Submitted:
20 May 2023
Posted:
22 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Design
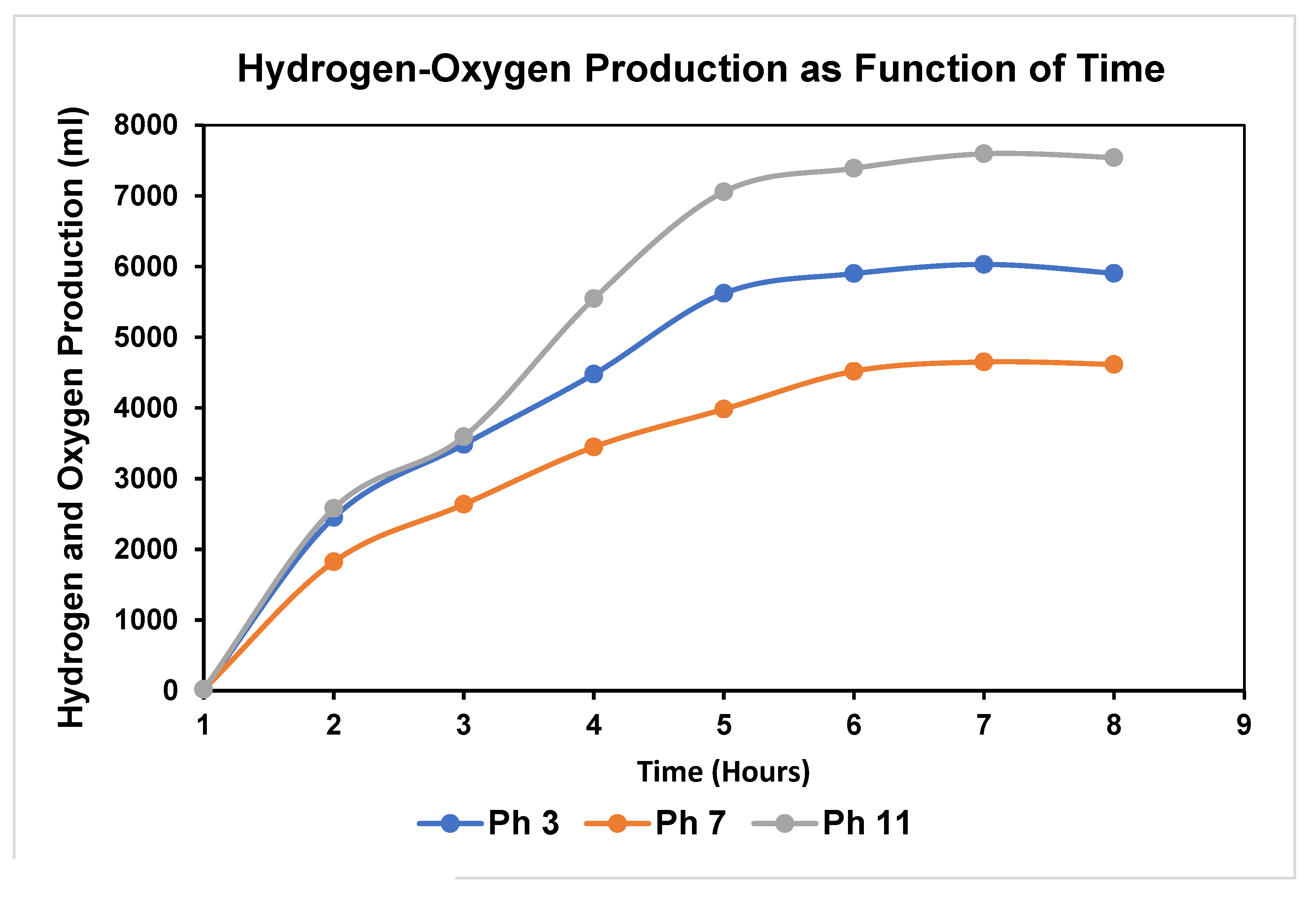
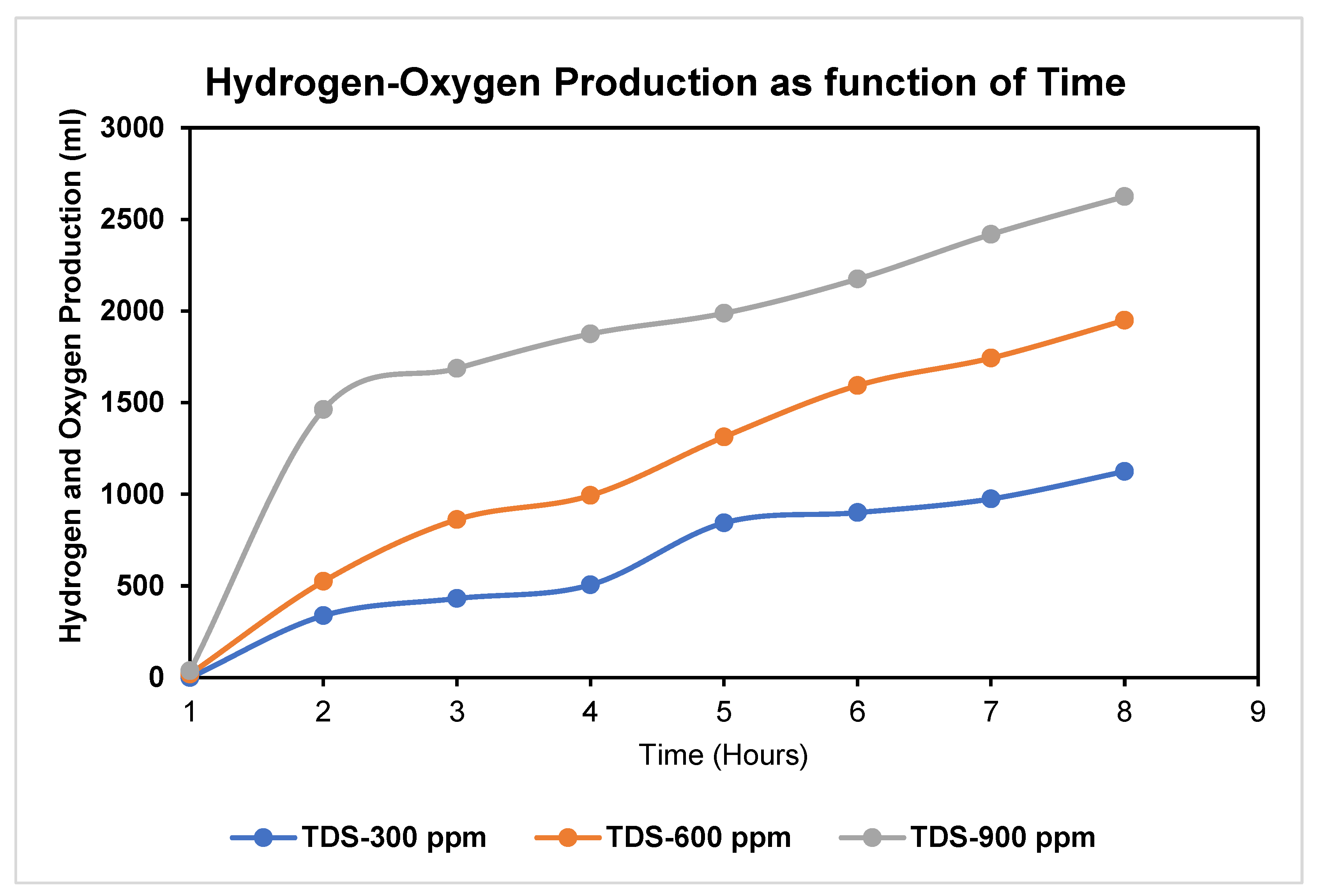
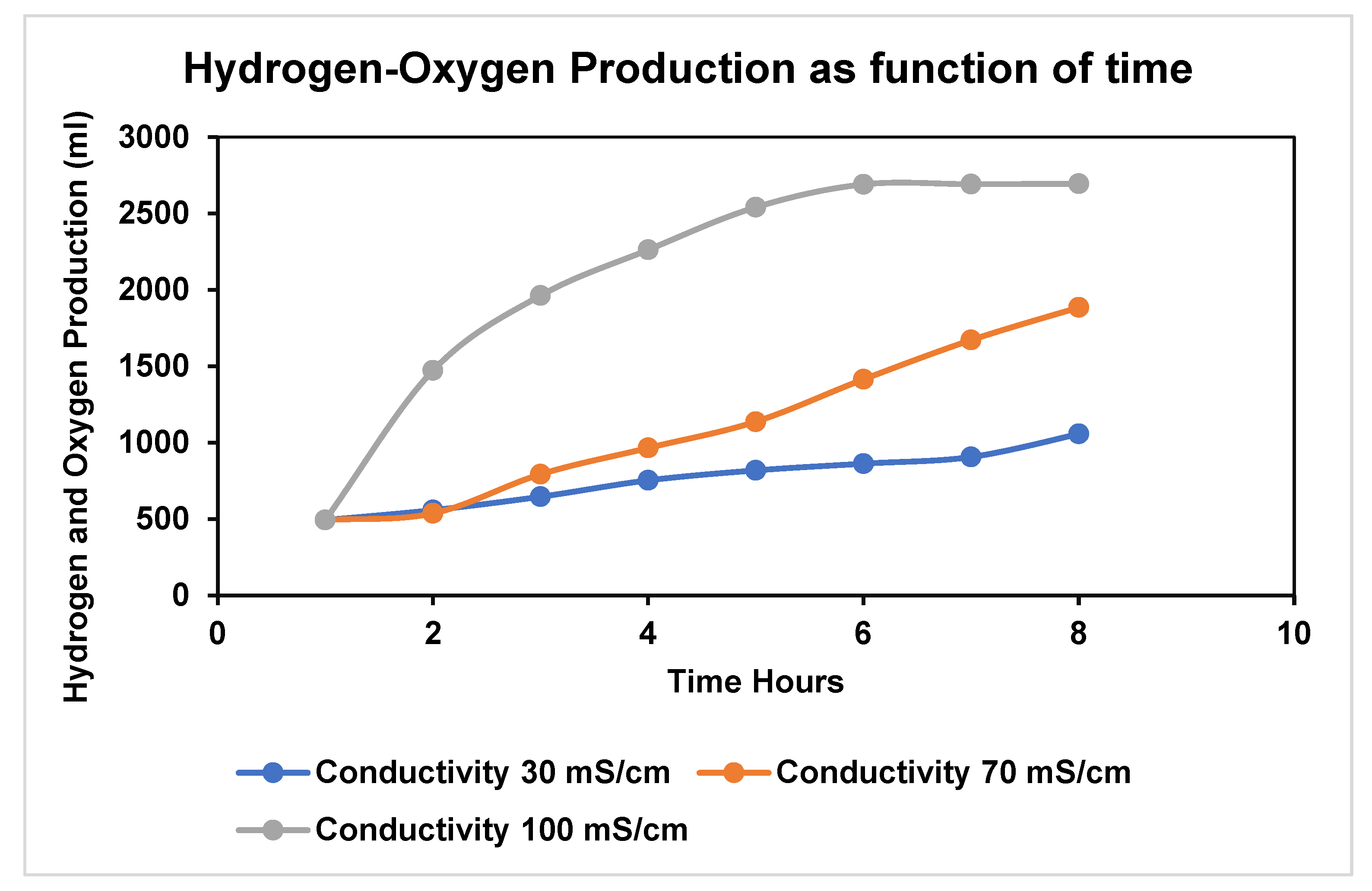
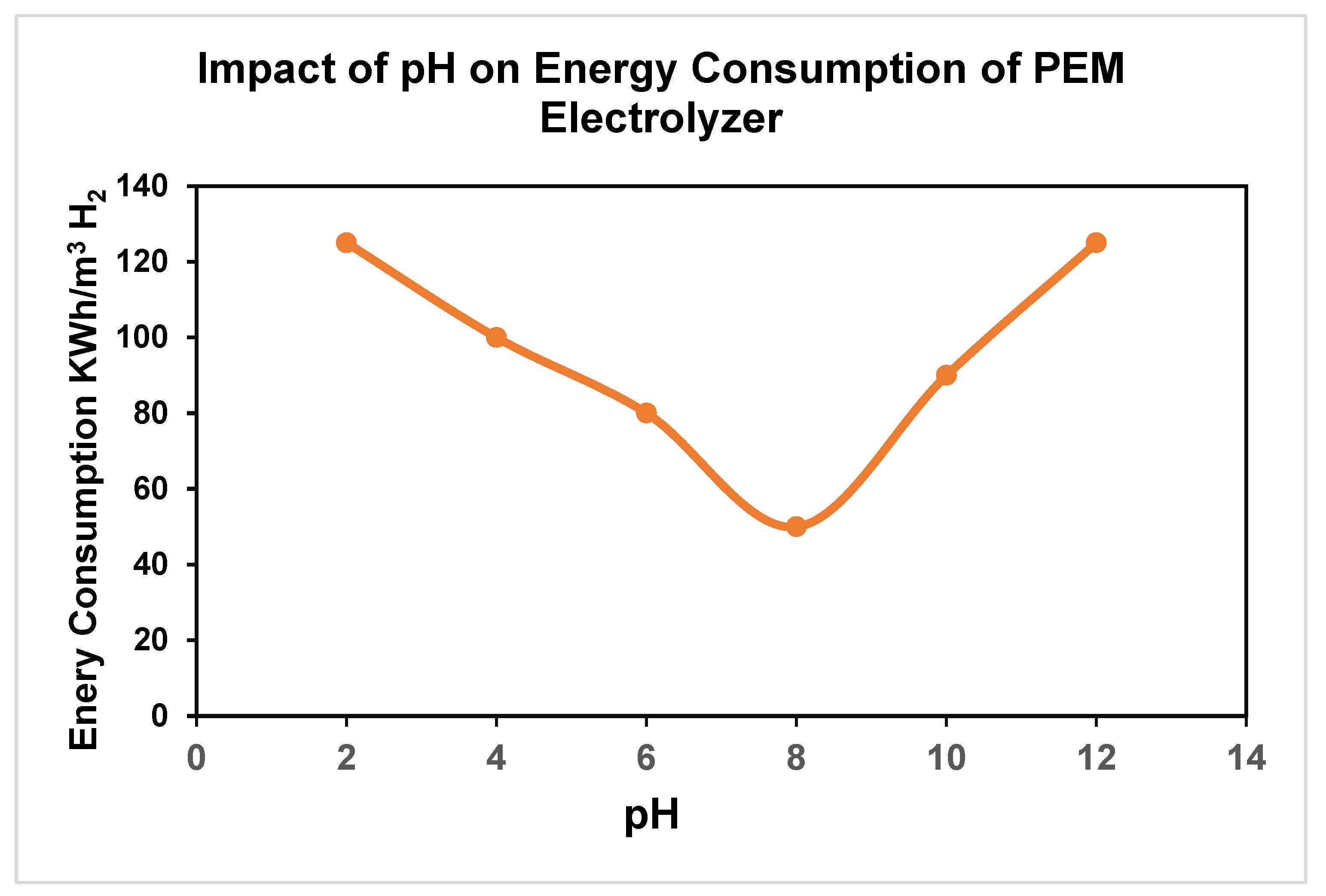
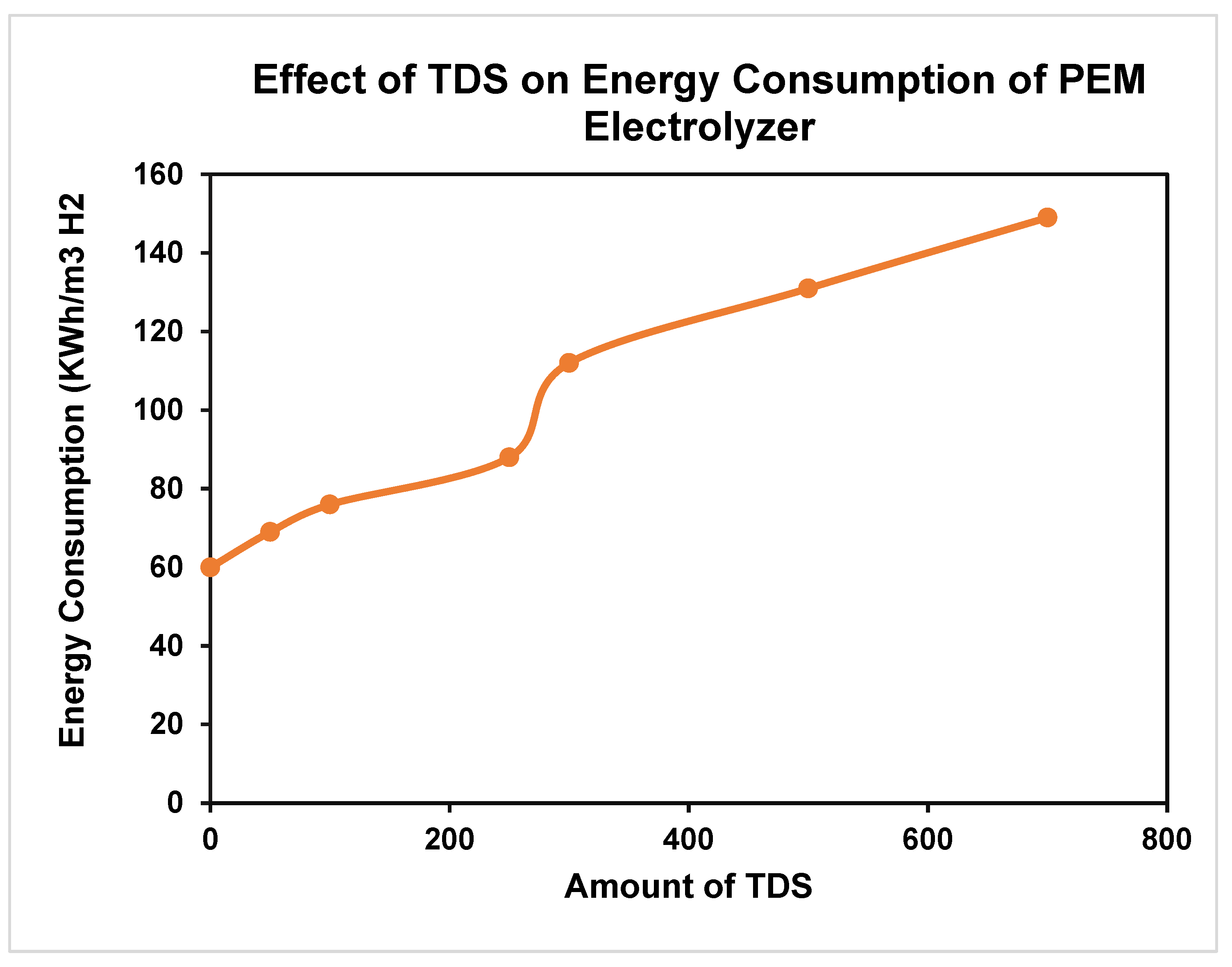
4. Expected Results
4.1. Hydrogen-Oxygen Production-Impact of pH:
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, M.; Ni, M.; Dennis, Y.C. Leung Electrochemistry Modeling of Proton Exchange Membrane (PEM) Water Electrolysis for Hydrogen Production. World Hydrogen Energy Conference 2006, 33–39. [Google Scholar]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Parametric Study of Solid Oxide