1. Introduction
Primary hyperaldosteronism (PHA), also known as Conn’s syndrome is the most common disease related to adrenal glands in cats [
1]. This disease are related to dysfunction of glomerular part of adrenal cortex, where the mineralocorticoids are synthetized [
2]. PHA is characterized by increase of aldosterone secretion, steroid hormone which regulates sodium, potassium, and intravascular fluids levels. Levels of aldosterone are usually regulated by renin-angiotensin system [
3], but in the PHA, aldosterone levels increases without renin-angiotensin system control [
4]. Hypersecretion of aldosterone could be as result of adrenocortical hyperplasia or neoplasia, which are adenocarcinomas or unilateral adenomas frequently [
5].
Prevalence of PHA is high in elderly cats, and the most common clinical signs are elevated blood pressures, low potassium levels, and metabolic alkalosis, which produces muscle weakness, apathy, anorexia, and even blindness [
6]. Diagnosis is carried out by measure of aldosterone plasma concentration or calculating the relationship between plasma levels of aldosterone and renin, so high levels of aldosterone plus low levels of renin indicated hypersecretion of aldosterone no related to renin-angiotensin system, characteristic of PHA [
1]. Other diagnosis method consists to administration of fludrocortisone, a synthetic corticosteroid with mineralocorticoid activity [
7]. Fludrocortisone suppress the renin-angiotensin-aldosterone system, so oral administration for four days to healthy cats suppressed the urinary aldosterone-to-creatinine ratio [
8]. A non-decrease aldosterone levels in urine and/or blood could be used as diagnosis of PHA in cats [
9].
The first-choice treatment in cats with PHA is adrenalectomy, but several patients presented adjacent structures invasion by tumor or metastasis [
10]. In these cases, chemical treatment including antihypertensives, potassium supplement, and aldosterone inhibitors, is the best option [
1,
11]. Diagnostic imaging is necessary to differentiate between tumors and non-tumors mineralocorticoids excess and is used to identify neoplasia and to evaluate surgical or non-surgical treatment. However, limitations to conventional diagnostic imaging to choose the optimal treatment strategy exists [
1].
The PHA is a underdiagnosed pathology in cats, with the prevalence is more elevated than other pets, since clinical signs are often confused with other disorders as chronic kidney disease [
