Submitted:
29 October 2025
Posted:
31 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
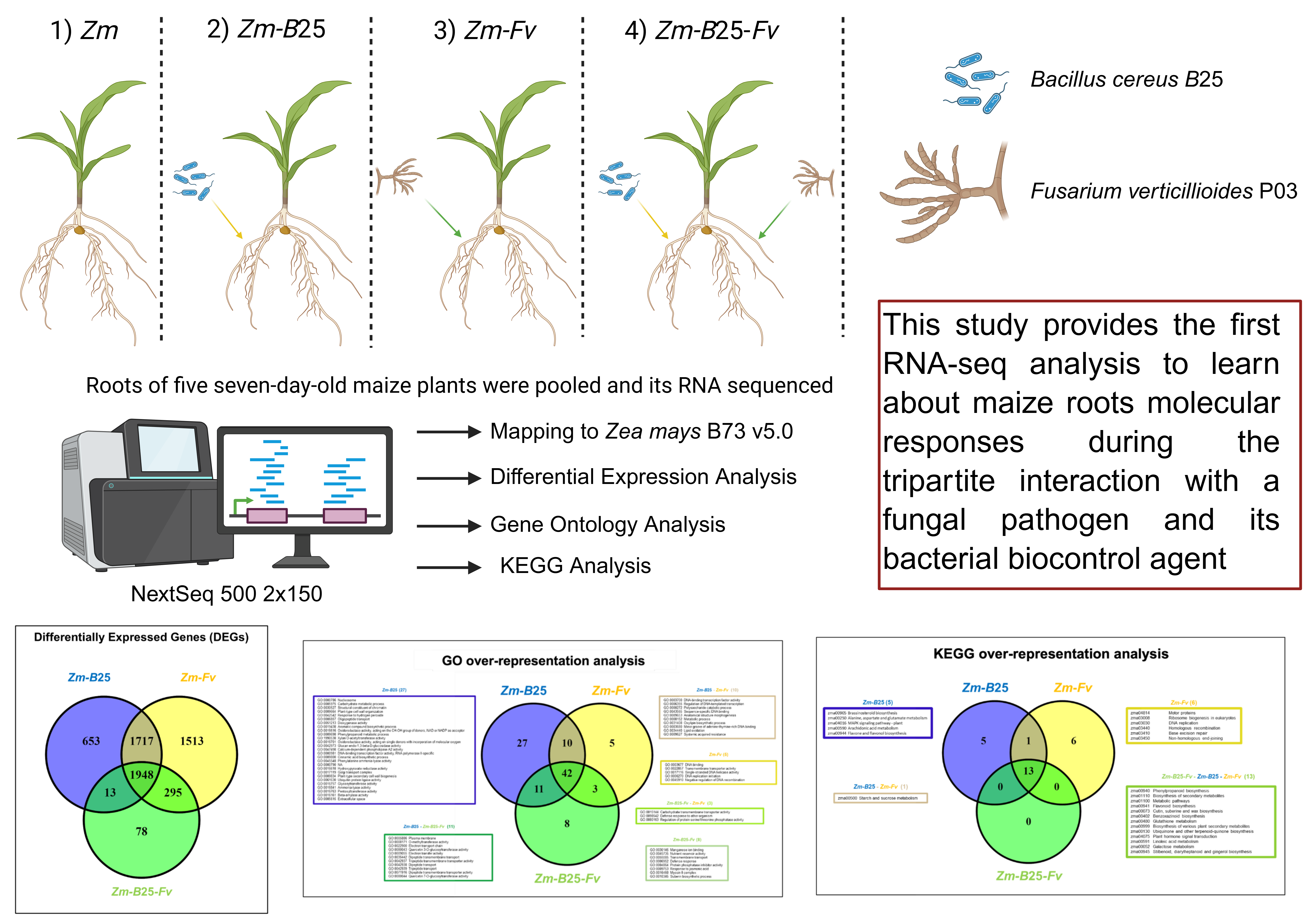
2.1. B25 Mediated the Biocontrol of Fv and Enhanced Maize Plant Growth in the Tripartite Interaction
2.2. RNA Sequencing of the Tripartite Assay
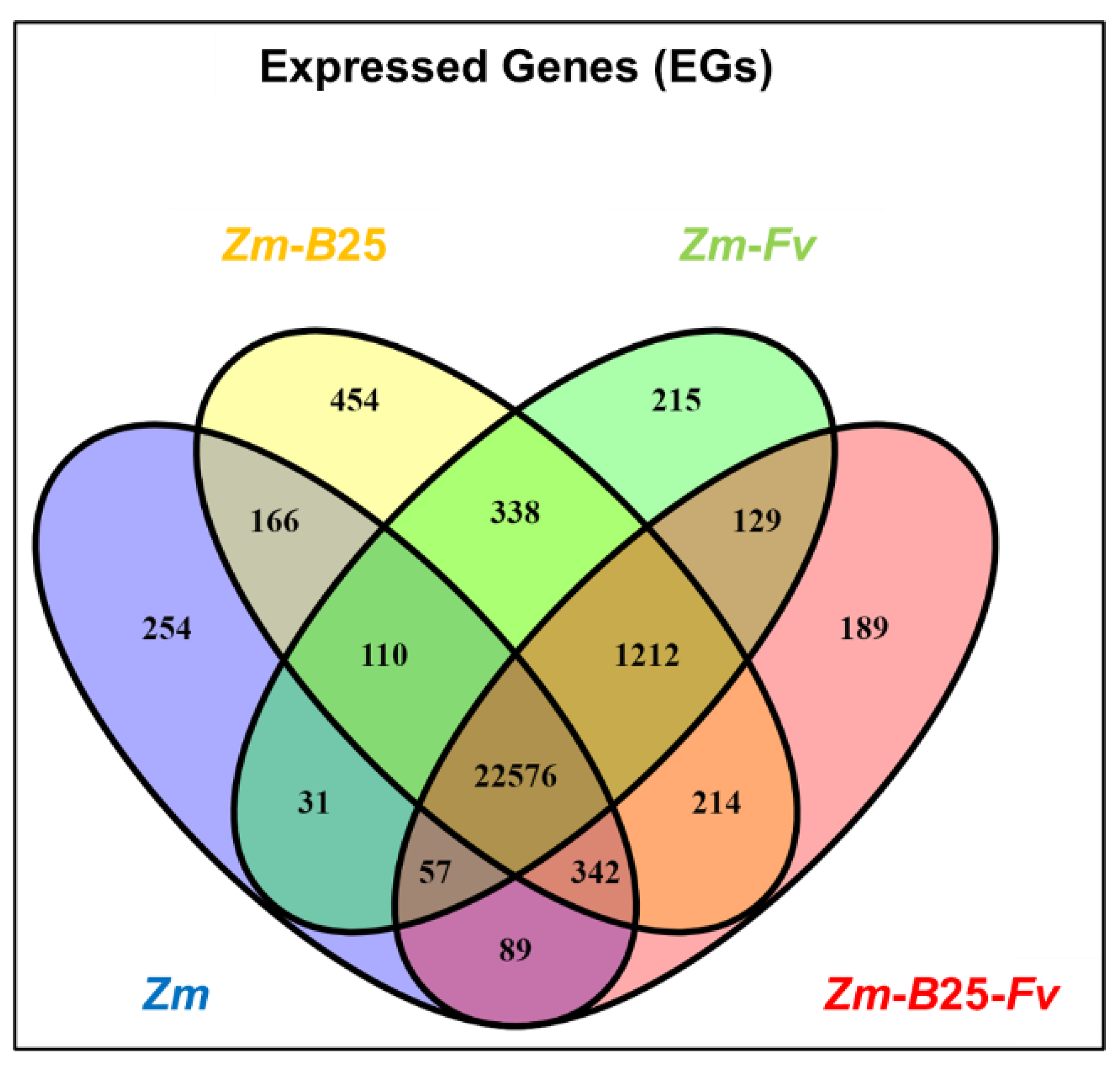
2.3. Global Transcriptome Profiles of Maize Roots from the Tripartite Assay
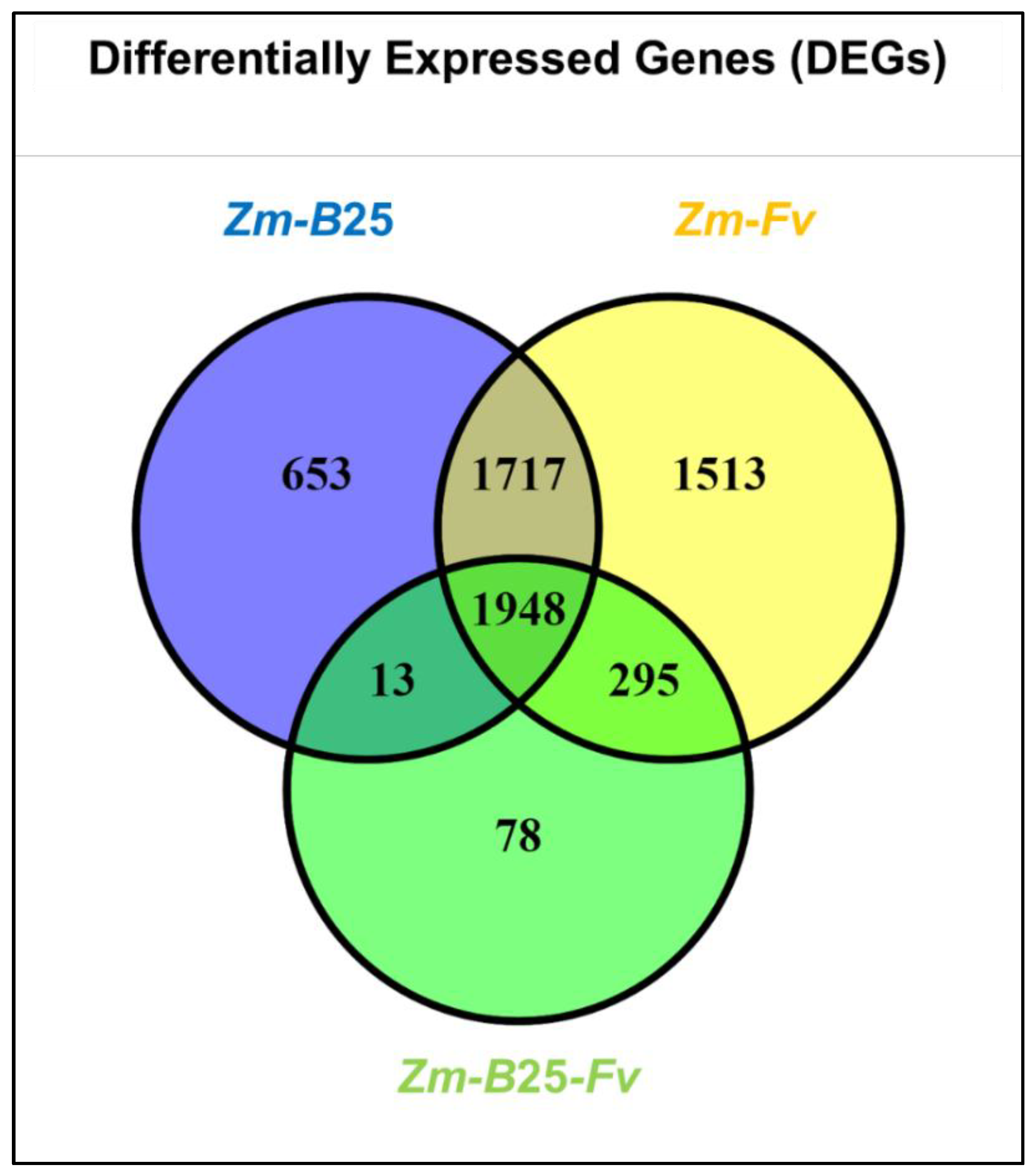
2.4. Differentially Expressed Genes Within and Among Maize Interactions
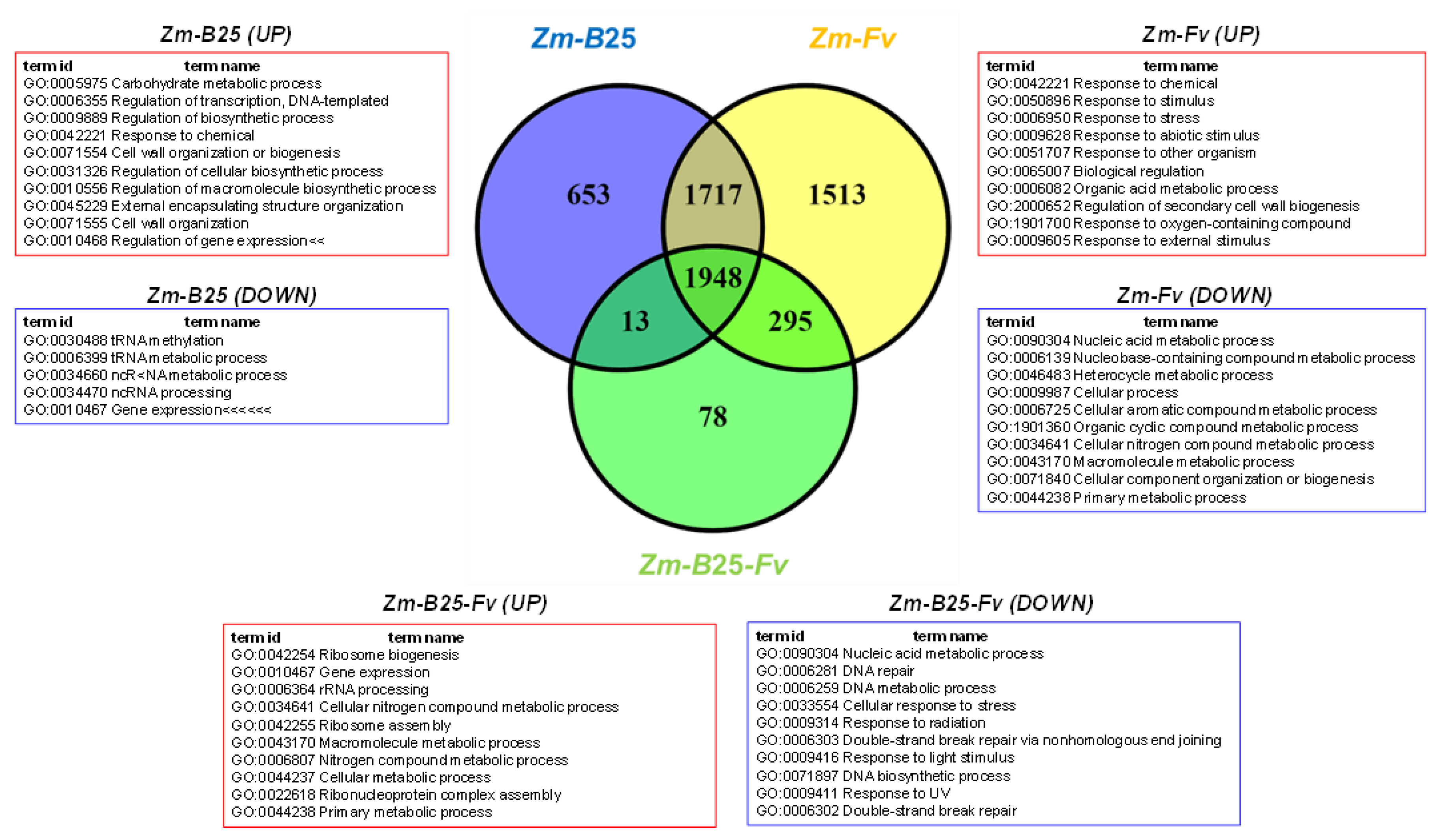
2.5. Overrepresentation Analysis: A General Approach
2.6. KEGG Analysis
2.7. Functional Gene Association Networks: Enriched Biological PROCESSES using A. thaliana Orthologous Genes
2.8. Gene Interaction Networks Based on Co-Expression
2.9. Validation of RNA Sequencing by qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Inoculum Preparation
4.2. Tripartite Assay: Maize-Bacteria-Fungus Interaction
4.3. Maize Root Colonization and Plant Protection Mediated by B25 Against Fv
4.4. RNA Extraction and Sequencing
4.5. Bioinformatic Analysis
4.6. Gene Function Annotation and Overrepresentation Analysis
4.7. Gene Set Enrichment Analyses: Networks and Clustering
4.8. RNA-Seq Validation by qRT-PCR
4.9. Statistical Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur 2022. [Google Scholar] [CrossRef]
- Butrón, A.; Reid, L.M.; Santiago, R.; Cao, A.; Malvar, R.A. Inheritance of Maize Resistance to Gibberella and Fusarium Ear Rots and Kernel Contamination with Deoxynivalenol and Fumonisins. Plant Pathol 2015, 64, 1053–1060. [Google Scholar] [CrossRef]
- Subedi, S. A Review on Important Maize Diseases and Their Management in Nepal. Journal of Maize Research and Development 2015, 1, 28–52. [Google Scholar] [CrossRef]
- Leyva-Madrigal, K.Y.; Larralde-Corona, C.P.; Apodaca-Sánchez, M.A.; Quiroz-Figueroa, F.R.; Mexia-Bolaños, P.A.; Portillo-Valenzuela, S.; Ordaz-Ochoa, J.; Maldonado-Mendoza, I.E. Fusarium Species from the Fusarium Fujikuroi Species Complex Involved in Mixed Infections of Maize in Northern Sinaloa, Mexico. Journal of Phytopathology 2015, 163, 486–497. [Google Scholar] [CrossRef]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium Verticillioides: Managing the Endophytic Association with Maize for Reduced Fumonisins Accumulation. Toxin Rev 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Deepa, N.; Nagaraja, H.; Sreenivasa, M.Y. Prevalence of Fumonisin Producing Fusarium Verticillioides Associated with Cereals Grown in Karnataka (India). Food Science and Human Wellness 2016, 5, 156–162. [Google Scholar] [CrossRef]
- Koul, B.; Chopra, M.; Lamba, S. Microorganisms as Biocontrol Agents for Sustainable Agriculture. Relationship Between Microbes and the Environment for Sustainable Ecosystem Services, Volume 1 2022, 45–68. [CrossRef]
