Submitted:
13 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
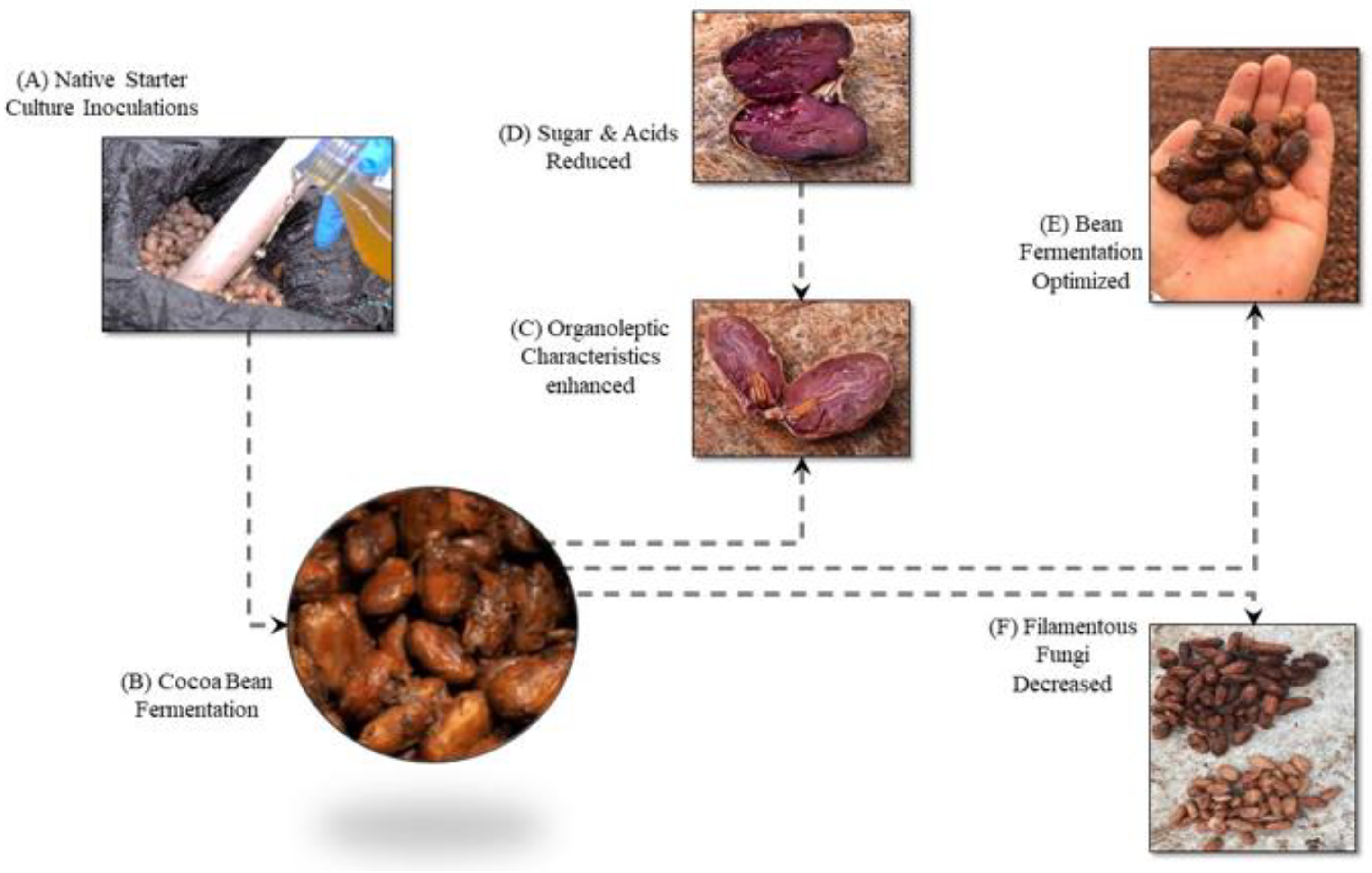
1. Introduction
2. Materials and Methods
2.1. Inoculum Preparation
2.2. Geographical Location of the Study
2.3. Inoculation and Beans Sampling during Fermentation
2.4. Temperature, pH, and Water Content
2.5. Quantification of Population Dynamics
2.6. Biochemical Characteristics
2.6.1. Sugar Concentration
2.6.2. Organic Acids Concentration
2.7. Analysis of Data
2.8. Total Polyphenol Content
2.9. Fermentation Percentage
2.10. Experimental Design
2.11. Statistic Analysis
3. Results
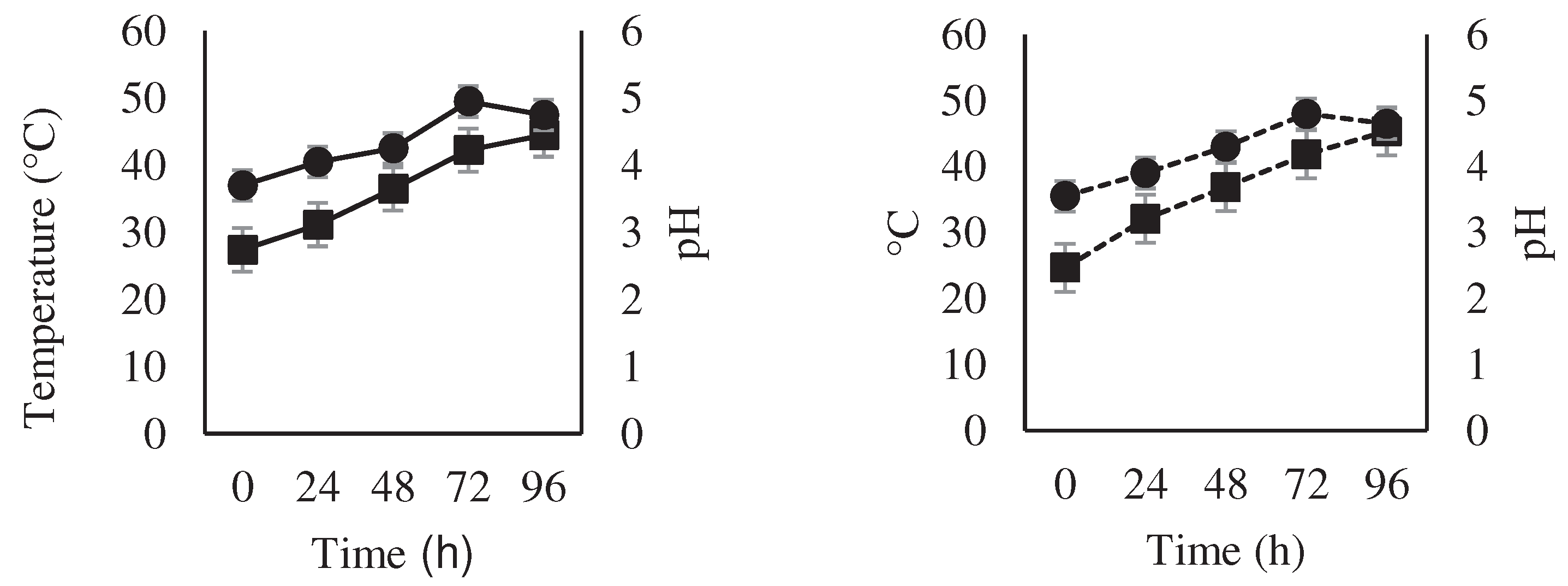
3.1. Variation of Temperature, pH and Water Content during Fermentation
3.2. Quantification of Population Dynamics during Fermentation
3.3. Variation in Concentrations of Sugars, Organic Acids, and Polyphenols
3.4. Fermentation Percentage
4. Discussion
4.1. Variation of Temperature, pH and Water Content during Fermentation
4.2. Quantification of Population Dynamics during Fermentation
4.3. Variation in Concentrations of Sugars, Organic Acids, and Polyphenols
44. Fermentation Percentage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ficha Sectorial, Cacao y Chocolate, Subgerencia de análisis de productos y servicios, Corporación Financiera Nacional (CFN). 2022. Available online: https://www.cfn.fin.ec/wp-content/uploads/downloads/biblioteca/2022/fichas-sectoriales-2-trimestre/Ficha-Sectorial-Cacao.pdf.
- Fowler, M.; Coutel, F. Cocoa beans: From tree to factory. In Beckett’s Industrial Chocolate Manufacture and Use, 5th ed.; Beckett, S., Fowler, M., Ziegler, G., Eds.; John Wiley & Sons Ltd., 2017. [Google Scholar] [CrossRef]
- Ganeswari, I.; Khairul Bariah, S.; Amizi, M.; Sim, K. Effects of different fermentation approaches on the microbiological and physicochemical changes during cocoa bean fermentation. Int. Food Res. J. 2015, 1, 70–76. [Google Scholar]
