1. Introduction
Multiple Sclerosis (MS) is a chronic, inflammatory, immune-mediated, demyelinating and neurodegenerative disease of the central nervous system (CNS) [
1,
2]. It can cause various symptoms (e.g. disturbances in movement, coordination, bulbar and visual functions; sensory deficits; cognitive impairment; fatigue and psychiatric conditions) depending on the localization of the lesions. The diagnosis of MS is based on physical examinations, neuroimaging, blood and cerebrospinal fluid (CSF) analysis [
1,
3]. The McDonald criteria, first published in 2001, were most recently updated in 2017 [
4]. In 2024 Xavier Montalban presented a new revised proposal at the ECTRIMS Congress in Copenhagen. In its 2017 version the presence of IgG oligoclonal bands (OCB) in CSF can substitute the criterion dissemination in time. Thus, this enables early MS diagnosis in patients experiencing a first CNS demyelinating event (clinically isolated syndrome, CIS) who meet the criteria for dissemination in space [
1,
4]. In the planned new version (2024) the Kappa Free Light Chain (κFLC) index is interchangeable with OCB. Using highly effective disease-modifying therapies (DMT) as early as possible provides an opportunity to delay long-term progression and maintain a good quality of life [
1]. New CSF and serum biomarkers can play an important role in the early diagnosis and in the estimation of prognosis [
1,
3]. The molecular biomarkers used in clinical practice and potential biomarkers in MS are summarized in
Table 1 (based on [
1,
3,
5,
6]).
B cells produce IgG and OCB intrathecally in MS [
7]. OCB positivity is usually defined by two or more IgG OCB in the CSF (without their appearance in the serum) [
8]. Laboratory testing methods for oligoclonal bands are isoelectric focusing followed by immunofixation [
9]. They are with high costs, need a specialist, and interpretation is also subjective [
1]. The sensitivity of CSF IgG OCB is 72.2% and the specificity is 95.2% [
10]. The presence of IgG OCB in the CSF indicates a higher risk of MS in patients with CIS [
8]. The intrathecal IgG synthesis can also be determined by quantitative methods, i.e., measurement of IgG in CSF and serum, followed by calculation of an intrathecal fraction. Different formulae have been suggested including the Auer and Hegen, the Reiber formulae or the IgG index. The IgG index >0.7 indicates a higher intrathecal B cell response and is associated with MS progression [
11]. In 2020, Zheng et al. [
12] and Simonsen et al. [
13] found the IgG index > 0.7 well predictive to OCB positivity, but it could not replace OCB in MS diagnosis [
14]. Quantitative methods show a lower diagnostic sensitivity than OCB.
Kappa free light chains are produced by B cells [
15,
16], and can be piled up in the CSF in case of inflammatory diseases of the CNS [
17]. Kappa free light chains (κFLC) can be measured by nephelometry or turbidimetry, which are reliable, cost-effective, and straightforward methods [
18]. κFLC concentrations can be measured both in the CSF and serum, followed by calculation of the κFLC index or an intrathecal κFLC fraction (formulas can be found in [
18]). Some centers just use the absolute CSF κFLC concentration. κFLC index has been recommended and a recent meta-analysis showed a diagnostic sensitivity of 88% and specificity of 89% which was similar to OCB with 85% sensitivity and 92% specificity [
18]. High CSF κFLC levels have been associated with predicting conversion from CIS to MS [
19,
20]. The limitation of the κFLC index is that there is no consensus about its diagnostic cut-off values [
3].
The components of Neurofilament proteins are light, medium or heavy chains, and among them Neurofilament Light Chain (NfL) is a marker of neurodegeneration. A promising biomarker in MS monitoring and treatment follow-up is the Neurofilament Light Chain. NfL is located in the neuronal cytoskeleton, it enters to the interstitial fluid in connection with axon injury and neurodegeneration [
21] (but NfL is not solely proof of neurodegeneration, the destruction of axons happens due to inflammation). In case of neuroaxonal injury its level is raised both in the CSF and serum. Electrochemiluminescence and single-molecule array assays are highly sensitive and they were developed to become eligible for serum NfL measurements [
22]. A summary about these methods in various MS clinical studies can be found in the review of Kouchaki et al. [
23]. Increased level of NfL (in the CSF and blood) can be found not only in MS, but also in other neurodegenerative disorders such as dementias (Alzheimer or frontotemporal type), ALS, brain injury, stroke, etc [
21,
24,
25,
26,
27,
28,
29,
30,
31,
32]. In MS serum NfL is an independent predictive biomarker for CIS conversion to clinically define MS [
33]. Blood NfL level is related to the MS disease activity and it has predictive value [
34]. In 2022 Benkert et al. [
35] aimed to assemble a reference database with age- and body mass index-corrected sNfL values in MS. The limitation of NfL measurement is that no consensus regarding NfL cut-off values in clinical practice [
23]. In addition, some studies report the usefulness of NfL in MS treatment follow-up, as nataliumab, ocrelizumab, rituximab, mitoxantron, and fingolimod were found to decrease the NfL levels [
34,
36,
37,
38,
39,
40,
41,
42,
43].
Since molecular biomarkers play a major role in establishing the diagnosis, in monitoring the progression of the disease, in making therapeutic decisions and assessing the effectiveness of therapy, strict adherence to international standards is essential, along with continued advancement (e.g. identification of new markers using mass spectrometry and other advanced analytical technologies and their introduction into clinical practice). This study aimed to determine a “snapshot” of the use of molecular biomarkers in MS in different Central-Eastern European Countries, which cover 107 million inhabitants.
2. Results
2.1. General Information About the Surveyed Centers
First, we assessed the organizational structure of the institutions. The results are summarized in
Table 2.
Most of the surveyed centers have neurology inbed patients and a specialized MS clinic. A research agenda focused on MS and biomarker development was present in ≥50% of the surveyed centers in Slovakia, Slovenia, and the reference centers.
Most of the centers treat 30-100 (numbers are marked with italic) or more than 100 MS patients per month (Croatia 3/5, 60% (treat 30-100 MS patients per month) or 1/5, 20% (treat more than 100 MS patients per month), Czech Republic 1/9, 11% or 8/9, 89%, Poland 8/20, 40% or 11/20, 55%, Romania 6/14, 43% or 6/14, 43%, Serbia 1/5, 20% or 4/5, 80%, Slovakia 3/5, 60% or 2/5, 40%, Slovenia 1/2, 50% or 1/2, 50%, reference centers 0/4, 0% or 4/4, 100%).
