Submitted:
29 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
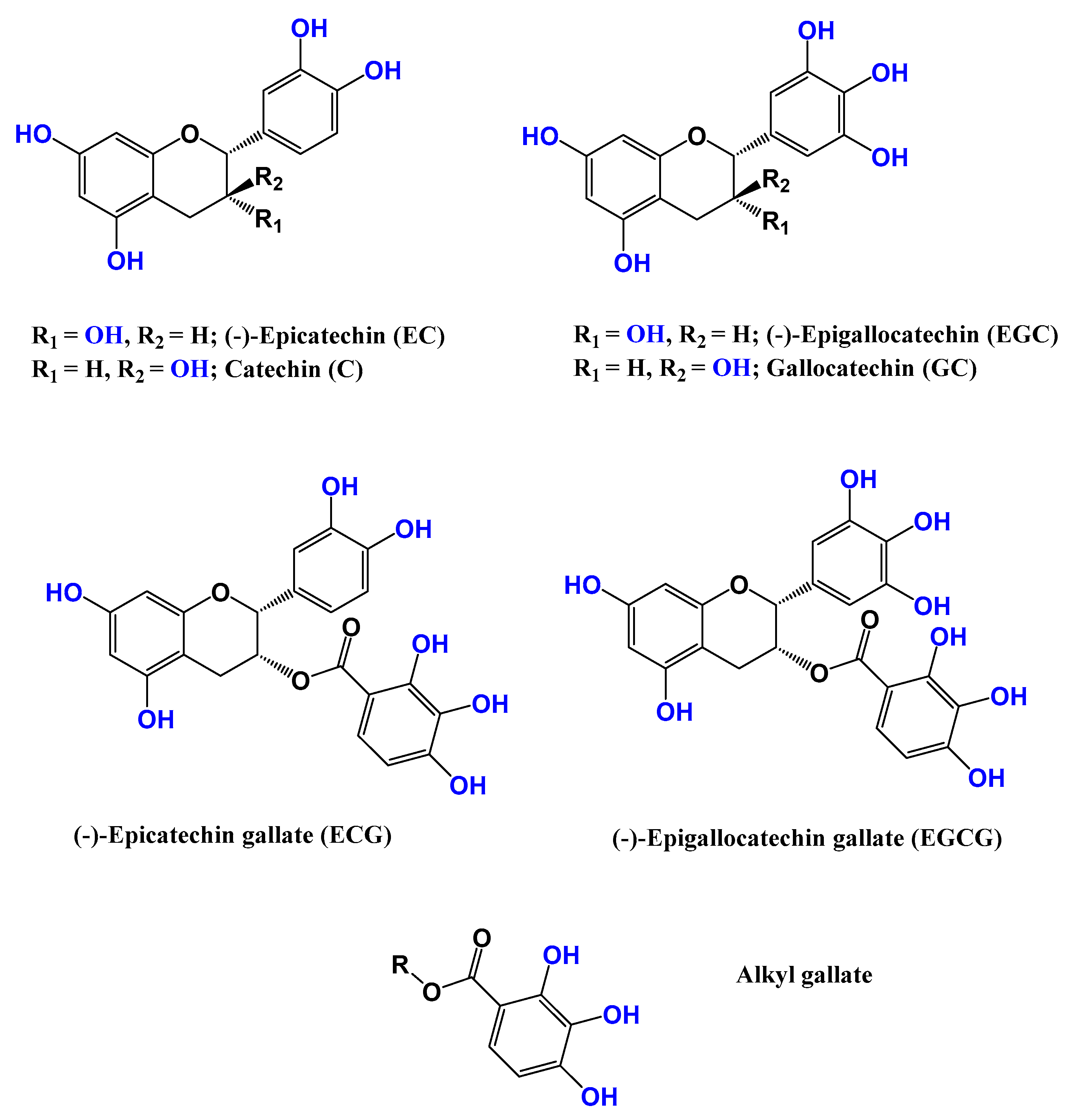
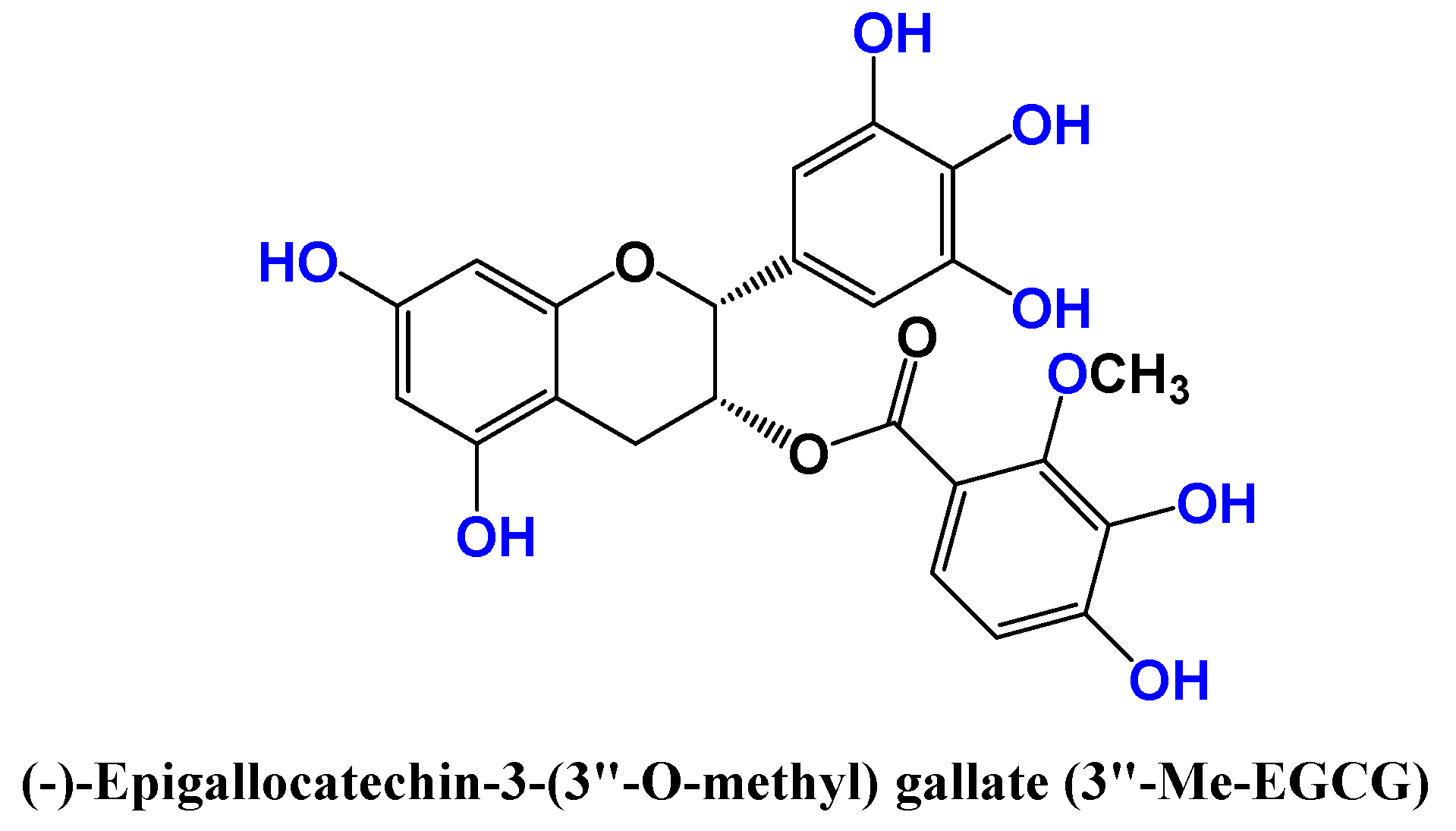
2.1. Camellia sinensis (Grenn tea)
2.2. Andrographis paniculata
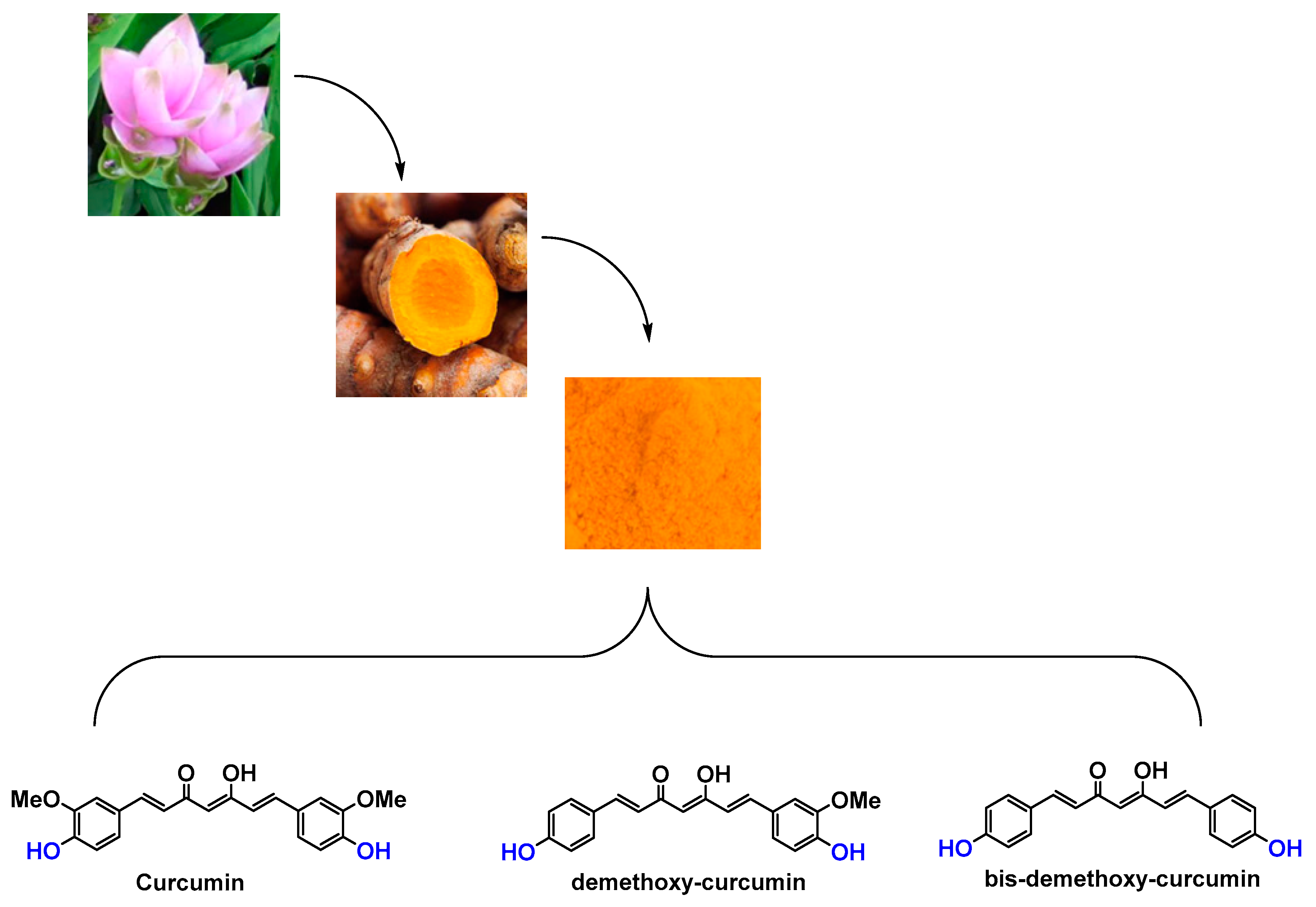
2.3. Curcuma longa
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cavinato, M.; Jansen-Dürr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Kondo, S.; Kono, T.; Sauder, D.N.; McKenzie, R.C. IL-8 Gene Expression and Production in Human Keratinocytes and Their Modulation by UVB. J. Investig. Dermatol. 1993, 101, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, N.; Moriwaki, S.; Sugiyama-Nakagiri, Y.; Haketa, K.; Takema, Y.; Imokawa, G. Neprilysin Is Identical to Skin Fibroblast Elastase its role in skin aging and UV responses. J. Biol. Chem. 2010, 285, 39819–39827. [Google Scholar] [CrossRef]
- Chang, T.-M.; Tsen, J.-H.; Yen, H.; Yang, T.-Y.; Huang, H.-C. Extract fromPeriostracum cicadaeInhibits Oxidative Stress and Inflammation Induced by Ultraviolet B Irradiation on HaCaT Keratinocytes. Evidence-Based Complement. Altern. Med. ECAM 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Khanam, S.; Prakash, A. Promising sources of antioxidants from herbs and spices: A review. Int. J. Adv. Res. 2021, 4, 188–195. [Google Scholar] [CrossRef]
- Mussard, E.; Cesaro, A.; Lespessailles, E.; Legrain, B.; Berteina-Raboin, S.; Toumi, H. Andrographolide, A natural Antioxydant : an Update. Antioxydants 2019, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, H.; Elmets, C.A. Photocarcinogenesis: mechanisms, models and human health implications. Photochem. Photobiol. 1996, 63, 356–357. [Google Scholar] [CrossRef]
- Kripke, M.L. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977, 37, 1395–1400. [Google Scholar] [PubMed]
- Kligman, L.H.; Akin, F.J.; Kligman, A.M. Sunscreens prevent ultraviolet photocarcinogenesis. J. Am. Acad. Dermatol. 1980, 3, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Goihman-Yahr, M. Skin aging and photoaging: an outlook. Clin. Dermatol. 1996, 14, 153–160. [Google Scholar] [CrossRef]
- Nadim, M.; Auriol, D.; Lamerant-Fayel, N.; Lefèvre, F.; Dubanet, L.; Redziniak, G.; Kieda, C.; Grillon, C. Improvement of polyphenol properties upon glucosylation in a UV-induced skin cell ageing model. Int. J. Cosmet. Sci. 2014, 36, 579–587. [Google Scholar] [CrossRef]