- Figueroa, C.; Mota, J.F.; Hernández, Z.; González, O.; Cocolin, L.; Suárez, M. The challenges and perspectives of the selection of starter cultures for fermented cocoa beans. Int. J. Food Microbiol. 2019, 301, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, G. Evaluación de la influencia del proceso de beneficio del cacao (Theobroma cacao) CCN-51 de altura en su calidad final, mediante el análisis físico, físico-químico y sensorial. Universidad Central del Ecuador. 2018. Available online: http://www.dspace.uce.edu.ec/bitstream/25000/16624/1/T-UCE-0008-CQU-044.pdf.
- Yánez-Mendizábal, V.; Falconi, C.E.; Palacios, H. Identification of microbial population in two fine cocoa varieties during the commercial fermentation process. Food Microbiol. Saf. accepted.
- Dutan, S.; Amaya, J.; Fredy, N.; Palacios, B. Plan de desarrollo y ordenamiento territorial 2015-2020. Gobierno Autónomo Descentralizado del cantón Vinces. 2015. Available online: http://app.sni.gob.ec/sni-link/sni/PORTAL_SNI/data_sigad_plus/sigadplusdiagnostico/1260001030001_Diagnostico%20Territorial%20del%20Cant%C3%B3n%20Vinces%202015_2020_04-04-2016_15-59-59.pdf.
- Gálvez, S.; Loiseau, G.; Paredes, J. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 2007, 114, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Ho, V.T.; Zhao, J.; Graham, F. Yeasts are essential for cocoa bean fermentation. Int. J. Food Microbiol. 2014, 174, 72–87. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M. HPLC Organic Acid Analysis in Different Citrus Juices under Reversed Phase Conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 44–48. [Google Scholar]
- Carrillo, L.; Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- INEN, INEN. NORMA TÉCNICA ECUATORIANA NTE INEN 176 Quinta revisión. 2018. Available online: https://www.normalizacion.gob.ec/buzon/normas/nte_inen_176-5.pdf.