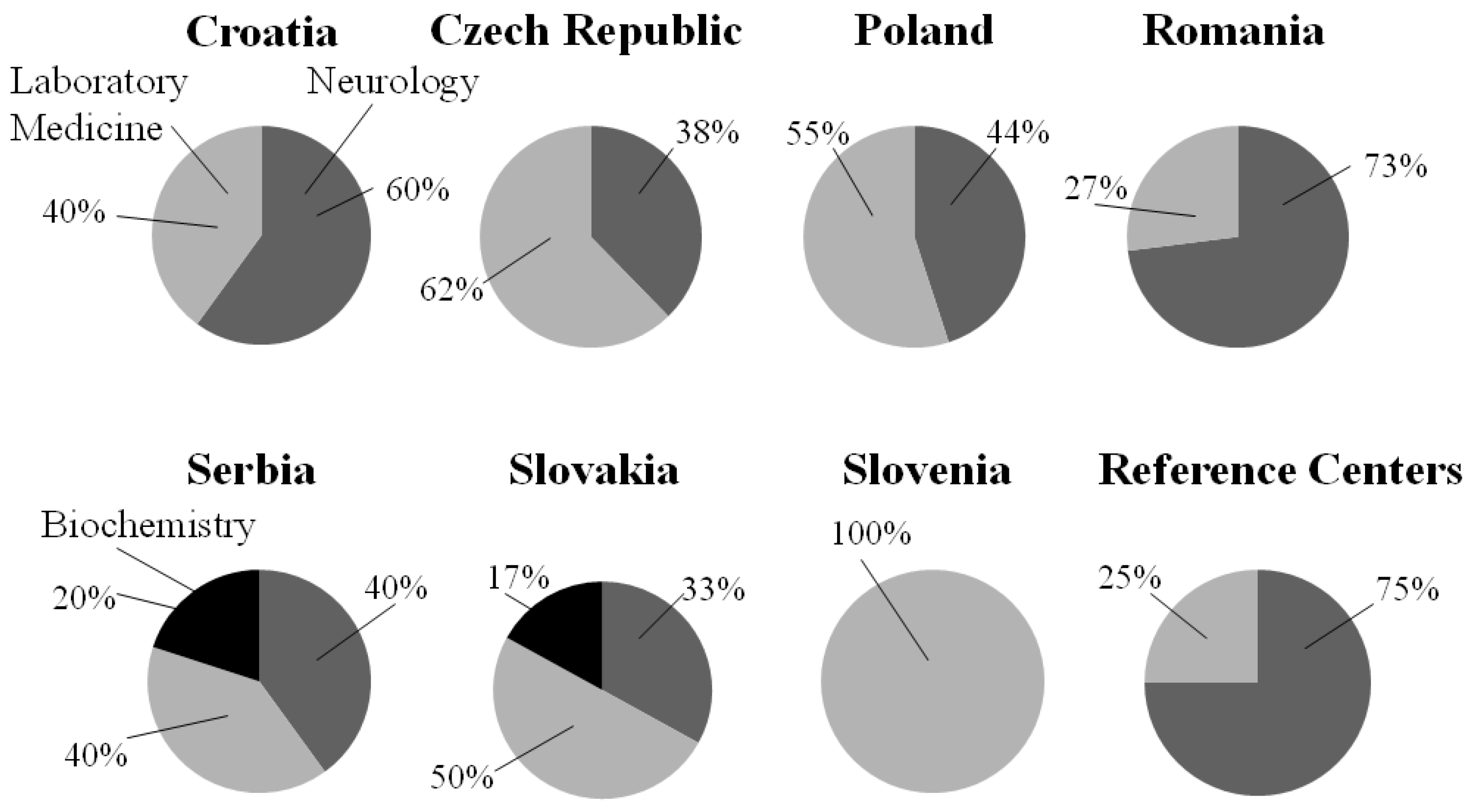
In all examined centers, the laboratory analysis of the CSF takes place in their institution, except for 3 centers in Romania (3/14, which is 21.5% of the Romanian participated centers), 1 in the Czech Republic (1/9, 11%), and 1 in Poland (1/20, 5%), where it is performed in a private laboratory, or in other hospital. The laboratory in these institutions is operated by Laboratory Medicine in 100% of the responding centers in Croatia (5/5), Romania (11/11), and Slovenia (2/2). In the Czech Republic, Poland and Serbia, it is mostly operated by Laboratory Medicine, only (1/8) 12.5%, (1/19) 5% and (1/5) 20% of the centers answered Neurology, and 1 (1/8, 12.5%) center in Czech Republic the Immunology, and one (1/19, 5%) in Poland the diagnostic unit. In Slovakia the centers marked Neurology (2/5, 40%), and Laboratory Medicine (2/5, 40%) in equal proportion, and one (1/5, 20%) denoted Biochemistry. The situation is quite different in reference centers, because it runs under Neurology in (3/4) 75%. In 1 reference center (1/4) (25%), it belongs to the Laboratory Medicine and Division of Neuropathology and Neurochemistry, Department of Neurology.
The answers to the question - of which discipline is responsible for the interpretation of CSF results (Neurology, Laboratory Medicine or Biochemistry) - can be seen in Figure. 1.
Figure 1.
The responsibility for the interpretation of CSF results in different countries. Neurogy is marked with dark gray, Biochemistry with black, and Laboratory Medicine with light gray.
Figure 1.
The responsibility for the interpretation of CSF results in different countries. Neurogy is marked with dark gray, Biochemistry with black, and Laboratory Medicine with light gray.
Besides this one center in Czech Republic marked Immunology, and one center in Austria answered Division of Neuropathology and Neurochemistry, Department of Neurology.
2.2. Cerebrospinal Fluid Analysis
All surveyed centers in all participating countries always perform lumbar puncture (LP) for CSF analysis in suspected MS cases, except in Romania and Poland. In Romania only (6/14) 43% of the centers do it always, (8/14) 57% of the Romanian centers carry out LP for CSF analysis when magnetic resonance imaging (MRI) is inconclusive, (6/14) 43% when clinical presentation is inconclusive in patients with suspected MS, and (1/14) 7% for research purposes. In Poland the majority of the centers do the LP always (17/20) 85%, and only (2/20) 10% when MRI is inconclusive, and (1/20) 5% when clinical presentation is inconclusive.
100% of the Croatian, Polish, Serbian, Slovakian, Slovenian and the reference centers answered that both the diagnosis and the differential diagnosis were the main reasons to perform CSF analysis in patients with suspected MS. One Czech center (1/9, 11%) performs it only for diagnostic and one Romanian center (1/14, 7%) only for differential diagnostic purposes, the other centers of these countries use it also for both reasons.
On the question of how long it takes to get the report on routine CSF parameters we see rather diverse results even within individual countries. In Croatia it is less than 1 day - 40% / 1 day (marked with italic) - 20% / more than 1 day - 40%; in Czech Republic 78% / 11% / 11%; in Poland 75% / 10% / 15%; in Romania 36% / 14% / 50%; in Serbia 80% / 20% / 0%; in Slovakia 40% / 20% / 40%; in Slovenia 50% / 0% / 50%; and in the reference centers 75% / 25% / 0%.
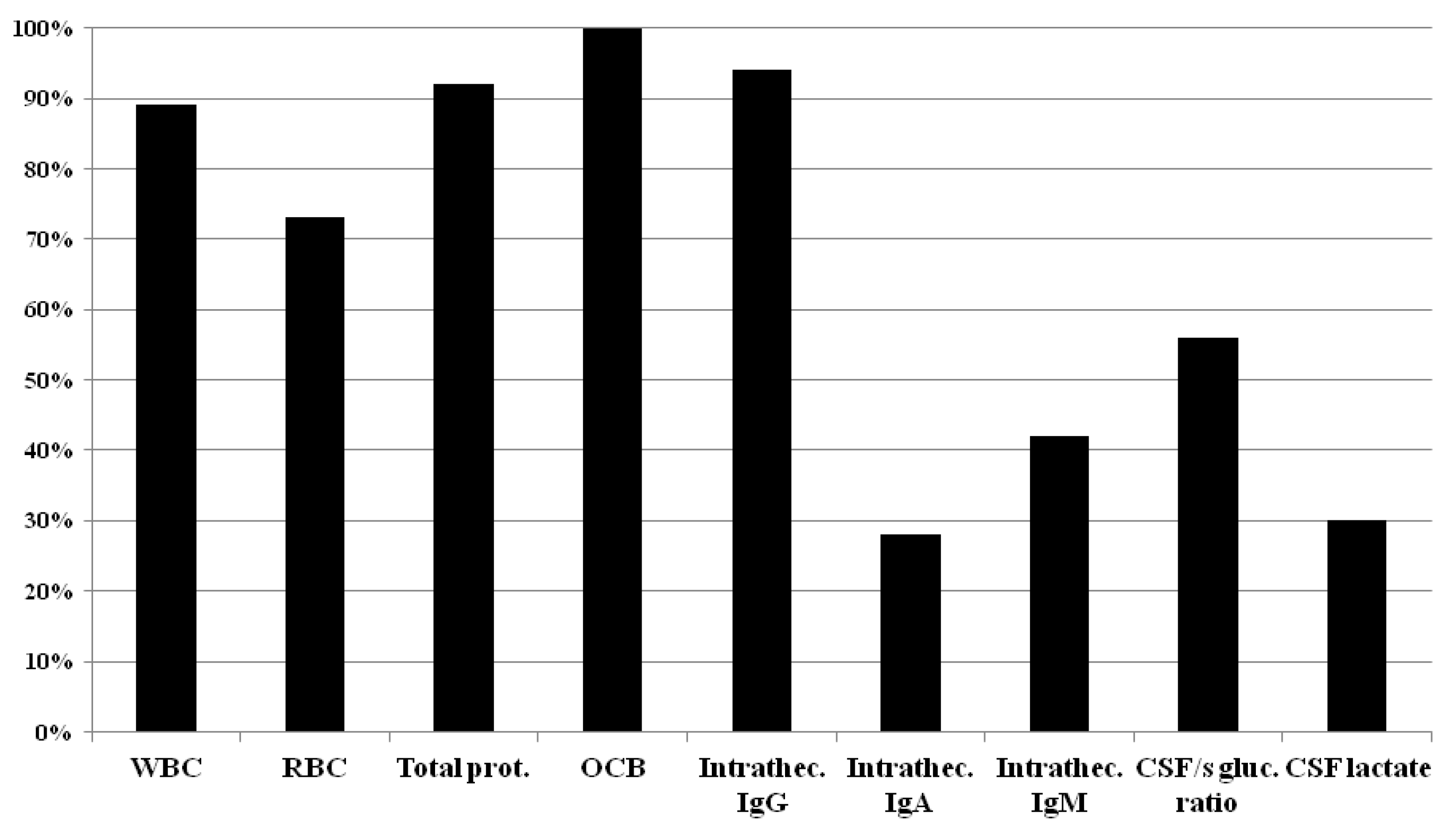
Routine CSF parameters usually requested by surveyed centers in suspected MS cases are summarized in
Figure 2.