2]. For this reason, the diagnosis of PHA remains a challenge in veterinary medicine.
The main of this systematic review is to describe the most common clinical presentation of PHA in cats, and to analyze the survival after surgery treatment.
2. Materials and Methods
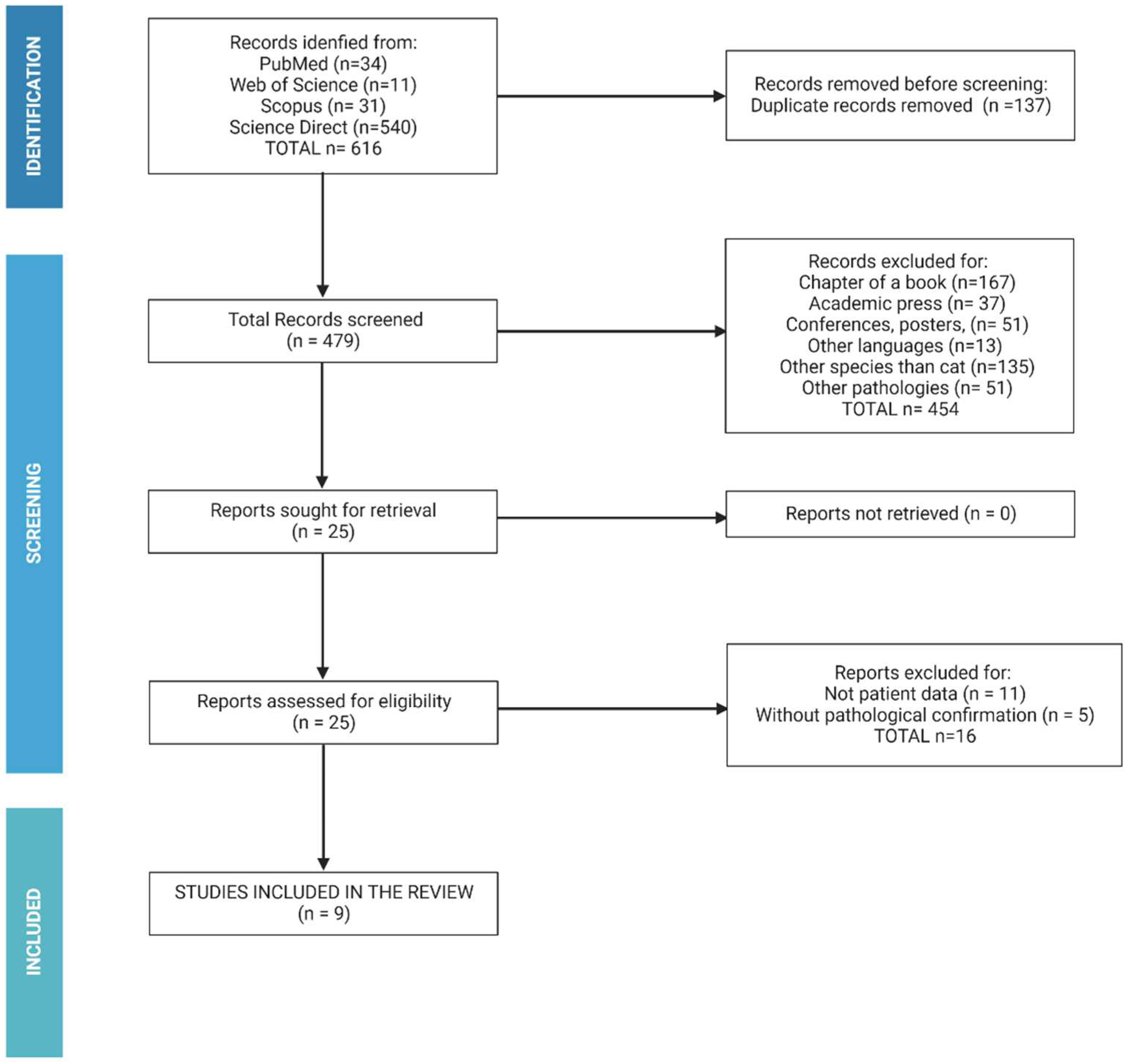
To carry out this study, an online bibliographic search was performed between 2012 to 2022 for studies relating to clinical cases and treatment of feline PHA. The preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines were followed to report this study. Articles were independently selected by two researchers (GR, SM). In case of disagreement, a third independent researcher (LL) was consulted. The search utilized the PubMed (
http://www.pubmed.gov/), Science Direct (
http://www.sciencedirect.com), Scopus (
http://www.scopus.com), and ISI Web of Science (
http://www.webofscience.com/wos/) databases, using the following terms: (primary AND Conn’s AND syndrome AND cat) OR (primary AND hyperaldosteronism AND cat).
Analysis was restricted to the English and German language, and the abstract were reviewed to evaluate the relevance of study to the question. Experimental and observational studies, editorial and book chapters were excluded. Also, case reports without detailed description of studied variables were eliminated. Studies meeting these criteria were reviewed by the primary author following the algorithm show in
Figure 1.
The following data were recorded to animals from each study: age, breed, sex, aldosterone and potassium levels, blood pressure, affected system (classified to cardiovascular, urinary, nervous, and ocular system), type of tumor (classified to adenocarcinoma, nodular hyperplasia, or adenoma), treatment (surgical or not surgical), and survival after six months of diagnosis.
Statistical analysis was performed by SAS statistical package (North Carolina State University). Normality and homoscedasticity of the quantitative variables (aldosterone and potassium blood levels, and blood pressure). Relationship between quantitative variables was studied by ANOVA test, whereas relationship between qualitative variables (sex, age (between 5 to 10 years-group A, between 11 to 15 years-group B, more than 15 years-group C), purebred and crossbreed, muscular weakness presence and type of tumor) was analyzed by Chi-square test. P-values < 0,05 were considered statistically significant.
