Submitted:
27 February 2023
Posted:
28 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fungus culture conditions and molecular Identification
2.2. Plant Growth-Promotion Assays
2.3. Agrobacterium-Mediated Transformation of B9
2.4. Fungal Interaction/Colonization Assays in Choy Sum Roots
2.5. Plant Growth Assay Under Low Pi Using P. citrinum Isolate(s)
2.6. Extraction And Purification Methods For Detection of Phytohormones in P. citrinum
2.7. Liquid Chromatography–Mass Spectrometry
2.8. Statistical Analysis
3. Results
3.1. Morphological characteristics and identification of fungal isolates B9 and FLP7
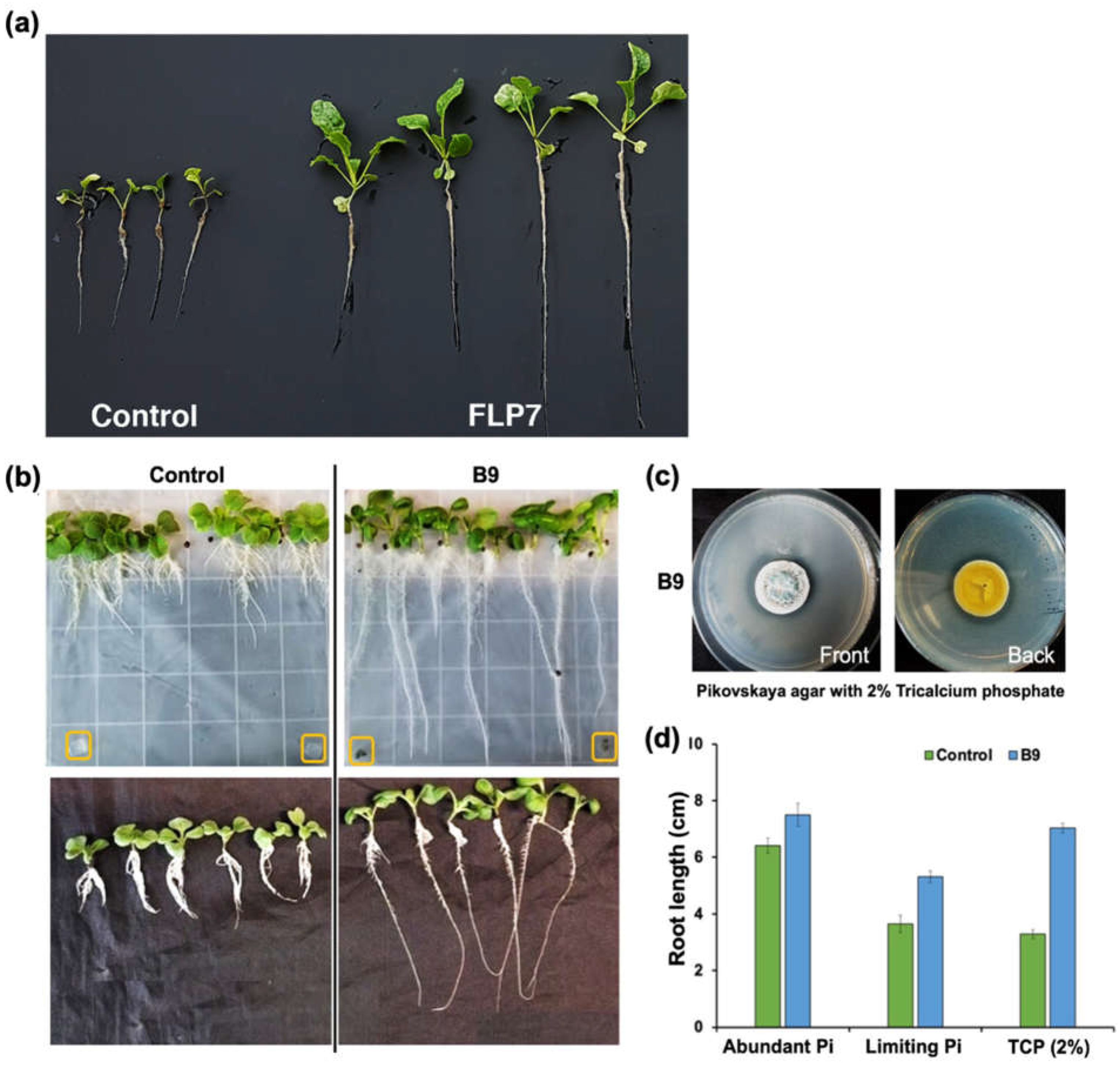
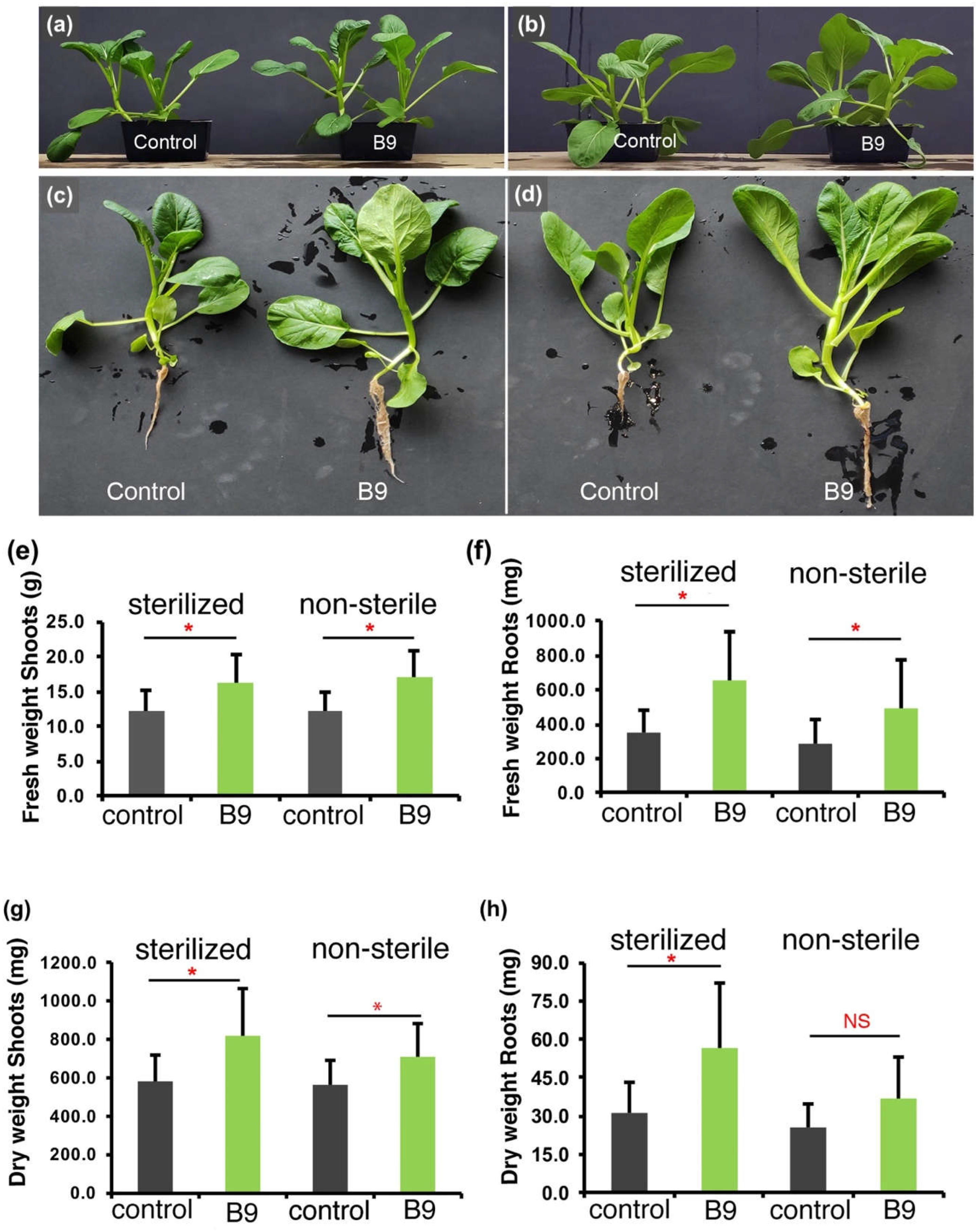
3.2. P. citrinum Improves Choy Sum Growth Under nutrient rich Conditions
3.3. P. citrinum Improves Choy Sum Growth Under Pi-limiting Conditions
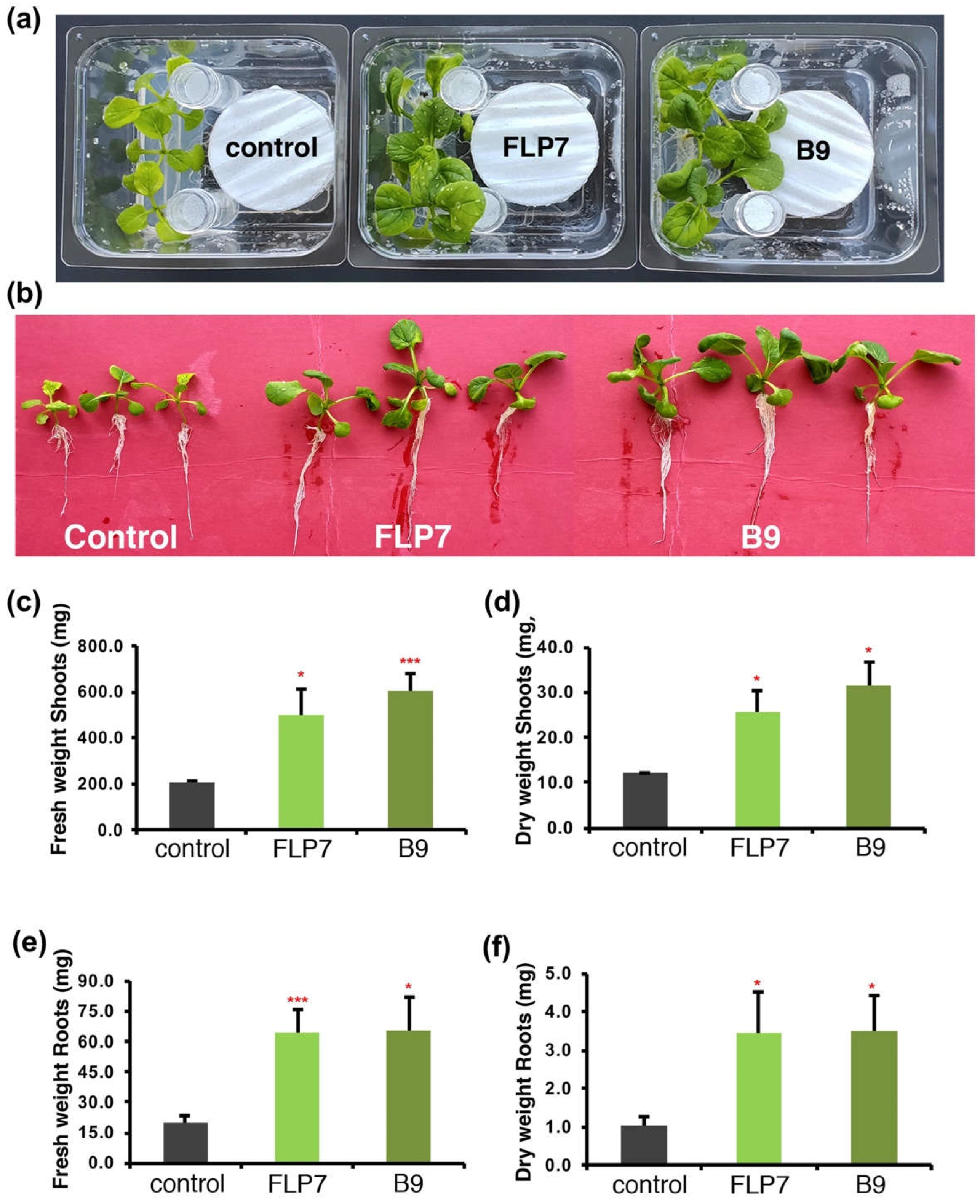
3.4. P. citrinum isolates enhance Choy Sum growth via volatile secondary metabolites
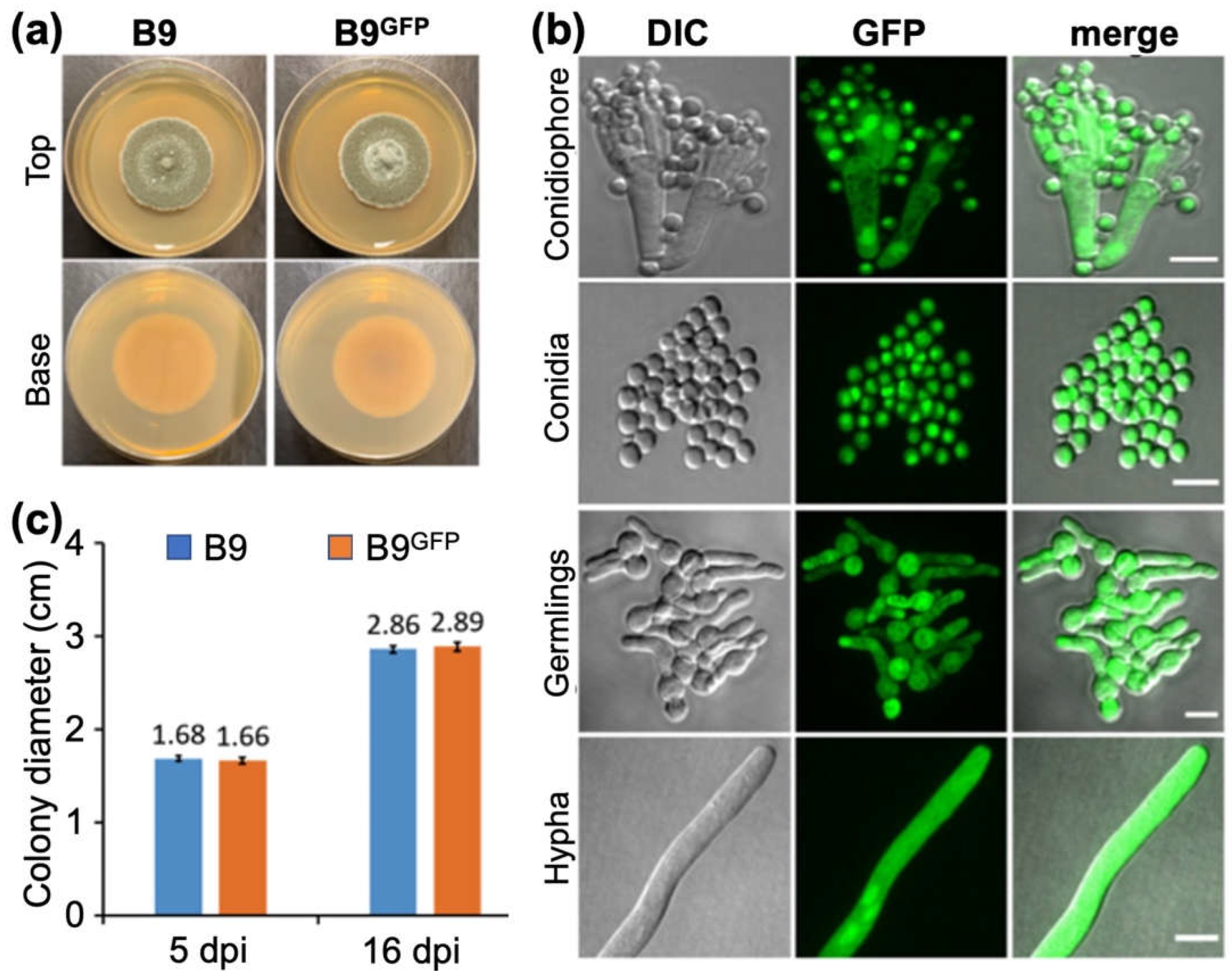
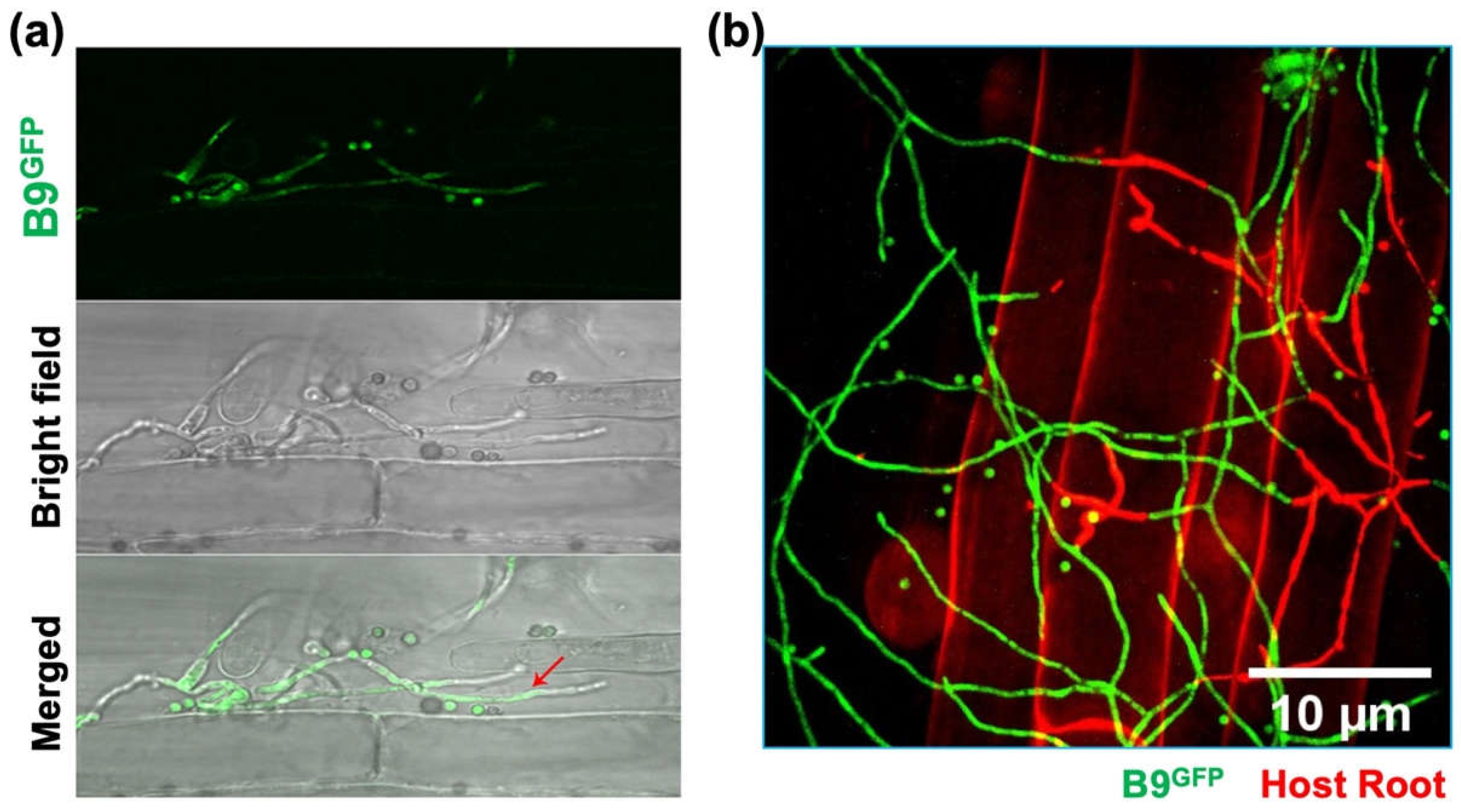
3.4. Analysing the colonization of plant roots by P. citrinum isolate B9
3.5. P. citrinum produces mimics of Gibberellins, and Cytokinins

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poveda, J.; Zabalgogeazcoa, I.; Soengas, P.; Rodriguez, V.M.; Cartea, M.E.; Abilleira, R.; Velasco, P. Brassica oleracea var. acephala (kale) improvement by biological activity of root endophytic fungi. Sci Rep 2020, 10, 20224. [Google Scholar] [CrossRef]
