Submitted:
12 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
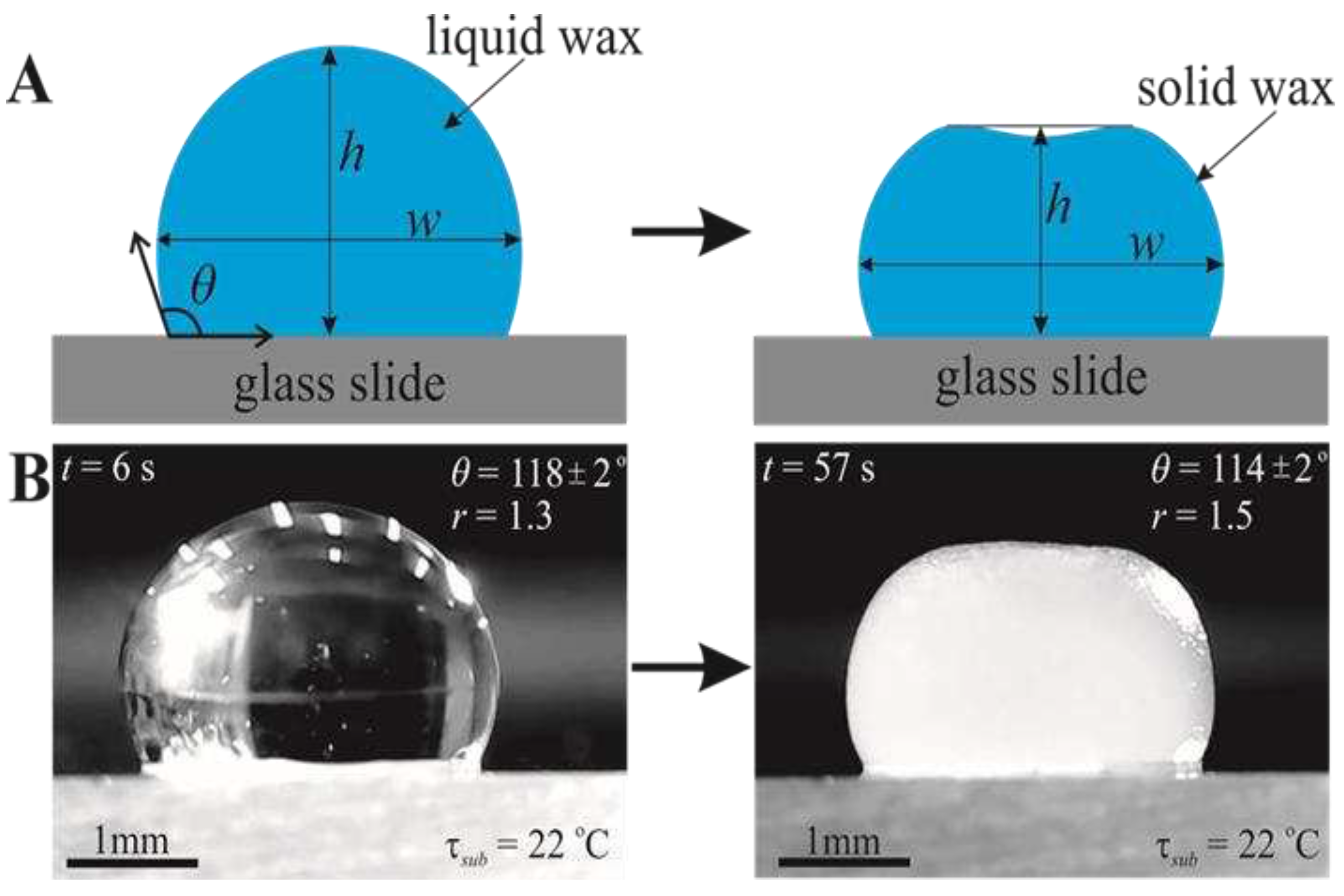
3. Results
3.1. In Situ Study of the Shape of Heated and Cooled Paraffin Wax Droplets