- De los Santos Villalobos, S.; Parra Cota, F.I.; Herrera Sepúlveda, A.; Valenzuela Aragón, B.; Estrada Mora, J.C. Colmena: Colección de Microorganismos Edáficos y Endófitos Nativos, Para Contribuir a La Seguridad Alimentaria Nacional. Rev Mex De Cienc Agric 2018, 9, 191–202. [Google Scholar] [CrossRef]
- Lizárraga-Sánchez, G.J.; Leyva-Madrigal, K.Y.; Sánchez-Peña, P.; Quiroz-Figueroa, F.R.; Maldonado-Mendoza, I.E. Bacillus Cereus Sensu Lato Strain B25 Controls Maize Stalk and Ear Rot in Sinaloa, Mexico. Field Crops Res 2015, 176, 11–21. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. In Planta Reduction of Maize Seedling Stalk Lesions by the Bacterial Endophyte Bacillus Mojavensis. Can J Microbiol 2011, 57, 485–492. [Google Scholar] [CrossRef]
- Pal, G.; Kumar, K.; Verma, A.; Verma, S.K. Seed Inhabiting Bacterial Endophytes of Maize Promote Seedling Establishment and Provide Protection against Fungal Disease. Microbiol Res 2022, 255, 126926. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, R.; Wu, X.; Xu, T.; Ahmad, S.; Zhang, X.; Zhao, J.; Liu, Y. An Endophytic Strain of the Genus Bacillus Isolated from the Seeds of Maize (Zea Mays L.) Has Antagonistic Activity against Maize Pathogenic Strains. Microb Pathog 2020, 142, 104074. [Google Scholar] [CrossRef]
- Figueroa-López, A.M.; Cordero-Ramírez, J.D.; Martínez-Álvarez, J.C.; López-Meyer, M.; Lizárraga-Sánchez, G.J.; Félix-Gastélum, R.; Castro-Martínez, C.; Maldonado-Mendoza, I.E. Rhizospheric Bacteria of Maize with Potential for Biocontrol of Fusarium Verticillioides. Springerplus 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities With Multifunctional Prospects. Front Bioeng Biotechnol 2020, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bolivar-Anillo, H.J.; González-Rodríguez, V.E.; Cantoral, J.M.; García-Sánchez, D.; Collado, I.G.; Garrido, C. Endophytic Bacteria Bacillus Subtilis, Isolated from Zea Mays, as Potential Biocontrol Agent against Botrytis Cinerea. Biology (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner Plant Values: Diversity, Colonization and Benefits from Endophytic Bacteria. Front Microbiol 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Figueroa-López, A.M. Caracterización de Los Mecanismos de Antagonismo Que Emplea Bacillus Cereus Seleccionado Para El Control de Fusarium Verticillioides. Doctoral Thesis, Instituto Politécnico Nacional: Guasave, Sinaloa, 2016. [Google Scholar]
- Martínez-Álvarez, J.C.; Castro-Martínez, C.; Sánchez-Peña, P.; Gutiérrez-Dorado, R.; Maldonado-Mendoza, I.E. Development of a Powder Formulation Based on Bacillus Cereus Sensu Lato Strain B25 Spores for Biological Control of Fusarium Verticillioides in Maize Plants. World J Microbiol Biotechnol 2016, 32. [Google Scholar] [CrossRef]