WBC: White blood cell count; RBC: Red blood cell count; Total protein; OCB: Oligoclonal bands; Intrathecal IgG synthesis; Intrathecal IgA synthesis; Intrathecal IgM synthesis; CSF/serum glucose ratio; CSF lactate
In
Table 3. the details of the routine CSF parameters are presented by countries.
As can be seen from the results, majority of the centers use white blood cell count, red blood cell count, total protein, OCB and intrathecal IgG. To our question of which CSF parameters are considered as MS specific, ≥50% of the responding centers marked the CSF-restricted OCB. Only in Czech Republic the denotation of the intrathecal Kappa Free Light Chain was (9/9) 100%, and in the other centers the choice of intrathecal Kappa Free Light Chains was ≤50% (
Table 4.).
On the question that “what is the number of CSF-OCB that is used by your laboratory to define OCB positivity?” we only received a homogeneous answer from the Czech Republic, where (9/9) 100% of the responding centers indicated ≥2 CSF bands. There is no clear consensus in the other countries (see the details in
Table 5.).
Most of the responding centers from all participated countries also apply the IgG index to calculate the intrathecal fraction of IgG (Croatia: (4/5) 80%; Czech Republic: (6/9) 67%; Poland (15/20) 75%; Romania: (11/14) 78.5%; Serbia: (4/5) 80%; Slovakia (3/5) 60%; Slovenia (1/2) 50%; reference centers: (3/4) 75%). The use of Reibergram is mostly below 50%, except Slovakia, where it is 100% (5/5).
It takes more than 3 days to get the report of OCB in the majority of the centers (Croatia: (4/5) 80%; Czech Republic: (7/9) 78%; Poland (18/20) 90%; Romania: (13/14) 93%; Serbia: (5/5) 100%; Slovenia (2/2) 100%; reference centers: (3/4) 75%). In Slovakia, 3 days or more and 3 days are equal part (2/5)-(2/5) 40%-40%.
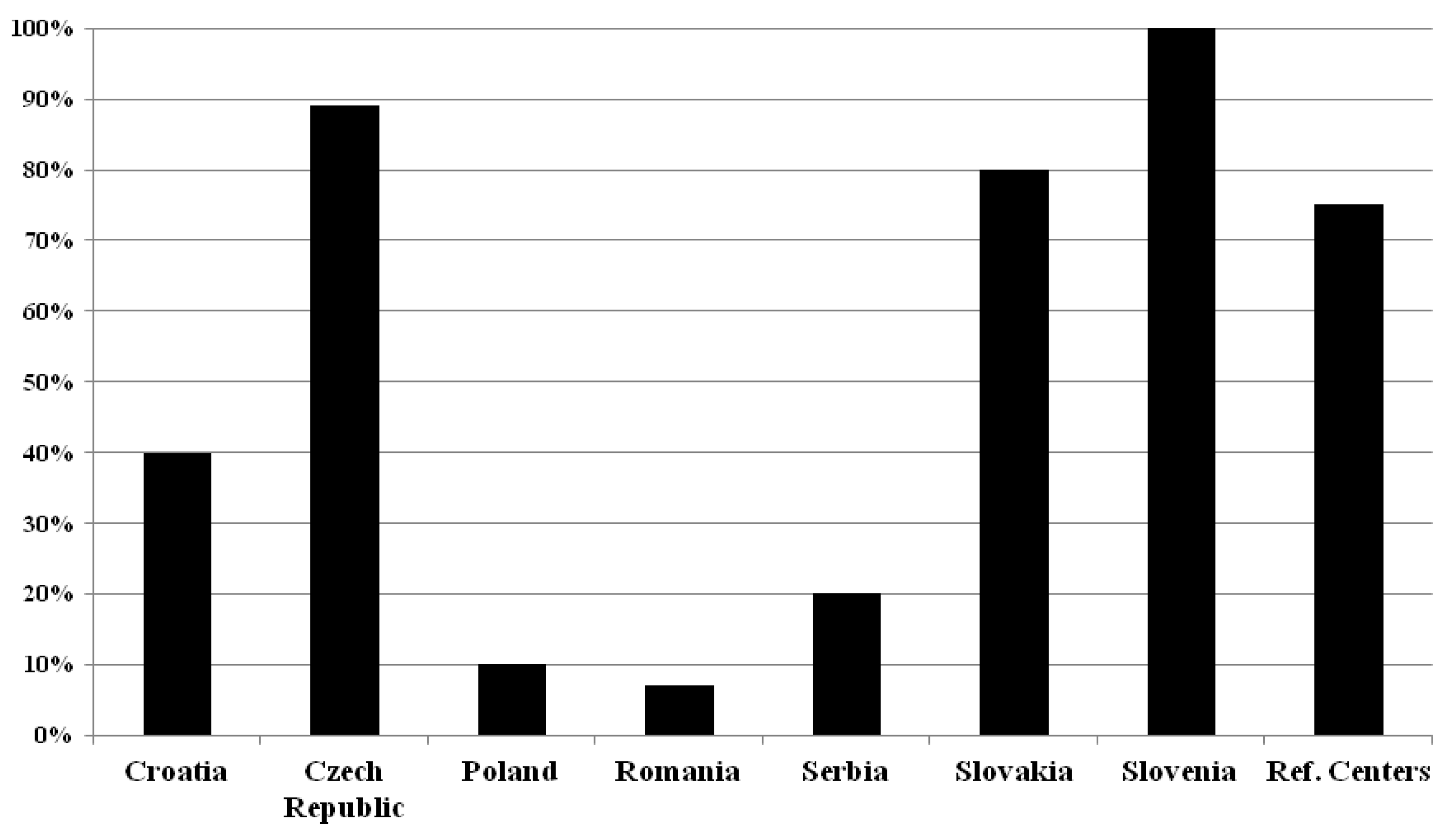
2.3. Determination of Kappa Free Light Chain (κFLC) in patients with suspected MS
Our results about the determination of κFLC in patients with suspected MS is summarized in
Figure 3.
In Croatia, only (2/5) 40% of the participated MS centers ask for the determination of κFLC. All of them preferred the κFLC index (2/2) (100%). In Czech Republic the majority of the centers (8/9) (89%) request the κFLC determination, (5/8) 62.5% of these respondents preferred the κFLC index, the rest the CSF κFLC concentration. In Poland, only 2 of the centers (2/20) (10%) ask to determine only the κFLC index. In Romania only one investigated center (1/14) (7%) uses CSF κFLC concentration (just in selected cases), the others do not ask for κFLC determination. In Serbia also only one of the surveyed centers (1/5) (20%) asks to determine the κFLC index. In Slovakia (4/5) 80% of the centers seek the determination of κFLC, and in the majority of the cases they asked the CSF κFLC concentration (3/4) (75%). In Slovenia, (2/2) 100% of the centers ask the determination of κFLC index and (1/2) 50% of CSF κFLC concentration. In the reference centers κFLC index is preferred only (3/3) (100%).