- Coexca. Instructivo control calidad/Coexca 2017. Available online: https://www.swisscontact.org/_Resources/Persistent/d/3/b/f/d3bfbb5a8d042f05cbf5533494e288f2c 52800b8 /Guia_de_buenas_practicas_de_poscosecha.pdf.
- Mayo, B.; Flórez, A. Lactic Acid Bacteria: Lactobacillus plantarum. In McNamara, Encyclopedia of Dairy Sciences, 3rd ed.; Paul, L.H., McSweeney, John, P., Eds.; Academic Press, 2022; pp. 206–217. [Google Scholar] [CrossRef]
- Muñoz, A.; Gómez, S. Análisis comparativo de la diversidad microbiana y la producción de compuestos bioquímicos de cacao (Theobroma cacao L.) Variedades nacional y trinitario ccn-51 durante la fermentación. Graduate Thesis, Universidad de las Américas, 2020. Available online: http://dspace.udla.edu.ec/handle/33000/12186.
- Sharmistha, S.; Tanmay, S.; Runu, C.; Maksim, R.; Mohammad, A.S.; Muthu, T.; Kannan, R.R.R. Dark chocolate: An overview of its biological activity, processing, and fortification approaches. Curr. Res. Food Sci. 2022, 5, 1916–1943. [Google Scholar] [CrossRef]
- Colonges, K.; Seguine, E.; Saltos, A.; Davrieux, F.; Minier, J.; Jimenez, J.C.; Lahon, M.C.; Calderon, D.; Subia, C.; Sotomayor, I.; et al. Diversity and determinants of bitterness, astringency, and fat content in cultivated Nacional and native Amazonian cocoa accessions from Ecuador. Plant Genome 2022, 15, e20218. [Google Scholar] [CrossRef]
- Janek, K.; Niewienda, A.; Wöstemeyer, J.; Voigt, J. The cleavage specificity of the aspartic protease of cocoa beans involved in the generation of the cocoa-specific aroma precursors. Food Chem. 2016, 211, 320–328. [Google Scholar] [CrossRef]
- Cempaka, L.; Aliwarga L y Purwo, S. Dynamics of Cocoa Bean Pulp Degradation during Cocoa Bean Fermentation: Effects of Yeast Starter Culture Addition. J. Math. Fundam. Sci. 2014, 46, 14–25. [Google Scholar] [CrossRef]
- Nielsen, D. The microbiology of Ghanaian cocoa fermentations; Denmark: Department of Food Science, The Royal Veterinary and Agricultural University, Denmark, 2006. [Google Scholar]
- Voigt, J. Chocolate and Cocoa Aroma. In Chocolate in Health and Nutrition; Humana Press, 2013; pp. 89–93. [Google Scholar] [CrossRef]
- Santander, M.; Rodríguez, J.; Vaillant, F.; Escobar, S. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. Crit. Rev. Food Sci. Nutr. 2019, 60, 1593–1613. [Google Scholar] [CrossRef] [PubMed]
- Sârbu, I.; Csutak, O. The microbiology of cocoa fermentation. Caffeinated Cocoa Based Beverages 2019, 8, 423–446. [Google Scholar] [CrossRef]
- Sandhya, M.V.S.; Yallappa, B.S.; Varadaraj, M.C.; Puranaik, J.; Jaganmohan Rao, L.; Janardhan, P.; Murthy Pushpa, S. Inoculum of the starter consortia and interactive metabolic process in enhancing quality of cocoa bean (Theobroma cacao) fermentation. LWT Food Sci. Technol. 2016, 65, 731–738. [Google Scholar] [CrossRef]
- Saunshi, Y.P.; Sagar Sandhya, M.V.; Rastogi, N.K.; Murthy, P.S. Starter consortia for on-farm cocoa fermentation and their quality attributes. Prep. Biochem. Biotechnol. 2020, 50, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Schwan, R.F. Cocoa Fermentations Conducted with a Defined Microbial Cocktail Inoculum. Appl. Environ. Microbiol. 1998, 44, 1477–1483. [Google Scholar] [CrossRef]
- Visintin, S.; Ramos, L.; Batista, N.; Dolci, P.; Schwan, F.; Cocolin, L. Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii starter cultures on cocoa beans fermentation. Int. J. Food Microbiol. 2017, 257, 31–40. [Google Scholar] [CrossRef]
- Illeghems, K.; Pelicaen, R.; De Vuyst, L.; Weckx, S. Assessment of the contribution of cocoa-derived strains of Acetobacter ghanensis and Acetobacter senegalensis to the cocoa bean fermentation process through a genomic approach. Food Microbiol. 2016, 58, 68–78. [Google Scholar] [CrossRef]
- Ramos, N.; Castro, A.; Juárez, J.; de la Cruz, O.; Rodríguez, N.; Blancas, J.; Navarro, A. Evaluación de ocratoxina a en Theobroma cacao l. “cacao blanco” durante el proceso de cosecha, fermentado, secado y almacenado. Rev. Soc. Química Perú. 2016, 82, 1810–634X. [Google Scholar]
- Ruggirello, M.; Nucera, D.; Cannoni, M.; Peraino, A.; Rosso, F.; Fontana, M.; Cocolin, L.; Dolci, P. Antifungal activity of yeasts and lactic acid bacteria isolated from cocoa bean fermentations. Food Res. Int. 2019, 115, 519–525. [Google Scholar] [CrossRef]
