Submitted:
25 March 2025
Posted:
25 March 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Database and Study Design
2.2. Pathological Data and Study Groups
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
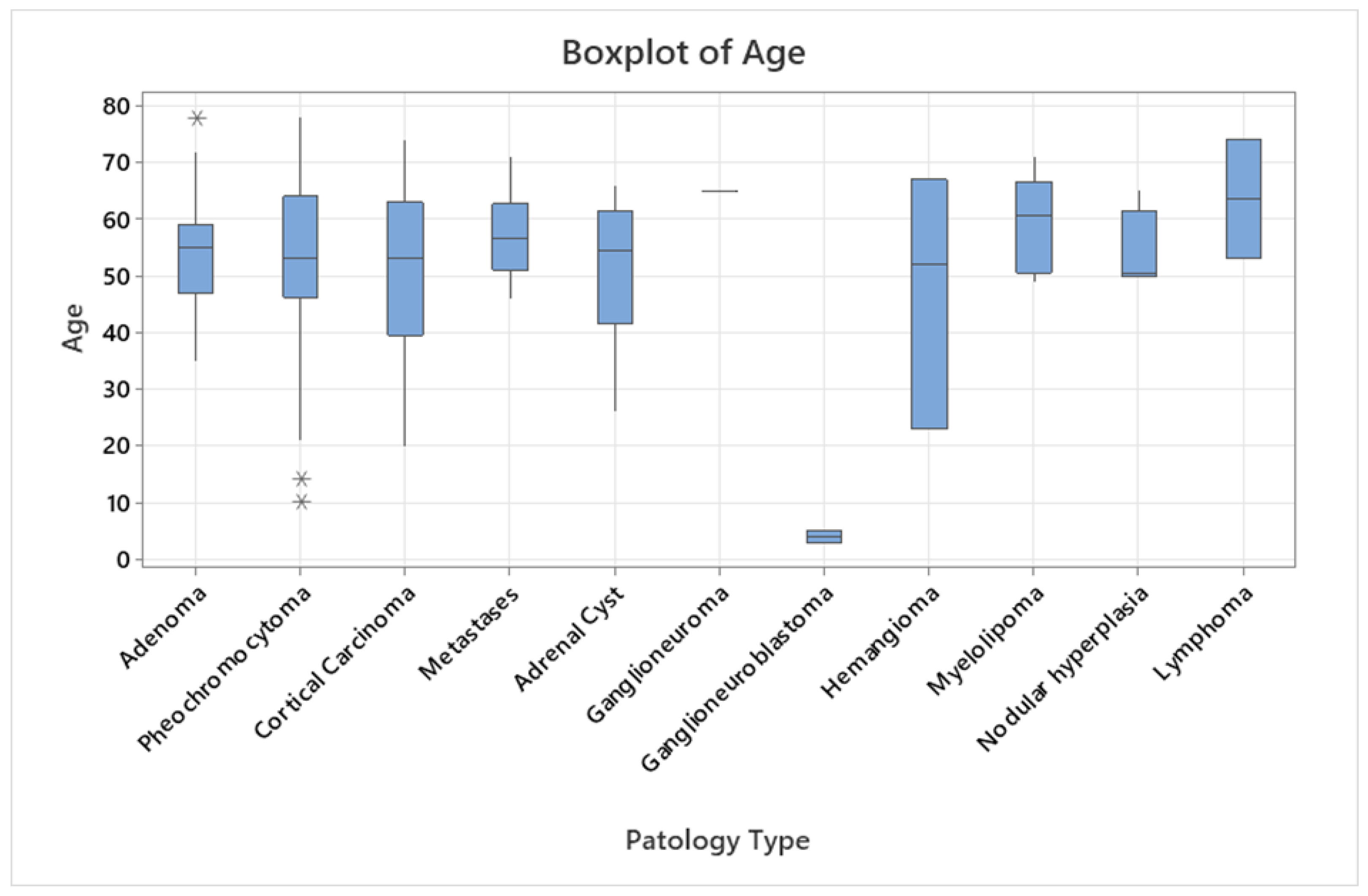
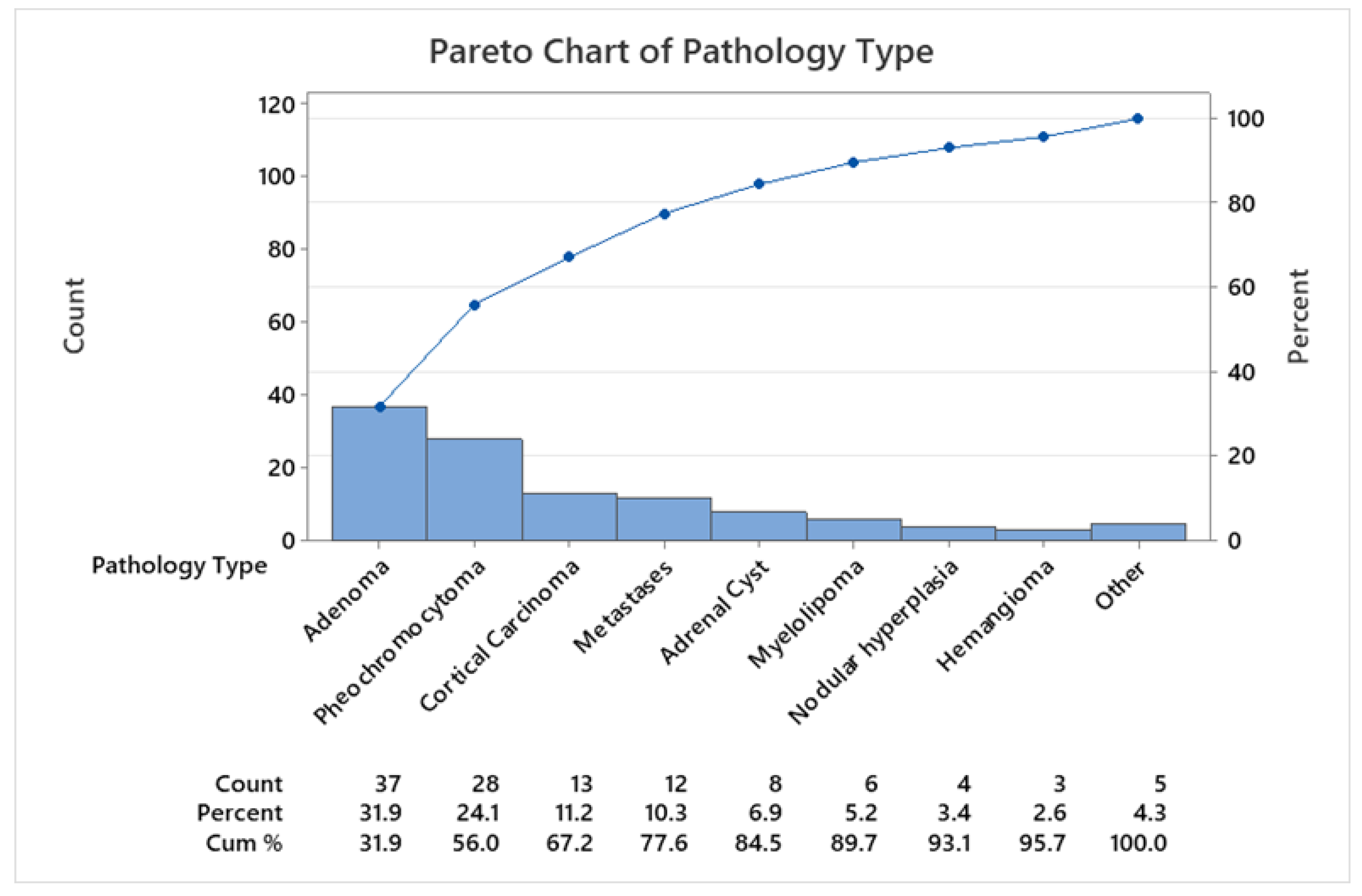
3.2. Pathological Data
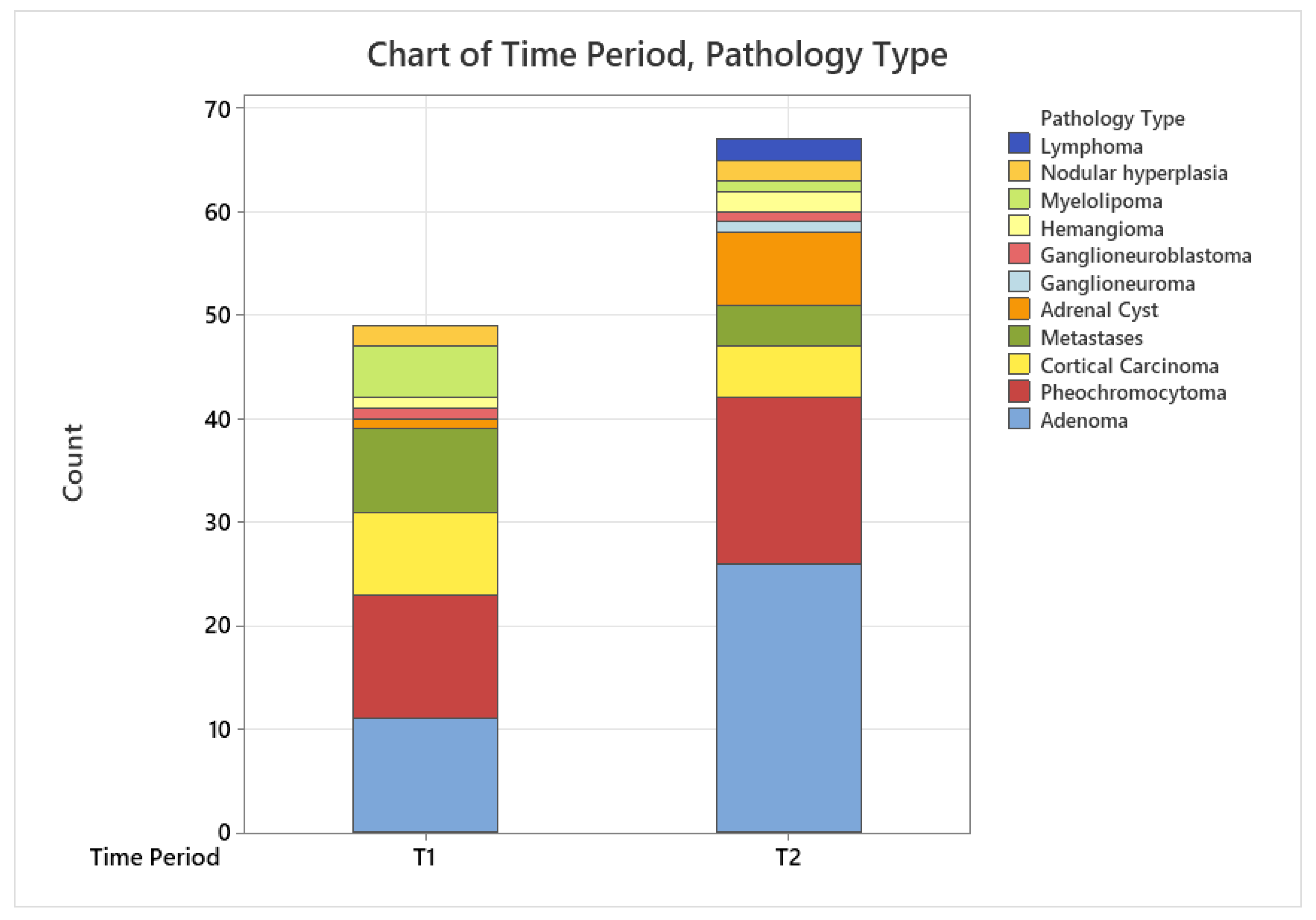
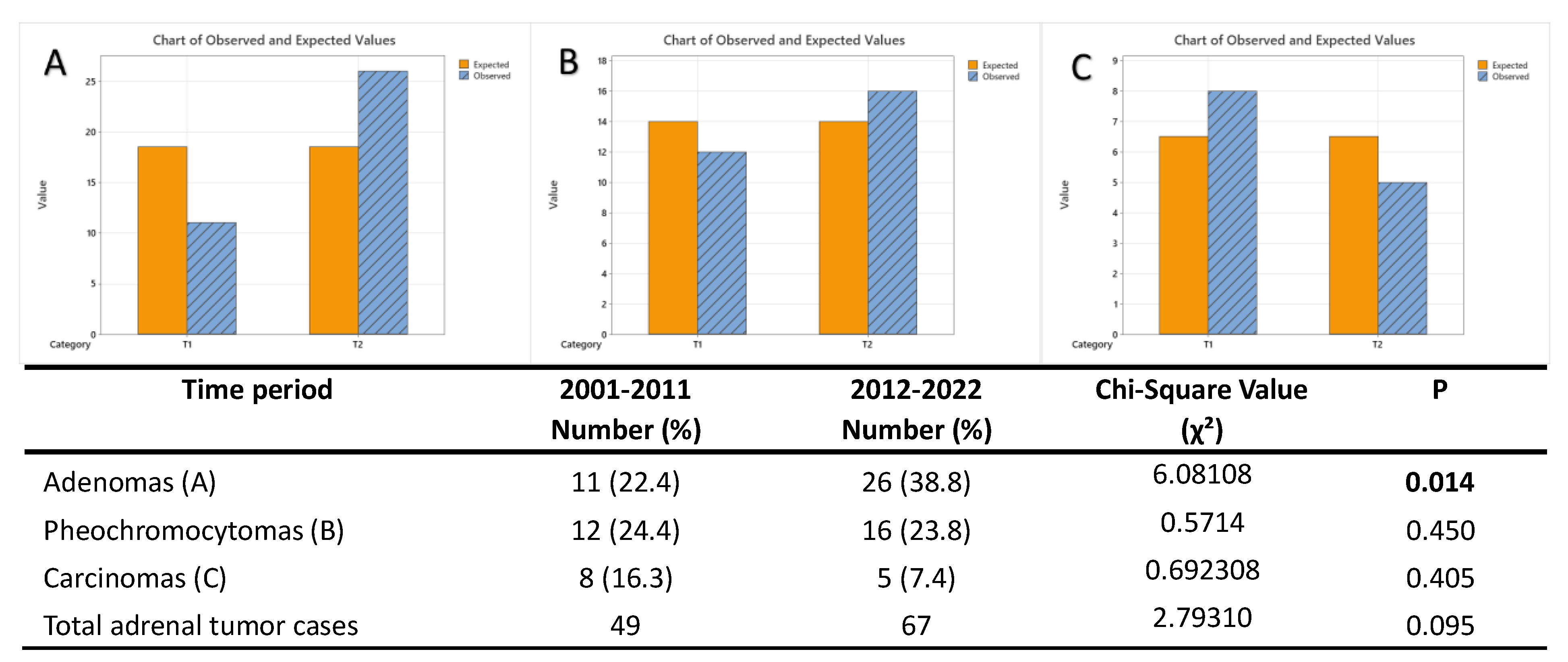
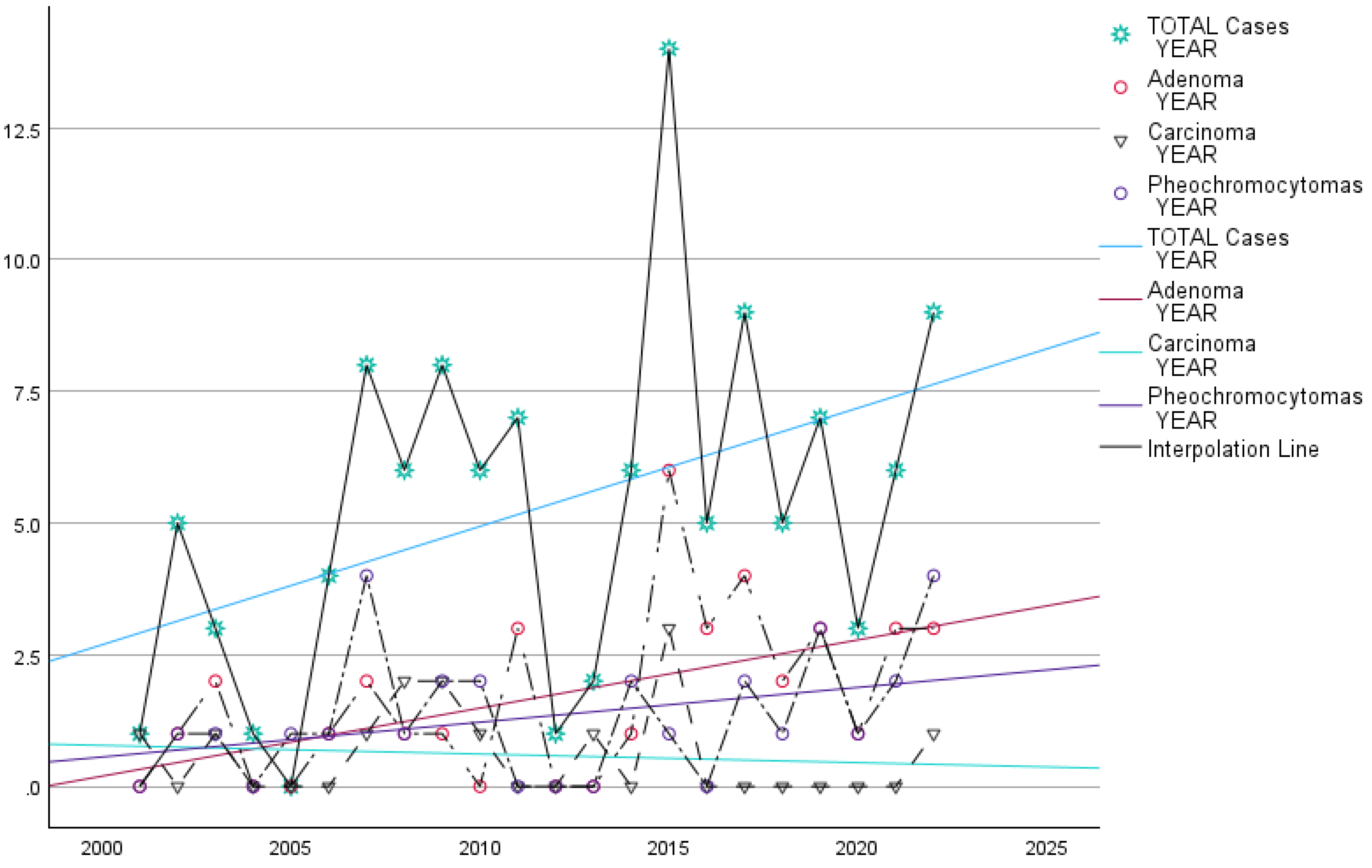
3.2. Time Trend Prevalence of Adrenal Tumors in Our