- Han, S.Y.; Kim, E.; Hwang, K.; Ratan, Z.A.; Hwang, H.; Kim, E.-M.; Kim, D.; Park, J.; Cho, J.Y. Cytoprotective Effect of Epigallocatechin Gallate (EGCG)-5’-O-α-Glucopyranoside, a novel EGCG derivative. Int. J. Mol. Sci. 2018, 19, 1466. [Google Scholar] [CrossRef]
- Gonzales-Alfonso, J.L.; Peñalver, P.; Ballesteros, A.O.; Morales, J.C.; Plou, F.J. Effect of α-glucosylation on the stability, antioxidant properties, toxicity and neuroprotective activity of (-)epigallocatechin gallate. Front. Nutr. 2019, 6, 30. [Google Scholar] [CrossRef]
- Mamenzigou, U.M.; Ikeda, T.; Morohoshi, T.; Nakayama, M.; Yui, K. The inclusion complex formation of epigallocatechin gallate in g-cyclodextrin and its effect on the antioxidant activity. Trans. Mat. Res. Soc. Japan 2013, 38, 681–685. [Google Scholar] [CrossRef]
- Chen, J.; Wei, N.; Lopez-Garcia, M.; Ambrose, D.; Lee, J.; Annelin, C.; Peterson, T. Development and evaluation of resveratrol, vitamin E and epigallocatechin gallate loaded lipid nanoparticles for skin care applications. Eur. J. Pharm. Biopharm. 2017, 117, 286–291. [Google Scholar] [CrossRef]
- Pereira, A.; Ramalho, M.J.; Silva, R.; Silva, V.; Marques-Oliveira, R.; Silva, A.C.; Pereira, M.C.; Loureiro, J.A. Vine cane compounds to prevent skin cells aging through solid lipid nanoparticles. Pharmaceutics, 2022, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Martini, N.; Wu, Z.; Chen, S.; Falconer, J.R.; Locke, M.; Zhang, Z.; Wen, J. Niosomal nanocarriers for enhanced dermal delivery of epigallocatechin gallate for protection against oxidative stress of the skin. Pharmaceutics, 2022, 14, 726. [Google Scholar] [CrossRef] [PubMed]
- Vale, E.P.; Morais, E.D.S.; Tavares, W.D.S.; Oliveira de Sousa, F.F. Epigallocatechin-3-gallate loaded-zein nanoparticles: characterization, stability and associated antioxidant, anti-tyrosinase and sun protection properties. J. Mol. Liq. 2022, 358, 119107. [Google Scholar] [CrossRef]
- Floyd, R.A. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc. Soc. Exp. Biol. Med. 1999, 222, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Perez-Torres, I.; Castrejon-Tellez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Angeloni, C. New mechanisms of action of natural antioxidants in health and disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Matsuoka, Y.; Sakai, K.; Fujiya, N.; Fujii, H.; Mano, J. Catechins in green tea powder (matcha) are heat-stable scavengers of acrolein, a lipid peroxide-derived reactive carbonyl species. Food Chem. 2021, 355, 129403. [Google Scholar] [CrossRef]