- Douriet-Gámez, N.R.; Maldonado-Mendoza, I.E.; Ibarra-Laclette, E.; Blom, J.; Calderón-Vázquez, C.L. Genomic Analysis of Bacillus Sp. Strain B25, a Biocontrol Agent of Maize Pathogen Fusarium Verticillioides. Curr Microbiol 2018, 75, 247–255. [Google Scholar] [CrossRef]
- Morales-Ruiz, E.; Priego-Rivera, R.; Figueroa-López, A.M.; Cazares-Álvarez, J.E.; Maldonado-Mendoza, I.E. Biochemical Characterization of Two Chitinases from Bacillus Cereus Sensu Lato B25 with Antifungal Activity against Fusarium Verticillioides P03. FEMS Microbiol Lett 2021, 368, 1–8. [Google Scholar] [CrossRef]
- Báez-Astorga, P.A.; Cázares-Álvarez, J.E.; Cruz-Mendívil, A.; Quiroz-Figueroa, F.R.; Sánchez-Valle, V.I.; Maldonado-Mendoza, I.E. Molecular and Biochemical Characterisation of Antagonistic Mechanisms of the Biocontrol Agent Bacillus Cereus B25 Inhibiting the Growth of the Phytopathogen Fusarium Verticillioides P03 during Their Direct Interaction in Vitro. Biocontrol Sci Technol 2022, 1–21. [Google Scholar] [CrossRef]
- Figueroa-López, A.M.; Leyva-Madrigal, K.Y.; Cervantes-Gámez, R.G.; Beltran-Arredondo, L.I.; Douriet-Gámez, N.R.; Castro-Martínez, C.; Maldonado-Mendoza, I.E. Induction of Bacillus Cereus Chitinases as a Response to Lysates of Fusarium Verticillioides. 2017, 22, 12722–12731. [Google Scholar]
- Park, Y.S.; Park, J.S.; Lee, S.; Jung, S.H.; Kim, S.K.; Ryu, C.M. Simultaneous Profiling of Arabidopsis Thaliana and Vibrio Vulnificus MO6-24/O Transcriptomes by Dual RNA-Seq Analysis. Comput Struct Biotechnol J 2021, 19, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Gheler, C.F.; Domingues Zucchi, T.; Soares de Melo, I. Biological Control of Phytopathogenic Fungi by Endophytic Actinomycetes Isolated from Maize (Zea Mays L.). Brazilian archives of Biology and Technology 2013, 56, 948–955. [Google Scholar] [CrossRef]
- Cazares-Álvarez, J.E.; Báez-Astorga, P.A.; Arroyo-Becerra, A.; Maldonado-Mendoza, I.E. Genome-Wide Identification of a Maize Chitinase Gene Family and the Induction of Its Expression by Fusarium Verticillioides (Sacc.) Nirenberg (1976) Infection. Genes (Basel) 2024, 15. [Google Scholar] [CrossRef] [PubMed]
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging Roles of β-Glucanases in Plant Development and Adaptative Responses. Plants 2022, 11. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Qiu, H. Flavones and Flavonols: Phytochemistry and Biochemistry. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp. 1821–1847. ISBN 978-3-642-22144-6. [Google Scholar]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The Role of Quercetin in Plants. Plant Physiology and Biochemistry 2021, 166, 10–19. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Lam, P.Y.; Dini-Andreote, F.; Dai, L.; Wei, Z. Multifaceted Roles of Flavonoids Mediating Plant-Microbe Interactions. Microbiome 2022, 10, 1–13. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, D.; Tang, Z.; Zhang, Y.; Zhang, K.; Dong, J.; Wang, F. Exogenous Brassinosteroids Promotes Root Growth, Enhances Stress Tolerance, and Increases Yield in Maize. Plant Signal Behav 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Hagen, G.; Guilfoyle, T. Auxin-Responsive Gene Expression: Genes, Promoters and Regulatory Factors. Plant Mol Biol 2002, 49, 373–385. [Google Scholar] [CrossRef]
- Franco, A.R.; Gee, M.A.; Guilfoyle, T.J. Induction and Superinduction of Auxin-Responsive MRNAs with Auxin and Protein Synthesis Inhibitors. J Biol Chem 1990, 265, 15845–15849. [Google Scholar] [CrossRef]
- Sowmya, R.S.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Effect of Amino Acids on Growth, Elemental Content, Functional Groups, and Essential Oils Composition on Hydroponically Cultivated Coriander under Different Conditions. Ind Crops Prod 2023, 197, 116577. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein Hydrolysates as Biostimulants in Horticulture. Sci Hortic 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Cámara-Zapata, J.M.; Simón, I.; Martínez-Nicolás, J.J.; Lidón, V.; García-Sánchez, F. Physiological, Nutritional and Metabolomic Responses of Tomato Plants After the Foliar Application of Amino Acids Aspartic Acid, Glutamic Acid and Alanine. Front Plant Sci 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Hassan, M.U.; Ishfaq, M.; Ripa, F.A.; Nadeem, F.; Ahmad, Z.; Xu, J.; Ning, P.; Li, X. Foliar Glutamine Application Improves Grain Yield and Nutritional Quality of Field-Grown Maize (Zea Mays L.) Hybrid ZD958. J Plant Growth Regul 2024, 43, 624–637. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S. Mitogen-Activated Protein Kinase Cascades in Signaling Plant Growth and Development. Trends Plant Sci 2015, 20, 56–64. [Google Scholar] [CrossRef]
- Lin, L.; Wu, J.; Jiang, M.; Wang, Y. Plant Mitogen-activated Protein Kinase Cascades in Environmental Stresses. Int J Mol Sci 2021, 22, 1–24. [Google Scholar] [CrossRef]
- Dedyukhina, E.G.; Kamzolova, S. V.; Vainshtein, M.B. Arachidonic Acid as an Elicitor of the Plant Defense Response to Phytopathogens. Chemical and Biological Technologies in Agriculture 2014, 1, 2–7. [Google Scholar] [CrossRef]
- Savchenko, T.; Walley, J.W.; Chehab, E.W.; Xiao, Y.; Kaspi, R.; Pye, M.F.; Mohamed, M.E.; Lazarus, C.M.; Bostock, R.M.; Dehesh, K. Arachidonic Acid: An Evolutionarily Conserved Signaling Molecule Modulates Plant Stress Signaling Networks. Plant Cell 2010, 22, 3193–3205. [Google Scholar] [CrossRef]