3. Results
A total of 479 studies without duplicated records have been used. Of them, chapter of books (n=167), academic press (n=37), conferences of congress (n=51), written in other languages (n=13), related to other species (n=135) and other pathologies (n=51) were removed. In addition, studies without patient data or without pathological confirmation (n=11) were excluded. Fourteen studies with the inclusion criteria were identified (
Table 1).
The total of animals included was 39. The epidemiological data of the cats included in this study shows in the
Table 2. Briefly, the most of cats included are males (61,54%), with age ranged from 11 to 15 years old (58,97%) and around of 90% are crossbreed (short- or longhair). Related to biochemical data, the mean of aldosterone levels was 2124,19 ±1471,17 pmol/L, being the normal range between 87 to 224 pmol/L. The blood potassium levels were 3,14 ± 0,44 mmol/L (normal range 3,5-5,8 mmol/L), and the mean of blood pressure was 198,45 ± 26,21 mmHg, with the normal range is 120-160mmHg.
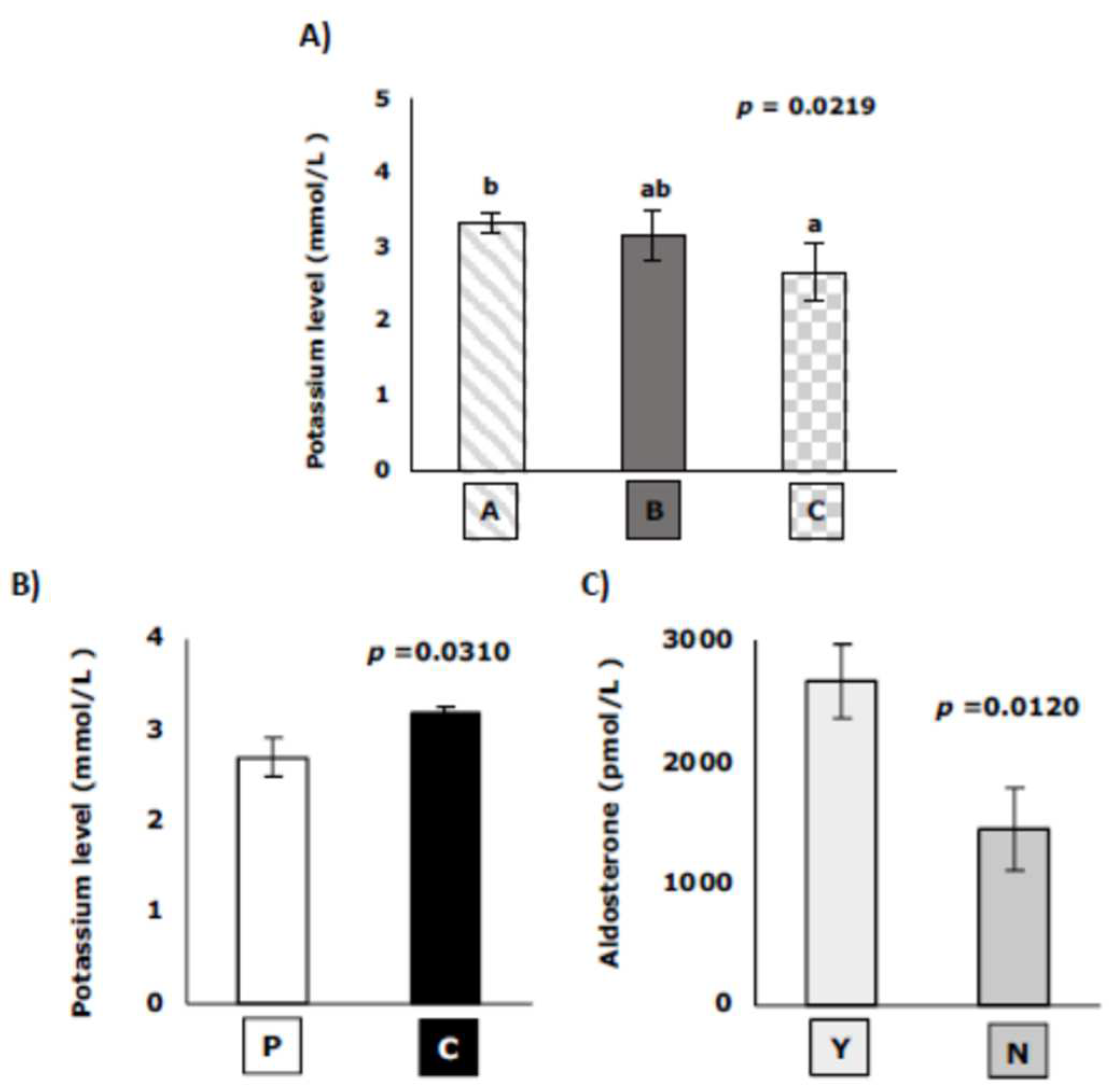
The blood potassium levels were statistically correlated with age and breed, being lower in elderly cats (more 15 years old) (
Figure 2A), and higher in crossbreed than purebred cats (
Figure 2B). Aldosterone levels only were statistically correlated with presence of muscular weakness, being higher in cats with muscular weakness (
Figure 2C).
