Submitted:
05 July 2023
Posted:
07 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
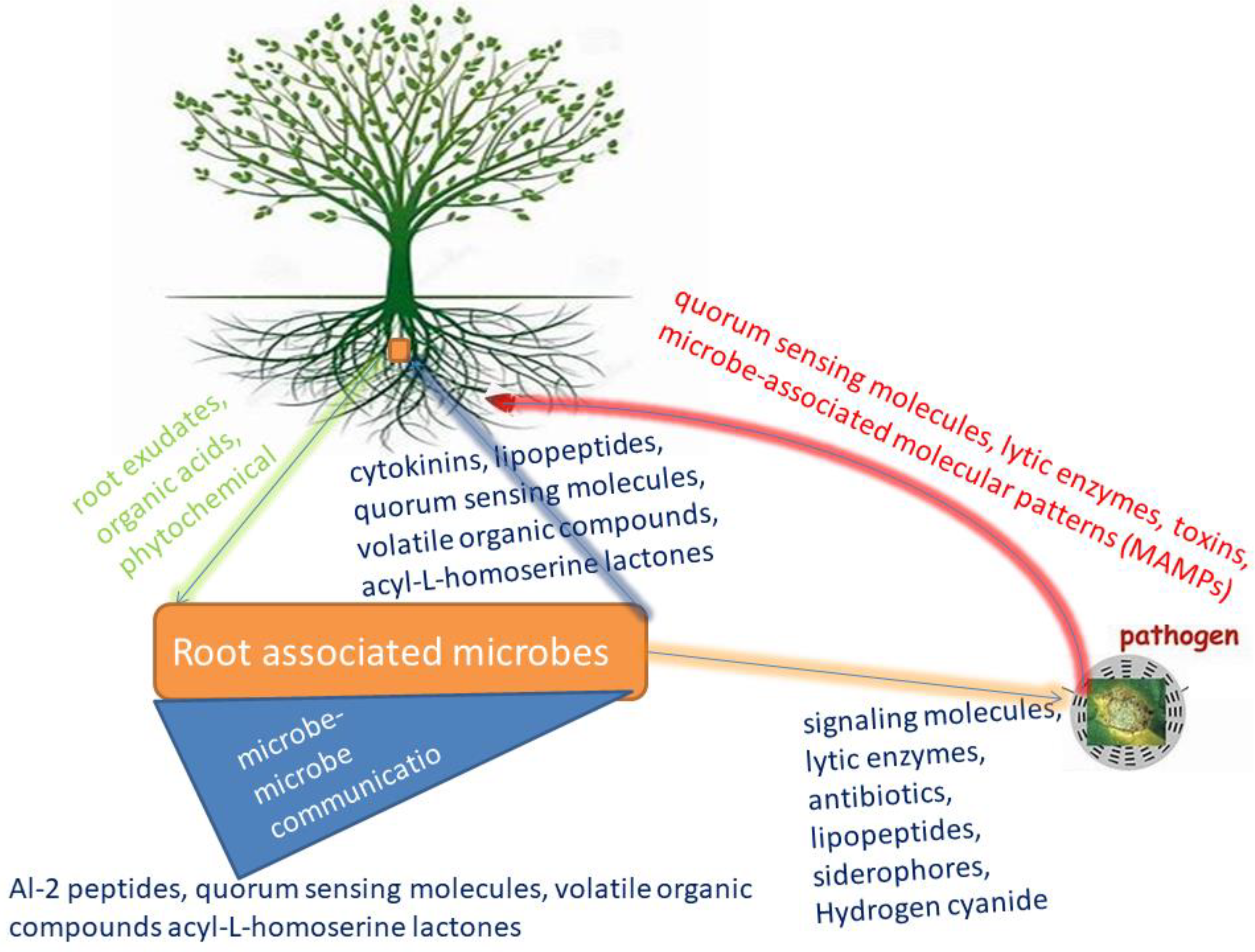
2. Communication between root microbiome and plant root
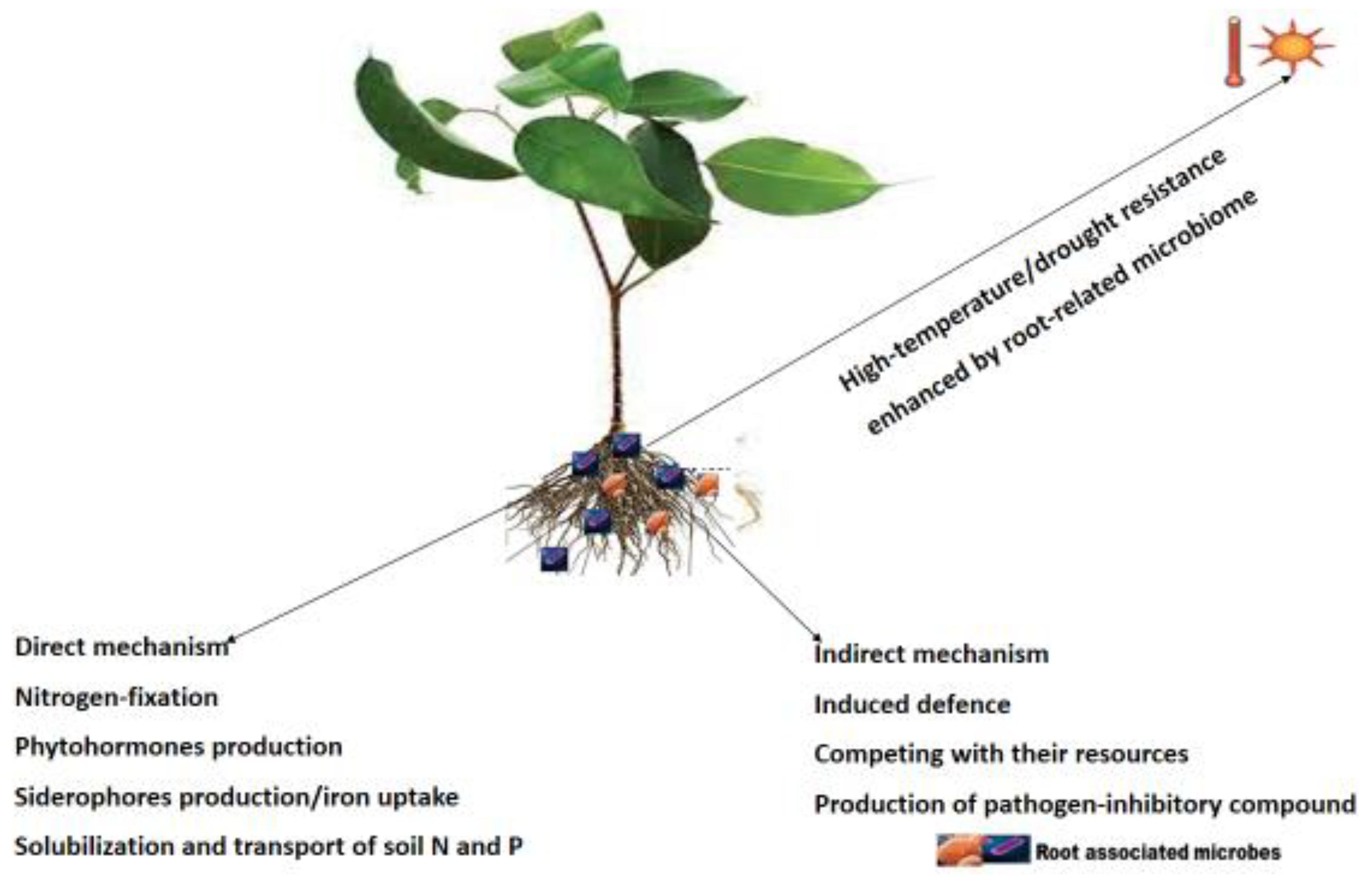
3. Applications of plant root-associated microbes in improving plant growth and yield
3.1. Root microbiome role in abiotic stress tolerance
| Stress type | Root associated microbes | Plant host | Inoculated with | Activities | The effect on plant | Reference |
|---|---|---|---|---|---|---|
| Drought | Enterobacter, Bacillus, Moraxella and Pseudomonas | Acacia arabica | Triticum aestivum L. | indole-3-carboxylic acid, Indole-3-lactic acid and indole-3-acetic acid production | Improvement in shoot length, tillers and number of spikelets and increases in spike length and seed weight of Triticum aestivum L. | [67] |
| Salt | Halomonas and one Bacillus |
Salicornia rubra, Sarcocornia utahensis, and Allenrolfea occidentalis |
Alfalfa | - | The total biomass of alfalfa increased, and root length were improved by 2.6 and 1.5 fold in Halomonas and Bacillus inoculated plants, respectively, compared with the uninoculated alfalfa | [68] |
| salt or drought | Bacillus amyloliquefaciens SB-9 | grapevine | Grapevine plantlet | melatonin secretion, 5-hydroxytryptophan, serotonin, and N-acetylserotonin | Lessen the antagonistic effects of salt- and drought-induced stress by decreasing the secretion of malondialdehyde, O2- and H2O2 (reactive oxygen species) in roots. | [69] |
| Heavy metal stress | Phialocephala fortinii, Rhizodermea veluwensis, and Rhizoscyphus sp | Clethra barbinervis | Clethra barbinervis seedling | Siderophores | Improved K absorption in shoots and decreased the concentrations of Cd, Zn, Pb, Cu, and Ni in roots. | [70] |
| Heavy metal | Penicillium ruqueforti Thom | Solanum surattense Burm | Wheat seedling | indole-3-acetic acid | It led to low concentrations of heavy metals in the root and shoot. In increase nutrient uptake and higher plant growth, | [71] |
| Heat | Thermomyces sp | Cullen plicata | cucumber | Increase in antioxidant enzyme activities, soluble proteins, flavonoids, saponins, and total sugars. | It maintains the optimal quantum efficiency of photosystem II, water use efficiency, and photosynthesis rate and increases the root length, induced accumulation of saponins, total sugars, soluble proteins, flavonoids, and antioxidant enzyme activities | [72] |
| High temperature, salinity, and glyphosate pollution | Ochrobactrum cytisi strain IPA7.2 | Solanum tuberosum L. | Solanum tuberosum L | .indole-3-acetic acid and type II 5-enolpyruvylshikimate-3-phosphate synthase | Improved the mitotic index of root meristem cells, the number of roots, the number of leaves and the length of shoots | [73] |