- Romanensa, E.; Freimüller, S.; Volland, A.; Stevens, M.; Krähenmann, U.; Isele, D.; Fischer, B.; Meile, L.; Schwenninger, S. Screening of lactic acid bacteria and yeast strains to select adapted antifungal co-cultures for cocoa bean fermentation. Int. J. Food Microbiol. 2019, 290, 262–272. [Google Scholar] [CrossRef]
- Schwan, R.; Wheals, A. The Microbiology of Cocoa Fermentation and its Role in Chocolate Quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.S.; Santos, M.F.S.; Gomes, L.P.; Leite, A.M.O.; Paschoalin, V.M.F.; Del Aguila, E.M. Analysis of the cocobiota and metabolites of Moniliophthora perniciosa-resistant (Theobroma cacao L.) beans during spontaneous fermentation in Southern Brazil. J. Sci Food Agric. 2018, 98, 4963–4970. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, S.L.; Loiseau, G.; Paredes, J.L.; Barel, M.; Guiraud, J.P. Study on the microflora and biochemistry of cocoa fermentation in the Dominican Republic. Int. J. Food Microbiol. 2007, 114, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bickel Haase, T.; Schweiggert-Weisz, U.; Ortner, E.; Zorn, H.; Naumann, S. Aroma Properties of Cocoa Fruit Pulp from Different Origins. Molecules 2021, 26, 7618. [Google Scholar] [CrossRef]
- Santander, M.; Rodríguez, J.; Vaillant, F.; y Escobar, S. An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. Crit. Rev. Food Sci. Nutr. 2019, 60, 1593–1613. [Google Scholar] [CrossRef]
- Papalexandratou, Z.; Dennis, S.; Nielsen, D.S. It's Gettin’ Hot in Here: Breeding Robust Yeast Starter Cultures for Cocoa Fermentation. Trends Microbiol. 2016, 24, 168–170. [Google Scholar] [CrossRef]
- Cempaka, L.; Rahmawati, E.A.; David, W.A. Sensory Profiles of Chocolate Drinks Made from Commercial Fermented Cocoa Powder and Unfermented Cocoa Beans. Curr. Res. Nutr. Food Sci. 2021, 9, 988–999. [Google Scholar] [CrossRef]
- Ooi, T.; Ting, A.; Siow, L. Influence of selected native yeast starter cultures on the antioxidant activities, fermentation index and total soluble solids of Malaysia cocoa beans: A simulation study. LWT Food Sci. Technol. 2020, 122, 1–8. [Google Scholar] [CrossRef]
- Ardhana, M.; Fleet, G. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 2003, 86, 87–99. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Quao, J.; Takrama, J.; Budu, A.S.; Saalia, F.K. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013, 50, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Weckx, S. The cocoa bean fermentation process: From ecosystem analysis to starter culture development. J. Appl. Microbiol. 2016, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Viesser, J.; Pereira, G.; de Carvalho, D.; Vandenberghe, L.; Azevedo, V.; Brenig, B.; de Carvalho, D.; Rogez, H.; Góes-Neto, A.; Soccol, C. Exploring the contribution of fructophilic lactic acid bacteria to cocoa beans fermentation: Isolation, selection and evaluation. Food Res. Int. 2020, 136. [Google Scholar] [CrossRef]
- Kresnowati, M.T.A.P.; Suryani, L.; Affifah, M. Improvement of cocoa beans fermentation by LAB starter addition. J. Med. Bioeng. 2013, 2, 274–278. [Google Scholar] [CrossRef]
- Puerari, C.; Teixeira Magalhães, K.; Freitas Schwan, R. New cocoa pulp-based kefir beverages: Microbiological, chemical composition and sensory analysis. Food Res. Int. 2012, 48, 634–640. [Google Scholar] [CrossRef]
- López, M.; Criollo, J.; Hernández, M.; Lozano, M. Physicochemical and microbiological dynamics of the fermentation of the CCN51 cocoa material in three maturity stages. Rev. Bras. Frutic. 2019, 41, 1–13. [Google Scholar] [CrossRef]
- El Salous, A.; Angulo, A.; Solís, L. Acceleration of cocoa fermentation through the action of bacteria (Acetobacter aceti) and yeast (Saccharomyces cerevisiae). Espirales Rev. Multidiscip. Investig. Científica 2019, 3, 1–20. [Google Scholar] [CrossRef]
- Fonseca, D.; López, D.; Ortíz, S.; Nuñez, C.; Lozano, D. Effect of Addition of a Specific Mixture of Yeast, Lactic and Acetic Bacteria in the Fermentation Process to Improve the Quality and Flavor of Cocoa Beans in Colombia. Pelita Perkeb. Coffee Cocoa Res. J. 2020, 36, 154–172. [Google Scholar] [CrossRef]
 ; BAL,
; BAL, ; BAA,
; BAA,  mesophilic
microorganisms,
mesophilic
microorganisms,  and filamentous
fungi
and filamentous
fungi  during the
fermentation of national variety cocoa beans (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a starter
culture.
during the
fermentation of national variety cocoa beans (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a starter
culture.