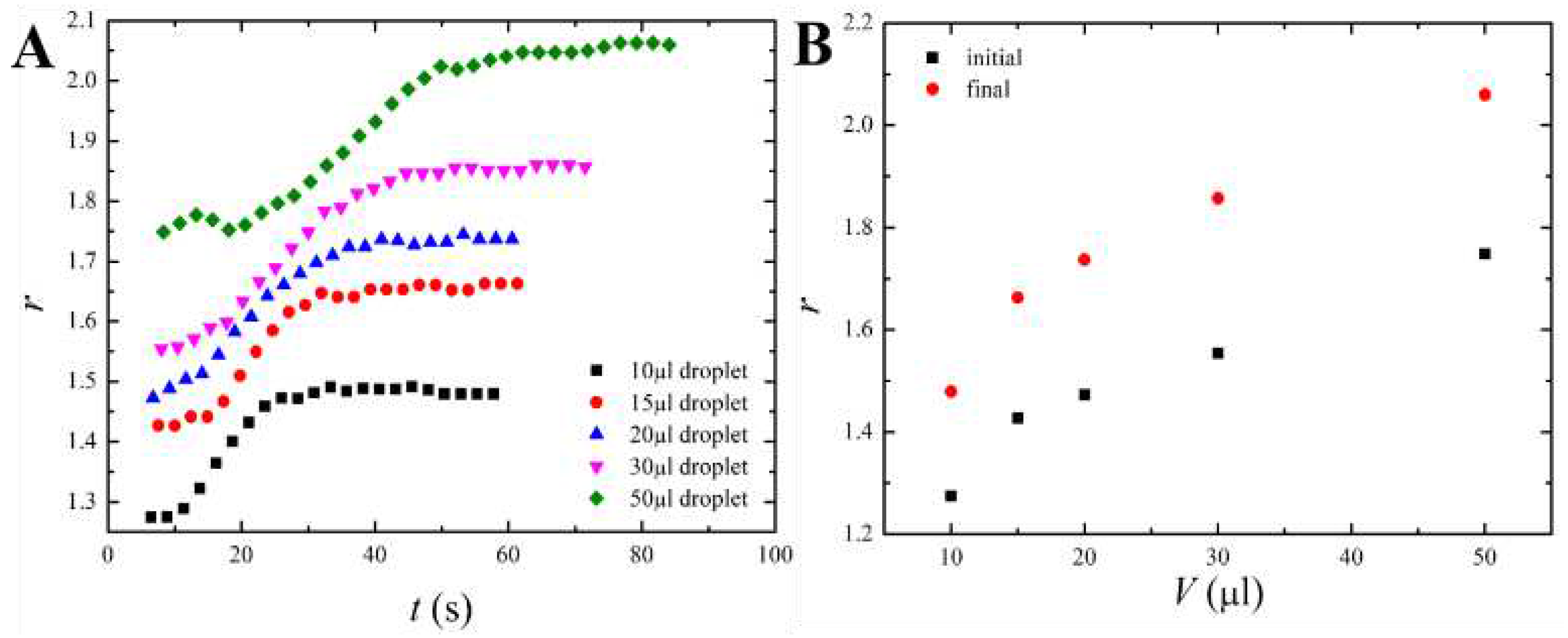
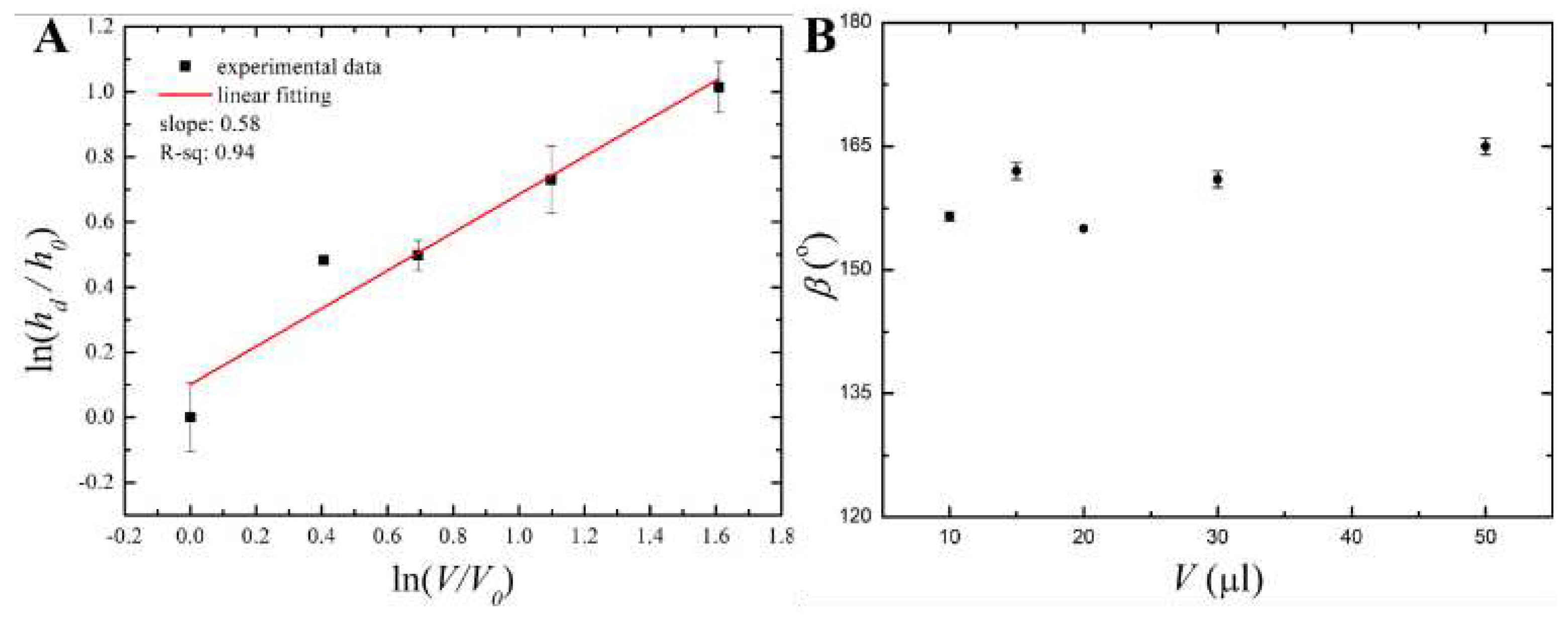
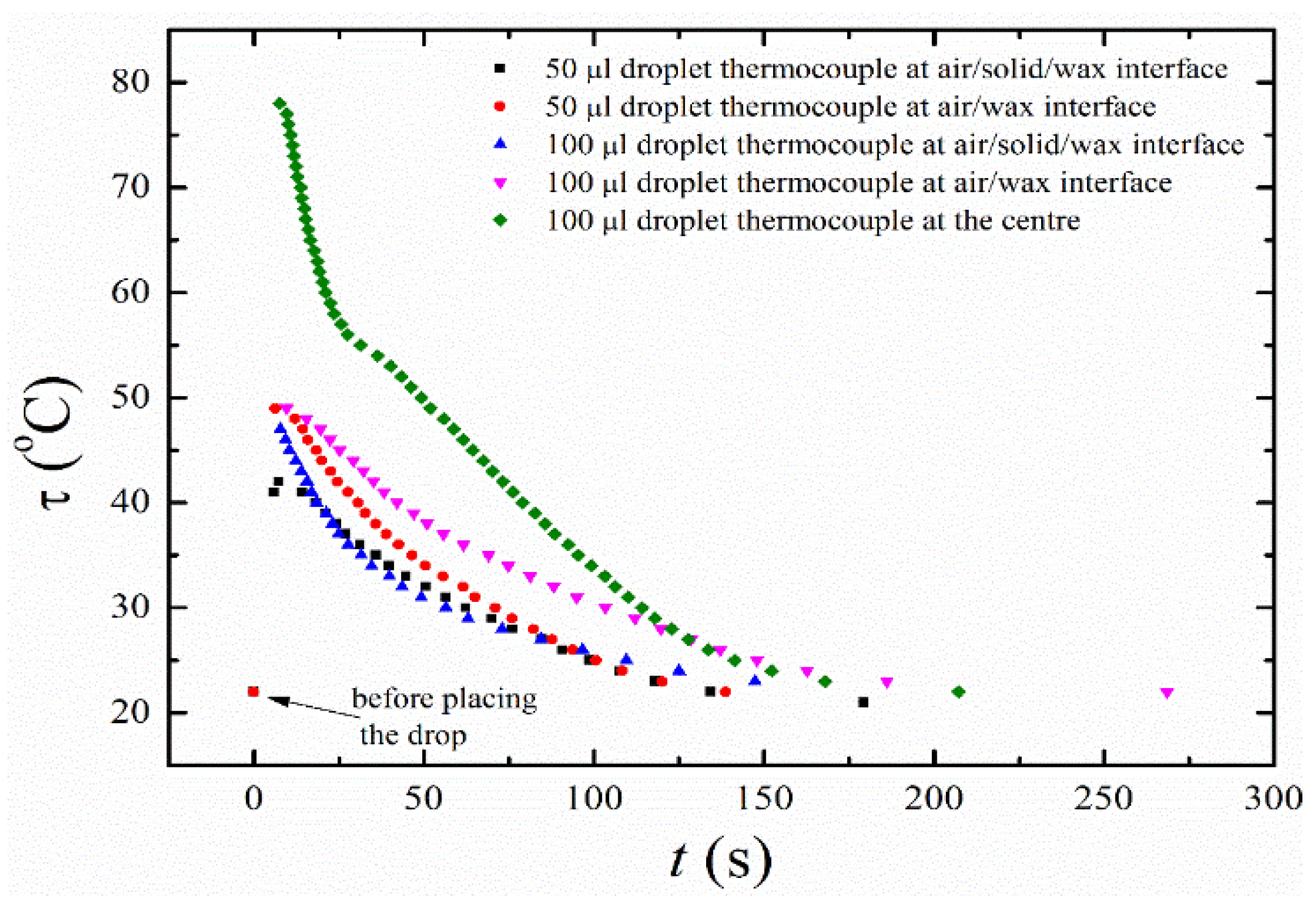
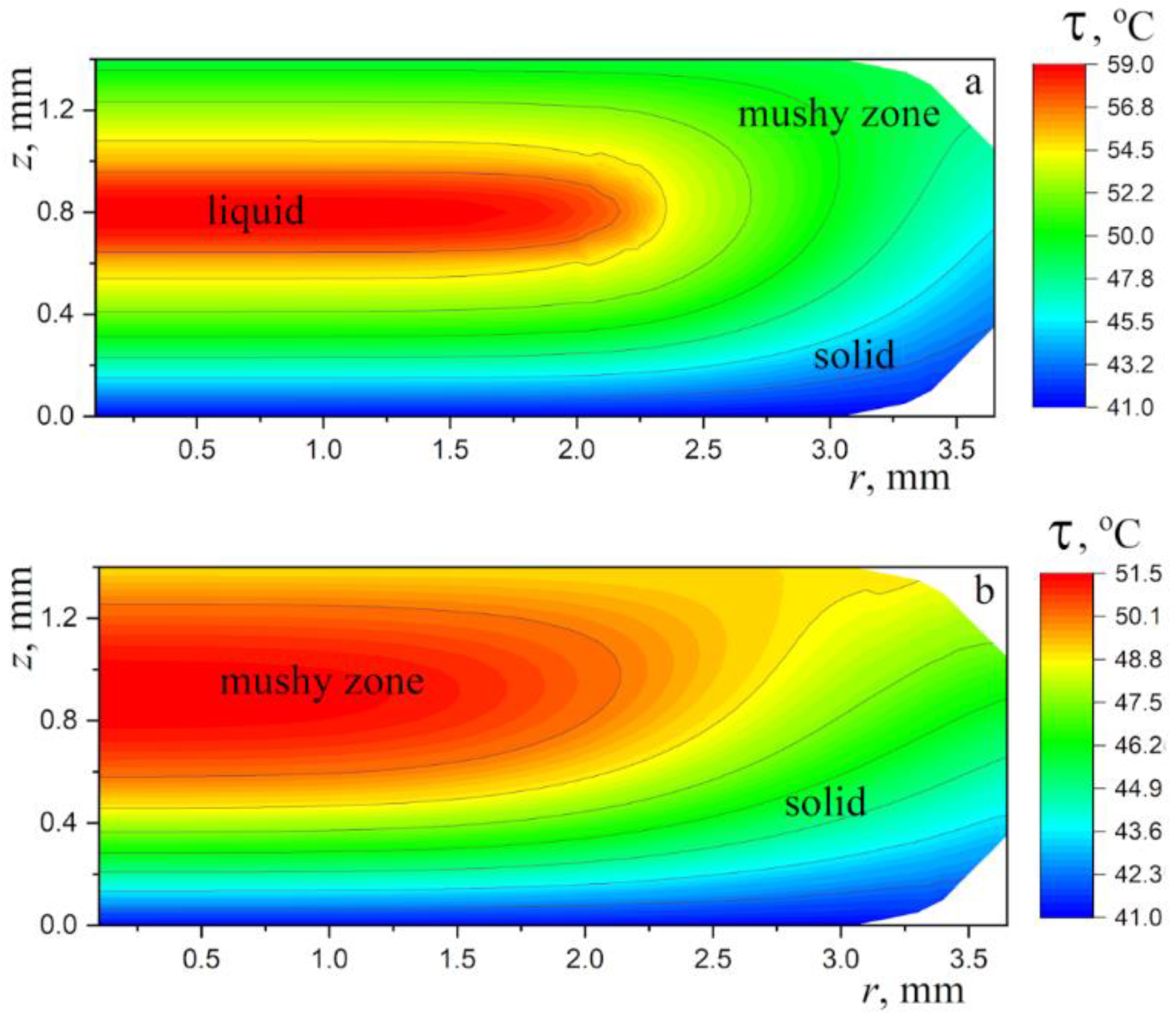
4. Discussion
4.1. Qualitative Analysis
4.2. A Model of Cooling and Solidification of a Paraffin Wax Droplet
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommerfeld, R.A.; LaChapelle, E. The Classification of Snow Metamorphism. J. Glaciol. 1970, 9 (55), 3–18. [CrossRef]
- LaChapelle, E. R. Field Guide to Snow Crystals. University of Washington Press: Seattle, USA, 1969.