| Flood | Klebsiella variicola AY13 | soybean | soybean | Indole acetic acid production | Plants' growth improved with enriched chlorophyll content and quantum efficiency of chlorophyll fluorescence | [74] |
3.2. Root microbiome role in nutrient acquisition
3.3. Root microbiome role in disease suppression/biocontrol
4. Conclusion and future prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Enagbonma, B.J.; Aremu, B.R.; Babalola, O.O. Profiling the functional diversity of termite mound soil bacteria as revealed by shotgun sequencing. Genes 2019, 10, 637. [Google Scholar] [CrossRef] [PubMed]
- Enagbonma, B.J.; Babalola, O.O. Unveiling plant-beneficial function as seen in bacteria genes from termite mound soil. J. Soil Sci. Plant Nutr. 2020, 1–10. [Google Scholar] [CrossRef]
- Babalola, O.O.; Alawiye, T.T.; Lopez, C.R.; Ayangbenro, A.S. Shotgun metagenomic sequencing data of sunflower rhizosphere microbial community in South Africa. Data in Brief 2020, 31. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Metagenomics methods for the study of plant-associated microbial communities: A review. J Microbiol Methods 2020, 170, 105860. [Google Scholar] [CrossRef] [PubMed]
- Regalado, J.; Lundberg, D.S.; Deusch, O.; Kersten, S.; Karasov, T.; Poersch, K.; Shirsekar, G.; Weigel, D. Combining whole-genome shotgun sequencing and rRNA gene amplicon analyses to improve detection of microbe–microbe interaction networks in plant leaves. ISME J. 2020, 14, 2116–2130. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Wilson, G.W.; Cobb, A.B.; Tang, S.; Guo, L.; Wang, K.; Deng, B. Plant–microbial interactions facilitate grassland species coexistence at the community level. Oikos 2020, 129, 533–543. [Google Scholar] [CrossRef]
- Qi, G.; Ma, G.; Chen, S.; Lin, C.; Zhao, X. Microbial network and soil properties are changed in bacterial wilt-susceptible soil. Appl. Environ. Microbiol. 2019, 85, e00162–00119. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Muller, D.; Babalola, O.O. A metagenomic lens into endosphere microbial communities, promises, and discoveries. Lett. Appl. Microbiol. 2023, 76, ovac030. [Google Scholar]
- Khan, M.W.; Bohannan, B.J.; Nüsslein, K.; Tiedje, J.M.; Tringe, S.G.; Parlade, E.; Barberán, A.; Rodrigues, J.L. Deforestation impacts network co-occurrence patterns of microbial communities in Amazon soils. FEMS Microbiol. Ecol. 2019, 95, fiy230. [Google Scholar] [CrossRef]
- Wakung’oli, M.; Amoo, A.E.; Enagbonma, B.J.; Babalola, O.O. Termite societies promote the taxonomic and functional diversity of archaeal communities in mound soils. Biology 2020, 9, 136. [Google Scholar] [CrossRef]