 ; BAL,
; BAL, ; BAA,
; BAA,  mesophilic
microorganisms,
mesophilic
microorganisms,  and filamentous
fungi
and filamentous
fungi  during the
fermentation of national variety cocoa beans (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a starter
culture.
during the
fermentation of national variety cocoa beans (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a starter
culture.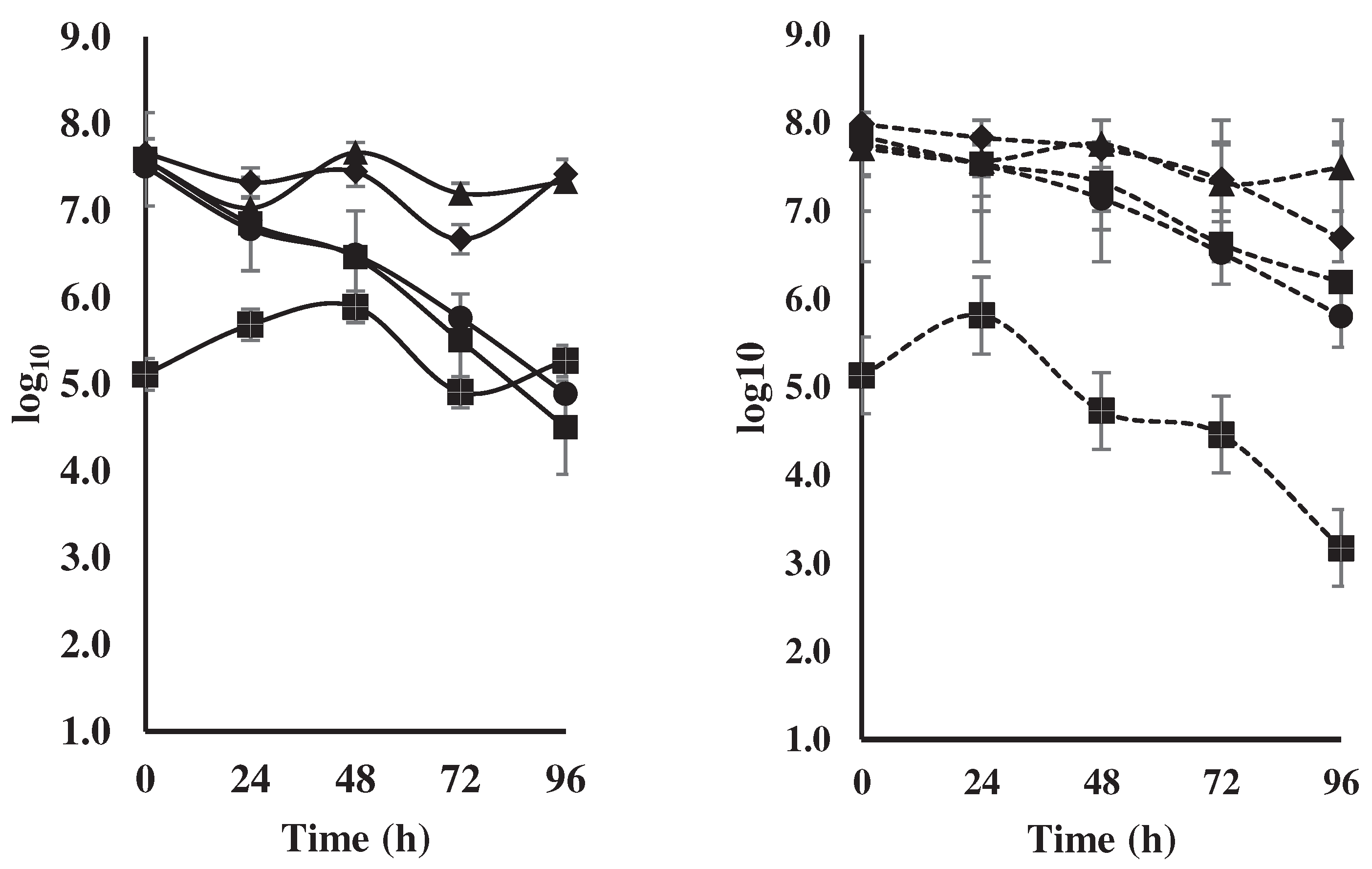
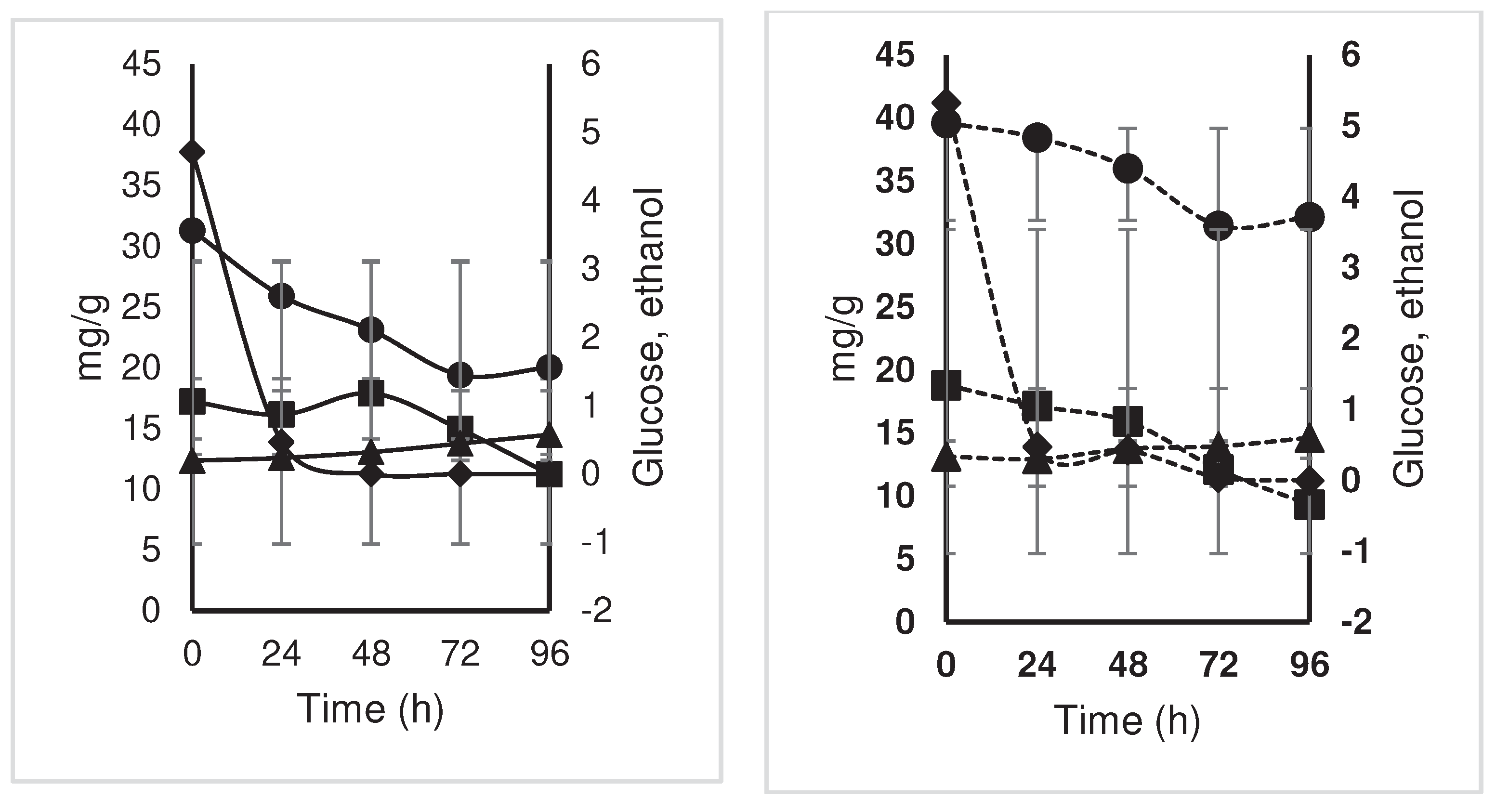
 ; acetic,
; acetic,  ; citric,
; citric, ; oxalic,
; oxalic, ; and malic,
