Submitted:
16 May 2023
Posted:
17 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Nutritional composition
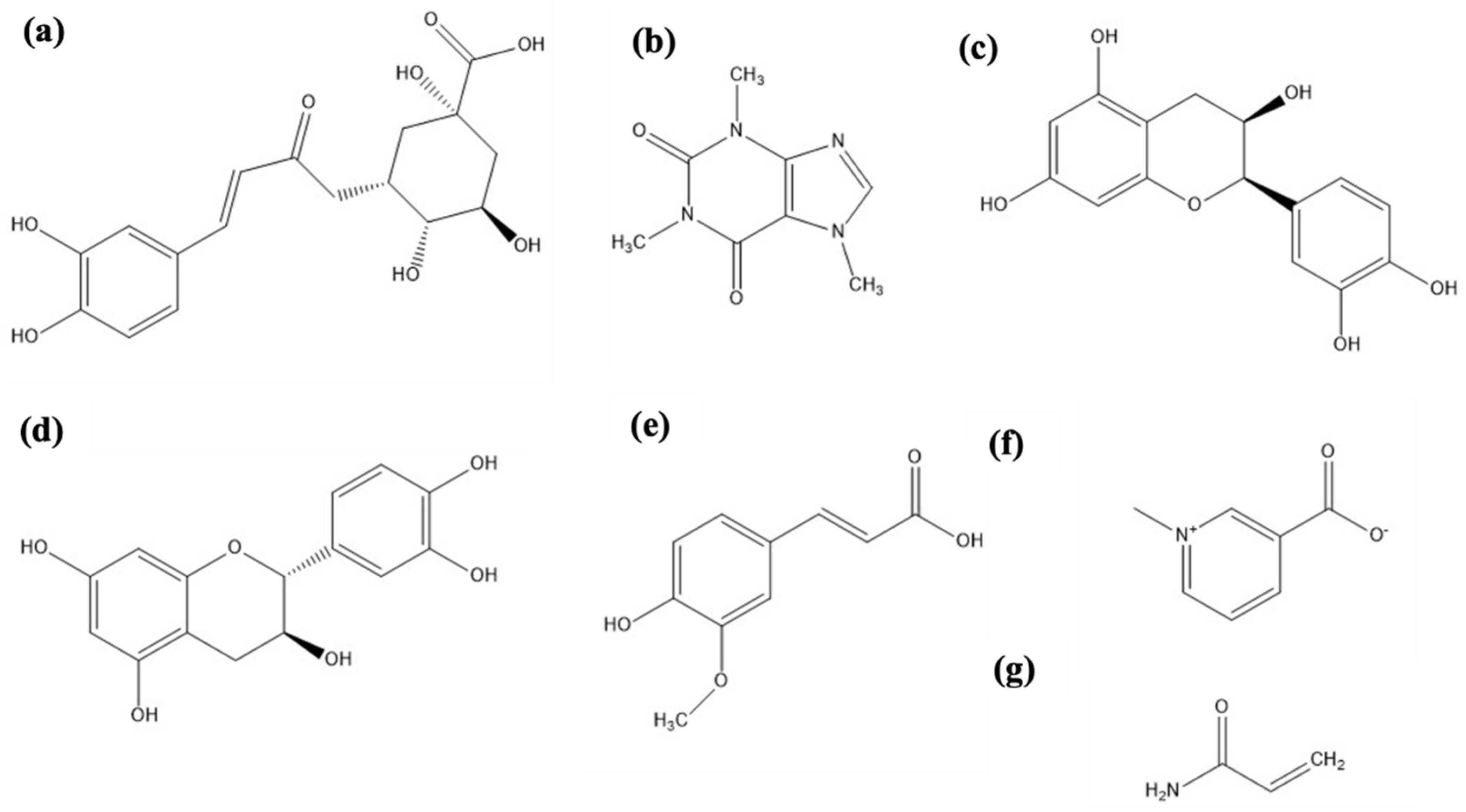
2.2. Chemical analysis
2.3. Melanoidins
2.4. Sensory evaluation
2.5. Biological activities
2.5.1. Antioxidant effect
2.5.2. Cytotoxic activity
3. Materials and Methods
3.1. Biological material
3.2. Bromatological analysis
3.3. Chemical analyses
3.3.1 Infusion preparation
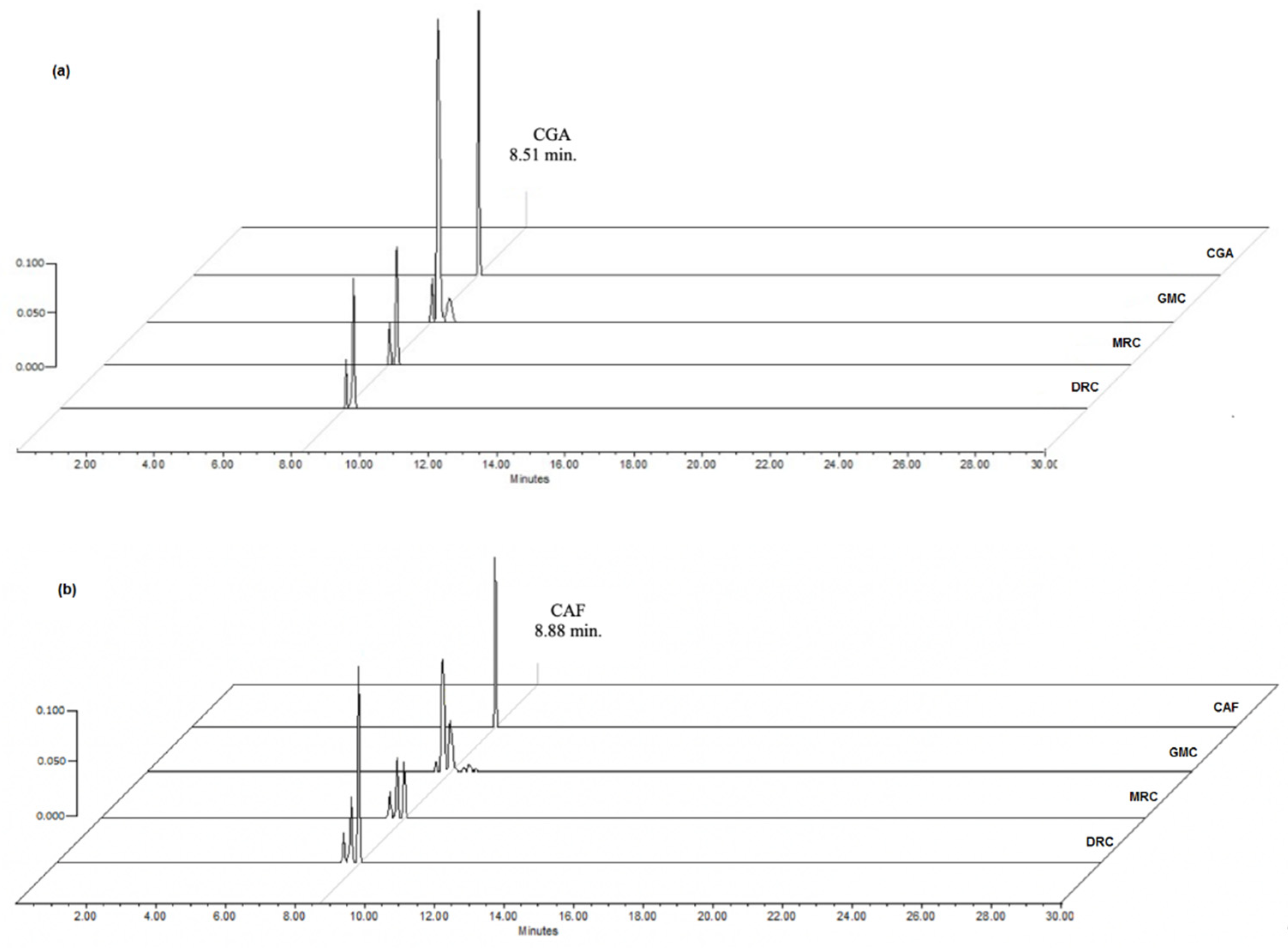
3.3.2. Chlorogenic acid and caffeine quantification
3.3.3. Chemical fractionation of MRC infusion
3.4. Melanoidins
3.5. Sensory assessment
3.6. Biological analyses
3.6.1. Antioxidant activity assays
Radical scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH)
ABTS (2,2′-Azinobis (3-ethylbenzothiazoline-6 sulfonic acid) radical
Ferric Reducing/Antioxidant Power (FRAP)
3.6.2. Cytotoxic evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization Exports of All Forms of Coffee by Exporting Countries to All Destinations January 2022. Available online: https://www.ico.org/prices/m1-exports.pdf (accessed on 10 May 2022).