- Simard, S.W. Mycorrhizal networks facilitate tree communication, learning, and memory. In: Memory and learning in plants. Springer; 2018, 191-213.
- Huang, X.-F.; Chaparro, J.M.; Reardon, K.F.; Zhang, R.; Shen, Q.; Vivanco, J.M. Rhizosphere interactions: Root exudates, microbes, and microbial communities. Botany 2014, 92, 267–275. [Google Scholar] [CrossRef]
- King, A.; Farrer, E.; Suding, K.; Schmidt, S. Co-occurrence patterns of plants and soil bacteria in the high-alpine subnival zone track environmental harshness. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Guo, L.; Bai, Y.; Liu, W.; Yan, J.; Bucher, M. Microbiomics and plant health: An interdisciplinary and international workshop on the plant microbiome. Mol. Plant 2019, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Nat Acad Sci 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Enebe, M.C.; Babalola, O.O. Effects of inorganic and organic treatments on the microbial community of maize rhizosphere by a shotgun metagenomics approach. Ann. Microbiol. 2020, 70, 1–10. [Google Scholar] [CrossRef]
- Gottel NR, Castro HF, Kerley M, Yang Z, Pelletier DA, Podar M, Karpinets T, Uberbacher E, Tuskan GA, Vilgalys R et al. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. Appl. Environ. Microbiol. 2011, 77, 5934–5944. [Google Scholar] [CrossRef]
- López-Lozano, N.E.; Echeverría Molinar, A.; Ortiz Durán, E.A.; Hernández Rosales, M.; Souza, V. Bacterial diversity and interaction networks of Agave lechuguilla rhizosphere differ significantly from bulk soil in the oligotrophic basin of cuatro cienegas. Front. Plant Sci. 2020, 11, 1028–1028. [Google Scholar] [CrossRef]
- Babalola, O.O.; Fadiji, A.E.; Enagbonma, B.J.; Alori, E.T.; Ayilara, M.S.; Ayangbenro, A.S. The nexus between plant and plant microbiome: Revelation of the networking strategies. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol Res 2020, 238, 126486. [Google Scholar] [CrossRef]
- Antar, M.; Gopal, P.; Msimbira, L.A.; Naamala, J.; Nazari, M.; Overbeek, W.; Backer, R.; Smith, D.L. Inter-organismal signaling in the rhizosphere. In: Rhizosphere Biology: Interactions Between Microbes and Plants. Edited by Gupta VVSR, Sharma AK: Springer Singapore; 2021, 255-293.
- Castro, R.O.; Bucio, J.L. Small molecules involved in transkingdom communication between plants and rhizobacteria. In: Molecular Microbial Ecology of the Rhizosphere. Edited by Bruijn FJd, vol. 1, John Wiley & Sons, Inc.; 2013, 295-307.
- Drogue, B.; Combes-Meynet, E.; Moënne-Loccoz, Y.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Control of the cooperation between plant growth-promoting rhizobacteria and crops by rhizosphere signals. In: Molecular Microbial Ecology of the Rhizosphere. Edited by Bruijn FJd, vol. 1: John Wiley & Sons, Inc.; 2013, 279-293.