- Wu, M.; Brown, A.C. Applications of Catechins in the Treatment of Bacterial Infections. Pathogens 2021, 10, 546. [Google Scholar] [CrossRef]
- Ahmadvand, H.; Khalatbary, A.R. Anti-Inflammatory Effect of the Epigallocatechin Gallate Following Spinal Cord Trauma in Rat. Iran Biomed J. 2011, 5, 31–37. [Google Scholar]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Dufresne, C.J.; Farnworth, E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001, 12, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.A.; Suarez, N.G.; Cappadocia, L.; Annabi, B. Functional targeting of the TGF-betaR1 kinase domain and downstream signaling: A role for the galloyl moiety of green tea-derived catechins in ES-2 ovarian clear cell carcinoma. J. Nutr. Biochem. 2021, 87, 108518. [Google Scholar] [CrossRef]
- Basati, G.; Ghanadi, P.; Abbaszadeh, S. A review of the most important natural antioxidants and effective medicinal plants in traditional medicine on prostate cancer and its disorders. J. HerbMed Pharmacol. 2020, 9, 112–120. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Cabrera, C.; Artacho, R.; Gimenez, R. ; Beneficial effects of green tea-a review. J. Am. Col. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Hossain Md., S.; Urbi, Z.; Sule, A.; Hafizur Rahman, K.M. Andrographis paniculata (Burm. f.) Wall. ex Nees: A Review of Ethnobotany, Phytochemistry, and Pharmacology. ScientificWorldJournal 2014, 274905. [Google Scholar] [CrossRef]
- Abu-Ghefreh, A.A.; Canatan, H.; Ezeamuzie, C.I. In vitro and in vivo anti-inflammatory effects of andrographolide. Int. Immunopharmacol. 2009, 9, 313–318. [Google Scholar] [CrossRef]
- Tan, W.S.D.; Liao, W.; Zhou, S.; Wong, W.S.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochemical Pharmacology 2017, 139, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Villedieu-Percheron, E.; Ferreira, V.; Campos, J.F.; Destandau, E.; Pichon, C.; Berteina-Raboin, S. Quantitative determination of andrographolide and related compounds in Andrographis Paniculata extracts and biological evaluation of their anti-inflammatory activity. Foods 2019, 8, 683. [Google Scholar] [CrossRef]
- Madav, S.; Tripathi, H.C.; Mishra, S.K. Analgesic, Antipyretic And Antiulcerogenic Effects Of Andrographolide. Indian Journal of Pharmaceutical Sciences 1995, 57, 121. [Google Scholar]