- Hutachok, N.; Angkasith, P.; Chumpun, C.; Fucharoen, S.; Mackie, I.J.; Porter, J.B.; Srichairatanakool, S. Anti-Platelet Aggregation and Anti-Cyclooxygenase Activities for a Range of Coffee Extracts (Coffea arabica). Molecules 2020, 26, 10. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Kalita, D.; Abshiru, N. Quantification of Major Bioactive Constituents, Antioxidant Activity, and Enzyme Inhibitory Effects of Whole Coffee Cherries (Coffea arabica) and Their Extracts. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Ignacio, J.; Nicasio-Torres, P.; Santibáñez, A.; Cabrera-Hilerio, S.L.; Cruz-Sosa, F. Ethnopharmacological Study of the Genus Coffea and Compounds of Biological Importance. Rev Mex Ing Quím 2022, 21, 2856. [Google Scholar] [CrossRef]
- Khochapong, W.; Ketnawa, S.; Ogawa, Y.; Punbusayakul, N. Effect of Invitro Digestion on Bioactive Compounds, Antioxidant and Antimicrobial Activities of Coffee (Coffea arabica L.) Pulp Aqueous Extract. Food Chem 2021, 348. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Matsuda, S. The Chemopreventive Effects of Chlorogenic Acids, Phenolic Compounds in Coffee, against Inflammation, Cancer, and Neurological Diseases. Molecules 2023, 28, 2381. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Elms, R. Coffee Anual Mexico; 2021. [Google Scholar]
- Flores, V.F. La Producción de Café En México: Ventana de Oportunidad Para El Sector Agrícola de Chiapas. Revista Espacio I+D Innovación más Desarrollo 2015, 4, 174–194. [Google Scholar] [CrossRef]
- Hidalgo-Espinosa, E. Coffee Origins: A Guide to Mexico - Perfect Daily Grind. Available online: https://perfectdailygrind.com/2020/03/coffee-origins-a-guide-to-mexico/ (accessed on 20 March 2022).
- Tablas, G.I.; Guerrero, R.J. de D.; Aceves, R.E.; Álvarez, C.N.M.; Loyo, E.L.; Olvera, H.J.I. El Cultivo de Café En Ojo de Agua de Cuauhtémoc, Malinaltepec, Guerrero. Rev Mex De Cienc Agric 2021, 12, 1031–1042. [Google Scholar] [CrossRef]
- Centro de Estudios para el Desarrollo Rural Sustentable y la Soberanía Alimentaria (CEDRSSA) El Café En México Diagnóstico y Perspectiva; México, 2018.