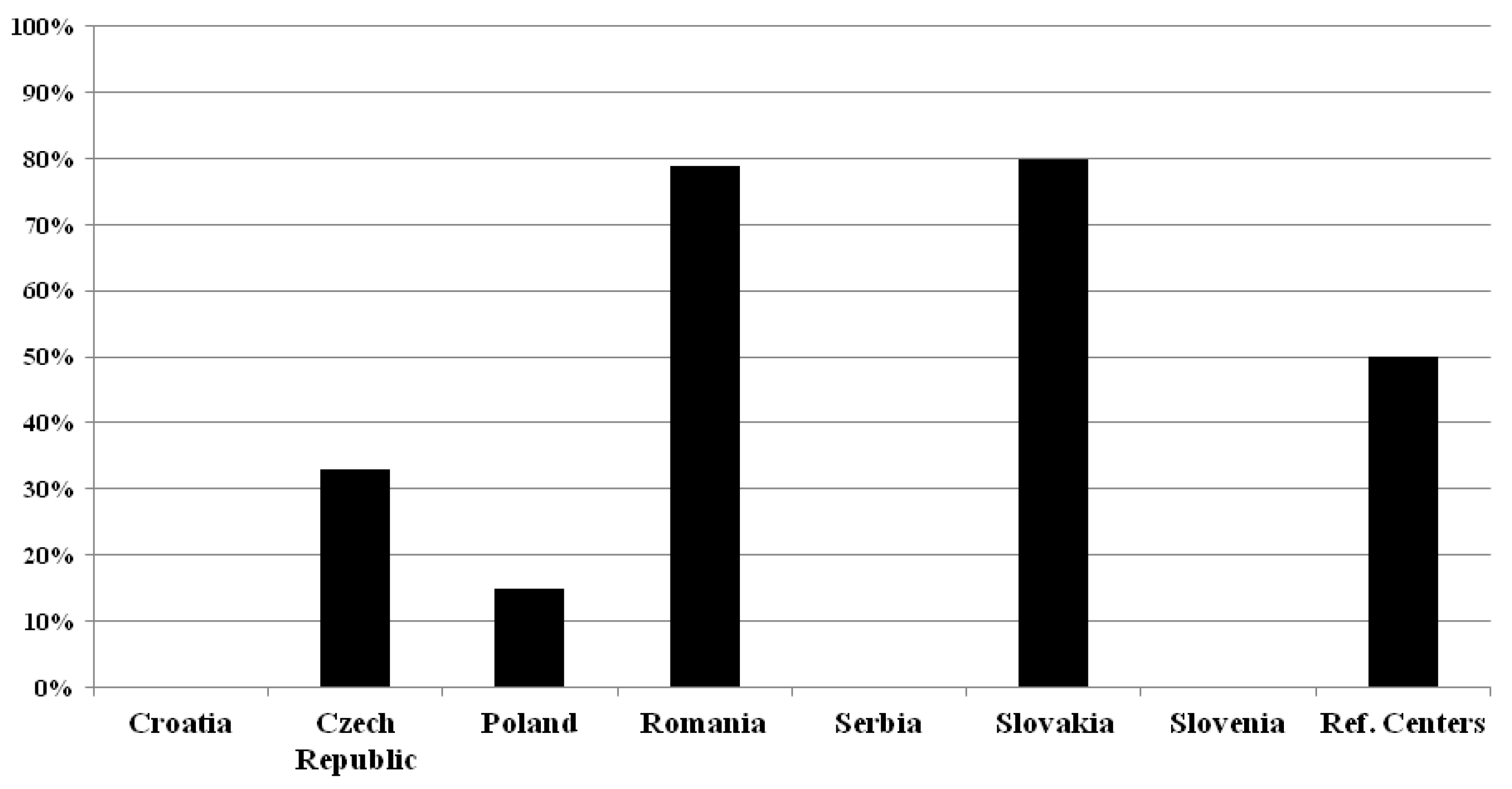
2.4. Specific CSF and/or Blood Biomarkers in Patients with MS
None of the surveyed Croatian, Serbian and Slovenian centers use Neurofilament Light Chain or any other specific CSF and/or blood biomarkers in patients with MS (see
Figure 4.).
In the Czech Republic, only (3/9) 33% of the respondents use NfL for MS, but no others. In Poland (3/20) 15% ask for the determination of NFL, and another one (1/20) (5%) use Ig anti AQP 4 and Ig anti-MOG in atypical cases, differentiation with NMOSD and MOGAD. In Romania, (11/14) 78.5% of respondents use NfL, and they also use IL17, miRNA, OCB and IgG index. In Slovakia (4/5) 80% of the participating centers utilize NfL, and one (1/5, 20%) the HLA-DQB1 and HLA-DRB1. Half of the reference centers (2/4, 50%) request NfL, in addition, one center also uses the phenotyping of the CSF cells, transcriptomics, cytokines and GFAP in the CSF. Most centers - which using NfL - request its determination from serum or sometimes from serum and CSF in parallel (Czech Republic: (2/3) 67% (from serum only) and (1/3) 33% (from both); Romania: (8/11) 73% and (3/11) 27%; Slovakia: (4/4) 100% and (0/4) 0%; reference centers: (1/2) 50% and (1/2) 50%), but two Polish centers (2/3, 67%) requests it from CSF alone. The utilization rate of raw NfL concentrations versus age- and body mass index-corrected NfL Z-scores was 3:1 in the Czech Republic, 2:1 in Poland, 4:6 in Romania; 4 : 0 in Slovakia; and 1 : 1 in reference centers. The purposes of the use of NfL are mostly the prognosis and monitoring of the disease course and evaluation of treatment response in Czech, Polish and Romanian centers, while Slovakian centers utilize it for monitoring disease course and evaluation of treatment response. Reference centers use it only for monitoring disease course and for research purposes (see the details in
Table 6).
Anti-drug antibodies (ADA) are determined regularly only by a smaller proportion of the centers, in contrast, the majority of the reference centers (3/4) (75%) use it. In Croatia, the ADA determination against Interferon-beta and Natalizumab is asked by only one center (1/5) (20%). The 44% (4/9) of the responding Czech centers request it, Natalizumab is asked for in the majority (4/4, 100%). In Poland - based on the received information- no center orders the ADA test against interferon beta, but in all centers that use natalizumab, ADA against natalizumab are determined regularly. In Romania, 3 centers (3/14, 21%) request, the Natalizumab determination in higher proportion (Natalizumab : Interferon-beta 3 : 1). None of the surveyed Serbian centers ask for ADA determination. In Slovakia, the ADA determination against Interferon-beta is asked only by one center (1/5, 20%). None of the surveyed centers ask for its determination regularly in Slovenia. In contrast, the majority of the reference centers (3/4) (75%) use it, in equal proportion against Interferon-beta and Natalizumab.
3. Discussion
In the first part of the survey, we examined the organizational and institutional structures of the participating centers. Our study mostly involved larger Multiple Sclerosis centers. CSF analysis is not mandatory for diagnosing MS, but it can aid in the diagnostic process [
44]. In McDonald criteria CSF examination is a “valuable diagnostic test”, mainly when clinical findings and MRI do not give enough proof for the diagnosis of MS, or in low-prevalence population or in case of primary progressive MS [
4,
45]. However, the centers we investigated considered it important as all surveyed centers of the participating countries always perform lumbar puncture for CSF analysis in patients with suspected MS, except the Romanian and Polish centers, where the opinions are somewhat divided. The centers primarily use it for both diagnostic and differential purposes. The routine CSF parameters usually requested by the surveyed centers in case of suspicion MS are white blood cell count, red blood cell count, total protein, OCB and intrathecal IgG. A short summary about routine CSF parameters can be found in the review of Deisenhammer et al. [
46]. White blood cell count may indicate inflammation, while total protein or the albumin quotient can suggest blood-brain barrier dysfunction. CSF glucose concentration is normal in MS [
47]. Lactate level in the CSF was found to be significantly elevated in MS patients compared to the control or to radiologically isolated syndrome [
48,
49], and it was positively correlated with the progression rate [
50]. Intrathecally produced IgM and IgA inversely correlated with the disease progression in primary progressive SM [
50].
Our responder centers use the CSF OCB determination. In the 2017 version of McDonald Criteria the presence of IgG oligoclonal bands in CSF can perform the requirement of dissemination in time, so it makes an early MS diagnosis in patients who fulfill the criteria for dissemination in space [
1,
4].
In our survey the opinions are quite divided on that “What is the number of CSF-OCB that is used by their laboratory to define OCB positivity”. Only in Czech Republic 100% of the participants marked ≥2 CSF bands as the cut-off of OCB positivity. The lack of evidence-based recommendations in CSF OCB detection makes its analysis process and interpretation various [
51]. In Higgins et al. [
51] survey, the majority of Canadian neurologists preferred a cut-off of ≥ 2 CSF specific bands as positive in agree with the 2017 McDonald criteria [
4].
To confirm intrathecal IgG synthesis, blood and CSF samples have to be analyzed in parallel. Quantitative IgG in CSF can be calculated by different formulae, such as IgG index [
9,
52,
53], Reibergram [
54,
55], or Auer and Hegen formula [
56] (Auer et al., 2016). In the calculation of intrathecal fraction most centers used the IgG index, but several also indicated Reibergram or other (Auer and Hegen formula).
Albumin quotient is a result of CSF albumin/serum albumin, and it characterise the blood-brain barrier functions. IgG index, Reiber and Auer and Hegen formulae include the determination of the Albumin quotient.
The IgG index value >0.7 seemed to be an adequate cut-off value of the increased IgG index for MS patient population [
14]. Its sensitivity was found to be lower than those of OCB [
9,
14].
The determination of κFLC in suspected MS by more than 50% of the centers is only in Czech Republic, Slovakia, Slovenia and in the reference centers and κFLC index is used by the majority opposite the absolute CSF κFLC concentration, except Romania and Slovakia. Previous studies have shown that both the absolute concentration of CSF-kappa and the kappa index were found to have good MS diagnostic and prognostic performances [
57,
58,
59,
60,
61]. κFree Light Chains are measured by turbidimetry or nephelometry and it is an easy, trustful and fast tool, but cut-off values have to be determined [
17,
18].