- Dye, S.M.; Bostock, R.M. Eicosapolyenoic Fatty Acids Induce Defense Responses and Resistance to Phytophthora Capsici in Tomato and Pepper. Physiol Mol Plant Pathol 2021, 114, 101642. [Google Scholar] [CrossRef]
- Lewis, D.C.; van der Zwan, T.; Richards, A.; Little, H.; Coaker, G.L.; Bostock, R.M. The Oomycete Microbe-Associated Molecular Pattern, Arachidonic Acid, and an Ascophyllum Nodosum-Derived Plant Biostimulant Induce Defense Metabolome Remodeling in Tomato. Phytopathology 2023, 113, 1084–1092. [Google Scholar] [CrossRef]
- Hyong, W.C.; Byung, G.L.; Nak, H.K.; Park, Y.; Chae, W.L.; Hyun, K.S.; Byung, K.H. A Role for a Menthone Reductase in Resistance against Microbial Pathogens in Plants. Plant Physiol 2008, 148, 383–401. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, L.J.; Wang, Y.; Liu, S.; Geng, Z.; Song, A.; Jiang, J.; Chen, S.; Chen, F. Functional Identification of a Flavone Synthase and a Flavonol Synthase Genes Affecting Flower Color Formation in Chrysanthemum Morifolium. Plant Physiology and Biochemistry 2021, 166, 1109–1120. [Google Scholar] [CrossRef]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The Style and Substance of Plant Flavonoid Decoration; towards Defining Both Structure and Function. Phytochemistry 2020, 174. [Google Scholar] [CrossRef] [PubMed]
- Manova, V.; Gruszka, D. DNA Damage and Repair in Plants – From Models to Crops. Front Plant Sci 2015, 6, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Nisa, M.U.; Huang, Y.; Benhamed, M.; Raynaud, C. The Plant DNA Damage Response: Signaling Pathways Leading to Growth Inhibition and Putative Role in Response to Stress Conditions. Front Plant Sci 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Grin, I.R.; Petrova, D. V.; Endutkin, A. V.; Ma, C.; Yu, B.; Li, H.; Zharkov, D.O. Base Excision DNA Repair in Plants: Arabidopsis and Beyond. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Szurman-Zubrzycka, M.; Jędrzejek, P.; Szarejko, I. How Do Plants Cope with DNA Damage? A Concise Review on the DDR Pathway in Plants. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Li, Y.; Xing, A.; Wu, C.; Li, D.; Wang, C.; de Bures, A.; Zhang, Y.; Guo, S.; et al. Arabidopsis SMO2 Modulates Ribosome Biogenesis by Maintaining the RID2 Abundance during Organ Growth. Plant Journal 2023, 114, 96–109. [Google Scholar] [CrossRef]
- Nebenführ, A.; Dixit, R. Kinesins and Myosins: Molecular Motors That Coordinate Cellular Functions in Plants. Annu Rev Plant Biol 2018, 69, 329–361. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Chong, K. The Novel Functions of Kinesin Motor Proteins in Plants. Protoplasma 2012, 249, 95–100. [Google Scholar] [CrossRef]