Study Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sherlock, M.; Scarsbrook, A.; Abbas, A.; Fraser, S.; Limumpornpetch, P.; Dineen, R.; Stewart, P.M. Adrenal Incidentaloma. Endocr Rev 2020, 41. [Google Scholar] [CrossRef] [PubMed]
- Bovio, S.; Cataldi, A.; Reimondo, G.; Sperone, P.; Novello, S.; Berruti, A.; Borasio, P.; Fava, C.; Dogliotti, L.; Scagliotti, G. V.; et al. Prevalence of Adrenal Incidentaloma in a Contemporary Computerized Tomography Series. J Endocrinol Invest 2006, 29. [Google Scholar] [CrossRef] [PubMed]
- Reimondo, G.; Castellano, E.; Grosso, M.; Priotto, R.; Puglisi, S.; Pia, A.; Pellegrino, M.; Borretta, G.; Terzolo, M. Adrenal Incidentalomas Are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. Journal of Clinical Endocrinology and Metabolism 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Chaudhry, F.S.; Mayo-Smith, W.W. The Incidental Adrenal Mass on CT: Prevalence of Adrenal Disease in 1,049 Consecutive Adrenal Masses in Patients with No Known Malignancy. American Journal of Roentgenology 2008, 190. [Google Scholar] [CrossRef]
- Ebbehoj, A.; Li, D.; Kaur, R.J.; Zhang, C.; Singh, S.; Li, T.; Atkinson, E.; Achenbach, S.; Khosla, S.; Arlt, W.; et