Neurofilaments are neuron-specific cytoskeletal proteins and can be measured in both CSF and serum [
23]. Our results showed that NfL is frequently used as a molecular biomarker for MS in Romania, Slovakia, and the reference centers. None of the surveyed Croatian, Serbian and Slovenian centers used Neurofilament Light Chain or any other specific CSF and/or blood biomarkers in patients with MS. Centers that use the NfL assay either measure serum only (dominantly), or both CSF and serum. They apply both NfL concentration or age- and body mass index-corrected Z scores in different proportions. Previous researches showed that NfL was higher both is CSF and blood in newly diagnosed MS patients and its concentrations correlated with the disease activity, prognosis and severity of the disease [9]. Higher NfL values are not specific to MS, as it can be found in other neurodegenerative disorders. It is also a possible biomarker to monitor treatments responses to DMTs [9]. The limitation is that the assays are not standardized, there are no accurate reference intervals or other factors defined for the result interpretation [9]. Different NfL cut-off values in clinical studies are summarized in the review of Kouchaki et al. [23]. NfL concentration as well as age-and body mass index corrected Z scores are used, this latter takes into account the modifying effect of age and body mass index.
Interferon-β and Natalizumab treatment may cause the production of antibodies. These interact with them and neutralize the aforementioned drugs, thus reducing their clinical and radiological effects [62,63]. In our survey, we found that anti-drug antibodies (ADA) were determined regularly only by a smaller proportion of the centers, in contrast, the majority of reference centers used it, both against Interferon-β and Natalizumab. Some European countries (but not all) used in the clinical practice the ADA testing [64]. A survey about the use of antibodies against Interferon-beta and Natalizumab in different countries (Sweden, Austria, Denmark, Germany, Switzerland and Spain) can be found in the paper of Link et al. [64]. Elevated baseline anti-Natalizumab antibody titers may predict reduced drug efficacy [65]. Monitoring anti-drug antibodies against DMTs can help in the prediction of later treatment-failure [66].
Our results show that the use of molecular biomarkers is currently not uniform between the examined centers and countries. Although, it can be seen, that the role of molecular biomarkers has recently increased in the diagnosis and follow up of MS. Further studies are needed to establish their possible role in the routine clinical practice [2]. In the future, with the rise of personalized medicine, biomarker packages will presumably be included in the clinical diagnosis and follow-up of MS along with MRI and other clinical patient data.
4. Materials and Methods
4.1. Participating Centers and Data Collection
This international study was coordinated by the Department of Neurology, Albert Szent-Györgyi Faculty of Medicine, Albert Szent-Györgyi Health Centre, University of Szeged, Hungary and by the International Danube Neurology Symposium for Neurological Sciences and Continuing Education.
A questionnaire (edited by Professor Thomas Berger and Dr. Harald Hegen), consisting of 17 questions on CSF analysis and molecular biomarkers in MS patients, was sent via email to essentially the same MS centers that participated in our previous study [67] in the following countries: Croatia, Czech Republic, Poland, Romania, Serbia, Slovakia and Slovenia. Participation rates were as follows (number of the participating centers/whole number of the MS centers; participation rate): Croatia (5/10; 50%), Czech Republic (9/15; 60%), Poland (20/129; 15.5%), Romania (14/15; 93.3%), Serbia (5/5; 100%), Slovakia (5/10; 50%) and Slovenia (2/3; 66.6%). In addition, two reference centers from Austria (Department of Neurology, Medical University of Vienna and Department of Neurology, Medical University of Innsbruck), one center from Denmark (Danish Multiple Sclerosis Center, University Hospital Copenhagen, Rigshospitalet) and one from Germany (Department of Neurology, School of Medicine and Health, Technical University Munich) also completed our questionnaire; together, these form the reference center group. Our study mostly surveyed larger MS centers, which typically treat 30-100 and more than 100 patients per month. Data collection occurred between February 2024 and January 2025.
4.2. Data Statistical Analysis
Descriptive statistics were used to analyze the data. Results are reported as the number of positively responding centers over the total number of responding centers in each country, expressed as percentages.
4.3. Ethical Approval
The study was approved by the Hungarian Medical Research Council (reference number: IV/5139-1/2021/EKU) in accordance with the Declaration of Helsinki.
5. Conclusions
Our study highlights the importance of CSF analysis in Multiple Sclerosis. Our surveyed centers request the determination of oligoclonal IgG bands in case of suspected MS, but there is no clear consensus in the number of CSF-restricted oligoclonal bands positivity within and between countries. In Czech Republic, in Slovakia, in Slovenia and in the reference centers the determination of κFLC in patients with suspected MS is required by more than half of the surveyed centers. In Croatia, in Serbia and in Slovenia none of the interviewed centers use NfL as biomarker in MS, in contrast, in Romania, in Slovakia and in the reference centers it is often used. In summary, besides the use of CSF-specific oligoclonal bands there is no consensus among countries regarding the use of molecular biomarkers in Multiple Sclerosis, although, neurologists find them useful. It is more likely a consequence of unavailability due to rembursement issues. The role of molecular biomarkers is significant for diagnosis, progression assessment, and the effectiveness of therapy. Early diagnosis and prompt initiation of highly effective disease-modifying therapies offer the opportunity to delay long-term progression and preserve quality of life. In the future, biomarker analysing methods and reference (cut-off) values should be standardized, and a clear, uniform statement on their use would be necessary. It will be worthwhile to repeat the survey after about 3 years.
Author Contributions
Conceptualization, L.V., A.J., T.B., and H.H.; methodology, L.V., K.B. and Zs.T.K; formal analysis, A.J. and L.V.; investigation, A.J.; T.B., H.H., B.H., H. B-P., V.B.K., A. B., J.D., M.H., D.H., A.H.L., E.K.H., M.M., K.R., C.T., P.T., and L.V.; writing—original draft preparation, A.J. and L.V.; writing—review and editing, T.B., H.H., B.H., H. B-P., V.B.K., A. B., J.D., M.H., D.H., A.H.L., E.K.H., M.M., K.R., C.T., P.T., K.B. and Zs.T.K; visualization, A.J.; supervision, L.V.; project administration, A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
Our thanks to Tanaka Masaru M.D., Ph.D. for the grammatical correction and the critical reading of the manuscript. Ágnes Szabó for the technical help in the figure preparation.
Additional thanks to the participants who filled out the questionnaire:
Croatia
Marija Ratković (OB „dr. Josip Benčević”, Slavonski Brod); Spomenka Kidemet-Piskac (General Hospital, Varazdin); Tea Mirosevic Zubonja (University Hospital Center Osijek)
Czech Republic
Eva Recmanová (KNTB, Zlin); Ivana Stetkarova (Department of Neurology, Charles University, Third School of Medicine and University Hospital Kralovske Vinohrady, Prague); Jana Houskova (Adamkova) (MS center, Hospital, C. Budejovice); Jana Libertinova (Neurology Department, Second Faculty of Medicine, Charles University and Motol University Hospital, Prague); Marek Peterka (Faculty Hospital Pilsen, Department of Neurology, Pilsen); Marta Vachova (MS Centre of Hospital, Teplice); Pavel Štourač (University Hospital Brno); Radek Ampapa (Nemocnice, Jihlava)