- Atkinson, S.; Williams, P. Quorum sensing and social networking in the microbial world. J. R. Soc. Interface 2009, 6, 959–978. [Google Scholar] [CrossRef]
- Phour, M.; Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Interkingdom signaling in plant-rhizomicrobiome interactions for sustainable agriculture. Microbiol Res 2020, 241, 126589. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Reverdy, A.; She, Q.; Sun, B.; Chai, Y. The role of rhizodeposits in shaping rhizomicrobiome. Env. Micro Rep. 2020, 12, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc Nat Acad Sci 2020, 117, 3874–3883. [Google Scholar] [CrossRef]
- Dekkers, L.C.; Van Der Bij, A.J.; Mulders, I.H.M.; Phoelich, C.C.; Wentwoord, R.A.R.; Glandorf, D.C.M.; Wijffelman, C.A.; Lugtenberg, B.J.J. Role of the O-antigen of lipopolysaccharide, and possible roles of growth rate and of NADH:ubiquinone oxidoreductase (nuo) in competitive tomato toot-tip colonization by Pseudomonas fluorescens WCS365. Mol Plant-Microbe Interact 1998, 11, 763–771. [Google Scholar] [CrossRef]
- Burdman, S.; Dulguerova, G.; Okon, Y.; Jurkevitch, E. Purification of the major outer membrane protein of Azospirillum brasilense, its affinity to plant roots, and its involvement in cell aggregation. Mol. Plant-Microbe Interact. ® 2001, 14, 555–561. [Google Scholar] [CrossRef]
- Ryu, C.-M. Promoting plant protection by root-associated microbes. Plant Pathol J 2013, 29, 123–124. [Google Scholar]
- Caddell, D.F.; Deng, S.; Coleman-Derr, D. Role of the Plant Root Microbiome in Abiotic Stress Tolerance. In: Seed Endophytes: Biology and Biotechnology. Edited by Verma SK, White JJF. Cham: Springer International Publishing; 2019, 273-311.
- Stringlis IA, Yu K, Feussner K, de Jonge R, Van Bentum S, Van Verk MC, Berendsen RL, Bakker PA, Feussner I, Pieterse CM. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Nat Acad Sci 2018, 115, E5213–E5222. [Google Scholar]
- Hu J, Wei Z, Friman V-P, Gu S-h, Wang X-f, Eisenhauer N, Yang T-j, Ma J, Shen Q-r, Xu Y-c et al. Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. Am. Soc. Microbiol. 2016, 7, e01790–e01716. [Google Scholar]
- Joo, H.-S.; Deyrup, S.T.; Shim, S.H.J.P.R. Endophyte-produced antimicrobials: A review of potential lead compounds with a focus on quorum-sensing disruptors. 2020, 1-26.
- Ortiz-Castro, R. Martinez-Trujillo, M. Lopez-Bucio J. N-acyl-L-homoserine lactones: A class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Env. 2008, 31, 1497–1509. [Google Scholar] [CrossRef]
- Heil, M.; Bostock, R.M. Induced systemic resistance (ISR) against pathogens in the context of induced plant defences. Ann Bot 2002, 89, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Junker, R.R.; Tholl, D. Volatile organic compound mediated interactions at the plant-microbe interface. J Chem Ecol 2013, 39, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.-M.; Farag, M.A.; Hu, C.-H.; Reddy, M.S.; Wei, H.-X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Biol Control 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, J.; Li, C.; Ma, Y. Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. MicrobiologyOpen 2019, 8, e00813. [Google Scholar] [CrossRef]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiological Research 2020, 126486. [Google Scholar] [CrossRef]
- Kawasaki, A.; Donn, S.; Ryan, P.R.; Mathesius, U.; Devilla, R.; Jones, A.; Watt, M. Microbiome and exudates of the root and rhizosphere of Brachypodium distachyon, a model for wheat. PLoS ONE 2016, 11, e0164533. [Google Scholar] [CrossRef]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. ProcNat Acad Sci 2020, 117, 3874–3883. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Jangu, O.; Sindhu, S. Differential response of inoculation with indole acetic acid producing Pseudomonas sp. in green gram (Vigna radiata L.) and black gram (Vigna mungo L.). Microbiol. J. 2011, 1, 159–173. [Google Scholar] [CrossRef]
- Park J-M, Radhakrishnan R, Kang S-M, Lee I-J. IAA producing Enterobacter sp. I-3 as a potent bio-herbicide candidate for weed control: A special reference with lettuce growth inhibition. Indian J Microbiol 2015, 55, 207–212. [Google Scholar] [CrossRef]
- Amara, U.; Khalid, R.; Hayat, R. Soil bacteria and phytohormones for sustainable crop production. In: Bacterial Metabolites in Sustainable Agroecosystem. Edited by D. M, vol. 12. Cham: Springer International Publishing; 2015, 87-103.
- Gao, X.; Zhang, Y.; He, Z.; Fu, X. Gibberellins. In: Hormone Metabolism and Signaling in Plants. Edited by Li J, Li C, Smith SM: Academic Press; 2017, 107-160.
- Enagbonma, B.J.; Babalola, O.O. Potentials of termite mound soil bacteria in ecosystem engineering for sustainable agriculture. Ann Microbiol 2019, 69, 211–219. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Enagbonma, B.J.; Babalola, O.O. Environmental sustainability: A review of termite mound soil material and its bacteria. Sustainability 2019, 11, 3847. [Google Scholar] [CrossRef]
- Adedeji, A.A.; Häggblom, M.M.; Babalola, O.O. Sustainable agriculture in Africa: Plant growth-promoting rhizobacteria (PGPR) to the rescue. Sci. Afr. 2020, 9, e00492. [Google Scholar] [CrossRef]
- Araújo, W.L.; Lacava, P.T.; Andreote, F.D.; Azevedo, J.L. Interaction between endophytes and plant host: Biotechnological aspects. Plant-Microbe Interact. 2008, 1, 1–21. [Google Scholar]
- Olanrewaju OS, Babalola OO. Bacterial consortium for improved maize (Zea mays L.) production. Microorganisms 2019, 7, 519. [Google Scholar] [CrossRef]
- Rho, H.; Hsieh, M.; Kandel, S.L.; Cantillo, J.; Doty, S.L.; Kim, S.-H. Do Endophytes Promote Growth of Host Plants Under Stress? A Meta-Analysis on Plant Stress Mitigation by Endophytes. Microb. Ecol. 2018, 75, 407–418. [Google Scholar] [CrossRef]
- Xu, L.; Coleman-Derr, D. Causes and consequences of a conserved bacterial root microbiome response to drought stress. Curr. Opin. Microbiol. 2019, 49, 1–6. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Cardoso, E.J.B.N. Isolation, selection and characterization of root-associated growth promoting bacteria in Brazil Pine (Araucaria angustifolia). Microbiol. Res. 2012, 167, 69–78. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, V.; Kumar, A.; Sayyed, R.; Hesham, A.E.-L.; Dhaliwal, H.S.; Saxena, A.K. Drought-tolerant phosphorus-solubilizing microbes: Biodiversity and biotechnological applications for alleviation of drought stress in plants. In: Plant growth promoting rhizobacteria for sustainable stress management. Springer; 2019, 255-308.