- Pokala, N.; Alasyam, N.; Rasamal, K. Evaluation and comparison of antipyretic activity of aqueous leaf extracts of Vitex negundo and Andrographis paniculata in rabbits. National Journal of Physiology, Pharmacy and Pharmacology 2019, 9, 1. [Google Scholar] [CrossRef]
- Pan, C.-W.; Yang, S.-X.; Pan, Z.-Z.; Zheng, B.; Wang, J.-Z.; Lu, G.-R.; Xue, Z.-X.; Xu, C.-L. Andrographolide ameliorates d-galactosamine/lipopolysaccharide-induced acute liver injury by activating Nrf2 signaling pathway. Oncotarget 2017, 8, 41202–41210. [Google Scholar] [CrossRef] [PubMed]
- Shukla, B.; Visen, P.K.; Patnaik, G.K.; Dhawan, B.N. Choleretic effect of andrographolide in rats and guinea pigs. Planta Med. 1992, 58, 146–149. [Google Scholar] [CrossRef]
- Chua, L.S. Review on Liver Inflammation and Antiinflammatory Activity of Andrographis paniculata for Hepatoprotection. Phytotherapy Research 2014, 28, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.K.; Roy, S.; Dey, S. Protective activity of andrographolide and arabinogalactan proteins from Andrographis paniculata Nees. against ethanol-induced toxicity in mice. J Ethnopharmacol 2007, 111, 13–21. [Google Scholar] [CrossRef]
- Lu, W.J.; Lin, K.H.; Hsu, M.J.; Chou, D.S.; Hsiao, G.; Sheu, J.R. Suppression of NF-κB signaling by andrographolide with a novel mechanism in human platelets: Regulatory roles of the p38 MAPK-hydroxyl radical-ERK2 cascade. Biochemical Pharmacology 2012, 84, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; Schmid, J.A. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Churiyah, *!!! REPLACE !!!*; Pongtuluran, O.B.; Rofaani, E. Tarwadi Antiviral and Immunostimulant Activities of Andrographis paniculata. HAYATI Journal of Biosciences 2015, 22, 67–72. [Google Scholar] [CrossRef]
- Puri, A.; Saxena, R.; Saxena, R.P.; Saxena, K.C.; Srivastava, V.; Tandon, J.S. Immunostimulant agents from Andrographis paniculata. J. Nat. Prod. 1993, 56, 995–999. [Google Scholar] [CrossRef]
- Manjula, S.; Kalaiarasi, C.; Pavan, M.S.; Hathwar, V.R.; Kumaradhas, P. Charge density and electrostatic potential of hepatitis C anti-viral agent andrographolide: an experimental and theoretical study. Acta Cryst B 2018, 74, 693–704. [Google Scholar] [CrossRef]
- Paemanee, A.; Hitakarun, A.; Wintachai, P.; Roytrakul, S.; Smith, D.R. A proteomic analysis of the anti-dengue virus activity of andrographolide. Biomedicine & Pharmacotherapy 2019, 109, 322–332. [Google Scholar] [CrossRef]