Poland
Alicja Kalinowska (Department of Neurology, Poznan University of Medical Sciences, Poznan); Alina Kulakowska (Department of Neurology, Medical University of Bialystok); Anna Jamroz-Wisniewska (Medical University of Lublin, Department of Neurology, Lublin); Anna Pokryszko-Dragan (Department of Neurology, University Clinical Hospital, Wroclaw Medical University); Arkadiusz Stęposz and Anetta Lasek-Bal (Department of Neurology, Medical University of Silesia in Katowice, Upper Silesian Medical Centre, Katowice); Beata Labuz-Roszak (Department of Neurology, St. Jadwiga Provincial Specialst Hospital, Institute of Medicine, University of Opole); Elżbieta Jasińska (Resmedica, Kielce); Ewa Krzystanek (Górnośląskie Centrum Medyczne, Medical University of Silesia, Katowice); Iwona Kurkowska-Jastrzębska (Institute of Psychaitry and Neurology, Warsaw); Izabela Domitrz and Paulina Fonderska (Department of Neurology, Faculty of Medicine and Dentistry, Medical University of Warsaw, Bielanski Hospital, Warsaw); Jacek Zaborski (Miedzyleski Szpital Specjalistyczny, Warszawa); Jarosław Sławek (St. Adalbert Hospital, Neurology and Stroke Department, Gdansk); Karolina Piasecka-Stryczynska (Department of Neurology, Poznan University of Science, Poznan); Marcin P. Mycko (Department of Neurology University of Warmia and Mazury in Olsztyn); Marius Stasiolek (Department of Neurology Medical University of Lodz); Marta Milewska-Jędrzejczak and Andrzej Głąbiński (Department of Neurology, University Clinical Hospital No. 2 of the Medical University, Lodz); Monika Adamczyk-Sowa (Department of Neurology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia in Katowice); Slawomir Wawrzyniak (10 Military Clinical Hospital with Polyclinic SP ZOZ, Bydgoszcz); Waldemar Brola (Department of Neurology, Collegium Medicum, Jan Kochanowski University, Kielce)
Romania
Adriana Dulamea (Fundeni Clinical Institute, Bucharest); Anabella Cioabla (St. Andrew Constanta Country Emergency Hospital, Constanta); Cristina Panea (Elias Emergency University Hospital, Bucarest); Davidescu Eugenia Irene (Colentina Clinical Hospital, Bucharest); Emilian-Bogdan Ignat (Spitalul Clinic de Recuperare Iasi, Iasi); Lungu Mihaela (Emergency Clinical Hospital, Galati); Lupu Raluca Miruna (Spitalul Clinic De Psihiatrie Si Neurologie, Brasov); Mihaela Simu (Clinical County Emergency Hospita „ Pius Brinzeu „ Timisoara Neurological Clinic 2; University of Medicine and Pharmacy „Victor Babes”, Timisoara); Rodica Balasa (Emergency University County Hospital, Targu Mures); Roman-Filip Corina (Neurology Clinic, Sibiu); Sirbu Carmen Adella (Central Military Emergency University Bucharest); Teleanu Raluca Ioana (Dr Victor Gomoiu Children’s Hospital, Bucharest); Vacaras Vitalie (Neurology II Clinic, Cluj Emergency County Hospital, Cluj-Napoca)
Serbia
Evica Dincic (Clinic of Neurology, Military Medical Academy, Belgrade); Dejan Aleksić (University Clinical Center Kragujevac, Clinic of Neurology, Kragujevac); Lorand Sakalas (Clinical center of Vojvodina, Clinic of neurology, Novi Sad); Slobodan Vojinovic (Clinic of Neurology, University Clinical center, Nis)
Slovakia
Egon Kurča (Neurologic Department, Martin); Georgi Krastev (Department of Neurology, MS Centrum, Complex Stroke Unit, University Hospital Trnava); Marianna Vitkova (Pavol Jozef Safarik University Kosice, Faculty of medicine, Louis Pasteur University Hospital, Kosice); Viera Hancinova (NK SZU UNB Ruzinov, Bratislava)
Slovenia
Jožef Magdič (Neurology division, University medical center, Maribor)
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADA |
Anti-drug antibodies |
| CIS |
Clinically isolated syndrome |
| CNS |
Central nervous system |
| CSF |
Cerebrospinal fluid |
| DMT |
Disease-modifying therapy |
| Ig |
Immunglobulin |
| κFLC |
Kappa Free Light Chain |
| LP |
Lumbar puncture |
| MRI |
Magnetic resonance imaing |
| MS |
Multiple Sclerosis |
| NfL |
Neurofilament Light Chain |
| OCB |
Oligoclonal band |
References
- Maroto-García, J.; Martínez-Escribano, A.; Delgado-Gil, V.; Mañez, M.; Mugueta, C.; Varo, N.; García de la Torre, Á.; Ruiz-Galdón, M. Biochemical biomarkers for multiple sclerosis. Clin. Chim. Acta 2023, 548, 117471. [Google Scholar] [CrossRef]
- Scalfari, A.; Traboulsee, A.; Oh, J.; Airas, L.; Bittner, S.; Calabrese, M.; Dominguez, J.M.G.; Granziera, C.; Greenberg, B.; Hellwig, K.; Illes, Z.; Lycke, J.; Popescu, V.; Bagnato, F.; Giovannoni, G. Smouldering-Associated Worsening in Multiple Sclerosis: An International Consensus Statement on Definition, Biology, Clinical Implications, and Future Directions. Ann. Neurol. 2024, 96(5), 826–845. [Google Scholar] [CrossRef]
- Maglio, G.; D’Agostino, M.; Caronte, F.P.; Pezone, L.; Casamassimi, A.; Rienzo, M.; Di Zazzo, E.; Nappo, C.; Medici, N.; Molinari, A.M.; Abbondanza, C. Multiple Sclerosis: From the Application of Oligoclonal Bands to Novel Potential Biomarkers. Int. J. Mol. Sci. 2024, 25(10), 5412. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; Fujihara, K.; Galetta, S.L.; Hartung, H.P.; Kappos, L.; Lublin, F.L.; Marrie, R.A.; Miller, A.E.; Miller, D.H.; Montalban, X.; Mowry, E.M.; Sorensen, P.S.; Tintoré, M.; Traboulsee, A.L.; Trojano, M.; Uitdehaag, B.M.J.; Vukusic, S.; Waubant, E.; Weinshenker, B.G.; Reingold, S.C.; Cohen, J.A. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17(2), 162–173. [Google Scholar] [CrossRef]
- Huang, J.; Khademi, M.; Fugger, L.; Lindhe, Ö.; Novakova, L.; Axelsson, M.; Malmeström, C.; Constantinescu, C.; Lycke, J.; Piehl, F.; Olsson, T.; Kockum, I. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc. Natl. Acad. Sci. U S A 2020, 117(23), 12952–12960. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.; Gaetani, L.; Centonze, D.; Hegen, H.; Kuhle, J.; Teunissen, C.E.; Tintoré, M.; Villar, L.M.; Willemse, E.A.J.; Zetterberg, H.; Parnetti, L. Fluid biomarkers in multiple sclerosis: from current to future applications. Lancet Reg. Health Eur. 2024, 44, 101009. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; George, W.; Yu, X. The elusive nature of the oligoclonal bands in multiple sclerosis. J. Neurol. 2024, 271(1), 116–124. [Google Scholar] [CrossRef] [PubMed]
- Link, H.; Huang, Y.M. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J. Neuroimmunol. 2006, 180, 17–28. [Google Scholar] [CrossRef]
- Saadeh, R.S.; Bryant, S.C.; McKeon, A.; Weinshenker, B.; Murray, D.L.; Pittock, S.J.; Willrich, M.A.V. CSF Kappa Free Light Chains: Cutoff Validation for Diagnosing Multiple Sclerosis. Mayo Clin. Proc. 2022, 97(4), 738–751. [Google Scholar] [CrossRef]
- Toscano, S.; Chisari, C.G.; Fermo, S.L.; Gulino, G.; Zappia, M.; Patti, F. A dynamic interpretation of κFLC index for the diagnosis of multiple sclerosis: a change of perspective. J. Neurol. 2023, 270(12), 6010–6020. [Google Scholar] [CrossRef]
- Izquierdo, G.; Angulo, S.; Garcia-Moreno, J.M.; Gamero, M.A.; Navarro, G.; Gata, J.M.; Ruiz-Peña, J.L.; Páramo, M.D. Intrathecal IgG synthesis: marker of progression in multiple sclerosis patients. Acta Neurol. Scand. 2002, 105(3), 158–63. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, M.-T.; Yang, F.; Zhou, J.-P.; Fang, W.; Shen, C.-H.; Zhang, Y.-X.; Ding, M.-P. IgG Index Revisited: Diagnostic Utility and Prognostic Value in Multiple Sclerosis. Front. Immunol. 2020, 11, 1799. [Google Scholar] [CrossRef]
- Simonsen, C.S.; Flemmen, H.O.; Lauritzen, T.; Berg-Hansen, P.; Moen, S.M.; Gulowsen Celius, E.G. The diagnostic value of IgG index versus oligoclonal bands in cerebrospinal fluid of patients with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6(1), 2055217319901291. [Google Scholar] [CrossRef]