; and malic, ) (mg/g) during the
fermentation of national variety cocoa almonds (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a
starter culture.
) (mg/g) during the
fermentation of national variety cocoa almonds (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a
starter culture.
 ; acetic,
; acetic,  ; citric,
; citric, ; oxalic,
; oxalic, ; and malic,
; and malic, ) (mg/g) during the
fermentation of national variety cocoa almonds (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a
starter culture.
) (mg/g) during the
fermentation of national variety cocoa almonds (a) by spontaneous
fermentation (control) and (b) by effect of a microbial cocktail as a
starter culture.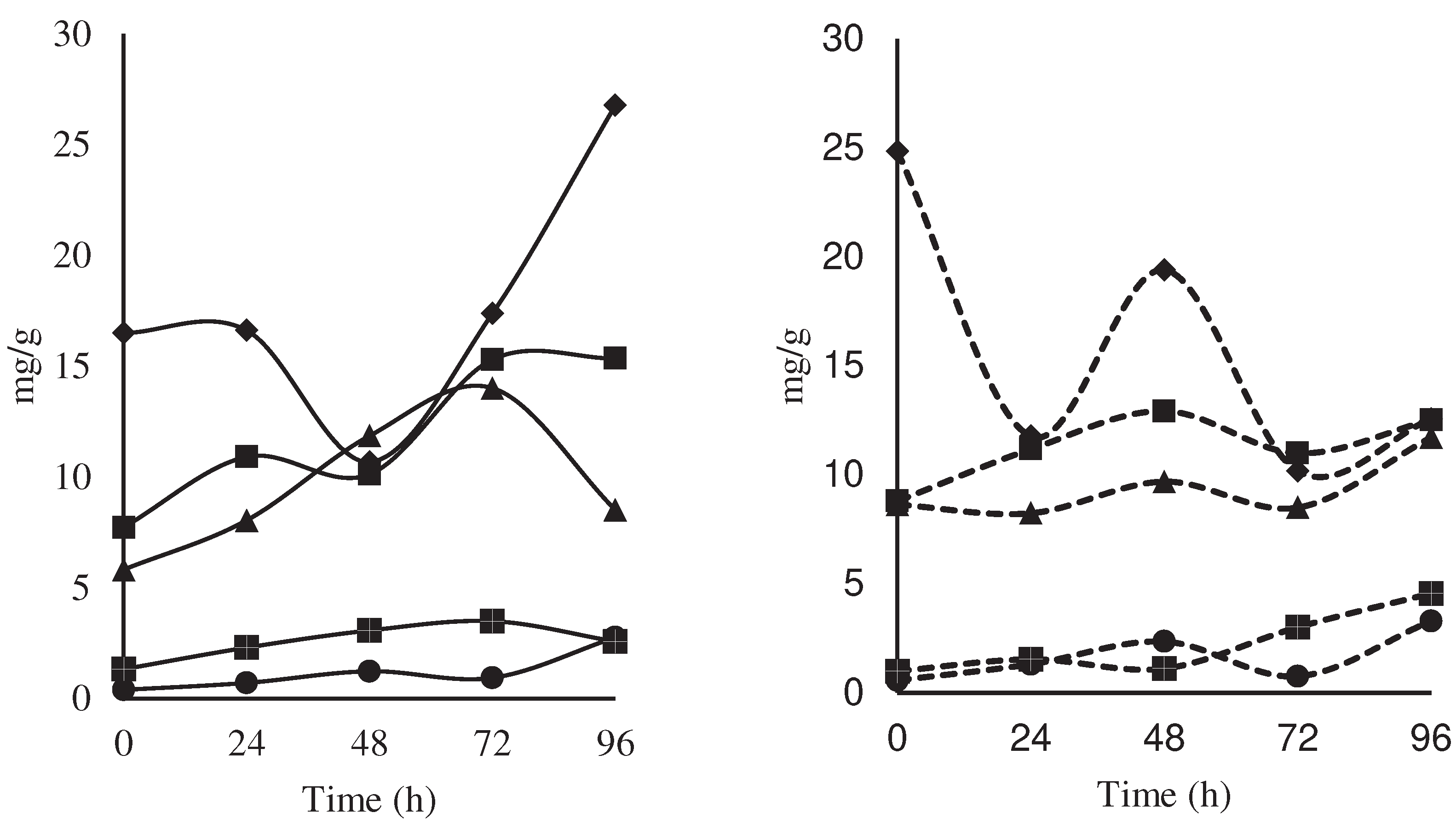
 ), compared to the
control by spontaneous fermentation (
), compared to the
control by spontaneous fermentation ( ).
).
 ), compared to the
control by spontaneous fermentation (
), compared to the
control by spontaneous fermentation ( ).
).
| Requirements | Unit | Natural Fermented |
Started Culture |
CBAS |
|---|---|---|---|---|