- Wintachai, P.; Kaur, P.; Lee, R.C.H.; Ramphan, S.; Kuadkitkan, A.; Wikan, N.; Ubol, S.; Roytrakul, S.; Chu, J.J.H.; Smith, D.R. Activity of andrographolide against chikungunya virus infection. Scientific Reports 2015, 5, 14179. [Google Scholar] [CrossRef]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and Its Bioactive Diterpenoids Against Inflammation and Oxidative Stress in Keratinocytes. Antioxydants 2020, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Matsushima, Y.; Okudaira, N.; Sakagami, H.; Shirataki, Y. Cytotoxic Components Against Human Oral Squamous Cell Carcinoma Isolated from Andrographis paniculata. Anticancer Res. 2016, 36, 5931–5935. [Google Scholar] [CrossRef]
- Liao, H.-C.; Chou, Y.-J.; Lin, C.-C.; Liu, S.-H.; Oswita, A.; Huang, Y.-L.; Wang, Y.-L.; Syu, J.-L.; Sun, C.-M.; Leu, C.-M.; Lin, C.H.; Fu, S.L. Andrographolide and its potent derivative exhibit anticancer effects against imatinib-resistant chronic myeloid leukemia cells by downregulating the Bcr-Abl oncoprotein. Biochemical Pharmacology 2019, 163, 308–320. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M. S.; Khalili, N.; Naimi, E.; Majeed, M.; Sahebkar, A. Antioxidant and anti-inflammatory effects of curcumi- noid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015, 34, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Yue, G.G.; Chan, B.C.; Hon, P.M.; Lee, M.Y.; Fung, K.P.; Leung, P.C.; Lau, C.B. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem. Toxicol. 2010, 48, 2011–2020. [Google Scholar] [CrossRef]
- Ogiwara, Kazutaka. Curcumine-encapsulated protein nanoparticles, pharmaceutical and cosmeticcosmetic compositions containing the same, and production thereof. Japan, JP2009249370 A 2009-10-2.
- Kamiya, Shunichi; Sakai, Yasushi; Kawasaki, Hideki; Osawa, Toshihiko. Production method for tetrahydrocurcumins. Japan, JP11235192 A 1999-08-3.
- Ochiai, Akio; Oosa, Takao. Skin preparations containing pungent substances. Japan, JP2006182680 A 2006-07-1.
- Aggarwal, B.; Deb, L.; Prasad, S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef]
- Kosuga, Masaki; Kosuga, Takao; Ando, Nobuhiro; Muramatsu, Nobue; Kawai, Michio Topical and cosmeticcosmetic preparations containing capsaicins, sinapines, or curcuminescurcumines for secretion stimulation. Japan, JP10120558 A 1998-05-12.