Fuel Cell Performance. Energy Conversion and Management 2007, 48, 1525–1535. [Google Scholar] [CrossRef]
- Koroneos, C.; Dompros, A.; Roumbas, G.; Moussiopoulos, N. Life Cycle Assessment of Hydrogen Fuel Production Processes. International Journal of Hydrogen Energy 2004, 29, 1443–1450. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renewable and Sustainable Energy Reviews 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Barbir, F. PEM Electrolysis for Production of Hydrogen from Renewable Energy Sources. Solar Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Aouali, F.Z.; Becherif, M.; Ramadan, H.S.; Emziane, M.; Khellaf, A.; Mohammedi, K. Analytical Modelling and Experimental Validation of Proton Exchange Membrane Electrolyser for Hydrogen Production. International Journal of Hydrogen Energy 2017, 42, 1366–1374. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Lufrano, F.; Staiti, P.; Aricò, A.S. Electrochemical Characterization of a PEM Water Electrolyzer Based on a Sulfonated Polysulfone Membrane. Journal of Membrane Sciences 2013, 448, 209–214. [Google Scholar] [CrossRef]
- MA, L.; SUI, S.; ZHAI, Y. Investigations on High Performance Proton Exchange Membrane Water Electrolyzer. International Journal of Hydrogen Energy 2009, 34, 678–684. [Google Scholar] [CrossRef]