- Tan, W.K.; Goenadie, V.; Lee, H.W.; Liang, X.; Loh, C.S.; Ong, C.N.; Tan, H.T.W. Growth and glucosinolate profiles of a common Asian green leafy vegetable, Brassica rapa subsp. chinensis var. parachinensis (choy sum), under LED lighting. Scientia Horticulturae 2020, 261, 108922. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, S.K. ; SpringerLink. Biotechnology of crucifers, 1st 2013. ed.; Springer: New York, 2013. [Google Scholar]
- Zou, L.; Tan, W.K.; Du, Y.; Lee, H.W.; Liang, X.; Lei, J.; Striegel, L.; Weber, N.; Rychlik, M.; Ong, C.N. Nutritional metabolites in Brassica rapa subsp. chinensis var. parachinensis (choy sum) at three different growth stages: Microgreen, seedling and adult plant. Food Chemistry 2021, 357, 129535. [Google Scholar] [CrossRef]
- Huang, J.J.; D'Souza, C.; Zhou, W. Light-Time-Biomass Response Model for Predicting the Growth of Choy Sum (Brassica rapa var. parachinensis) in Soil-Based LED-Constructed Indoor Plant Factory for Efficient Seedling Production. Front Plant Sci 2021, 12, 623682. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moenne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dye, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci 2013, 4, 356. [Google Scholar] [CrossRef]
- Mabood, F.; Zhou, X.; Smith, D.L. Microbial signaling and plant growth promotion. Canadian Journal of Plant Science 2014, 94, 1051–1063. [Google Scholar] [CrossRef]