- Graham, H.N. ; Green tea composition, consumption and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Ousji, O.; Sleno, L. Structural elucidation of novel stable and reactive metabolites of green tea catechins and alkyl gallates by LCMS/MS. Antioxidants, 2022, 11, 1635. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. Factors affecting polyphenol content and composition of fresh and processed tea leaves. Akademik Gida 2009, 7, 29–40. [Google Scholar]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Campos, J. F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol as new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Campos, J. F.; Berteina-Raboin, S. Eucalyptol as bio-based solvent for Migita–Kosugi–Stille coupling reaction on O,S,N-heterocycle. Catal. Today 2020, 348, 138–142. [Google Scholar] [CrossRef]
- Wisuitiprot, W.; Somsiri, A.; Ingkaninan, K.; Waranuch, N. In vitro human skin permeation and cutaneous metabolism of catechins from green tea extract and green tea extract-loaded chitosan microparticles. Int. J. Cosmet. Sci. 2011, 33, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Hwang, K.; Lee, J.; Han, S.Y.; Kim, E.-M.; Park, J.; Cho, J.Y. Skin Protective Effect of Epigallocatechin Gallate. Int. J. Mol. Sci. 2018, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Han, S.Y.; Hwang, K.; Kim, D.; Kim, E.-M.; Hossain, M.A.; Kim, J-H.; Cho, J.Y. Antioxidant and Cytoprotective Effects of (-)-Epigallocatechin-3-(3”-O-methyl) Gallate. Int. J. Mol. Sci. 2019, 20, 3993. [Google Scholar] [CrossRef]
- Dal Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M.B.G.; Marty, J.P. Skin penetration of epigallocatechin-3-gallate and quercetin from green tea and ginkgo biloba extracts vehiculated in cosmetic formulations. Skin Pharmacol. Physiol. 2009, 22, 299–304. [Google Scholar] [CrossRef]
- Yücel, C.; Karatoprak, G.S.; Yalçintaş, S.; Böncü, T.E. Ethosomal (-)-epigallocatechin-3-gallate as a novel approach to enhance antioxidant, anti-collagenase and anti-elastase effects. Beilstein J. Nanotechnol. 2022, 13, 491–502. [Google Scholar] [CrossRef]
- Maheshwari, R.G.S.; Tekade, R.K.; Sharma, P.A.; Darwhekar, G.; Tyagi, A.; Patel, R.P.; Jain, D.K. Ethosomes and ultradeformable liposomes for transdermal delivery of clotrimazole: A comparative assessment. Saudi Pharm. J. 2012, 20, 161–170. [Google Scholar] [CrossRef]
- Li, D.; Martini, N.; Wu, Z.; Chen, S.; Falconer, J.R.; Locke, M.; Zhang, Z.; Wen, J. Niosomal Nanocarriers for Enhanced Dermal Delivery of Epigallocatechin Gallate for Protection against Oxidative Stress of the Skin. Pharmaceutics 2022, 14, 726. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.; Basha, M.; Abd, S.H. Development of a novel vesicular system using a binary mixture of sorbitan monostearate and polyethylene glycol fatty acid esters for rectal delivery of rutin. J. Liposome Res. 2013, 23, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Muzzalupo, R.; Picci, N.; De, C.B. Co-encapsulation of lipophilic antioxidants into niosomal carriers: Percutaneous permeation studies for cosmeceutical applications. Colloids Surf. B 2014, 114, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Marchetti, N.; Bianchi, A. Comparative Evaluation of Different Co-Antioxidants on the Photochemical- and Functional-Stability of Epigallocatechin-3-gallate in Topical Creams Exposed to Simulated Sunlight. Molecules 2013, 18, 574–587. [Google Scholar] [CrossRef]
- Frasheri, L.; Schielein, M.C.; Tizek, L.; Mikschl, P.; Biedermann, T.; Zink, A. Great green tea ingredient? A narrative literature review on epigallocatechin gallate and its biophysical properties for topical use in dermatology. Phytother. Res. 2020, 34, 2170–2179. [Google Scholar] [CrossRef]
- Silva, A. R.; Seidl, C.; Furusho, A. S.; Boeno, M. M. S.; Dieamant, G. C.; Weffort-Santos, A. M. In vitro evaluation of the efficacy of commercial green tea extracts in UV protection. Int. J. Cosmet. Sci. 2013, 35, 69–77. [Google Scholar] [CrossRef]
- Chen, H.-W.; Huang, C.-S.; Li, C.-C.; Lin, A.-H.; Huang, Y.-J.; Wang, T.-S.; Yao, H.-T.; Lii, C.-K. Bioavailability of andrographolide and protection against carbon tetrachloride-induced oxidative damage in rats. Toxicol. Appl. Pharmacol. 2014, 280, 1–9. [Google Scholar] [CrossRef]