Muscular weakness appears in 56,41% of animals, and adenoma and adrenal adenocarcinoma are the most frequent cause of PHA development, being the percentage of affected animals 30,77 % for both types. Related to system affected, the most frequent affectation is in ocular system (47,07%), followed by urinary system affectation (34,48%) and cardiovascular system (24,14%). The neurological signs are low frequency (13,79%). In ocular system, retinal detachment and subsequent blindness appears in 62,5% of cats. The cardiovascular signs as gallop rhythms, tachycardia, systolic murmurs, and ventricular hypertrophy are described (
Table 3).
With surgical treatment (adrenalectomy), the free survival after six months is 83,33%, whereas without surgical treatment, life expectancy is lower than six months in all cases, regardless of pre-intervention aldosterone levels.
4. Discussion
In this systematic review, literature regarding the clinical signs, prognosis factors, treatment, and efficacy in feline PHA have been analyzed. This disease in more frequent in older cats. However, it is underdiagnosed because clinical signs are often confused with other disorders as chronic kidney disease. For this reason, this systematic review could serve to improve the decision tools for the diagnosis and treatment of this disease in feline veterinary medicine.
A total of fourteen studies with 39 individual clinical cases have been included. Despite these small numbers, the quality of information provided, and the strict inclusion criteria used in this review, ensure concrete and effective conclusions for use in the feline PHA diagnosis and treatment. Epidemiological data shows that the most common patients with PHA are elderly (more than ten years old) and crossbreed animals. Mean values of aldosterone levels and blood pressure were elevated, whereas potassium levels were low comparing with normal standard range, according with the pathogenesis of disease. In fact, the renin-angiotensin-aldosterone system regulates blood pressure, and renal retention of sodium and water, so dysregulation of this system provokes high levels of blood pressure and low levels of potassium in blood [
25,
26]. Potassium has an antihypertensive effect by promoting sodium excretion, so low levels of potassium are reflected in a blood pressure increase [
27].
Potassium levels are lower in cats with more than 15 years old. These results are in accordance with results observed in chronic kidney disease, where age is a relevant factor of hypokalemia [
28]. Related to breed, crossbred cats show higher potassium levels than purebred cats. Up to now, no study relates potassium levels to feline breed, although some papers have found genetic factors associated with hypokalemia, mainly in Burmese breed [
29] and in human [
30]. Aldosterone levels only present correlation with muscular weakness, so that high levels of aldosterone increase the probability of suffering muscular weakness, typical clinical sign of PHA. This result indicate that an increase of aldosterone levels regardless renin-angiotensin system could be a prognostic factor of PHA development. Also, these levels are not related to age, sex, or breed, so could be a good prognostic factor.
Type of tumor which provokes the PHA disease are not related with none of epidemiological or biochemical factors studied. According to results of [
6], the most common tumor is adenocarcinoma, although nodular hyperplasia are underdiagnosed, so imaging diagnosis are not able to visualize adrenal gland mass.
