Submitted:
29 June 2023
Posted:
30 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
| AQP | Function | Tissue Distribution |
|---|---|---|
| AQP0 | Also known as MIP, major intrinsic protein forms water channels in lens fiber cells and contributes to lens transparency | Lens fiber cells |
| AQP1 | Water transport | Kidney (proximal tubule, thin descending limb of Henle’s loop), Brain (astrocytes), red blood cells, lung (alveolar epithelium), and vascular endothelium |
| AQP2 | Water reabsorption | Kidney (principal cells of the collecting ducts) |
| AQP3 | Skin hydration and water transport | Kidney (proximal tubule), skin (basal and supra-basal layers), gastrointestinal tract, and other tissues |
| AQP4 | Water movement across the blood-brain barrier and plays a role in brain edema and water homeostasis | Brain (astrocyte end-feet at blood-brain barrier), spinal cord, skeletal muscle, and other tissues |
| AQP5 | Water transport | Salivary, lacrimal and other exocrine glands, respiratory tract submucosal glands |
| AQP6 | Possible role in acid-base homeostasis in the kidney. | Kidney (collecting ducts, intercalated cells) |
| AQP7 | Glycerol transport | Adipose tissue, liver, kidney (proximal tubule), and other tissues |
| AQP8 | Transport of water, urea, and other small solutes | Kidney (proximal tubule, thin descending limb of Henle’s loop), liver, and other tissues |
| AQP9 | Transport of water, urea, and other small solutes | Liver (hepatocytes), kidney (proximal tubule), testis, and other tissues |
| AQP10 | Water and solute transport in the gastrointestinal tract and kidney | Tissue distribution: Intestine (colon, ileum, duodenum), kidney (proximal tubule), liver, and other tissues |
| AQP11 | Glycerol channel activity and water channel activity, transport hydrogen peroxide | In several organs such as liver, kidney and brain |