- Kour, D.; Rana, K.L.; Kaur, T.; Sheikh, I.; Yadav, A.N.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal. Agric. Biotechnol. 2020, 23, 101501. [Google Scholar] [CrossRef]
- Yuwono, T.; Handayani, D.; Soedarsono, J. The role of osmotolerant rhizobacteria in rice growth under different drought conditions. Aust. J Agric Res 2005, 56, 715–721. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Collados, C.; Barea, J.M.; Azcón, R. Arbuscular mycorrhizal symbiosis can alleviate drought-induced nodule senescence in soybean plants. 2001, 151, 493–502. 2001, 151, 493–502. [Google Scholar]
- Qin, Y.; Druzhinina, I.S.; Pan, X.; Yuan, Z. Microbially Mediated Plant Salt Tolerance and Microbiome-based Solutions for Saline Agriculture. Biotechnol. Adv. 2016, 34, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- del Carmen Orozco-Mosqueda M, Glick BR, Santoyo G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environmental Experimental Botany 2020, 104124. [Google Scholar] [CrossRef]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L). following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Barka, E.A.; Nowak, J.; Clément, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef]
- Raheem A, Shaposhnikov A, Belimov AA, Dodd IC, Ali B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arc Agron Soil Sci 2018, 64, 574–587. [Google Scholar] [CrossRef]
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-tolerant halophyte rhizosphere bacteria stimulate growth of alfalfa in salty soil. Frontier in Microbiology 2019, 10, 1849. [Google Scholar] [CrossRef]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-producing endophytic bacteria from grapevine roots promote the abiotic stress-induced production of endogenous melatonin in their hosts. Front Plant Sci 2016, 7, 1387. [Google Scholar] [CrossRef]
- Yamaji, K.; Watanabe, Y.; Masuya, H.; Shigeto, A.; Yui, H.; Haruma, T. Root fungal endophytes enhance heavy-metal stress tolerance of clethra barbinervis growing naturally at mining sites via growth enhancement, promotion of nutrient uptake and decrease of heavy-metal concentration. PLoS ONE 2016, 11, e0169089. [Google Scholar] [CrossRef]
- Ikram M, Ali N, Jan G, Jan FG, Rahman IU, Iqbal A, Hamayun M. IAA producing fungal endophyte Penicillium roqueforti Thom., enhances stress tolerance and nutrients uptake in wheat plants grown on heavy metal contaminated soils. PLoS ONE 2018, 13, e0208150. [Google Scholar]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Burygin, G.L.; Kargapolova, K.Y.; Kryuchkova, Y.V.; Avdeeva, E.S.; Gogoleva, N.E.; Ponomaryova, T.S.; Tkachenko, O.V.J.W.J.o.M. Biotechnology: Ochrobactrum cytisi IPA7. 2 promotes growth of potato microplants and is resistant to abiotic stress. 2019, 35, 55. [Google Scholar]
- Kim A-Y, Shahzad R, Kang S-M, Seo C-W, Park Y-G, Park H-J, Lee I-J. biotechnology: IAA-producing Klebsiella variicola AY13 reprograms soybean growth during flooding stress. J Crop Sci 2017, 20, 235–242. [Google Scholar]
- Jog, R.; Pandya, M.; Nareshkumar, G.; Rajkumar, S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology 2014, 160, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Otieno N, Lally RD, Kiwanuka S, Lloyd A, Ryan D, Germaine KJ, Dowling DN. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar]
- Li G, Kronzucker HJ, Shi W. The response of the root apex in plant adaptation to iron heterogeneity in soil. Front. Plant Sci. 2016, 7, 344. [Google Scholar]
- Gaiero JR, McCall CA, Thompson KA, Day NJ, Best AS, Dunfield KE. Inside the root microbiome: Bacterial root endophytes and plant growth promotion. Am. J. Bot. 2013, 100, 1738–1750. [Google Scholar] [CrossRef]
- Behie SW, Padilla-Guerrero IE, Bidochka MJ. Nutrient transfer to plants by phylogenetically diverse fungi suggests convergent evolutionary strategies in rhizospheric symbionts. Commun. Integr. Biol. 2013, 6, e22321. [Google Scholar] [CrossRef]
- Fadiji AE, Babalola OO. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Rascovan N, Carbonetto B, Perrig D, Díaz M, Canciani W, Abalo M, Alloati J, González-Anta G, Vazquez MP. Integrated analysis of root microbiomes of soybean and wheat from agricultural fields. Sci. Rep. 2016, 6, 28084. [Google Scholar] [CrossRef] [PubMed]
- Suman A, Yadav AN, Verma P. Endophytic microbes in crops: Diversity and beneficial impact for sustainable agriculture. In: Microbial inoculants in sustainable agricultural productivity, New Delhi. Springer pp 117-143; 2016, 117-143.