- Ryan, J.M.; Nebenführ, A. Update on Myosin Motors: Molecular Mechanisms and Physiological Functions. Plant Physiol 2018, 176, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Ge, L.; Ye, X.; Xu, L.; Si, W.; Ding, T. ZmGLP1, a Germin-like Protein from Maize, Plays an Important Role in the Regulation of Pathogen Resistance. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Khare, D.; Choi, H.; Huh, S.U.; Bassin, B.; Kim, J.; Martinoia, E.; Sohn, K.H.; Paek, K.H.; Lee, Y.; Chrispeels, M.J. Arabidopsis ABCG34 Contributes to Defense against Necrotrophic Pathogens by Mediating the Secretion of Camalexin. Proc Natl Acad Sci U S A 2017, 114, E5712–E5720. [Google Scholar] [CrossRef]
- Li, M.R.; Qi, X.; Li, D.; Wu, Z.; Liu, M.; Yang, W.; Zang, Z.; Jiang, L. Comparative Transcriptome Analysis Highlights Resistance Regulatory Networks of Maize in Response to Exserohilum Turcicum Infection at the Early Stage. Physiol Plant 2024, 176. [Google Scholar] [CrossRef]
- Shumayla; Madhu; Singh, K.; Upadhyay, S.K. LysM Domain-Containing Proteins Modulate Stress Response and Signalling in Triticum Aestivum L. Environ Exp Bot. 2021, 189. [Google Scholar] [CrossRef]
- Giovannoni, M.; Lironi, D.; Marti, L.; Paparella, C.; Vecchi, V.; Gust, A.A.; De Lorenzo, G.; Nürnberger, T.; Ferrari, S. The Arabidopsis Thaliana LysM-Containing Receptor-Like Kinase 2 Is Required for Elicitor-Induced Resistance to Pathogens. Plant Cell Environ 2021, 44, 3545–3562. [Google Scholar] [CrossRef]
- Kumar, R.; Meghwanshi, G.K.; Marcianò, D.; Ullah, S.F.; Bulone, V.; Toffolatti, S.L.; Srivastava, V. Sequence, Structure and Functionality of Pectin Methylesterases and Their Use in Sustainable Carbohydrate Bioproducts: A Review. Int J Biol Macromol 2023, 244. [Google Scholar] [CrossRef]
- Zhou, S.; Richter, A.; Jander, G. Beyond Defense: Multiple Functions of Benzoxazinoids in Maize Metabolism. Plant Cell Physiol 2018, 59, 1528–1533. [Google Scholar] [CrossRef]
- Lee, J.R.; Boltz, K.A.; Lee, S.Y. Molecular Chaperone Function of Arabidopsis Thaliana Phloem Protein 2-A1, Encodes a Protein Similar to Phloem Lectin. Biochem Biophys Res Commun 2014, 443, 18–21. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, H.; Zheng, X.; Duan, Y.; Yu, X. PP2 Gene Family in Phyllostachys Edulis: Identification, Characterization, and Expression Profiles. BMC Genomics 2024, 25. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Song, W.M.; Pan, J.; Jiang, C.M.; Srivastava, R.; Li, B.; Zhu, L.Y.; Su, H.Y.; Gao, X.S.; Liu, H.; et al. Overexpression of the NDR1/HIN1-Like Gene NHL6 Modifies Seed Germination in Response to Abscisic Acid and Abiotic Stresses in Arabidopsis. PLoS One 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.; Youssef, R.; McDonald, M.; Brewer, E.; Beard, H.; Matthews, B. Overexpression of Four Arabidopsis Thaliana NHL Genes in Soybean (Glycine Max) Roots and Their Effect on Resistance to the Soybean Cyst Nematode (Heterodera Glycines). Physiol Mol Plant Pathol 2014, 86, 1–10. [Google Scholar] [CrossRef]