| AQP12 | Digestive enzyme secretion such as maturation and exocytosis of secretory granules | Pancreatic acinar cells |
2. Autoimmune Response to AQPs of the CNS
2.1. Autoimmunity to AQP4
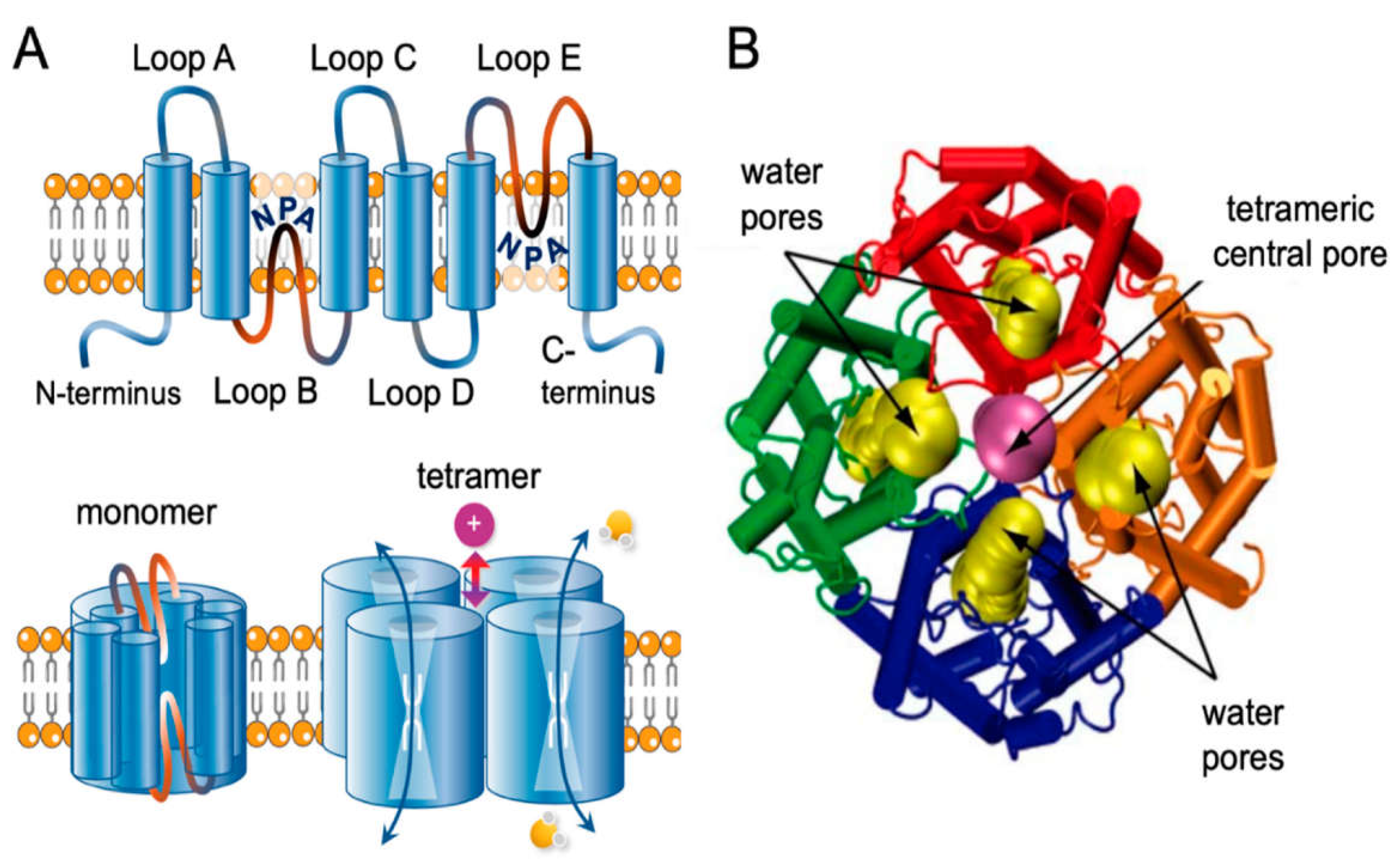
2.2. AQP1 in Health and Disease
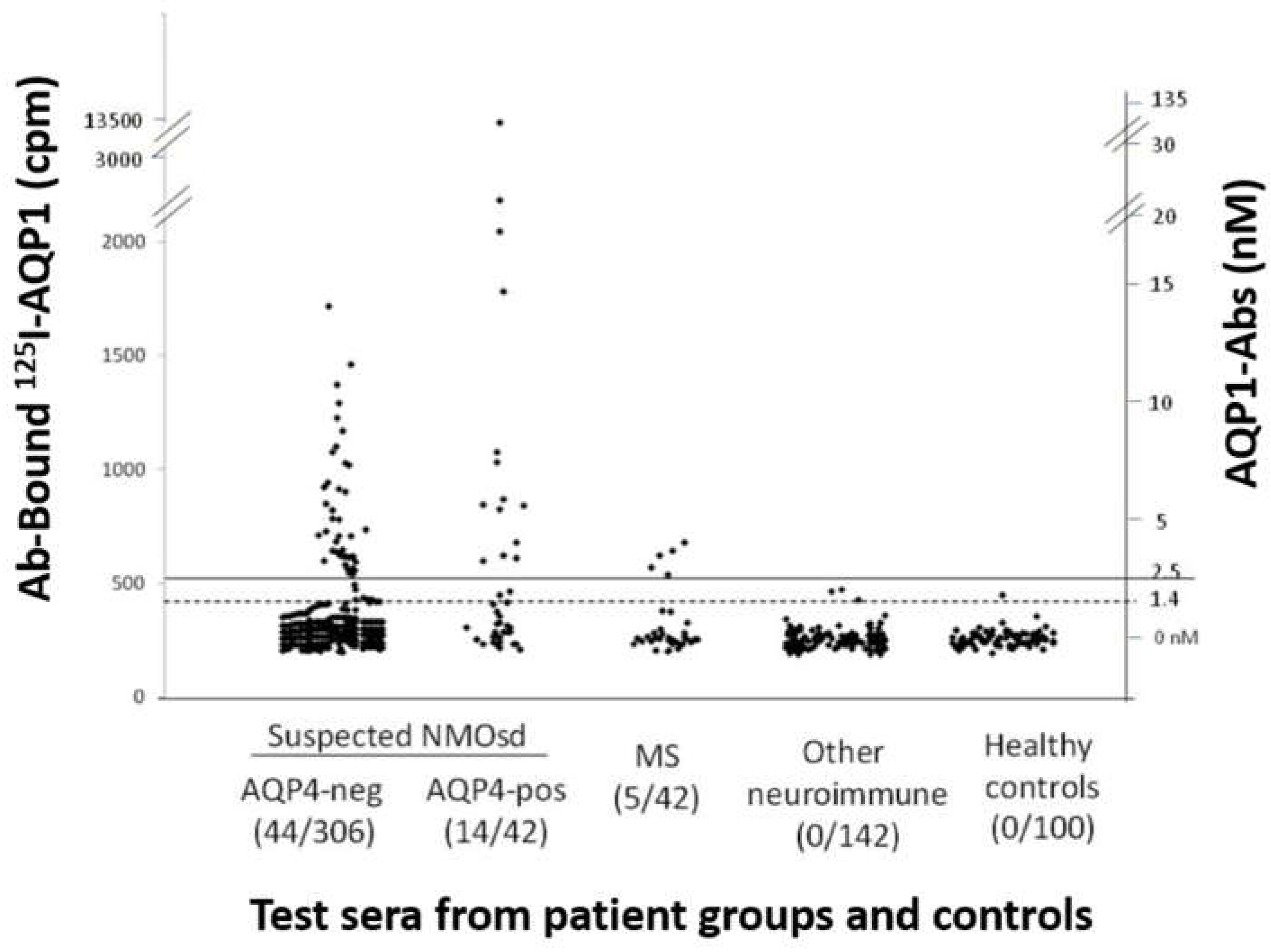
2.3. AQP1 Antibodies in NMOSD Phenotype
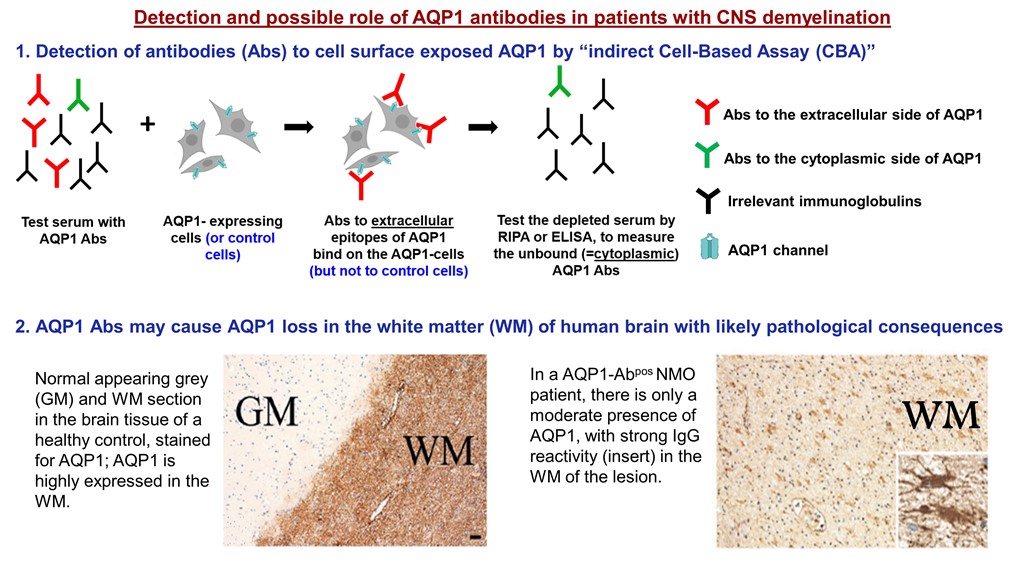
2.3.1. Assays for the Detection of Antibodies against AQP1
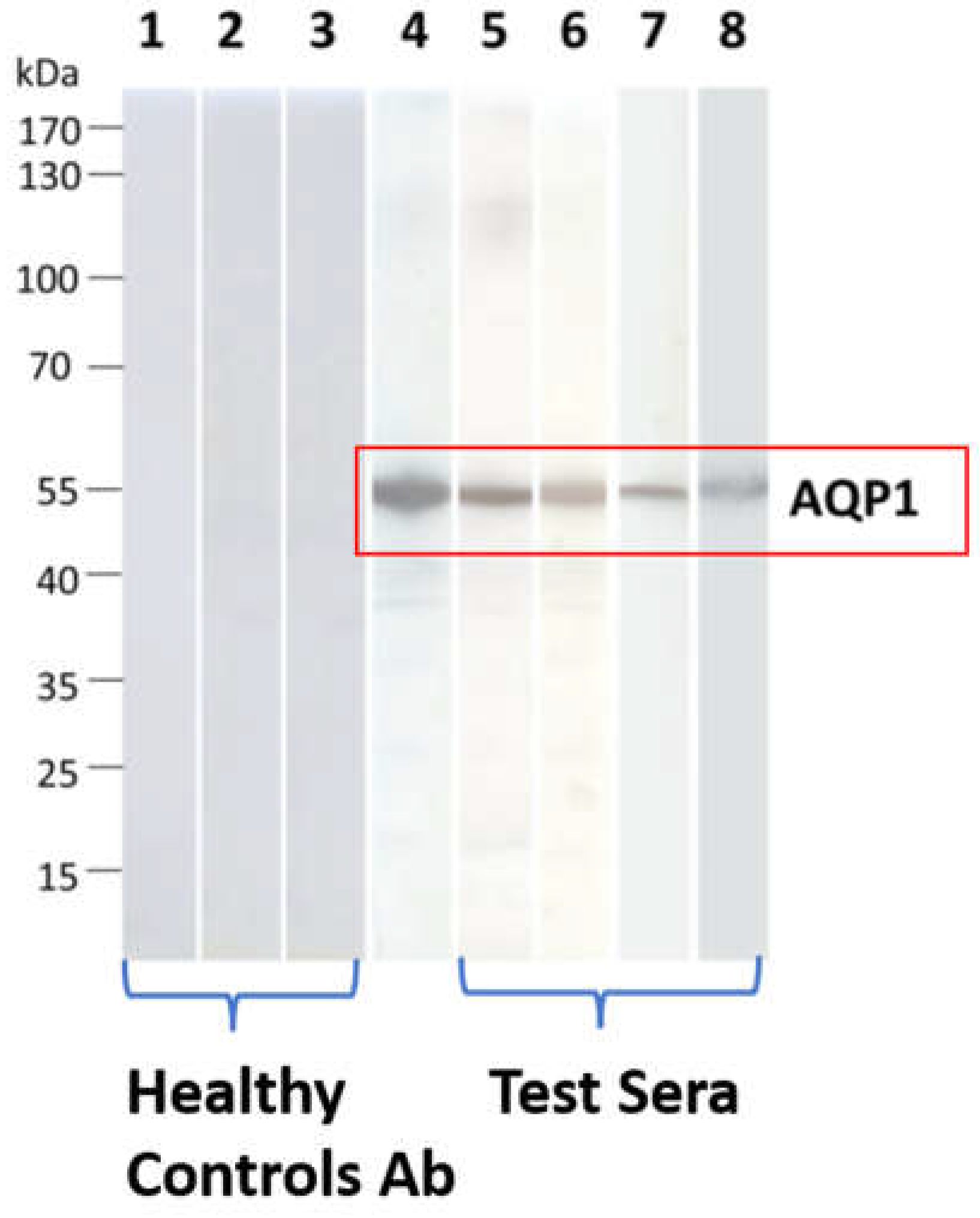
2.3.1.1. Assays for Antibody Binding to Cell-Free AQP1
- a.
- Radioimmunoprecipitation assay (RIPA)
- b.
- Western blotting with SDS-denatured AQP1 polypeptides
- c.
- ELISA with intact AQP1
- d.
- ELISA with AQP1 synthetic peptides (extracellular vs intracellular location of the epitopes)
2.3.1.2. Assays for the Detection of the Autoantibody Binding to the Cell-Embedded AQP1
- a.
- Direct CBA
- b.