A 47,07% of cats presented ocular affectation, including retinal detachment. The retinal detachment is associated to elevated blood pressure, and other clinical ocular signs are related in PHA patients, as intraocular hemorrhages, hyphemia, and mydriasis, all related to blood pressure [
6]. [
31] found retinal detachment as the most common sign, and related hypertension with renal failure progression. The second system altered was urinary system. Likewise, [
13] shows urinary system failure. However, lack correlation between blood pressure and renal failure indicates that other etiologies could be the cause, as hypokalemia, or increase of aldosterone or angiotensinogen II levels [
5]. a continuous monitoring of renal function is necessary in these patients, regardless the blood pressure. An increase in aldosterone levels over time can cause fibrotic damage to the kidney and heart [
1]. If aldosterone levels do not completely suppress the renin-angiotensin-aldosterone system, the increased of angiotensinogen II can trigger renal fibrosis, which could explain the urinary system alterations. The same mechanism is described as triggering fibrosis of the heart [
6], explaining the cardiovascular problems encountered in our results. In addition to failures in the ocular, renal, and cardiovascular systems, the observed results also showed neurological problems associated with PHA, as ataxia, and multifocal intravascular thrombi around the brainstem and cerebellum. These neurological signs are related to chronic hypertension (Mau et al., 2020), and between 29 to 46% of hypertensive cats presents neurological failure [
31]. Related to hypokalemia, other neurological signs, as hyporeflexia, ventroflexion of the neck, flaccid paresis, and muscle hypotonia has been described in the literature [
6].
If it is possible, the adrenalectomy is carried out, with free survival after six months in 83,33% of the cases. However, if the surgical treatment is not possible, the prognosis is lower, and in all the cases, the patients do not survive more than six months. These results are similar to human, where if surgical treatment is not possible, treatment with mineralocorticoid receptor antagonists is the election treatment [
32]. Low-sodium diet is recommended to maintain a high-normal serum potassium concentration to mitigate the clinical signs [
33]. This systematic review has limitations related mainly with the number of studies and the number of clinical cases included. This factor should be taken into account when interpreting the results of this systematic review.
5. Conclusions
In conclusion, the high prevalence of feline PHA is in elderly cats, and the most frequent clinical signs are polyuria, polydipsia, blindness, and muscular weakness. On physical examination, cardiac murmur and arrhythmias are common. Adenocarcinomas are the most usual cause, and provokes high aldosterone concentrations, hypertension, and low levels of potassium. These levels are statistically correlated to age and feline breed, whereas high levels of aldosterone could indicate muscular weakness. Finally, high levels of aldosterone are not related to survival after adrenalectomy, which is the treatment with better prognosis.
Author Contributions
Conceptualization, G.R. and S.M.; methodology, G.R. and J.R.; analysis, P-J.M-G. and L.LL.; data curation, P-J.M-G. and L.LL; writing—original draft preparation, L.LL.; writing—review and editing, L.LL.; supervision, L.LL.; funding acquisition, P-J.M-G. and L.LL. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the Veterinary Medicine Faculty of Universidad Cardenal Herrera-CEU.