- Block, A.K.; Tang, H. V.; Hopkins, D.; Mendoza, J.; Solemslie, R.K.; du Toit, L.J.; Christensen, S.A. A Maize Leucine-Rich Repeat Receptor-like Protein Kinase Mediates Responses to Fungal Attack. Planta 2021, 254. [Google Scholar] [CrossRef]
- Ding, N.; Zhao, Y.; Wang, W.; Liu, X.; Shi, W.; Zhang, D.; Chen, J.; Ma, S.; Sun, Q.; Wang, T.; et al. Transcriptome Analysis in Contrasting Maize Inbred Lines and Functional Analysis of Five Maize NAC Genes under Drought Stress Treatment. Front Plant Sci 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, M.; Wang, D.; Gong, Y.; Sha, Q.; Lv, P.; Yang, J.; Chu, P.; Guo, S. Research Progress on the Roles of Actin-Depolymerizing Factor in Plant Stress Responses. Front Plant Sci 2023, 14. [Google Scholar] [CrossRef]
- Shigeyama, T.; Watanabe, A.; Tokuchi, K.; Toh, S.; Sakurai, N.; Shibuya, N.; Kawakami, N. α-Xylosidase Plays Essential Roles in Xyloglucan Remodelling, Maintenance of Cell Wall Integrity, and Seed Germination in Arabidopsis Thaliana. J Exp Bot 2016, 67, 5615–5629. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Rubinchik, S.; Voelkel-Johnson, C. Transformation, Translation and TRAIL: An Unexpected Intersection. Cytokine Growth Factor Rev 2008, 19, 167–172. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Yu, X.; Lin, Y.; Zhu, Y.; Chen, J.; Xie, H.; Zhang, Q.; Wang, L.; Wei, Y.; et al. SH3P2, an SH3 Domain-Containing Protein That Interacts with Both Pib and AvrPib, Suppresses Effector-Triggered, Pib-Mediated Immunity in Rice. Mol Plant 2022, 15, 1931–1946. [Google Scholar] [CrossRef]
- Jasim, B.; Joseph, A.A.; John, C.J.; Mathew, J.; Radhakrishnan, E.K. Isolation and Characterization of Plant Growth Promoting Endophytic Bacteria from the Rhizome of Zingiber Officinale. 3 Biotech 2014, 4, 197–204. [Google Scholar] [CrossRef]
- Aranda, P.S.; Lajoie, D.M.; Jorcyk, C.L. Bleach Gel: A Simple Agarose Gel for Analyzing RNA Quality. Electrophoresis 2012, 33, 366–369. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Woodhouse, M.R.; Cannon, E.K.; Portwood, J.L.; Harper, L.C.; Gardiner, J.M.; Schaeffer, M.L.; Andorf, C.M. A Pan-Genomic Approach to Genome Databases Using Maize as a Model System. BMC Plant Biol 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Martin, F.J.; Amode, M.R.; Aneja, A.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Becker, A.; Bennett, R.; Berry, A.; Bhai, J.; et al. Ensembl 2023. Nucleic Acids Res 2023, 51, D933–D941. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol 2010, 11. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. AgriGO v2.0: A GO Analysis Toolkit for the Agricultural Community, 2017 Update. Nucleic Acids Res 2017, 45, W122–W129. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA Prediction Server: Biological Network Integration for Gene Prioritization and Predicting Gene Function. Nucleic Acids Res 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Lin, F.; Jiang, L.; Liu, Y.; Lv, Y.; Dai, H.; Zhao, H. Genome-Wide Identification of Housekeeping Genes in Maize. Plant Mol Biol 2014, 86, 543–554. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