- Indirect CBA
2.3.1.3. Combination of Assays for the Detection of AQP1 Antibodies
2.3.2. Are AQP1-Abs Pathogenic?
3. Conclusions
Author Contributions
Conflicts of Interest
References
- M. C. Papadopoulos and A. S. Verkman, "Aquaporin water channels in the nervous system," Nat Rev Neurosci, vol. 14, no. 4, pp. 265-77, Apr 2013. [CrossRef]
- Z. Zhou et al., "The Water Transport System in Astrocytes-Aquaporins," Cells, vol. 11, no. 16, Aug 18 2022. [CrossRef]
- M. Xu, M. Xiao, S. Li, and B. Yang, "Aquaporins in Nervous System," Adv Exp Med Biol, vol. 969, pp. 81-103, 2017. [CrossRef]
- T. Litman, R. Sogaard, and T. Zeuthen, "Ammonia and urea permeability of mammalian aquaporins," Handb Exp Pharmacol, no. 190, pp. 327-58, 2009. [CrossRef]
- K. Azad, T. Raihan, J. Ahmed, A. Hakim, T. H. Emon, and P. A. Chowdhury, "Human Aquaporins: Functional Diversity and Potential Roles in Infectious and Non-infectious Diseases," Front Genet, vol. 12, p. 654865, 2021. [CrossRef]
- S. Filippidis, R. B. Carozza, and H. L. Rekate, "Aquaporins in Brain Edema and Neuropathological Conditions," Int J Mol Sci, vol. 18, no. 1, Dec 28 2016. [CrossRef]
- T. Itoh et al., "Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells," Biochem Biophys Res Commun, vol. 330, no. 3, pp. 832-8, May 13 2005. [CrossRef]
- S. H. Lin et al., "Two novel aquaporin-2 mutations responsible for congenital nephrogenic diabetes insipidus in Chinese families," J Clin Endocrinol Metab, vol. 87, no. 6, pp. 2694-700, Jun 2002. [CrossRef]
- Kavanagh and N. S. Uy, "Nephrogenic Diabetes Insipidus," Pediatr Clin North Am, vol. 66, no. 1, pp. 227-234, Feb 2019. [CrossRef]
- Y. Noda and S. Sasaki, "Updates and Perspectives on Aquaporin-2 and Water Balance Disorders," Int J Mol Sci, vol. 22, no. 23, Nov 30 2021. [CrossRef]
- L. Tradtrantip, B. J. Jin, X. Yao, M. O. Anderson, and A. S. Verkman, "Aquaporin-Targeted Therapeutics: State-of-the-Field," Adv Exp Med Biol, vol. 969, pp. 239-250, 2017. [CrossRef]
- M. Wingerchuk et al., "International consensus diagnostic criteria for neuromyelitis optica spectrum disorders," Neurology, vol. 85, no. 2, pp. 177-89, Jul 14 2015. [CrossRef]
- J. S. Tzartos et al., "Autoimmune hemolytic anemia, demyelinating relapse, and AQP1 antibodies after alemtuzumab infusion," Neurol Neuroimmunol Neuroinflamm, vol. 7, no. 3, May 2020. [CrossRef]
- N. Landegren et al., "Autoantibodies Targeting a Collecting Duct-Specific Water Channel in Tubulointerstitial Nephritis," J Am Soc Nephrol, vol. 27, no. 10, pp. 3220-3228, Oct 2016. [CrossRef]
- J. S. Tzartos et al., "Antibodies to aquaporins are frequent in patients with primary Sjogren's syndrome," Rheumatology (Oxford), vol. 56, no. 12, pp. 2114-2122, Dec 1 2017. [CrossRef]
- S. Jarius et al., "Update on the diagnosis and treatment of neuromyelits optica spectrum disorders (NMOSD) - revised recommendations of the Neuromyelitis Optica Study Group (NEMOS). Part I: Diagnosis and differential diagnosis," J Neurol, Apr 6 2023. [CrossRef]
- Valencia-Sanchez and D. M. Wingerchuk, "Emerging Targeted Therapies for Neuromyelitis Optica Spectrum Disorders," BioDrugs, vol. 35, no. 1, pp. 7-17, Jan 2021. [CrossRef]
- G. C. Rosu et al., "Distribution of Aquaporins 1 and 4 in the Central Nervous System," Curr Health Sci J, vol. 45, no. 2, pp. 218-226, Apr-Jun 2019. [CrossRef]
- J. L. Trillo-Contreras, R. Ramirez-Lorca, J. Villadiego, and M. Echevarria, "Cellular Distribution of Brain Aquaporins and Their Contribution to Cerebrospinal Fluid Homeostasis and Hydrocephalus," Biomolecules, vol. 12, no. 4, Mar 31 2022. [CrossRef]
- V. A. Lennon et al., "A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis," Lancet, vol. 364, no. 9451, pp. 2106-12, Dec 11-17 2004. [CrossRef]
- V. A. Lennon, T. J. Kryzer, S. J. Pittock, A. S. Verkman, and S. R. Hinson, "IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel," J Exp Med, vol. 202, no. 4, pp. 473-7, Aug 15 2005. [CrossRef]