- Roman-Maldonado, Y.; Gutiérrez-Salomón, A.L.; Jaimez-Ordaz, J.; García-Barrón, S.E.; Barajas-Ramírez, J.A. Drivers of Liking to Predict Consumers’ Acceptance of Local Coffee from Indigenous Mexican Regions. European Food Research and Technology 2022, 248, 467–475. [Google Scholar] [CrossRef]
- Poisson, L.; Blank, I.; Dunkel, A.; Hofmann, T. The Chemistry of Roasting—Decoding Flavor Formation. The Craft and Science of Coffee 2017, 273–309. [Google Scholar] [CrossRef]
- Duangjai, A.; Saokaew, S.; Goh, B.-H.; Phisalprapa, P. Shifting of Physicochemical and Biological Characteristics of Coffee Roasting Under Ultrasound-Assisted Extraction. Front Nutr 2021, 8, 1–8. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic Compounds in Coffee. Brazilian Journal of Plant Physiology 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Pan, T.; Ren, F.; Luo, H.; Zhang, H. Synthesis, Characterization and Effect of Alkyl Chain Unsaturation on the Antioxidant Activities of Chlorogenic Acid Derivatives. LWT 2022, 162, 113325. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic Acids, Caffeine Content and Antioxidant Properties of Green Coffee Extracts: Influence of Green Coffee Bean Preparation. European Food Research and Technology 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its in Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef] [PubMed]
- Lazcano-Sánchez, E.; Trejo-Márquez, Ma.A.; Vargas-Martinez, Ma.G.; Pascual-Bustamante, S. Contenido de Fenoles, Cafeina y Capacidad Antioxidante de Granos de Café Verdes y Tostados de Diferentes Estados de México. Revista Iberoamericana de Tecnología Postcosecha 2015, 16, 293–298. [Google Scholar]
- Tsai, C.-F.; Jioe, I.P.J. The Analysis of Chlorogenic Acid and Caffeine Content and Its Correlation with Coffee Bean Color under Different Roasting Degree and Sources of Coffee (Coffea arabica Typica). Processes 2021, 9, 2040. [Google Scholar] [CrossRef]
- Cwiková, O.; Komprda, T.; Šottníková, V.; Svoboda, Z.; Simonová, J.; Slováček, J.; Jůzl, M. Effects of Different Processing Methods of Coffee arabica on Colour, Acrylamide, Caffeine, Chlorogenic Acid, and Polyphenol Content. Foods 2022, 11, 3295. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The Content of Polyphenols in Coffee Beans as Roasting, Origin and Storage Effect. European Food Research and Technology 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Farah, A.; dePaula Lima, J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan Coffee Brew Chemical Analysis in Comparison to Espresso, Moka and American Brews. 2014. [Google Scholar] [CrossRef]
- Pietsch, A. Decaffeination-Process and Quality. In The Craft and Science of Coffee; Elsevier Inc., 2017; pp. 225–143. ISBN 9780128035580. [Google Scholar]
- Endeshaw, H.; Belay, A. Optimization of the Roasting Conditions to Lower Acrylamide Content and Improve the Nutrient Composition and Antioxidant Properties of Coffea Arabica. PLoS One 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Várady, M.; Ślusarczyk, S.; Boržíkova, J.; Hanková, K.; Vieriková, M.; Marcinčák, S.; Popelka, P. Heavy-Metal Contents and the Impact of Roasting on Polyphenols, Caffeine, and Acrylamide in Specialty Coffee Beans. Foods 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Rufián-Henares, J.A.; Pastoriza, S. Melanoidins in Coffee. In Coffee in Health and Disease Prevention; Elsevier, 2015; pp. 183–188. ISBN 978-0-12-409517-5. [Google Scholar]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee Melanoidins: Structures, Mechanisms of Formation and Potential Health Impacts. Food Funct 2012, 3, 903. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, M.L.; Chávez-Quiroz, K.; Medina-Juárez, L.Á.; Gámez, M.N. Compuestos Fenólicos, Melanoidinas y Actividad Antioxidante de Café Verde y Procesados de Las Especies Coffea arabica y Coffea canephora. Revista de Ciencias Biológicas y de la Salud 2013, XV, 51–56. [Google Scholar] [CrossRef]
- De Melo Pereira, G. v.; de Carvalho Neto, D.P.; Magalhães Júnior, A.I.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the Impacts of Postharvest Processing on the Aroma Formation of Coffee Beans–A Review. Food Chem 2019, 272, 441–452. [Google Scholar] [CrossRef]
- Cortés-Macías, E.T.; López, C.F.; Gentile, P.; Girón-Hernández, J.; López, A.F. Impact of Post-Harvest Treatments on Physicochemical and Sensory Characteristics of Coffee Beans in Huila, Colombia. Postharvest Biol Technol 2022, 187. [Google Scholar] [CrossRef]
- Stefanello, N.; Spanevello, R.M.; Passamonti, S.; Porciúncula, L.; Bonan, C.D.; Olabiyi, A.A.; Teixeira da Rocha, J.B.; Assmann, C.E.; Morsch, V.M.; Schetinger, M.R.C. Coffee, Caffeine, Chlorogenic Acid, and the Purinergic System. Food and Chemical Toxicology 2019, 123, 298–313. [Google Scholar] [CrossRef]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic Acid Attenuates High-Carbohydrate, High-Fat Diet–Induced Cardiovascular, Liver, and Metabolic Changes in Rats. Nutrition Research 2019, 62, 78–88. [Google Scholar] [CrossRef]
- Dos Santos, de S.L.; Carrero, H.I.P.; De Souza Rosa, L.R.; Barbosa, L.L.G.; Santos, da R.J.; Montenegro, J.; Da Silva, S.L.; Nana, de C.R.B.; Freitas-Silva, O.; Teodoro, J.A. Effect of the Roasting Levels of Coffea arabica L. Extracts on Their Potential Antioxidant Capacity and Antiproliferative Activity in Human Prostate Cancer Cells. RSC Adv 2020, 10, 30115–30126. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Phytochemical Profile and Antioxidant Capacity of Coffee Plant Organs Compared to Green and Roasted Coffee Beans. Antioxidants 2020, 9. [Google Scholar] [CrossRef]
- United States Department Agriculture (USDA). Coffee: World Markets and Trade; 2021. [Google Scholar]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The Sources and Mechanisms of Bioactive Ingredients in Coffee. Food Funct 2019, 10, 3113–3126. [Google Scholar] [CrossRef]
- Cordell, G.A.; Farnsworth, N.R.; Beecher, C.W.W.; Soejarto, D.D.; Kinghorn, A.D.; Pezzuto, J.M.; Wall, M.E.; Wani, M.C.; Cobb, R.R.; O’Neill, M.J.; et al. Novel Strategies for the Discovery of Plant-Derived Anticancer Agents. Anticancer Drug Discovery and Development: Natural Products and New Molecular Models 1994, 63–83. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Endeshaw, H.; Belay, A. Optimization of the Roasting Conditions to Lower Acrylamide Content and Improve the Nutrient Composition and Antioxidant Properties of Coffea arabica. PLoS One 2020, 15, e0237265. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Gökmen, V. Formation of Acrylamide in Coffee. Curr Opin Food Sci 2022, 45, 100842. [Google Scholar] [CrossRef]
- Cereals & Grains Association Approved Methods of Analysis. Available online: https://www.cerealsgrains.org/resources/methods/Pages/default.aspx (accessed on 11 March 2023).