- Hyakumachi, M. Plant-Growth-Promoting Fungi from Turfgrass Rhizosphere with Potential for Disease Suppression. Soil Microorganisms 1994, 44, 53–68. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Miyazawa, M.; Hyakumachi, M. Plant growth-promoting fungus Penicillium spp. GP15-1 enhances growth and confers protection against damping-off and anthracnose in the cucumber. J Oleo Sci 2014, 63, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu Rev Phytopathol 2010, 48, 21–43. [Google Scholar] [CrossRef]
- Hossain, M.; Sultana, F.; Islam, S. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance. 2017; pp. 135-191.
- Murali, M.; Naziya, B.; Ansari, M.A.; Alomary, M.N.; AlYahya, S.; Almatroudi, A.; Thriveni, M.C.; Gowtham, H.G.; Singh, S.B.; Aiyaz, M.; et al. Bioprospecting of Rhizosphere-Resident Fungi: Their Role and Importance in Sustainable Agriculture. J Fungi (Basel) 2021, 7. [Google Scholar] [CrossRef]
- Yin, Z.; Shi, F.; Jiang, H.; Roberts, D.P.; Chen, S.; Fan, B. Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil. Can J Microbiol 2015, 61, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.Y.; Rim, S.O.; Lee, J.H.; Lee, J.M.; Lee, I.J.; Cho, K.J.; Rhee, I.K.; Kwon, J.B.; Kim, J.G. Isolation of Gibberellins-Producing Fungi from the Root of Several Sesamum indicum Plants. 2005, v. 15.
- Babu, A.G.; Kim, S.W.; Yadav, D.R.; Hyum, U.; Adhikari, M.; Lee, Y.S. Penicillium menonorum: A Novel Fungus to Promote Growth and Nutrient Management in Cucumber Plants. Mycobiology 2015, 43, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Dovana, F.; Mucciarelli, M.; Mascarello, M.; Fusconi, A. In Vitro Morphogenesis of Arabidopsis to Search for Novel Endophytic Fungi Modulating Plant Growth. PLoS One 2015, 10, e0143353. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Lee, I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Luan, Y.; An, L.; Yu, K. Arbuscular mycorrhizae formed by Penicillium pinophilum improve the growth, nutrient uptake and photosynthesis of strawberry with two inoculum-types. Biotechnol Lett 2008, 30, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Khalmuratova, I.; Kim, H.; Nam, Y.J.; Oh, Y.; Jeong, M.J.; Choi, H.R.; You, Y.H.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; et al. Diversity and Plant Growth Promoting Capacity of Endophytic Fungi Associated with Halophytic Plants from the West Coast of Korea. Mycobiology 2015, 43, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Kang, S.-M.; Baek, I.-Y.; Lee, I.-J. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. Journal of Plant Interactions 2014, 9, 754–762. [Google Scholar] [CrossRef]
- Selvaraj, P.; Shen, Q.; Yang, F.; Naqvi, N.I. Cpk2, a Catalytic Subunit of Cyclic AMP-PKA, Regulates Growth and Pathogenesis in Rice Blast. Front Microbiol 2017, 8, 2289. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, J.; He, Y.; Xie, Q.; Chen, A.; Zheng, H.; Shi, L.; Zhao, X.; Zhang, C.; Huang, Q.; et al. Retromer Is Essential for Autophagy-Dependent Plant Infection by the Rice Blast Fungus. PLoS Genet 2015, 11, e1005704. [Google Scholar] [CrossRef]