- Yadav, A.; Rana, K.; Kumar, V.; Dhaliwal, H. Phosphorus solubilizing endophytic microbes: Potential application for sustainable agriculture. EU Voice 2016, 2, 21–22. [Google Scholar]
- Yadav, A.N.; Verma, P.; Kour, D.; Rana, K.L.; Kumar, V.; Singh, B.; Chauahan, V.S.; Sugitha, T.; Saxena, A.K.; Dhaliwal, H.S. Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int. J. Environ. Sci. Nat. Resour. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, M.; Verma, S.; Choudhary, P.; Chakdar, H. Plant Microbiome: Trends and Prospects for Sustainable Agriculture. In Plant Microbe Symbiosis; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer: Cham, 2002; pp. 129–151. [Google Scholar] [CrossRef]
- Sengupta, S.; Ganguli, S.; Singh, P.K. Metagenome analysis of the root endophytic microbial community of Indian rice (O. sativa L.). Genom. Data 2017, 12, 41–43. [Google Scholar] [CrossRef]
- Ji SH, Gururani MA, Chun S-C. Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol. Res. 2014, 169, 83–98. [Google Scholar] [CrossRef]
- Gyaneshwar P, Hirsch AM, Moulin L, Chen W-M, Elliott GN, Bontemps C, Estrada-de los Santos P, Gross E, dos Reis Jr FB, Sprent JI. Legume-nodulating betaproteobacteria: Diversity, host range, and future prospects. Mol. Plant-Microbe Interact. 2011, 24, 1276–1288. [Google Scholar] [CrossRef]
- Oldroyd GE, Murray JD, Poole PS, Downie JA. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- da Silva Fonseca E, Peixoto RS, Rosado AS, de Carvalho Balieiro F, Tiedje JM, da Costa Rachid CTC. The microbiome of Eucalyptus roots under different management conditions and its potential for biological nitrogen fixation. Microb. Ecol. 2018, 75, 183–191. [Google Scholar] [CrossRef]
- Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK, Suman A. Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann. Microbiol. 2015, 65, 1885–1899. [Google Scholar] [CrossRef]
- Singh D, Geat N, Rajawat MVS, Mahajan MM, Prasanna R, Singh S, Kaushik R, Singh RN, Kumar K, Saxena AK. Deciphering the mechanisms of endophyte-mediated biofortification of Fe and Zn in wheat. J. Plant Growth Regul. 2018, 37, 174–182. [Google Scholar] [CrossRef]
- Oliveira Ad, Urquiaga S, Döbereiner J, Baldani J. The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 2002, 242, 205–215. [Google Scholar] [CrossRef]
- Subramanian P, Kim K, Krishnamoorthy R, Sundaram S, Sa T. Endophytic bacteria improve nodule function and plant nitrogen in soybean on co-inoculation with Bradyrhizobium japonicum MN110. Plant Growth Regul. 2015, 76, 327–332. [Google Scholar] [CrossRef]
- Zhao L, Xu Y, Lai X. Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz. J. Microbiol. 2018, 49, 269–278. [Google Scholar] [CrossRef]
- Perin L, Martínez-Aguilar L, Paredes-Valdez G, Baldani J, Estrada-De Los Santos P, Reis V, Caballero-Mellado J. Burkholderia silvatlantica sp. nov., a diazotrophic bacterium associated with sugar cane and maize. Int. J. Syst. Evol. Microbiol. 2006, 56, 1931–1937.
- Ramesh A, Sharma SK, Sharma MP, Yadav N, Joshi OP. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Puri A, Padda KP, Chanway CP. Nitrogen-fixation by endophytic bacteria in agricultural crops: Recent advances. Nitrogen in agriculture IntechOpen, London, GBR 2018, 73–94. [Google Scholar]
- Reis V, Estrada-De Los Santos P, Tenorio-Salgado S, Vogel J, Stoffels M, Guyon S, Mavingui P, Baldani V, Schmid M, Baldani J. Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 2155–2162.
- Mbai F, Magiri E, Matiru V, Nganga J, Nyambati V. Isolation and characterisation of bacterial root endophytes with potential to enhance plant growth from kenyan basmati rice. Am. Int. J. Contemp. Res. 2013, 3, 25–40. [Google Scholar]
- Govindarajan M, Balandreau J, Kwon S-W, Weon H-Y, Lakshminarasimhan C. Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of rice. Microb. Ecol. 2008, 55, 21–37. [Google Scholar] [CrossRef]
- Kumar P, Dubey R, Maheshwari D, Bajpai V. ACC deaminase producing Rhizobium leguminosarum rpn5 isolated from root nodules of Phaseolus vulgaris L. Bangladesh J. Bot. 2016, 45, 477–484. [Google Scholar]
- Joseph B, Ranjan Patra R, Lawrence R. Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int. J. Plant Prod. 2012, 1, 141–152. [Google Scholar]
- Singh D, Rajawat MVS, Kaushik R, Prasanna R, Saxena AK. Beneficial role of endophytes in biofortification of Zn in wheat genotypes varying in nutrient use efficiency grown in soils sufficient and deficient in Zn. Plant Soil 2017, 416, 107–116. [Google Scholar] [CrossRef]
- Puri A, Padda KP, Chanway CP. Can a diazotrophic endophyte originally isolated from lodgepole pine colonize an agricultural crop (corn) and promote its growth? Soil Biol. Biochem. 2015, 89, 210–216. [Google Scholar] [CrossRef]
- Sandhya V, Shrivastava M, Ali SZ, Prasad VSSK. Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ. Agric. Sci. 2017, 43, 22–34. [Google Scholar] [CrossRef]
- Tanvir KLRDK, Yadav KRDAN, Anil NYHSD, Saxena K. Endophytic microbes: Biodiversity, plant growth-promoting mechanisms and potential applications for agricultural sustainability. 2020, 113, 1075–1107.