- M. Wingerchuk, W. F. Hogancamp, P. C. O'Brien, and B. G. Weinshenker, "The clinical course of neuromyelitis optica (Devic's syndrome)," Neurology, vol. 53, no. 5, pp. 1107-14, Sep 22 1999. [CrossRef]
- D. M. Wingerchuk, V. A. Lennon, C. F. Lucchinetti, S. J. Pittock, and B. G. Weinshenker, "The spectrum of neuromyelitis optica," Lancet Neurol, vol. 6, no. 9, pp. 805-15, Sep 2007. [CrossRef]
- Zekeridou and V. A. Lennon, "Aquaporin-4 autoimmunity," Neurol Neuroimmunol Neuroinflamm, vol. 2, no. 4, p. e110, Aug 2015. [CrossRef]
- J. Sugie, M. Intaglietta, and L. A. Sung, "Water transport and homeostasis as a major function of erythrocytes," Am J Physiol Heart Circ Physiol, vol. 314, no. 5, pp. H1098-H1107, May 1 2018. [CrossRef]
- J. L. Trillo-Contreras, J. J. Toledo-Aral, M. Echevarria, and J. Villadiego, "AQP1 and AQP4 Contribution to Cerebrospinal Fluid Homeostasis," Cells, vol. 8, no. 2, Feb 24 2019. [CrossRef]
- R. Turkoglu, H. Lassmann, F. V. Aker, J. Tzartos, S. Tzartos, and E. Tuzun, "Recurrent tumefactive demyelinating lesions: a pathological study," Clin Neuropathol, vol. 36, no. 4, pp. 195-198, Jul/Aug 2017. [CrossRef]
- T. Walz et al., "The three-dimensional structure of aquaporin-1," Nature, vol. 387, no. 6633, pp. 624-7, Jun 5 1997. [CrossRef]
- S. Khan, C. Ricciardelli, and A. J. Yool, "Targeting Aquaporins in Novel Therapies for Male and Female Breast and Reproductive Cancers," Cells, vol. 10, no. 2, Jan 22 2021. [CrossRef]
- J. Yu, A. J. Yool, K. Schulten, and E. Tajkhorshid, "Mechanism of gating and ion conductivity of a possible tetrameric pore in aquaporin-1," Structure, vol. 14, no. 9, pp. 1411-23, Sep 2006. [CrossRef]
- J. S. Tzartos, C. Stergiou, K. Kilidireas, P. Zisimopoulou, T. Thomaidis, and S. J. Tzartos, "Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders," PLoS One, vol. 8, no. 9, p. e74773, 2013. [CrossRef]
- K. Schanda et al., "Antibodies to aquaporin-1 are not present in neuromyelitis optica," Neurol Neuroimmunol Neuroinflamm, vol. 2, no. 6, p. e160, Dec 2015. [CrossRef]
- Sanchez Gomar et al., "Comparative Analysis for the Presence of IgG Anti-Aquaporin-1 in Patients with NMO-Spectrum Disorders," Int J Mol Sci, vol. 17, no. 8, Jul 23 2016. [CrossRef]
- Y. Long et al., "Development of a cell-based assay for the detection of anti-aquaporin 1 antibodies in neuromyelitis optica spectrum disorders," J Neuroimmunol, vol. 273, no. 1-2, pp. 103-10, Aug 15 2014. [CrossRef]
- M. Jasiak-Zatonska, S. Michalak, K. Osztynowicz, W. Kozubski, and A. Kalinowska-Lyszczarz, "Relationship between blood-brain permeability and antibodies against aquaporins in neuromyelitis optica spectrum disorders and multiple sclerosis patients," Neurol Neurochir Pol, vol. 56, no. 4, pp. 308-317, 2022. [CrossRef]
- Vincent et al., "Antibodies identified by cell-based assays in myasthenia gravis and associated diseases," Ann N Y Acad Sci, vol. 1274, pp. 92-8, Dec 2012. [CrossRef]
- P. D. Burbelo, M. J. Iadarola, J. M. Keller, and B. M. Warner, "Autoantibodies Targeting Intracellular and Extracellular Proteins in Autoimmunity," Front Immunol, vol. 12, p. 548469, 2021. [CrossRef]
- R. J. Ludwig et al., "Mechanisms of Autoantibody-Induced Pathology," Front Immunol, vol. 8, p. 603, 2017. [CrossRef]
- "<41. Letter. Antibodies to surface aquaporin-1 are present in NMO but low AQP1 expression prohibits use of CBA.pdf>.".
- R. Halverson and T. Peyrard, "A review of the Colton blood group system," Immunohematology, vol. 26, no. 1, pp. 22-6, 2010. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/20795314.
- R. B. Covin, K. S. Evans, R. Olshock, and H. W. Thompson, "Acute hemolytic transfusion reaction caused by anti-Coa," Immunohematology, vol. 17, no. 2, pp. 45-9, 2001. [Online]. Available: https://www.ncbi.nlm.nih.gov/pubmed/15373591.