| Maximum humidity | % | 60,64 | 58 | 7 |
| 100 grain weight | g | 106,5 | 109,9 | 100-120 |
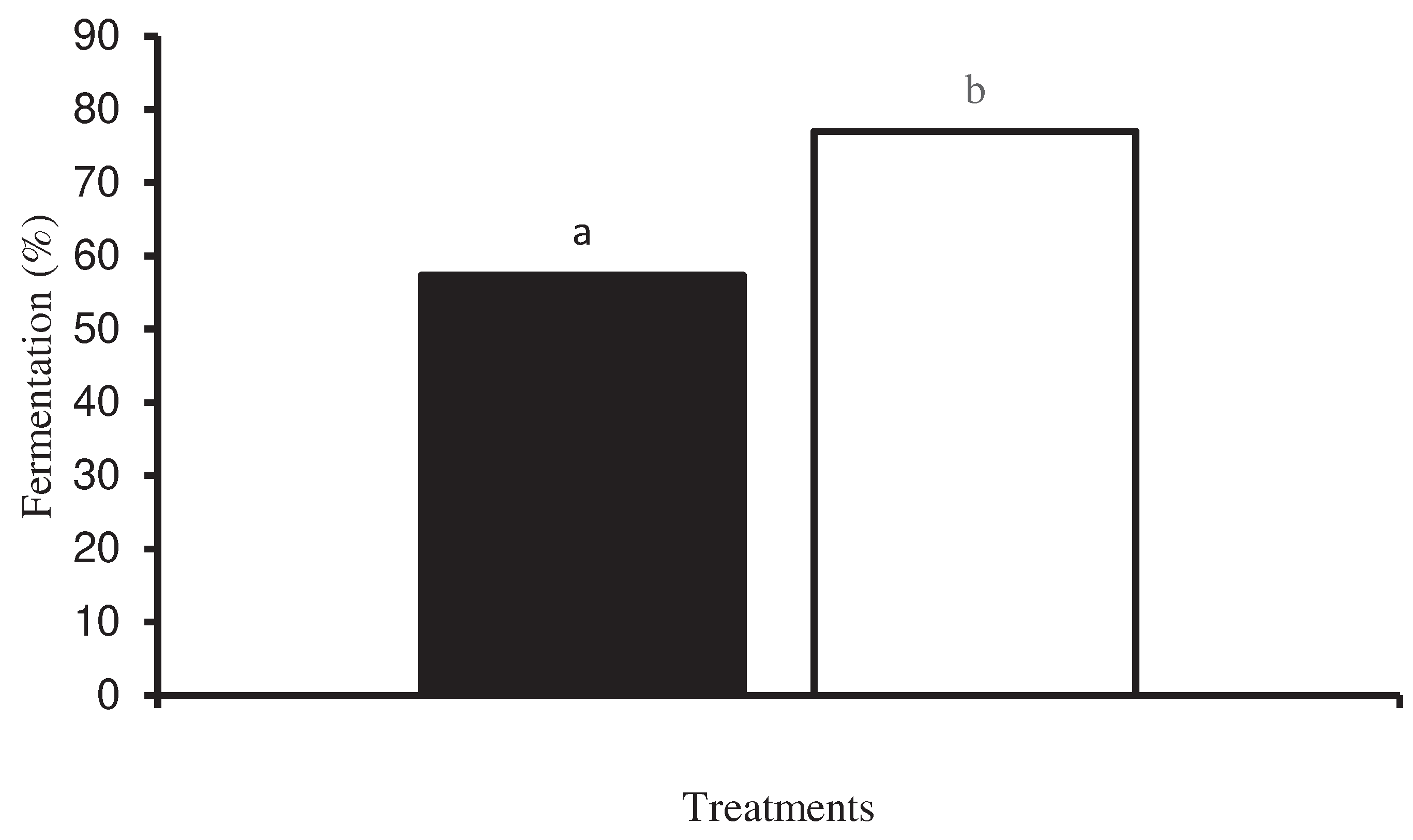
| Fermented grains, minimal | % | 57 | 77 | 53 |
| Violet grains, maximum | % | 43 | 20 | 25 |
| Slaty grains, maximum | % | 0 | 3 | 18 |
| Moldy grains, maximum | % | 0 | 0 | 4 |
| Totals (analysis in 100 grams) | % | 100 | 100 | 100 |
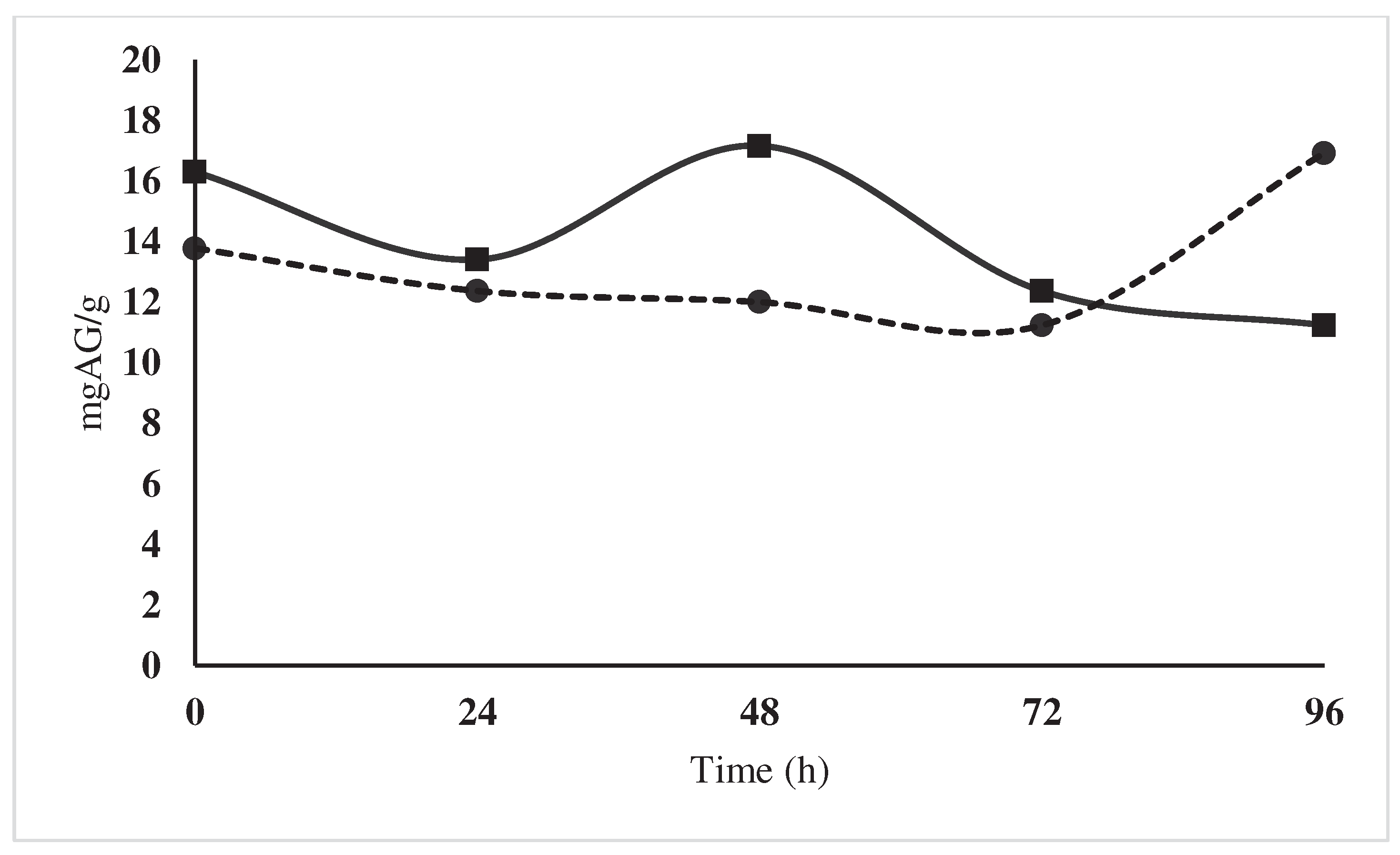
| Polyphenol content | mgAG/g | 11,23 | 16,92 | 25-40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