- Fritz, M.; Jakobsen, I.; Lyngkjær, M.F.; Thordal-Christensen, H.; Pons-Kühnemann, J. Arbuscular mycorrhiza reduces susceptibility of tomato to Alternaria solani. Mycorrhiza 2006, 16, 413–419. [Google Scholar] [CrossRef]
- Ortega RA, Mahnert A, Berg C, Müller H, Berg G. The plant is crucial: Specific composition and function of the phyllosphere microbiome of indoor ornamentals. FEMS Microbiology Ecology 2016, 92, fiw173. [Google Scholar] [CrossRef]
- Bordiec S, Paquis S, Lacroix H, Dhondt S, Ait Barka E, Kauffmann S, Jeandet P, Mazeyrat-Gourbeyre F, Clément C, Baillieul F. Comparative analysis of defence responses induced by the endophytic plant growth-promoting rhizobacterium Burkholderia phytofirmans strain PsJN and the non-host bacterium Pseudomonas syringae pv. pisi in grapevine cell suspensions. J. Exp. Bot. 2011, 62, 595–603. [Google Scholar] [CrossRef]
- Higginbotham SJ, Arnold AE, Ibañez A, Spadafora C, Coley PD, Kursar TA. Bioactivity of fungal endophytes as a function of endophyte taxonomy and the taxonomy and distribution of their host plants. PLoS ONE 2013, 8, e73192. [Google Scholar]
- Ding L, Maier A, Fiebig H-H, Lin W-H, Hertweck C. A family of multicyclic indolosesquiterpenes from a bacterial endophyte. Org. Biomol. Chem. 2011, 9, 4029–4031. [Google Scholar] [CrossRef] [PubMed]
- Ezra D, Castillo UF, Strobel GA, Hess WM, Porter H, Jensen JB, Condron MA, Teplow DB, Sears J, Maranta M. Coronamycins, peptide antibiotics produced by a verticillate Streptomyces sp.(MSU-2110) endophytic on Monstera sp. Microbiology 2004, 150, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Mendes R, Kruijt M, De Bruijn I, Dekkers E, van der Voort M, Schneider JH, Piceno YM, DeSantis TZ, Andersen GL, Bakker PA. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Klein E, Ofek M, Katan J, Minz D, Gamliel A. Soil suppressiveness to Fusarium disease: Shifts in root microbiome associated with reduction of pathogen root colonization. Phytopathology 2013, 103, 23–33. [Google Scholar] [CrossRef]
- Asghari, S.; Harighi, B.; Mozafari, A.A.; Esmaeel, Q.; Barka, E.A. Screening of endophytic bacteria isolated from domesticated and wild growing grapevines as potential biological control agents against crown gall disease. BioControl 2019, 64, 723–735. [Google Scholar] [CrossRef]
- Dalal J, Kulkarni N, Bodhankar M. Antagonistic and plant growth promoting potentials of indigenous endophytic fungi of soybean (Glycine max (L) Merril). Indian J. Adv. Plant Res. 2014, 1, 9–16. [Google Scholar]
- Kumar, V.; Jain, L.; Jain, S.K.; Chaturvedi, S.; Kaushal, P. Bacterial endophytes of rice (Oryza sativa L.) and their potential for plant growth promotion and antagonistic activities. South African Journal of Botany 2020. [Google Scholar] [CrossRef]
- Shehata H, Lyons E, Jordan K, Raizada M. Bacterial endophytes from wild and ancient maize are able to suppress the fungal pathogen Sclerotinia homoeocarpa. J. Appl. Microbiol. 2016, 120, 756–769. [Google Scholar] [CrossRef]
- Ardebili ZO, Ardebili NO, Mahdi Hamdi SM. Physiological effects of'Pseudomonas fluorescens' CHA0 on tomato ('Lycopersicon esculentum'Mill.) plants and its possible impact on Fusarium oxysporum f. sp.'Lycopersici'. Aust. J. Crop Sci. 2011, 5, 1631. [Google Scholar]
- Falahian F, Ardebili ZO, Fahimi F, Khavarinejad R. Effect of mycorrhizal fungi on some defense enzymes against Gaeumannomyces gaminis in wheat. Pak. J. Biol. Sci. 2007, 10, 2418–2422. [Google Scholar] [CrossRef]
- Lee, J.; Seo, M.; Kim, H. Isolation and characterization of an antagonistic endophytic bacterium Bacillus velezensis CB3 the control of citrus green mold pathogen Penicillium digitatum. Korean J. Mycol. 2012, 40, 118–123. [Google Scholar] [CrossRef]
- Omomowo IO, Fadiji AE, Omomowo OI. Assessment of bio-efficacy of Glomus versiforme and Trichoderma harzianum in inhibiting powdery mildew disease and enhancing the growth of cowpea. Ann. Agric. Sci. 2018, 63, 9–17. [Google Scholar] [CrossRef]
- Yang F, Zhang R, Wu X, Xu T, Ahmad S, Zhang X, Zhao J, Liu YJMP. An endophytic strain of the genus Bacillus isolated from the seeds of maize (Zea mays L.) has antagonistic activity against maize pathogenic strains. Microb. Pathog. 2020, 142, 104074. [Google Scholar] [CrossRef] [PubMed]
- Irabor A, Mmbaga M. Evaluation of Selected Bacterial Endophytes for Biocontrol Potential against Phytophthora Blight of Bell Pepper (Capsicum annuum L.) J. Plant Pathol. Microbiol. 2017, 8, 31–34.
- Kushwaha, P.; Kashyap, P.L.; Srivastava, A.K.; Tiwari, R.K. Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum). Braz. J. Microbiol. 2020, 51, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Wang, Q.; Li, Y. Suppression of Magnaporthe oryzae and interaction between Bacillus subtilis and rice plants in the control of rice blast. SpringerPlus 2016, 5, 1238. [Google Scholar] [CrossRef]
- 128. Nourozian J, Etebarian HR, Khodakaramian G. Biological control of Fusarium graminearum on wheat by antagonistic bacteria. Songklanakarin Journal of Science and Technology 2006, 28 Suppl. 1, 29–38.