- Snoeijer, J.H.; Brunet, P. Pointy ice-drops: How water freezes into a singular shape. Am. J. Phys.2012 , 80 (9), 764–771. [CrossRef]
- Marín, A.G.; Enríquez, O.R.; Brunet, P.; Colinet, P.; Snoeijer, J.H. Universality of tip singularity formation in freezing water drops. Phys. Rev. Lett. 2014, 113 (5), 054301 . [CrossRef] [PubMed]
- Schetnikov, A.; Matiunin, V.; Chernov, V. Conical shape of frozen water droplets. Am. J. Phys. 2015, 83 (1), 36–38. [Google Scholar] [CrossRef]
- Ismail, M.F.; Waghmare, P.R. Universality in freezing of an asymmetric drop. Appl. Phys. Lett. 2016, 109 (23), 234105. [CrossRef]
- Kolasinski, K.W. Solid structure formation during the liquid/solid phase transition. Curr. Opin. Solid State Mater. Sci. 2007, 11 (5-6), 76–85. [CrossRef]
- Starostin, A.; Strelnikov, V.; Dombrovsky, L.A.; Shoval, S.; Bormashenko, E. On the universality of shapes of the freezing water droplets. Colloids & Interface Sci. Comm. 2022, 47, 100590. [CrossRef]
- Starostin, A.; Strelnikov, V.; Dombrovsky, L.A.; Shoval, S.; Gendelman, O.; Bormashenko, E. Effect of asymmetric cooling of sessile droplets on orientation of the freezing tip. J. Colloid & Interface Sci. 2022, 620, 179–186. [CrossRef]
- Gandee, H.; Zhou, Y.; Lee, J.; Chomali, J.; Xu, H.; Adera, S. Unique ice dendrite morphology on state-of-the-art oil-impregnated surfaces. PNAS 2023, 120 (1) e2214143120. [CrossRef]
- Shang, Y.; Liu, X.; Bai, B.; Zhong, X. Central-pointy to central-concave icing transition of an impact droplet by increasing surface subcooling. Int. Comm. Heat & Mass Transfer 2019, 108, 104326. [CrossRef]
- Hu, M.; Wang, F.; Tao, Q.; Chen, L.; Rubinstein, S.M.; Deng, D. Frozen patterns of impacted droplets: From conical tips to toroidal shapes. Phys. Rev. Fluids 2020, 5 (8), 081601(R). [CrossRef]
- Ma, Z.; Xiong, W.; Cheng, P. 3D Lattice Boltzmann simulations for water droplet’s impact and transition from central-pointy icing pattern to central-concave icing pattern on supercooled surfaces. Part I: Smooth surfaces. Int. J. Heat & Mass Transfer 2021, 171, 121097. [Google Scholar] [CrossRef]
- Fang, W.-Z.; Zhu, F.; Tao, W.-Q.; Yang, C. How different freezing morphologies of impacting droplets form. J. Colloid & Interface Sci. 2021, 584, 403–410. [Google Scholar] [CrossRef]
- Oldenburg, D.W.; Brune, J.N. Ridge transform fault spreading pattern in freezing wax. Science 1972, 178 (4058), 301–304. [CrossRef] [PubMed]
- Himran, S.; Suwono, A.; Mansoori, A. Characterization of alkanes and paraffin waxes for application as phase change energy storage medium. Energy Sources 1994, 16 (1), 117–128. [CrossRef]
- Akgün, M.; Aydın, O.; Kaygusuz, K. Experimental study on melting/solidification characteristics of a paraffin as PCM. Energy Convers. Manag. 2007, 48 (2), 669–678. [CrossRef]
- Akgün, M.; Aydın, O.; Kaygusuz, K. Thermal energy storage performance of paraffin in a novel tube-in-shell system. Appl. Therm. Eng. 2008, 28 (5-6), 405–413. [CrossRef]
- Vakhshouri, A.R. Paraffin as phase change material. Chapter 5 in “Paraffin – an Overview” edited by F. S. Soliman, IntechOpen. 2020. [CrossRef]
- Mishra, D.K.; Bhowmik, C.; Bhowmik, S.; Pandey, K.M. Property-enhanced paraffin-based composite phase change material for thermal energy storage: a review. Environ. Sci. & Pollut. Res. 2022, 29, 43556–43587. [Google Scholar] [CrossRef]