- Shaw, F.; Chadwick, C. The diagnostic utility of IgG index and oligoclonal bands for multiple sclerosis in a neurology hospital patient population. Ann. Clin. Biochem. 2023, 60(5), 353–355. [Google Scholar] [CrossRef]
- Nakano, T.; Matsui, M.; Inoue, I.; Awata, T.; Katayama, S.; Murakoshi, T. Free immunoglobulin light chain: its biology and implications in diseases. Clin. Chim. Acta 2011, 412, 843–849. [Google Scholar] [CrossRef]
- Esparvarinha, M.; Nickho, H.; Mohammadi, H.; Aghebati-Maleki, L.; Abdolalizadeh, J.; Majidi, J. The role of free kappa and lambda light chains in the pathogenesis and treatment of inflammatory diseases. Biomed. Pharmacother. 2017, 91, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Berek, K.; Deisenhammer, F. Cerebrospinal fluid kappa free light chains as biomarker in multiple sclerosis-from diagnosis to prediction of disease activity. Wien Med. Wochenschr. 2022, 172, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Hegen, H.; Walde, J.; Berek, K.; Arrambide, G.; Gnanapavan, S.; Kaplan, B.; Khalil, M.; Saadeh, R.; Teunissen, C.; Tumani, H.; Villar, L.M.; Willrich, M.A.V.; Zetterberg, H.; Deisenhammer, F. Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. 2023, 29(2), 169–181. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.M.; Espiño, M.; Costa-Frossard, L.; Muriel, A.; Jiménez, J.; Alvarez-Cermeño, J.C. High levels of cerebrospinal fluid free kappa chains predict conversion to multiple sclerosis. Clin. Chim. Acta 2012, 413, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Makshakov, G.; Nazarov, V.; Kochetova, O.; Surkova, E.; Lapin, S.; Evdoshenko, E. Diagnostic and Prognostic Value of the Cerebrospinal Fluid Concentration of Immunoglobulin Free Light Chains in Clinically Isolated Syndrome with Conversion to Multiple Sclerosis. PLoS One 2015, 10(11), e0143375. [Google Scholar] [CrossRef]
- Gaetani, L.; Blennow, K.; Calabresi, P.; Di Filippo, M.; Parnetti, L.; Zetterberg, H. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 2019, 90(8), 870–881. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; Petzold, A.; Blennow, K.; Zetterberg, H.; Kuhle, J. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14(10), 577–589. [Google Scholar] [CrossRef]
- Kouchaki, E.; Dashti, F.; Mirazimi, S.M.A.; Alirezaei, Z.; Jafari, S.H.; Hamblin, M.R.; Mirzaei, H. Neurofilament light chain as a biomarker for diagnosis of multiple sclerosis. EXCLI J 2021, 20, 1308–1325. [Google Scholar] [CrossRef]
- Pijnenburg, Y.A.L.; Janssen, J.C.; Schoonenboom, N.S.M.; Petzold, A.; Mulder, C.; Stigbrand, T.; Norgren, N.; Heijst, H.; Hack, C.E.; Scheltens, P.; Teunissen, C.E. CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer’s disease and controls. Dement. Geriatr. Cogn. Disord. 2007, 23(4), 225–30. [Google Scholar] [CrossRef]
- Lu, C.-H.; Macdonald-Wallis, C.; Gray, E.; Pearce, N.; Petzold, A.; Norgren, N.; Giovannoni, G.; Fratta, P.; Sidle, K.; Fish, M.; Orrell, R.; Howard, R.; Talbot, K.; Greensmith, L.; Kuhle, J.; Turner, M.R.; Malaspina, A. Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 2015, 84(22), 2247–57. [Google Scholar] [CrossRef] [PubMed]
- Neselius, S.; Brisby, H.; Marcusson, J.; Zetterberg, H.; Blennow, K.; Karlsson, T. Neurological assessment and its relationship to CSF biomarkers in amateur boxers. PLoS One 2014, 9(6), e99870. [Google Scholar] [CrossRef] [PubMed]
- Gattringer, T.; Pinter, D.; Enzinger, C.; Seifert-Held, T.; Kneihsl, M.; Fandler, S.; Pichler, A.; Barro, C.; Gröbke, S.; Voortman, M.; Pirpamer, L.; Hofer, E.; Ropele, S.; Schmidt, R.; Kuhle, J.; Fazekas, F.; Khalil, M. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 2017, 89(20), 2108–2114. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Tao, Q.; Lu, P.; Meng, F.; Zhuang, L.; Qiao, S.; Zhang, Y.; Luo, B.; Liu, Y.; Peng, G. Diagnostic value of isolated plasma biomarkers and its combination in neurodegenerative dementias: A multicenter cohort study. Clin. Chim. Acta 2024, 558, 118784. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Liu, K.; Lin, J.; Yang, T.; Xiao, Y.; Wei, Q.; Hou, Y.; Li, C.; Zhang, L.; Jiang, Z.; Zhao, B.; Chen, X.; Song, W.; Wu, Y.; Shang, H. Relationship between plasma NFL and disease progression in Parkinson’s disease: a prospective cohort study. J. Neurol. 2024, 271(4), 1837–1843. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawarabayashi, T.; Shibata, M.; Kasahara, H.; Makioka, K.; Sugawara, T.; Oka, H.; Ishizawa, K.; Amari, M.; Ueda, T.; Kinoshita, S.; Miyamoto, Y.; Kaito, K.; Takatama, M.; Ikeda, Y.; Shoji, M. High levels of plasma neurofilament light chain correlated with brainstem and peripheral nerve damage. J. Neurol. Sci. 2024, 15, 463:123137. [Google Scholar] [CrossRef]
- Rosén, C.; Mitre, B.; Nellgård, B.; Axelsson, M.; Constantinescu, R.; Munch Andersen, P.; Dalla, K.; Blennow, K.; Nilsson, G.; Zetterberg, H.; Rosén, H. High levels of neurofilament light and YKL-40 in cerebrospinal fluid are related to poor outcome in ALS. J. Neurol. Sci. 2024, 463, 123112. [Google Scholar] [CrossRef]
- Skillbäck, T.; Farahmand, B.; Bartlett, J.W.; Rosén, C.; Mattsson, N.; Nägga, K.; Kilander, L.; Religa, D.; Wimo, A.; Winblad, B.; Rosengren, L.; Schott, J.M.; Blennow, K.; Eriksdotter, M.; Zetterberg, H. CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 2014, 83(21), 1945–53. [Google Scholar] [CrossRef]
- Martínez, M.A.; Olsson, B.; Bau, L.; Matas, E.; Cobo Calvo, Á.; Andreasson, U.; Blennow, K.; Romero-Pinel, L.; Martínez-Yélamos, S.; Zetterberg, H. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult. Scler. 2015, 21(5), 550–61. [Google Scholar] [CrossRef]
- Kuhle, J.; Kropshofer, H.; Haering, D.A.; Kundu, U.; Meinert, R.; Barro, C.; Dahlke, F.; Tomic, D.; Leppert, D.; Kappos, L. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019, 92(10), e1007–e1015. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; Lalive, P.H.; Mueller, C.; Müller, S.; Naegelin, Y.; Oksenberg, J.R.; Pot, C.; Salmen, A.; Willemse, E.; Kockum, I.; Blennow, K.; Zetterberg, H.; Gobbi, C.; Kappos, L.; Wiendl, H.; Berger, K.; Sormani, M.P.; Granziera, C.; Piehl, F.; Leppert, D.; Kuhle, J.; NfL Reference Database in the Swiss Multiple Sclerosis Cohort Study Group. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef]
- Højsgaard Chow, H.; Petersen, E.R.; Olsson, A.; Hejgaard Laursen, J.; Bredahl Hansen, M.; Bang Oturai, A.; Soelberg Sørensen, P.; Bach Søndergaard, H.; Sellebjerg, F. Age-corrected neurofilament light chain ratio decreases but does not predict relapse in highly active multiple sclerosis patients initiating natalizumab treatment. Mult. Scler. Relat. Disord. 2024, 88, 105701. [Google Scholar] [CrossRef]
- Pape, K.; Rolfes, L.; Steffen, F.; Muthuraman, M.; Korsen, M.; Meuth, S.G.; Zipp, F.; Bittner, S. Comparative effectiveness of natalizumab versus ocrelizumab in multiple sclerosis: a real-world propensity score-matched study. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221142924. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, M.; Malmeström, C.; Axelsson, M.; Sundström, P.; Dahle, C.; Vrethem, M.; Olsson, T.; Piehl, F.; Norgren, N.; Rosengren, L.; Svenningsson, A.; Lycke, J. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol. 2011, 69(1), 83–9. [Google Scholar] [CrossRef] [PubMed]
- Novakova, L.; Axelsson, M.; Khademi, M.; Zetterberg, H.; Blennow, K.; Malmeström, C.; Piehl, F.; Olsson, T.; Lycke, J. Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult. Scler. 2017, 23(1), 62–71. [Google Scholar] [CrossRef]
- de Flon, P.; Gunnarsson, M.; Laurell, K.; Söderström, L.; Birgander, R.; Lindqvist, T.; Krauss, W.; Dring, A.; Bergman, J.; Sundström, P.; Svenningsson, A. Reduced inflammation in relapsing-remitting multiple sclerosis after therapy switch to rituximab. Neurology 2016, 87(2), 141–7. [Google Scholar] [CrossRef]