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kooistra, H.S. Primary Hyperaldosteronism in Cats: An Underdiagnosed Disorder. Vet Clin North Am Small Anim Pract 2020, 50, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Langlois, D.K.; Mazaki-Tovi, M.; Harro, C.C.; Refsal, K.R. Multiple Corticosteroid Abnormalities in Cats with Hyperaldosteronism. J Vet Intern Med 2021, 35, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.H. Aldosterone Biosynthesis, Regulation, and Classical Mechanism of Action. Heart Fail Rev 2005, 10, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Edinga-Melenge, B.E.; Ama Moor, V.J.; Nansseu, J.R.N.; Nguetse Djoumessi, R.; Mengnjo, M.K.; Katte, J.-C.; Noubiap, J.J.N.; Sobngwi, E. Renin Angiotensin Aldosterone System Altered in Resistant Hypertension in Sub-Saharan African Diabetes Patients without Evidence of Primary Hyperaldosteronism. JRSM Cardiovasc Dis 2017, 6, 2048004017695006. [Google Scholar] [CrossRef] [PubMed]
- Attipa, C.; Beck, S.; Lipscomb, V.; English, K.; Carvalho, S.; Kiupel, M.; Szladovits, B.; Peters, L.M. Aldosterone-Producing Adrenocortical Carcinoma with Myxoid Differentiation in a Cat. Vet Clin Pathol 2018, 47, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Djajadiningrat-Laanen, S.; Galac, S.; Kooistra, H. Primary Hyperaldosteronism: Expanding the Diagnostic Net. J Feline Med Surg 2011, 13, 641–650. [Google Scholar] [CrossRef]
- Djajadiningrat-Laanen, S.C.; Galac, null; Boevé, M.H.; Boroffka, S. a. E.B.; Naan, E.C.; IJzer, J.; Kooistra, H.S. Evaluation of the Oral Fludrocortisone Suppression Test for Diagnosing Primary Hyperaldosteronism in Cats. J Vet Intern Med 2013, 27, 1493–1499. [CrossRef]
- Djajadiningrat-Laanen, S.C.; Galac, S.; Cammelbeeck, S.E.; van Laar, K.J.C.; Boer, P.; Kooistra, H.S. Urinary Aldosterone to Creatinine Ratio in Cats before and after Suppression with Salt or Fludrocortisone Acetate. J Vet Intern Med 2008, 22, 1283–1288. [Google Scholar] [CrossRef]
- Matsuda, M.; Behrend, E.N.; Kemppainen, R.; Refsal, K.; Johnson, A.; Lee, H. Serum Aldosterone and Cortisol Concentrations before and after Suppression with Fludrocortisone in Cats: A Pilot Study. J VET Diagn Invest 2015, 27, 361–368. [Google Scholar] [CrossRef]
- Griffin, S. Feline Abdominal Ultrasonography: What’s Normal? What’s Abnormal? The Kidneys and Perinephric Space. J Feline Med Surg 2020, 22, 409–427. [Google Scholar] [CrossRef]
- Rose, S.A.; Kyles, A.E.; Labelle, P.; Pypendop, B.H.; Mattu, J.S.; Foreman, O.; Rodriguez, C.O.; Nelson, R.W. Adrenalectomy and Caval Thrombectomy in a Cat with Primary Hyperaldosteronism. J Am Anim Hosp Assoc 2007, 43, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Leshinsky, J.; Beatty, J.A.; Fawcett, A.; Voss, K.; Makara, M.; Krockenberger, M.B.; Barrs, V.R. Aldosterone and Progesterone-Secreting Adrenocortical Adenocarcinoma in a Cat with a Concurrent Meningioma. JFMS Open Rep 2016, 2, 2055116915624448. [Google Scholar] [CrossRef]
- Kirkwood, N.; Boland, L.; Brunel, L.; Wardman, A.; Barrs, V.R. Acute Adrenal Haemorrhage in Two Cats with Aldosterone-Secreting Adenocarcinomas. JFMS Open Rep 2019, 5, 2055116919840828. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, K.; Barrs, V.R.; Foster, D.F.; Beatty, J.A. Hyperaldosteronism and Hyperprogesteronism in a Cat. J Feline Med Surg 2009, 11, 758–762. [Google Scholar] [CrossRef]
- Lopez, W.A.; Ayers, J.C.; Bertran, J.; Fox-Alvarez, S.A.; Vilaplana Grosso, F.R. What Is Your Diagnosis? J Am Vet Med Assoc 2022, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Guerios, S.D.; Souza, C.H. de M.; Bacon, N.J. Adrenocortical Tumor in a Cat Secreting More than One Type of Corticosteroid. JFMS Open Rep 2015, 1, 2055116915617970. [Google Scholar] [CrossRef]
- Ischaemic Myelopathy in a Cat with Chronic Kidney Disease, Hyperthyroidism, and Hyperaldosteronism - Silvestrini - 2021 - Veterinary Record Case Reports - Wiley Online Library. Available online: https://bvajournals.onlinelibrary.wiley.com/doi/full/10.1002/vrc2.11 (accessed on 8 December 2022).