- Khan, A.; Hamayun, M.; Kim, Y.-H.; Kang, S.-M.; Lee, J.-H.; Lee, I.-J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. PROCESS BIOCHEMISTRY 2011, 46, 440–447. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kang, S.M.; Kim, Y.H.; Jung, H.Y.; Lee, J.H.; Lee, I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol 2012, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.N.; Knowles, S.; Hayward, A.; Thorn, R.G.; Saville, B.J.; Emery, R.J.N. Detection of phytohormones in temperate forest fungi predicts consistent abscisic acid production and a common pathway for cytokinin biosynthesis. Mycologia 2015, 107, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Umashankar, S.; Liang, X.; Lee, H.W.; Swarup, S.; Ong, C.N. Characterization of Plant Volatiles Reveals Distinct Metabolic Profiles and Pathways among 12 Brassicaceae Vegetables. Metabolites 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, K.; Gerlach, N.; Sacristan, S.; Nakano, R.T.; Hacquard, S.; Kracher, B.; Neumann, U.; Ramirez, D.; Bucher, M.; O'Connell, R.J.; et al. Root Endophyte Colletotrichum tofieldiae Confers Plant Fitness Benefits that Are Phosphate Status Dependent. Cell 2016, 165, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Bennett, J.W. Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecology 2013, 6, 19–26. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kimura, M.; Miyazawa, M.; Hyakumachi, M. Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma sp. GS8-3 for growth promotion effects on tobacco. Microbes Environ 2013, 28, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Jalali, F.; Zafari, D.; Salari, H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecology 2017, 29, 67–75. [Google Scholar] [CrossRef]