- Axelsson, M.; Malmeström, C.; Gunnarsson, M.; Zetterberg, H.; Sundström, P.; Lycke, J.; Svenningsson, A. Immunosuppressive therapy reduces axonal damage in progressive multiple sclerosis. Mult. Scler. 2014, 20(1), 43–50. [Google Scholar] [CrossRef]
- Ashkar, A.A.; Ali Baig, M.M.; Arif, A.; Mazhar Ali, M.; Yousuf, F.; Ashkar, R. Prognostic significance of neurofilament light in Fingolimod therapy for Multiple Sclerosis: A systemic review and meta-analysis based on randomized control trials. Mult. Scler. Relat. Disord. 2023, 69, 104416. [Google Scholar] [CrossRef]
- Piehl, F.; Kockum, I.; Khademi, M.; Blennow, K.; Lycke, J.; Zetterberg, H.; Olsson, T. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult. Scler. 2018, 24(8), 1046–1054. [Google Scholar] [CrossRef]
- Shahan, B.; Choi, E.Y.; Nieves, G. Cerebrospinal Fluid Analysis. Am. Fam. Physician 2021, 103(7), 422–428. [Google Scholar]
- Lo Sasso, B.; Agnello, L.; Bivona, G.; Bellia, C.; Ciaccio, M. Cerebrospinal Fluid Analysis in Multiple Sclerosis Diagnosis: An Update. Medicina (Kaunas) 2019, 55(6), 245. [Google Scholar] [CrossRef] [PubMed]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The Cerebrospinal Fluid in Multiple Sclerosis. Front. Immunol. 10, 726. [CrossRef]
- Osenbrück, M.; Rao, M.L.; Quednau, H.D. Pattern of albumin, immunoglobulins, and glucose in cerebrospinal fluid and serum of patients with disorders of the central nervous system. Eur. Neurol. 1985, 24(1), 16–22. [Google Scholar] [CrossRef]
- Haarmann, A.; Hähnel, L.; Schuhmann, M.K.; Buttmann, M. Age-adjusted CSF β2-microglobulin and lactate are increased and ACE is decreased in patients with multiple sclerosis, but only lactate correlates with clinical disease duration and severity. J. Neuroimmunol. 2018, 15, 323:19–27. [Google Scholar] [CrossRef]
- Tarhan, G.; Domaç, S.F.; Selek, S.; Gül, A.Z.; Demir, S. Utilizing metabolomic profiling as a supportive diagnostic tool for radiologically isolated syndrome. Mult. Scler. Relat. Disord. 2025, 94, 106250. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Hottenrott, T.; Mayer, C.; Hintereder, G.; Zettl, U.K.; Stich, O.; Tumani, H. CSF profile in primary progressive multiple sclerosis: Re-exploring the basics. PLoS One 2017, 12(8), e0182647. [Google Scholar] [CrossRef] [PubMed]
- Higgins, V.; Parker, M.L.; Beriault, D.L.; Mostafa, A.; Estey, M.P.; Agbor, T.; Ismail, O.Z. A survey of Canadian neurologists’ perspectives and preferences for laboratory reporting of CSF oligoclonal banding. Clin. Biochem. 2025, 135, 110855. [Google Scholar] [CrossRef] [PubMed]
- Link, H.; Tibbling, G. Principles of albumin and IgG analyses in neurological disorders. III. Evaluation of IgG synthesis within the central nervous system in multiple sclerosis. Scand. J. Clin. Lab. Invest. 1977, 37(5), 397–401. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Takahashi, T.; Fujihara, K.; Misu, T.; Nishiyama, S.; Takai, Y.; Fujimori, J.; Abe, M.; Ishii, T.; Aoki, M.; Nakashima, I. Impact of intrathecal IgG synthesis on neurological disability in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102382. [Google Scholar] [CrossRef]
- Reiber, H.; Felgenhauer, K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin. Chim. Acta 1987, 163(3), 319–28. [Google Scholar] [CrossRef]
- Karamehic, J.; Delic-Sarac, M.; Subasic, D.; Jukic, T.; Coric, J.; Panjeta, M.; Drace, Z.; Zecevic, L.; Mutevelic, S.; Dzananovic, N.; Grcic, N.; Kesmer, A. Reibergram and oligoclonal bands in diagnosis of multiple sclerosis. Med. Arch. 2012, 66(4), 222–5. [Google Scholar] [CrossRef]
- Auer, M., Hegen, H., Zeileis, A., Deisenhammer, F. Quantitation of intrathecal immunoglobulin synthesis - a new empirical formula. Eur. J. Neurol. 2016, 23, 713–721. [CrossRef]
- Bernardi, G.; Biagioli, T.; Malpassi, P.; De Michele, T.; Vecchio, D.; Repice, A.M.; Lugaresi, A.; Mirabella, M.; Torri Clerici, V.; Ilaria Crespi, I. The contribute of cerebrospinal fluid free light-chain assay in the diagnosis of multiple sclerosis and other neurological diseases in an Italian multicenter study. Mult. Scler. 2022, 28(9), 1364–1372. [Google Scholar] [CrossRef]
- Castillo-Villalba, J.; Gil-Perotín, S.; Gasque-Rubio, R.; Cubas-Nuñez, L.; Carratalà-Boscà, S.; Alcalá, C.; Quintanilla-Bordás, C.; Pérez-Miralles, F.; Ferrer, C.; Martínez, A.C.; Tortosa, J.; Solís-Tarazona, L.; Campos, L.; Leivas, A.; Marro, B.L.; Casanova, B. High Levels of Cerebrospinal Fluid Kappa Free Light Chains Relate to IgM Intrathecal Synthesis and Might Have Prognostic Implications in Relapsing Multiple Sclerosis. Front. Immunol. 2022, 7, 13:827738. [Google Scholar] [CrossRef]
- Christiansen, M.; Gjelstrup, M.C.; Stilund, M.; Christensen, T.; Petersen, T.; Møller, H.J. Cerebrospinal fluid free kappa light chains and kappa index perform equal to oligoclonal bands in the diagnosis of multiple sclerosis. Clin. Chem. Lab. Med. 2018, 57(2), 210–220. [Google Scholar] [CrossRef]
- Domingues, R.B.; Dos Santos, M.V.; Salomão, D.; Senne, C. Concordance rate between oligoclonal bands and the Kappa index in patients with suspected multiple sclerosis (MS). Arq. Neuropsiquiatr. 2024, 82(3), 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.; Bedin, R.; Natali, P.; Franciotta, D.; Smolik, K.; Santangelo, M.; Immovili, P.; Camera Vitetta, F.; Gastaldi, M.; Trenti, T.; Meletti, S.; Sola, P. Kappa Index Versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders. Diagnostics (Basel) 2020, 10(10), 856. [Google Scholar] [CrossRef]
- Deisenhammer, F. Neutralizing antibodies to interferon-beta and other immunological treatments for multiple sclerosis: prevalence and impact on outcomes. CNS Drugs 2009, 23(5), 379–96. [Google Scholar] [CrossRef]
- Hegen, H.; Auer, M.; Deisenhammer, F. Predictors of Response to Multiple Sclerosis Therapeutics in Individual Patients. Drugs 2016, 76(15), 1421–1445. [Google Scholar] [CrossRef]
- Link, J.; Ramanujam, R.; Auer, M.; Ryner, M.; Hässler, S.; Bachelet, D.; Mbogning, C.; Warnke, C.; Buck, D.; Jensen, P.E.H.; Sievers, C.; Ingenhoven, K.; Fissolo, N.; Lindberg, R.; Grummel, V.; Donnellan, N.; Comabella, M.; Montalban, X.; Kieseier, B.; Sørensen, P.S.; Hartung, H.P.; Derfuss, T.; Lawton, A.; Sikkema, D.; Pallardy, M.; Hemmer, B.; Deisenhammer, F.; Broët, P.; Dönnes, P.; Davidson, J. ; Fogdell-Hahn, A; ABIRISK Consortium Clinical practice of analysis of anti-drug antibodies against interferon beta and natalizumab in multiple sclerosis patients in Europe: A descriptive study of test results. PLoS One 2017, 12(2), e0170395. [Google Scholar] [CrossRef] [PubMed]
- Deisenhammer, F.; Jank, M.; Lauren, A.; Sjödin, A.; Ryner, M.; Fogdell-Hahn, A.; Sievers, C.; Lindberg, R.; Jensen, P.E.; Sellebjerg, F.; Christodoulou, L.; Birchler, M.; Pallardy, M.; Auer, M.; Liblau, R. ; ABIRISK consortium Prediction of natalizumab anti-drug antibodies persistency. Mult. Scler. 2019b, 25(3), 392–398. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Asardag, A.N.; Quinn, O.A.; Efimov, A.; Kang, A.S. Anti-drug antibodies to antibody-based therapeutics in multiple sclerosis. Hum. Antibodies 2021, 29(4), 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kokas, Zs.; Járdánházy, A.; Sandi, D.; Biernacki, T.; Fricska-Nagy, Zs.; Füvesi, J.; Bartosik-Psujek, H.; Basic Kes, V.; Berger, T.; Berthele, A.; Drulovic, J.; Hemmer, B.; Horakova, D.; Horvat Ledinek, A.; Kubala Havrdova, E.; Magyari, M.; Rejdak, K.; Tiu, C.; Turcani, P.; Klivényi, P.; Kincses, Zs.T.; Vécsei, L.; Bencsik, K. Real-world operation of multiple sclerosis centres in Central-Eastern European countries covering 107 million inhabitants. Mult. Scler. Relat. Disord. 2023, 69, 104406. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).