- Smith, R.R.; Mayhew, P.D.; Berent, A.C. Laparoscopic Adrenalectomy for Management of a Functional Adrenal Tumor in a Cat. J Am Vet Med Assoc 2012, 241, 368–372. [Google Scholar] [CrossRef]
- Yu, J.; Lenord, J.; Lau, M.; Brunel, L.; Gray, R.; Donahoe, S.L.; Boland, L. Gynaecomastia in a Male Neutered Cat with an Adrenal Tumour and Associated Hyperprogesteronism, Hypercortisolism and Hyperaldosteronism. JFMS Open Rep 2021, 7, 20551169211045640. [Google Scholar] [CrossRef]
- Koutinas, C.K.; Soubasis, N.C.; Djajadiningrat-Laanen, S.C.; Kolia, E.; Theodorou, K. Urinary Aldosterone/Creatinine Ratio After Fludrocortisone Suppression Consistent with PHA in a Cat. J Am Anim Hosp Assoc 2015, 51, 338–341. [Google Scholar] [CrossRef]
- Mau, J.; Dietere, K.; Rohwedder, T.; Deutschland, M.; Böttcher, P.; Klopfleish, R.; Bertram, C. Conn’s Syndrome in Two Cats with Unusual Complications. Praktische Tierarzt 101, 342–356.
- Willi, B.; Kook, P.H.; Quante, S.; Boretti, F.; Sieber-Ruckstuhl, N.S.; Grest, P.; Scherrer, O.; Riond, B.; Hofmann-Lehmann, R.; Nussberger, J.; et al. [Primary hyperaldosteronism in cats]. Schweiz Arch Tierheilkd 2012, 154, 529–537. [Google Scholar] [CrossRef]
- Lo, A.J.; Holt, D.E.; Brown, D.C.; Schlicksup, M.D.; Orsher, R.J.; Agnello, K.A. Treatment of Aldosterone-Secreting Adrenocortical Tumors in Cats by Unilateral Adrenalectomy: 10 Cases (2002-2012). J Vet Intern Med 2014, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, P.; Piviani, M.; Sanchez Masian, D. Ischaemic Myelopathy in a Cat with Chronic Kidney Disease, Hyperthyroidism, and Hyperaldosteronism. Veterinary Record Case Reports 2021, 9, e11. [Google Scholar] [CrossRef]
- Javadi, S.; Slingerland, L.I.; van de Beek, M.G.; Boer, P.; Boer, W.H.; Mol, J.A.; Rijnberk, A.; Kooistra, H.S. Plasma Renin Activity and Plasma Concentrations of Aldosterone, Cortisol, Adrenocorticotropic Hormone, and Alpha-Melanocyte-Stimulating Hormone in Healthy Cats. J Vet Intern Med 2004, 18, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J Am Soc Nephrol 2017, 28, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Giebisch, G.; Wang, W. Potassium Transport: From Clearance to Channels and Pumps. Kidney Int 1996, 49, 1624–1631. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, S.; Raphael, K.L. Hyperkalemia and Hypokalemia in CKD: Prevalence, Risk Factors, and Clinical Outcomes. Adv Chronic Kidney Dis 2017, 24, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, B.; Gruffydd-Jones, T.J.; Malik, R.; Cortes, A.; Jones, B.R.; Helps, C.R.; Prinzenberg, E.M.; Erhardt, G.; Lyons, L.A. First WNK4-Hypokalemia Animal Model Identified by Genome-Wide Association in Burmese Cats. PLoS One 2012, 7, e53173. [Google Scholar] [CrossRef]
- Mou, L.; Wu, F. Simultaneous Homozygous Mutations in SLC12A3 and CLCNKB in an Inbred Chinese Pedigree. Genes (Basel) 2021, 12, 369. [Google Scholar] [CrossRef]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM Consensus Statement: Guidelines for the Identification, Evaluation, and Management of Systemic Hypertension in Dogs and Cats. J Vet Intern Med 2018, 32, 1803–1822. [Google Scholar] [CrossRef]
- Reincke, M.; Bancos, I.; Mulatero, P.; Scholl, U.I.; Stowasser, M.; Williams, T.A. Diagnosis and Treatment of Primary Aldosteronism. Lancet Diabetes Endocrinol 2021, 9, 876–892. [Google Scholar] [CrossRef]
- Young, W.F. Diagnosis and Treatment of Primary Aldosteronism: Practical Clinical Perspectives. J Intern Med 2019, 285, 126–148. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).