- Fletcher, R.A.; Sopher, C.R.; Natraj, N.; Vettakkorumakankav. Modulation of gibberellins protects plants from environmental stresses (India). 2000, v. 5.
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol 2008, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C. Gibberellin signaling in plants - the extended version. Front Plant Sci 2011, 2, 107. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: still a mystery unresolved. Plant Signal Behav 2013, 8. [Google Scholar] [CrossRef]
- Hasan, H.A.H. Gibberellin and auxin production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Plant, Soil and Environment 2002, 48, 101–106. [Google Scholar] [CrossRef]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biology and Fertility of Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Rim, S.O.; Lee, J.H.; Choi, W.Y.; Hwang, S.K.; Suh, S.J.; Lee, I.J.; Rhee, I.K; Kim, J.G. Fusarium proliferatum KGL0401 as a New Gibberellin-Producing Fungus. 2005, v. 15.
- Claeys, H.; De Bodt, S.; Inze, D. Gibberellins and DELLAs: central nodes in growth regulatory networks. Trends Plant Sci 2014, 19, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Roldan, A.; Albacete, A.; Pascual, J.A. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry 2011, 72, 223–229. [Google Scholar] [CrossRef]
- Vadassery, J.; Ritter, C.; Venus, Y.; Camehl, I.; Varma, A.; Shahollari, B.; Novak, O.; Strnad, M.; Ludwig-Muller, J.; Oelmuller, R. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol Plant Microbe Interact 2008, 21, 1371–1383. [Google Scholar] [CrossRef]
- Kai, M.; Piechulla, B. Plant growth promotion due to rhizobacterial volatiles--an effect of CO2? FEBS Lett 2009, 583, 3473–3477. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Faubert, P.; Hagen, M.; Zu Castell, W.; Polle, A.; Schnitzler, J.P.; Rosenkranz, M. Volatile profiles of fungi--chemotyping of species and ecological functions. Fungal Genet Biol 2013, 54, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hiltpold, I.; Turlings, T.C. Belowground chemical signaling in maize: when simplicity rhymes with efficiency. J Chem Ecol 2008, 34, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Ditengou, F.A.; Muller, A.; Rosenkranz, M.; Felten, J.; Lasok, H.; van Doorn, M.M.; Legue, V.; Palme, K.; Schnitzler, J.P.; Polle, A. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat Commun 2015, 6, 6279. [Google Scholar] [CrossRef]
- Kobayashi, K. Plant methyl salicylate induces defense responses in the rhizobacterium Bacillus subtilis. Environ Microbiol 2015, 17, 1365–1376. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cellular & Developmental Biology - Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hung, R.; Yap, M.; Bennett, J.W. Age matters: the effects of volatile organic compounds emitted by Trichoderma atroviride on plant growth. Archives of Microbiology 2015, 197, 723–727. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