- Bera, R.; Ahmed, S.K.M.; Sarkar, L.; Sen, T.; Karmakar, S. Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC-MS/MS method. Pharm Biol 2014, 52, 321–329. [Google Scholar] [CrossRef]
- Sareer, O.; Ahmad, S.; Umar, S. Andrographis paniculata: a critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat. Prod. Res. 2014, 28, 2081–2101. [Google Scholar] [CrossRef]
- Casamonti, M.; Risaliti, L.; Vanti, G.; Piazzini, V.; Bergonzi, M.C.; Bilia, A.R. Andrographolide Loaded in Micro- and Nano-Formulations: Improved Bioavailability, Target-Tissue Distribution, and Efficacy of the “King of Bitters.”. Engineering 2019, 5, 69–75. [Google Scholar] [CrossRef]
- Yang, T.; Sheng, H.-H.; Feng, N.-P.; Wei, H.; Wang, Z.-T.; Wang, C.-H. Preparation of andrographolide-loaded solid lipid nanoparticles and their in vitro and in vivo evaluations: characteristics, release, absorption, transports, pharmacokinetics, and antihyperlipidemic activity. J Pharm Sci 2013, 102, 4414–4425. [Google Scholar] [CrossRef]
- Yan, H.; Huang, Z.; Bai, Q.; Sheng, Y.; Hao, Z.; Wang, Z.; Ji, L. Natural product andrographolide alleviated APAP-induced liver fibrosis by activating Nrf2 antioxidant pathway. Toxicology 2018, 396–397, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.L.; Wu, S.J.; Lee, S.C.; Ng, L.T. Antioxidant, antioedema and analgesic activities of Andrographis paniculata extracts and their active constituent andrographolide. Phytother Res 2009, 23, 958–964. [Google Scholar] [CrossRef]
- Peng, S.; Gao, J.; Liu, W.; Jiang, C.; Yang, X.; Sun, Y.; Guo, W.; Xu, Q. Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget 2016, 7, 80262–80274. [Google Scholar] [CrossRef] [PubMed]
- Sheeja, K.; Shihab, P.K.; Kuttan, G. Antioxidant and anti-inflammatory activities of the plant Andrographis paniculata Nees. Immunopharmacol Immunotoxicol 2006, 28, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Mussard, E.; Jousselin, S.; Cesaro, A.; Legrain, B.; Lespessailles, E.; Esteve, E.; Berteina-Raboin, S.; Toumi, H. Andrographis paniculata and Its Bioactive Diterpenoids Protect Dermal Fibroblasts against Inflammation and Oxidative Stress. Antioxidants 2020, 9, 432. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Su, Z.; Sun, C.; Zhang, X.; Zhao, X.; Lai, X.; Su, Z.; Li, Y.; Zhan, J.Y. Enhanced anti-tumor activity and reduced toxicity by combination andrographolide and bleomycin in ascitic tumor-bearing mice. Eur. J. Pharmacol. 2016, 776, 52–63. [Google Scholar] [CrossRef]
- Zhan, J.Y.-X.; Wang, X.-F.; Liu, Y.-H.; Zhang, Z.-B.; Wang, L.; Chen, J.-N.; Huang, S.; Zeng, H.-F.; Lai, X.-P. Andrographolide Sodium Bisulfate Prevents UV-Induced Skin Photoaging through Inhibiting Oxidative Stress and Inflammation. Mediators Inflamm. 2016, 2016, 3271451. [Google Scholar] [CrossRef]
- Yu, A.-L.; Lu, C.-Y.; Wang, T.-S.; Tsai, C.-W.; Liu, K.-L.; Cheng, Y.-P.; Chang, H.C.; Lii, C.-K.; Chen, H.-W. Induction of heme oxygenase 1 and inhibition of tumor necrosis factor alpha-induced intercellular adhesion molecule expression by andrographolide in EA.hy926 cells. J. Agric. Food Chem. 2010, 58, 7641–7648. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.; Su, S.-L.; Lu, C.-Y.; Lin, A.-H.; Lin, W.-C.; Liu, C.-S.; Yang, Y.-C.; Wang, H.-M.; Lii, C.-K.; Chen, H.-W. Andrographolide inhibits hypoxia-induced HIF-1α-driven endothelin 1 secretion by activating Nrf2/HO-1 and promoting the expression of prolyl hydroxylases 2/3 in human endothelial cells. Environ. Toxicol. 2017, 32, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.P.; Tee, W.; Ng, D.S.W.; Chan, T.K.; Peh, H.Y.; Ho, W.E.; Cheng, C.; Mak, J.C.; Wong, W.S.F. Andrographolide protects against cigarette smoke-induced oxidative lung injury via augmentation of Nrf2 activity. Br. J. Pharmacol. 2013, 168, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Tseng, C.-K.; Young, K.-C.; Sun, H.-Y.; Wang, S.-W.; Chen, W.-C.; Lin, C.-K.; Wu, Y.-H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014, 171, 237–252. [Google Scholar] [CrossRef]