- Wang, S.; Hu, T.; Jiao, Y.; Wei, J.; Cao, K. Isolation and characterization of Bacillus subtilis EB-28, an endophytic bacterium strain displaying biocontrol activity against Botrytis cinerea Pers. Front. Agric. China 2009, 3, 247–252. [Google Scholar] [CrossRef]
- Fiorilli V, Vannini C, Ortolani F, Garcia-Seco D, Chiapello M, Novero M, Domingo G, Terzi V, Morcia C, Bagnaresi P. Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci. Rep. 2018, 8, 1–18. [Google Scholar]
- Mustafa, G.; Khong, N.G.; Tisserant, B.; Randoux, B.; Fontaine, J.; Magnin-Robert, M.; Reignault, P.; Sahraoui, A.L.-H. Defence mechanisms associated with mycorrhiza-induced resistance in wheat against powdery mildew. Funct. Plant Biol. 2017, 44, 443–454. [Google Scholar] [CrossRef]
- Behn, O. Influence of Pseudomonas fluorescens and arbuscular mycorrhiza on the growth, yield, quality and resistance of wheat infected with Gaeumannomyces graminis. J. Plant Dis. Prot. 2008, 115, 4–8. [Google Scholar] [CrossRef]
| Root Microbiomes | Host plant | Phosphorus (P) | Potassium (K) | Nitrogen fixers (N2F) | Siderophore (Sid) | Zinc (Zn) | References |
|---|---|---|---|---|---|---|---|
| B. amyloliquefacien | Rice | + | + | + | + | + | [91] |
| A. sulfonivorans | Wheat | - | - | - | + | + | [92] |
| A. amazonense | Sugarcane | - | - | + | - | - | [93] |
| B. megaterium | Soybean | + | - | + | + | - | [94] |
| P. agglomerans | Rice | + | - | + | - | - | [91] |
| P. putida | Soybean | - | - | + | + | - | [95] |
| B. silvatlantica | Sugarcane | - | - | + | - | - | [96] |
| B. aryabhattai | Soybean | - | - | - | - | + | [97] |
| K. pneumoniae | Rice | - | - | - | + | - | [98] |
| B. tropica | Sugarcane | - | - | + | - | - | [99] |
| P. putida | Rice | + | - | - | - | - | [100] |
| P. dispersa | Wheat | - | - | - | + | + | [91] |
| B. vietnamiensis | Rice | - | - | + | - | - | [101] |
| R. leguminosarum | Beans | + | - | - | + | + | [102] |
| B. licheniformis | Chickpea | + | - | - | - | - | [103] |
| B. subtilis | Soybean | - | - | + | + | - | [104] |
| P. polymyxa | Maize | - | - | + | - | - | [105] |
| P. thivervalensis | Maize | - | - | - | + | - | [106] |
| E. asburiae | Maize | - | - | - | + | - | [106] |
| R. endophyticum | Beans | + | - | - | - | - | [107] |
| R. irregularis | Tomato | + | - | - | - | - | [108] |
| Root Microbiomes | Host plant | Pathogens active against | Activities and metabolites secreted/Induced | References |
|---|---|---|---|---|
| Pseudomonas sp., Pantoea sp. | Grapevine | A. tumefaciens, A. vitis | - | [116] |
| A. calcoaceticus | Soybean | P. sojae 01 | Siderophore and indole acetic acid | [95] |
| Bacillus sp. | Soybean |
C. truncatum, R. solani, F oxysporum, S. rolfsii, A. alternata, and M. phaseolina |
Siderophore and Hydrogen cyanide. |
[117] |
| B. subtilis | Rice |
R. solani, F. verticelloides, and S. rolfsii |
Lipopeptides | [118] |
| B. gladioli 3A12 | Maize | S. homoeocarpa | - | [119] |
| P. fluorescens 63-28 | Pea |
P. ultimum and F. oxysporum f. sp. pisi |
Induced peroxidase, polyphenoloxisae, Superoxide dismutase and phenylalanine amonialyase. | [120] |
| P. aeruginosa FTR | Maize |
F. oxysporium, P. aphanidermatum, Alternaria sp., R solani, M. phaseolina, Alternaria sp. and S. rolfii, |
- | [106] |
| Glomus etunicatum | Wheat | G. graminis | Isozyme | [121] |
| B. velezensis CB3 | Citrus | P. digitatum | - | [122] |
| G. versiforme and T harzianum | Cowpea | E. flexuosa | - | [123] |
| B. velezensis | Maize |
T. funiculosus, P. oxalicum, and F. verticillioides |
Lipopeptide | [124] |
| R. leguminosarum RPN5 | Beans |
M. phaseolina, F. oxysporum, S. sclerotiorum and F. solani. |
- | [102] |
| Serratia (B17B), Enterobacter (E), and Bacillus (IMC8, Y, Ps, Psl, and Prt) | Papaya and Bean | P. capsici | - | [125] |
| Acremonium sp., Leptosphaeria sp., T. flavus, and P. simplicissimum. | Cotton | V. dahliae strain Vd080 | - | [107] |
| Bacillus sp. | Millet |
R. solani, S. rolfsii, and F. solani |
Antimicrobial peptides | [126] |
| B. subtilis | Rice | M. oryzae | Enhanced activity of peroxidase, polyphenol oxidase and superoxide dismutase | [127] |
| Pseudomonas sp. | Wheat | F. graminearum | - | [128] |
| B. subtilis EB-28 | Tomato | B. cinerea | - | [129] |
| F. mosseae | Wheat | X. translucens | - | [130] |
| R. irregularis | Tomato | A. solani | - | [108] |
| F. mosseae | Wheat | B. graminis | - | [131] |
| F. mosseae and P. fluorescens | Wheat | G. graminis | - | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