- Lubarda, V.A.; Talke, K.A. Analysis of the equilibrium droplet shape based on an ellipsoidal droplet model, Langmuir 2011, 27 (17), 10705–10713. [CrossRef]
- Whyman, G.; Bormashenko, Ed. Oblate spheroid model for calculation of the shape and contact angles of heavy droplets. J. Colloid Interface Sci. 2009, 331 (1), 174–177. [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Angewandte Chemie 1998, 37 (5), 550–575. [CrossRef]
- Jasper, J.J. The surface tension of pure liquid compounds. J. Phys. Chem. Ref. Data 1972, 1, 841. [Google Scholar] [CrossRef]
- Rossetti, F.; Ranalli, G.; Faccenna, C. Rheological properties of paraffin as an analogue material for viscous crustal deformation. J. Struct. Geol. 1999, 21, 413–417. [Google Scholar] [CrossRef]
- Starostin, A.; Strelnikov, V.; Dombrovsky, L.A.; Shoval, S.; Bormashenko, E. Three scenarios of freezing of liquid marbles. Colloids Surf., A 2022, 636, 128125. [Google Scholar] [CrossRef]
- Ferrer, G.; Gschwander, S.; Solé, A.; Barreneche, C.; Fernández, A.I.; Schossig, P.; Cabeza, L.F. Empirical equation to estimate viscosity of paraffin. J. Energy Storage 2017, 11, 154–161. [Google Scholar] [CrossRef]
- Dombrovsky, L.A.; Nenarokomova, N.B.; Tsiganov, D.I.; Zeigarnik, Yu.A. Modeling of repeating freezing of biological tissues and analysis of possible microwave monitoring of volumetric phase changes. Int. J. Heat & Mass Transfer 2015, 89, 894–902. [Google Scholar] [CrossRef]
- Dombrovsky, L.A.; Kokhanovsky, A.A.; Randrianalisoa, J. H. On snowpack heating by solar radiation: A computational model. J. Quant. Spectrosc. Radiat. Transfer 2019, 227, 72–85. [Google Scholar] [CrossRef]
- Starostin, A.; Strelnikov, V.; Dombrovsky, L.A.; Shoval, S.; Gendelman, O.; Bormashenko, E. Effects of asymmetric cooling and surface wettability on the orientation of the freezing tip. Surface Innovations 2201081, published online: Jan. 24, 2023. [CrossRef]
- Kumar, M.; Krishna, D.J. Influence of mushy zone constant on thermohydraulics of PCM, Energy Procedia 2017, 109, 314–321. 109. [CrossRef]
- Soliman, A.S.; Sultan, A.A.; Sultan, M.A. Effect of mushy zone parameter on phase change behavior of different configurations storage unit: Numerical simulation and experimental validation. Sustainability 2022, 14, 14540. [Google Scholar] [CrossRef]
- Fedorets, A.A.; Dombrovsky, L.A. Generation of levitating droplet clusters above the locally heated water surface: A thermal analysis of modified installation. Int. J. Heat & Mass Transfer 2017, 104, 1268–1274. [Google Scholar] [CrossRef]
- Frenkel, M.; Dombrovsky, L.A.; Multanen, V.; Danchuk, V.; Legchenkova, I.; Shoval, S.; Bormashenko, Y.; Binks, B.P.; Bormashenko, E. Self-propulsion of water-supported liquid marbles filled with sulfuric acid. J. Phys. Chem. B 2018, 122 (32), 7936–7942. [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: materials and applications. Energy Convers. Manage. 2004, 45 (9-10), 1597–1615. [CrossRef]
- Aguenim, F.; Hewitt, N.; Eams, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renewable Sustainable Energy Rev. 2010, 14 (2), 615–628. [CrossRef]
- Grigor’ev, I.S.; Dedov, A.V.; Eletskii, A.V. Phase change materials and power engineering. Therm. Eng. 2021, 68 (4), 257–269. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