- Yen, T.-L.; Chen, R.-J.; Jayakumar, T.; Lu, W.-J.; Hsieh, C.-Y.; Hsu, M.-J.; Yang, C.-H.; Chang, C.-C.; Lin, Y.-K.; Lin, K.-H.; Sheu, J.R. Andrographolide stimulates p38 mitogen-activated protein kinase-nuclear factor erythroid-2-related factor 2-heme oxygenase 1 signaling in primary cerebral endothelial cells for definite protection against ischemic stroke in rats. Transl Res 2016, 170, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.D.; Peh, H.Y.; Liao, W.; Pang, C.H.; Chan, T.K.; Lau, S.H.; Chow, V.T.; Wong, W.S.F. Cigarette Smoke-Induced Lung Disease Predisposes to More Severe Infection with Nontypeable Haemophilus influenzae: Protective Effects of Andrographolide. J. Nat. Prod. 2016, 79, 1308–1315. [Google Scholar] [CrossRef]
- Pan, C.-W.; Yang, S.-X.; Pan, Z.-Z.; Zheng, B.; Wang, J.-Z.; Lu, G.-R.; Xue, Z.-X.; Xu, C.-L. Andrographolide ameliorates d-galactosamine/lipopolysaccharide-induced acute liver injury by activating Nrf2 signaling pathway. Oncotarget 2017, 8, 41202–41210. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.Y.X.; Wang, X.F.; Liu, Y.H.; Zhang, Z.B.; Wang, L.; Chen, J.N.; Lai, X.P. Andrographolide Sodium Bisulfate Prevents UV-Induced Skin Photoaging through Inhibiting Oxidative Stress and Inflammation. Med. Inflamm. 2016, 2016, 3271451. [Google Scholar] [CrossRef]
- Fu, S.; Sun, C.; Tao, X.; Ren, Y. Anti-inflammatory effects of active constituents extracted from Chinese medicinal herbs against Propionibacterium acnes. Nat. Prod. Res. 2012, 26, 1746–1749. [Google Scholar] [CrossRef] [PubMed]
- Sirisha Mulukuri, N.V.L.; Kumar, P.; Satheesh Madhav, N.V.; Kusumdevi, V.; Nagajyothi. An insight review on andrographolide from the king of bitters and its therapeutic potential for skin cancer and cosmeceutical applications. Annals of Phytomedicine 2021, 10, 280–285. [Google Scholar]
- Asasutjarit, R.; Sooksai, N.; Fristiohady, A.; Lairungruang, K.; Ng, S. F.; Fuongfuchat, A. Optimization of production parameters for andrographolide-loaded nanoemulsion preparation by micro fluidization and evaluations of its bioactivities in skin cancer cells and UVB radiation-exposed skin. Pharmaceutics, 2021, 13, 1290. [Google Scholar] [CrossRef]
- Cantelli, M.; Ferrillo, M.; Donnarumma, M.; Emanuele, E.; Fabbrocini, G. A new proprietary gel containing glabridin, andrographolide, and apolactoferrin improves the appearance of epidermal melasma in adult women: A 6-month pilot, uncontrolled open-label study. J. Cosmet. Dermatol. 2020, 19, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K. I. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Heerdink, E. R.; Leufkens, H. G.; Herings, R. M. C.; Ottervanger, J. P.; Stricker, B. H. C.; Bakker, A. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics Arch. Intern. Med. 1998, 158, 1108–1112. [Google Scholar] [CrossRef]
- Nagappan, A. S.; Varghese, J.; Pranesh, G. T.; Jeyaseelan, V.; Jacob, M. Indomethacin inhibits activation of endothelial nitric oxide synthase in the rat kidney: possible role of this effect in the pathogenesis of indomethacin-induced renal damage. Chem. Biol. Interact. 2014, 221, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; Burr, A.M.; Zhao, W.W.; Kent, J.D.; Lefkowith, J.B.; Verburg, K.M.; Geis, G.S. Jama. 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Yue, Y.; Zhang, K.; Chen, Q.; Wang, H.; Lu, Y.; Huang, M.; Zheng, Xi.; Du, Z. Synthesis and Biological Evaluation of Curcumin Derivatives Containing NSAIDs for Their Anti-inflammatory Activity. Bioorganic & Medicinal Chemistry Letters 2015, 25, 3044–3051. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Chow, S.-C.; Chiu, S.-T. A note on design and analysis of clinical trials. Drug Des. Open Access 2013, 2, 102. [Google Scholar] [CrossRef]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark-side of curcumin. Int.J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
| Name | Structure and details |
|---|---|
| Andrographolide | 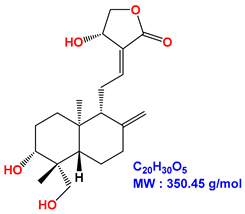 |
| Neoandrographolide | 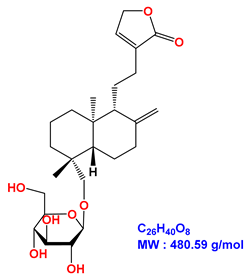 |
| 14-deoxyandrographolide | 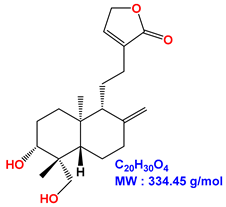 |
| 14-deoxy-11,12-didehydroandrographolide | 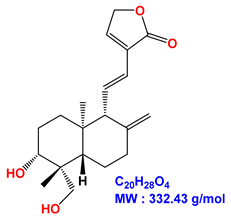 |
| 7,8-dimethoxy-2’-hydroxy-5-O-β-glucopyranosyloxyflavone | 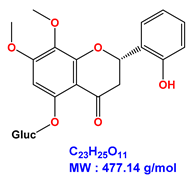 |
| Luteolin | 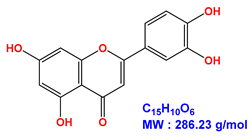 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