- Binninger, T.; Heinritz, A.; Mohamed, R. Kinetic Reference Potential, PH-Effect, and Energy Recovery in Electrolysis of Water. Physical Chemistry 2020. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis – a Review. Materials Science for Energy Technologies 2019, 2. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, M.G. Seawater Electrolysis for Hydrogen Production: A Solution Looking for a Problem? Energy & Environmental Science. [CrossRef]
- Abdallah, S.; Yousef, E.; Abdullah, I.; Tamime, A. The Effect of TDS on the Hydrogen and Oxygen Production Using Photovoltaic Power Generation System Available online:. Available online: https://www.semanticscholar.org/paper/The-Effect-of-TDS-on-the-Hydrogen-and-Oxygen-Using-Abdallah-Yousef/4bb62514951dca4847b1dfbb90fc0f6e51900acd (accessed on 19 May 2023).
- GRIGORIEV, S.; POREMBSKY, V.; FATEEV, V. Pure Hydrogen Production by PEM Electrolysis for Hydrogen Energy. International Journal of Hydrogen Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- LINKOUS, C. Development of New Proton Exchange Membrane Electrolytes for Water Electrolysis at Higher Temperatures. International Journal of Hydrogen Energy 1998, 23, 525–529. [Google Scholar] [CrossRef]
- Kowsari, E.; Zare, A.; Ansari, V. Phosphoric Acid-Doped Ionic Liquid-Functionalized Graphene Oxide/Sulfonated Polyimide Composites as Proton Exchange Membrane. International Journal of Hydrogen Energy 2015, 40, 13964–13978. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