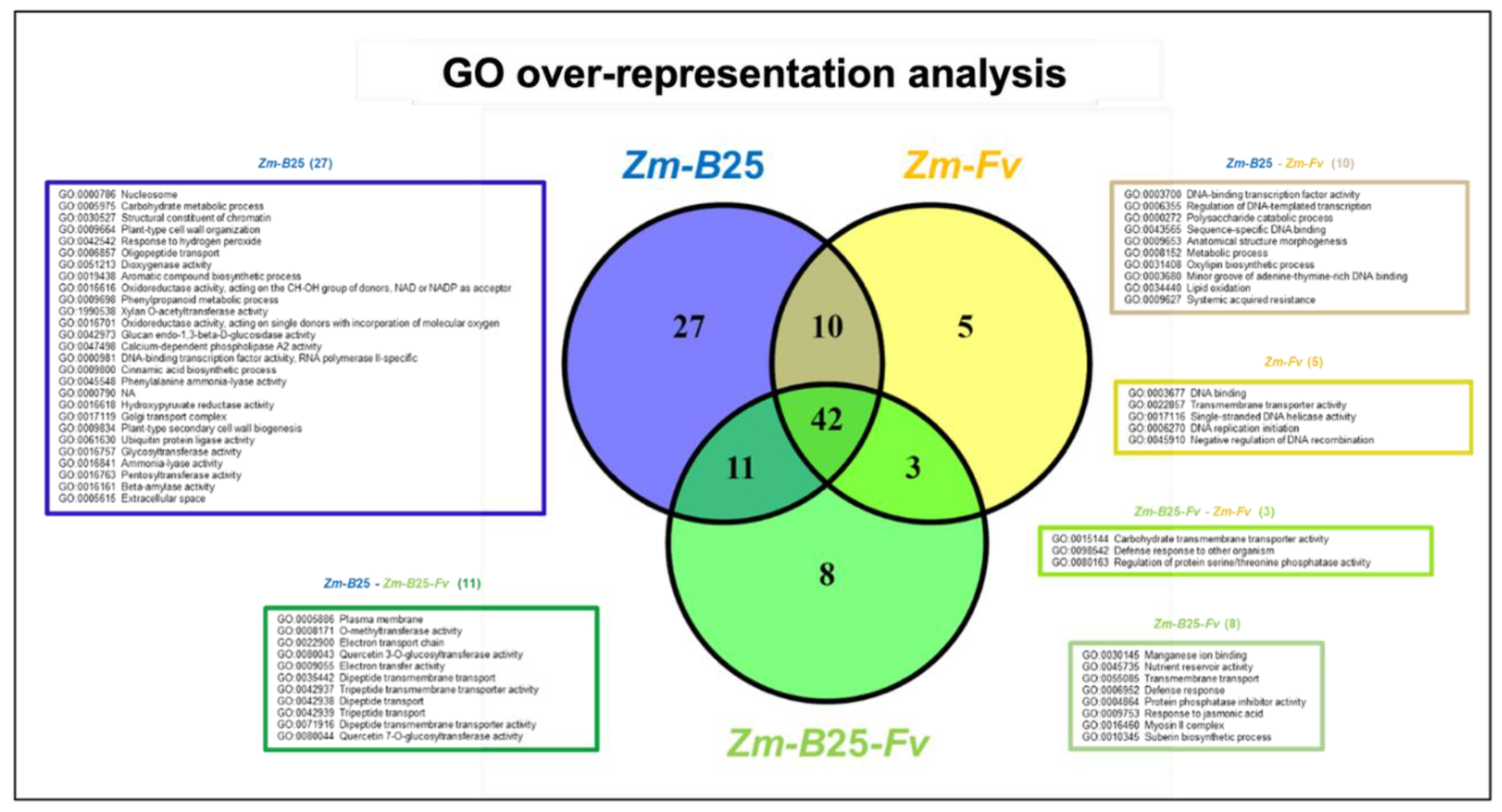
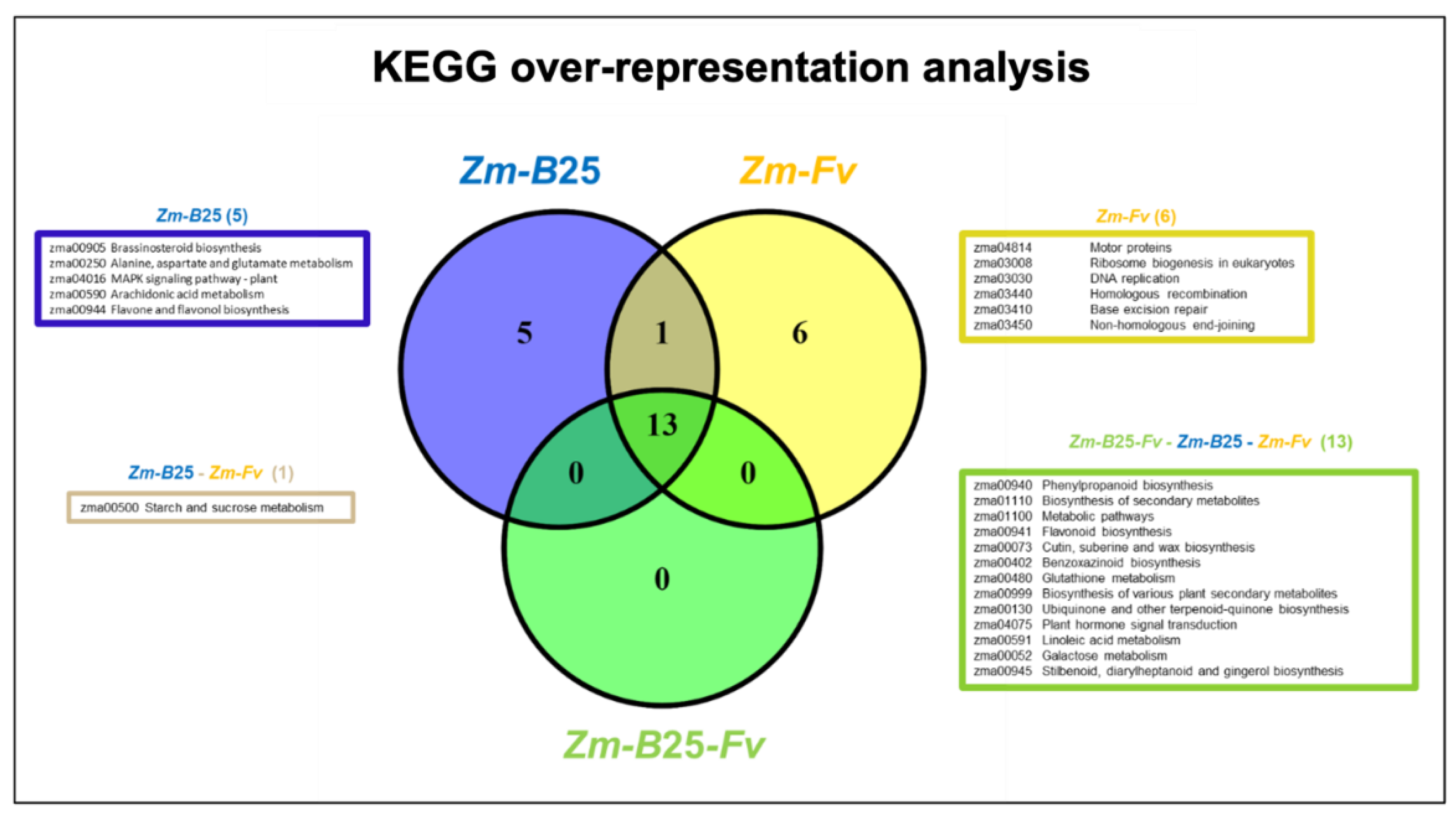
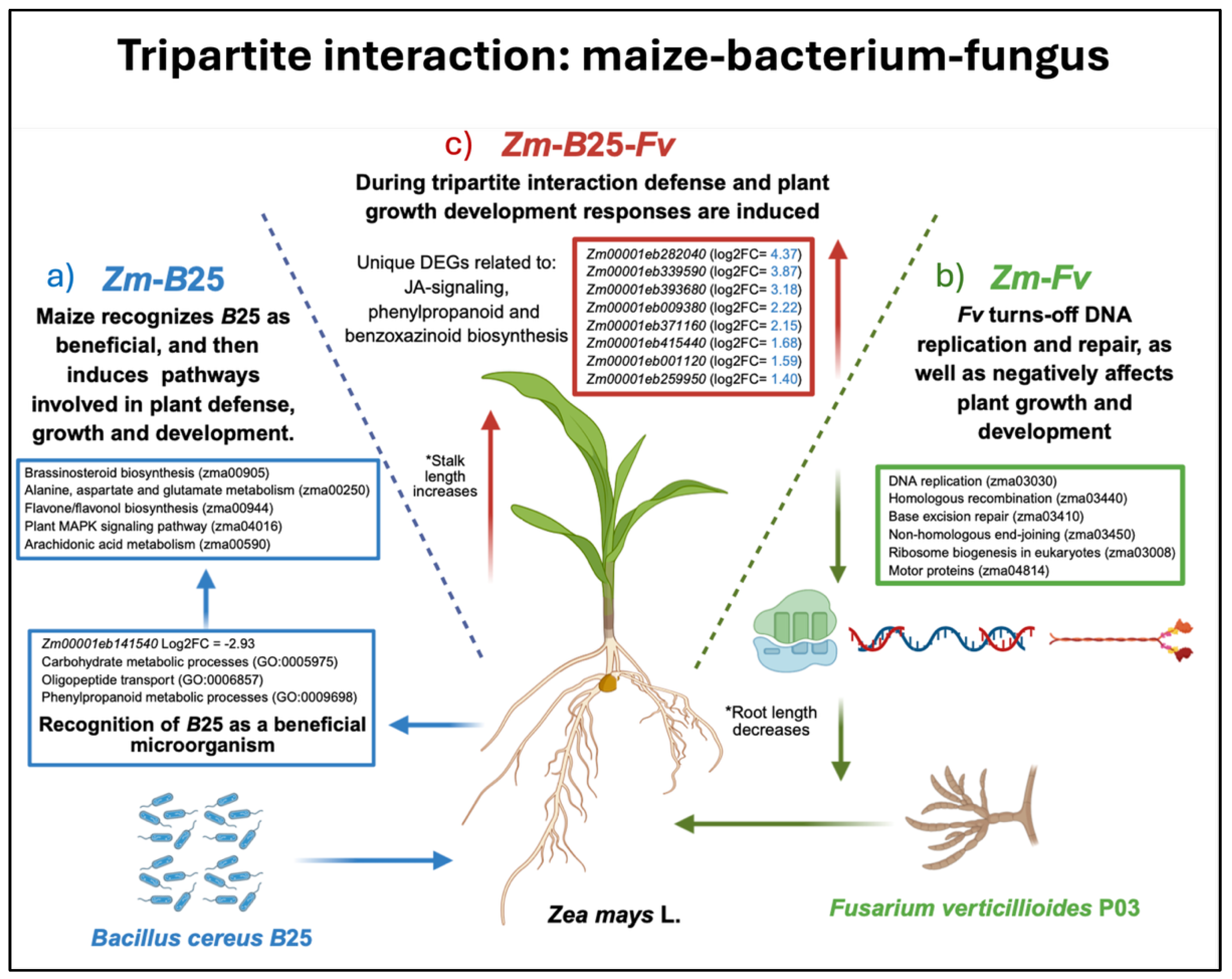
| Condition | Length (cm) | Fresh weight (g) | |||
| Leaf | Root | Leaf | Root | ||
| Zm | 11.92b | 13.07a | 0.38b | 0.78a | |
| Zm-B25 | 11.27b | 12.52a | 0.34b | 0.78a | |
| Zm-Fv | 11.52b | 9.86b | 0.34b | 0.64b | |
| Zm-B25-Fv | 14.28a | 13.80a | 0.47a | 0.77a | |
| Sample | Raw Reads | Filtered Reads (Q>20) | Unique Mapped Reads | ||
| Zm-2 | 13,742,216 | 13,534,709 | (98.49%) | 12,542,341 | (91.27%) |
| Zm-3 | 21,273,970 | 20,839,981 | (97.96%) | 17,042,555 | (80.11%) |
| Zm-B25-1 | 22,841,141 | 22,498,524 | (98.50%) | 19,719,075 | (86.33%) |
| Zm-B25-2 | 27,395,643 | 27,003,885 | (98.57%) | 24,442,305 | (89.22%) |
| Zm-Fv-1 | 19,155,891 | 18,880,046 | (98.56%) | 17,497,335 | (91.34%) |
| Zm-Fv-3 | 18,080,786 | 17,815,902 | (98.54%) | 14,513,113 | (80.27%) |
| Zm-B25-Fv-1 | 18,442,655 | 18.199,212 | (98.68%) | 16,495,370 | (89.44%) |
| Zm-B25-Fv-3 | 21,465,145 | 21,166,779 | (98.61%) | 19,373,477 | (90.26%) |
| Average | 20,299,681 | 19,992,380 | (98.49%) | 17,703,196 | (87.28%) |
| Condition | Total EGs | Percentage of the genomea |
| Zm | 23,625 | 53.33% |
| Zm-B25 | 25,412 | 57.36% |
| Zm-Fv | 24,668 | 55.68% |
| Zm-B25-Fv | 24,808 | 56.00% |
| Average | 24,628 | 55.59% |
| Condition | Total DEGs | Up-regulated | Down-regulated |
| Zm-B25 | 4331 | 3884 (89.68%) | 447 (10.32%) |
| Zm-Fv | 5473 | 4076 (74.47%) | 1397 (25.53%) |
| Zm-B25-Fv | 2334 | 2077 (88.98%) | 257 (11.02%) |
| Condition | IDa | Gene | Fold Change vs control | |
| FC RNA-seq | FC qRT-PCR | |||
| Zm-B25-Fv | Zm00001eb375460 | Glutaredoxin | 11.49 | 4.11 |
| Zm00001eb229260 | Protein kinase domain | 2.18 | 2.24 | |
| Zm00001eb251380 | Cyclic nucleotide-binding domain | -2.23 | -7.56 | |
| Zm00001eb029490 | Gdt1 family | -2.41 | -4.37 | |
| Zm00001eb397080 | CRAL/TRIO, N-terminal domain | -2.61 | -2.74 | |
| Zm-B25 | Zm00001eb124940 | Multi antimicrobial extrusion protein | 2.87 | 2.92 |
| Zm-Fv | Zm00001eb041100 | Hydroxycinnamoyltransferase 9 | 13.1 | 7.3 |
| Zm00001eb419890 | Deoxymugineic acid synthase 6 | 5.09 | 5.57 | |
| Zm00001eb285030 | Putative cytochrome P450 superfamily protein | 7.07 | 3.72 | |
| Zm00001eb241870 | Small auxin up RNA54 | 9.69 | 24.98 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





