk-Draper, L.; Bhattarai, N.; Wilber, A.; Ran, S. Th2 cytokines IL-4, IL-13, and IL-10 promote differentiation of pro-lymphatic progenitors derived from bone marrow myeloid precursors. Stem Cells Dev. 2022, 31, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Ogino, R.; Hayashida, K.; Yamakawa, S.; Morita, E. Adipose-derived stem cells promote intussusceptive lymphangiogenesis by restricting dermal fibrosis in irradiated tissue of mice. Int J Mol Sci. 2020, 21, 3885. [Google Scholar] [CrossRef]
- Patan, S.; Tanda, S.; Roberge, S.; Jones, R.C.; Jain, R.K.; Munn, L.L. Vascular morphogenesis and remodeling in a human tumor xenograft: blood vessel formation and growth after ovariectomy and tumor implantation. Circ Res. 2001, 89, 732–739. [Google Scholar] [CrossRef]
- Rossi-Schneider, T.R.; Verli, F.D.; Marinho, S.A.; Yurgel, L.S.; De Souza, M.A. Study of intussusceptive angiogenesis in inflammatory regional lymph nodes by scanning electron microscopy. Microsc Res Tech. 2010, 73, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Flores, L.; Gutiérrez, R.; Gayoso, S.; García, M.P.; González-Gómez, M.; Díaz-Flores, L Jr. ; Sánchez, R.; Carrasco, J.L.; Madrid, J.F. Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. Histol Histopathol. 2020, 35, 1083–1103. [Google Scholar] [CrossRef] [PubMed]
- Burri, P.H.; Hlushchuk, R.; Djonov, V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004, 231, 474–488. [Google Scholar] [CrossRef]
- Hayashida, K.; Yoshida, S.; Yoshimoto, H.; Fujioka, M.; Saijo, H.; Migita, K.; Kumaya, M.; Akita, S. Adipose-derived stem cells and vascularized lymph node transfers successfully treat mouse hindlimb secondary lymphedema by early reconnection of the lymphatic system and lymphangiogenesis. Plast Reconstr Surg. 2017, 139, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Ogino, R.; Yokooji, T.; Hayashida, M.; Suda, S.; Yamakawa, S.; Hayashida, K. Emerging anti-inflammatory pharmacotherapy and cell-based therapy for lymphedema. Int J Mol Sci. 2022, 23, 7614. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).