- Tagliazucchi, D.; Elena, V.; Angela, C. Effect of Dietary Melanoidins on Lipid Peroxidation during Simulated Gastric Digestion: Their Possible Role in the Prevention of Oxidative Damage. J Agric Food Chem 2010, 58, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Specialty Coffee Association of America SCAA. SCAA Protocols. Cupping Specialty Coffee; 2015. [Google Scholar]
| Coffee | Content in percentage (%) | ||||
|---|---|---|---|---|---|
| Humidity | Ash | Fats | Proteins | Carbohydrates | |
| GCM | 8.48 ± 0.13** | 4.54 ± 0.06** | 5.09 ± 0.89 | 12.34 ± 0.29 | 69.56 ± 1.06 |
| MRC | 4.23 ± 0.14* | 3.84 ± 0.11 | 6.48 ± 0.34* | 13.04 ± 0.28 | 72.41 ± 1.26 |
| DRC | 3.59 ± 0.12 | 4.44 ± 0.08** | 8.15 ± 0.63** | 13.01 ± 0.38 | 70.81 ± 1.11 |
| Coffee beans | CGA | Caffeine | Melanoidins | ||
|---|---|---|---|---|---|
| Unclarified | Clarified | ||||
| mg/g coffee | Kmix Lg-1cm-1 | ||||
| GCM | 30.81 ± 2.22 | 0.87± 0.09 | 15.41±1.15 | 2.04 ±0.88 | 0.07 |
| Bourbon-GC | 55.75 ± 2.31** | 1.78± 0.12** | - | - | - |
| Oro Azteca-GC | 54.63 ± 2.43** | 1.77± 0.15** | - | - | - |
| Typica-GC | 36.81 ± 0.10 | 1.16 ± 0.18 | - | - | - |
| MRC | 30.26 ± 0.45** | 2.52±0.17* | 85.51±5.99* | 18.95 ±1.9** | 1.586 |
| DRC | 14.52 ± 0.65 | 3.88±0.23** | 96.79±3.44** | 29.06 ± 7.7** | 1.614 |
| Coffee beans | MRC | DRC |
|---|---|---|
| Aroma | 8.00 ± 0.16* | 7.75 ± 0.20 |
| Taste | 7.75 ± 0.29 | 7.75 ± 0.29 |
| Aftertaste | 8.00 ± 0.20 | 8.00 ± 0.20 |
| Acidity | 8.00 ± 0.61 | 8.00 ± 0.13 |
| Body | 8.25 ± 0.20* | 8.00 ± 0.13 |
| Balance | 8.00 ± 0.29* | 7.25 ± 0.29 |
| Uniformity | 10 ± 0 | 10 ± 0 |
| Clean cup | 10 ± 0 | 10 ± 0 |
| Sweetness | 10 ± 0 | 10 ± 0 |
| Taster score | 8.25 ± 0.29* | 7.5 ± 0.41 |
| Total Score | 86.25 | 84.25 |
| Sample | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|
| eq CGA | eq Trolox | eq CGA | eq Trolox | eq CGA | eq Trolox | eq FeSO4 | |
| MRC | 1.60 ± 0.27* | 52.74 ± 4.84* | 16.09 ± 0.33* | 14.39 ± 1.16 | 16.22 ± 1.04* | 14.59 ± 2.35* | 54.68 ± 1.46* |
| DRC | 1.12 ± 0.37 | 42.52 ± 1.91 | 12.49 ± 0.46 | 12.15 ± 0.49 | 8.82 ± 0.94 | 6.38 ± 1.40 | 33.30 ± 0.63 |
| Assay | IC50 | |||||
|---|---|---|---|---|---|---|
| MRC | DRC | MRC | DRC | CGA | Trolox | |
| mg/mL extract | μg/mL CGA content | μg/mL standard | ||||
| DPPH | 2.22 ± 0.08 | 2.59 ± 0.05* | 56.92 ± 1.90 | 66.20 ± 1.46* | 28.18 ± 0.83 | 91.88 ± 3.75* |
| ABTS | 0.38 ± 0.02 | 0.49 ± 0.02* | 9.69 ± 0.35 | 12.67 ± 0.44* | 6.51 ± 0.16* | 6.29 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