- S. Siritho, I. Nakashima, T. Takahashi, K. Fujihara, and N. Prayoonwiwat, "AQP4 antibody-positive Thai cases: clinical features and diagnostic problems," Neurology, vol. 77, no. 9, pp. 827-34, Aug 30 2011. [CrossRef]
- S. J. Pittock and V. A. Lennon, "Aquaporin-4 autoantibodies in a paraneoplastic context," Arch Neurol, vol. 65, no. 5, pp. 629-32, May 2008. [CrossRef]
- T. Misu et al., "Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica," Acta Neuropathol, vol. 125, no. 6, pp. 815-27, Jun 2013. [CrossRef]
- P. A. Desai, C. M. Romere, L. Nguyen, A. Saksena, S. J. Abdullah, and A. E. Diaz, "Severe Coombs Positive Autoimmune Hemolytic Anemia after Alemtuzumab Infusion for Relapsing Remitting Multiple Sclerosis. What Can We Learn?," Blood, vol. 132, no. Supplement 1, pp. 2331-2331, 2018. [CrossRef]
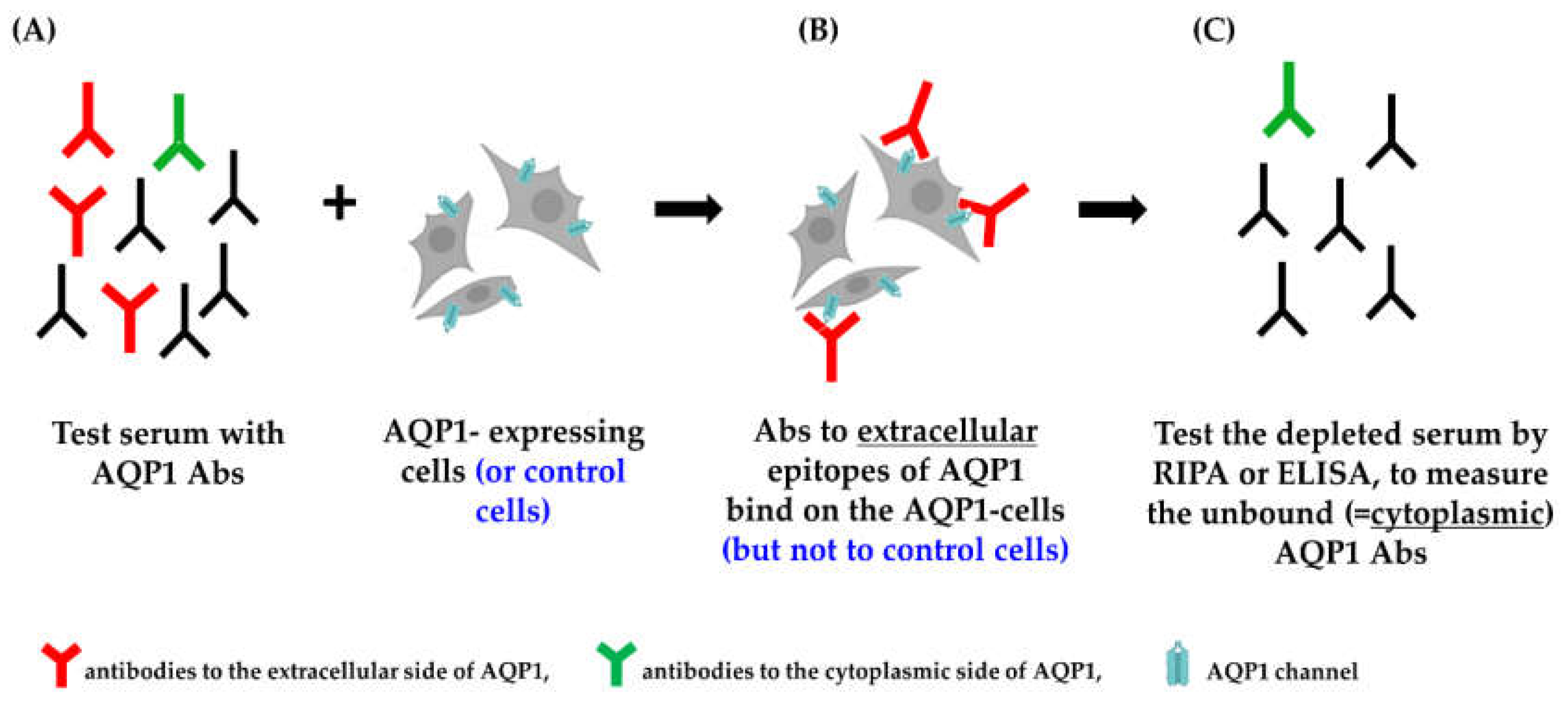
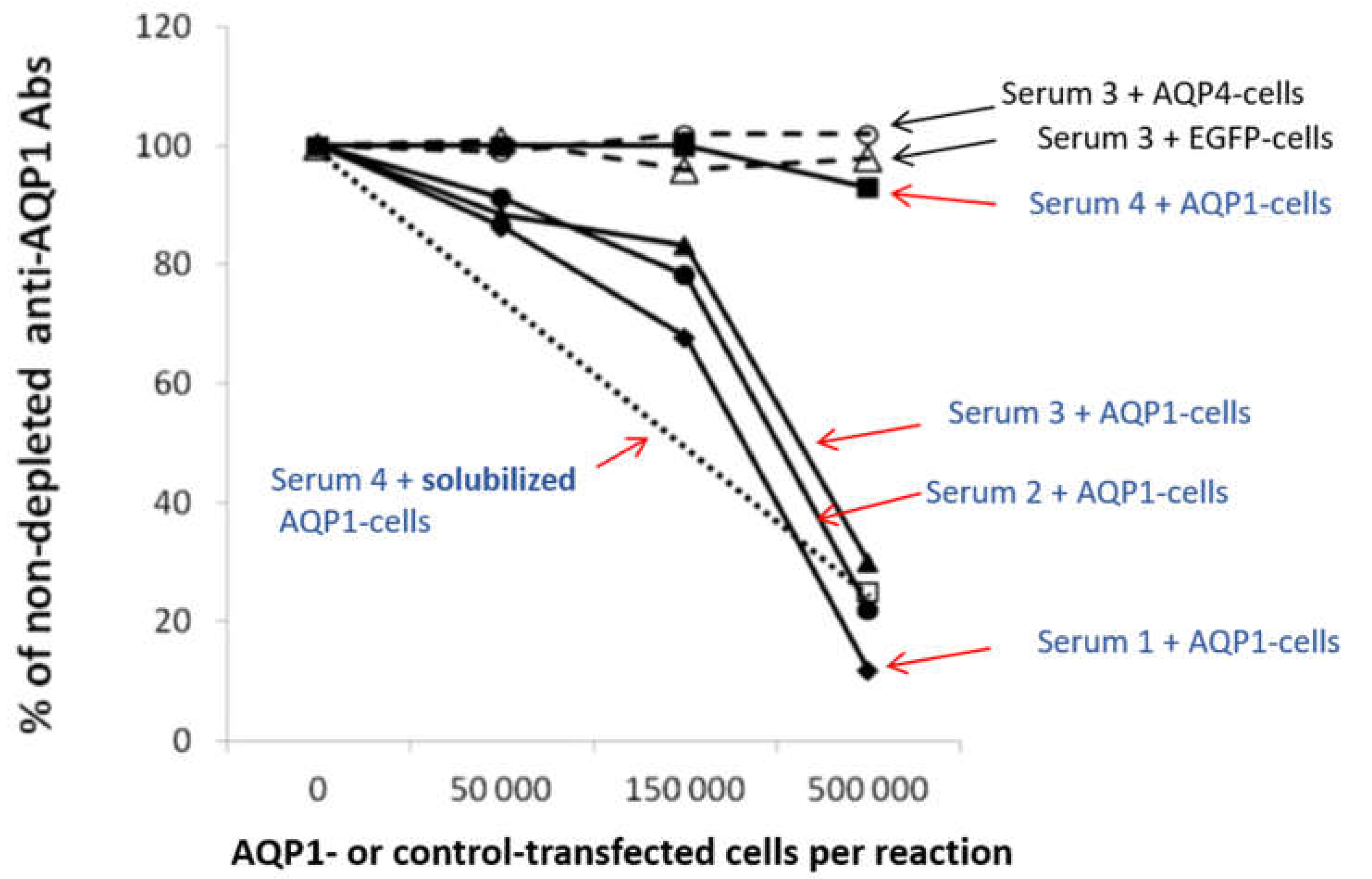
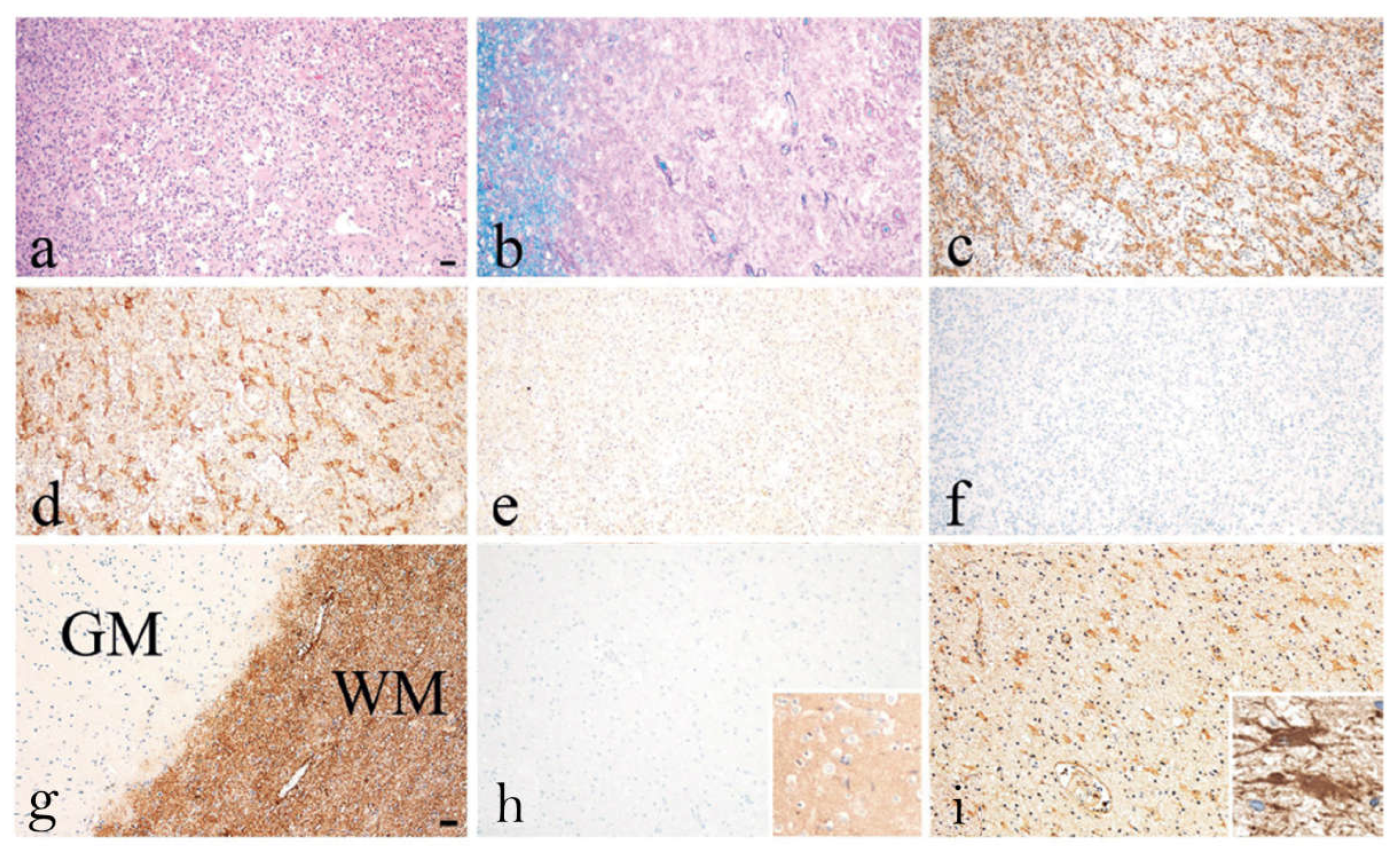
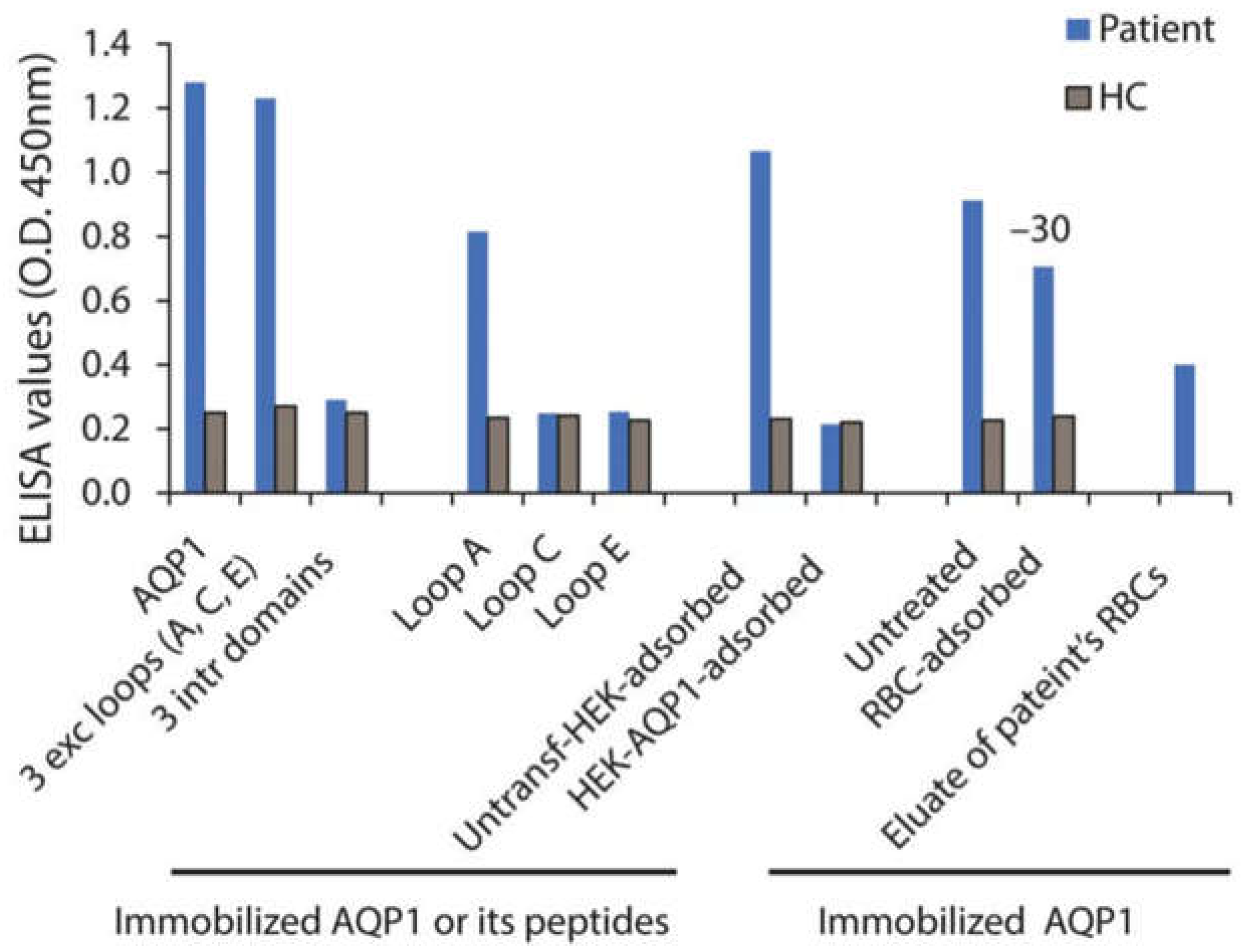
| Patienta | Sex | Epitopesb | Specific Binding on Live AQP1-Cells (“Indirect CBA”)c | MRI & Clinical Data (& Neoplasms) |
|---|---|---|---|---|
| 1 | F | Cytopl | ΝΤ | LETM and optic neuritis (NMO) |
| 2 | M | NT | Few antibodies (5%) | LETM and optic neuritis (NMO) |
| 3 | F | Extr-C | Most antibodies | LETM and optic neuritis (NMO) |
| 4 | F | Extr-A | Most antibodies | LETM and optic neuritis (NMO) |
| 5 | F | Extr-A | Most antibodies | LETM and optic neuritis (NMO) |
| 6 | F | Cytopl | NT | LETM |
| 7 | F | Cytopl | Few antibodies (16%) | LETM |
| 8 | F | Extr-E | Some antibodies (30%) | LETM |
| 9 | M | Extr-A | Most antibodies | LETM |
| 10 | M | Extr-A | Most antibodies | LETM |
| 11 | F | Extr-A | Most antibodies | LETM |
| 12 | F | Extr-A | Few antibodies (16%) | LETM |
| 13 | F | Extr-A | Most antibodies | LETM |
| 14 | F | Extr-A | Most antibodies | LETM |
| 15 | M | Extr & Cytopl | Few antibodies (19%) | LETM & Hodgkin lymphoma |
| 16 | M | Extr-A | Most antibodies | LETM & brain lesions (fulfilled Barkhof criteria) |
| 17 | M | Extr-A | Most antibodies | Transverse myelitis & kidney neoplasm |
| 18 | F | Extr-E | Most antibodies | MS & predominant spinal cord lesions |
| 19 | F | Extr-A | Most antibodies | MS & predominant spinal cord lesions & breast cancer |
| 20 | F | Cytopl | No antibodies | MS |
| 21 | F | Cytopl | No antibodies | MS |
| 22 | F | Cytopl | No antibodies | MS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