al. Epidemiology of Adrenal Tumors - a Population-Based Study in Olmsted County, Minnesota. Lancet Diabetes Endocrinol 2020, 8. [Google Scholar] [CrossRef]
- Li, L.; Dou, J.; Gu, W.; Yang, G.; Du, J.; Yang, L.; Zang, L.; Wang, X.; Jin, N.; Ouyang, J.; et al. Clinical Analysis of 4 049 Hospitalized Cases of Adrenal Lesions. Zhonghua Yi Xue Za Zhi 2014, 94. [Google Scholar]
- Sharma, S.T.; Nieman, L.K.; Feelders, R.A. Cushing’s Syndrome: Epidemiology and Developments in Disease Management. Clin Epidemiol 2015, 7. [Google Scholar] [CrossRef]
- Savas, M.; Mehta, S.; Agrawal, N.; Van Rossum, E.F.C.; Feelders, R.A. Approach to the Patient: Diagnosis of Cushing Syndrome. Journal of Clinical Endocrinology and Metabolism 2022, 107. [Google Scholar] [CrossRef]
- Pillai, K.; Fares, A.; Dargham, S.; Al Suwaidi, J.; Jayyousi, A.; Abi Khalil, C. Primary Hyperaldosteronism Is Associated with Increased Mortality and Morbidity in Patients with Hypertension and Diabetes. Front Endocrinol (Lausanne) 2023, 14. [Google Scholar] [CrossRef]
- Hundemer, G.L.; Vaidya, A. Primary Aldosteronism Diagnosis and Management: A Clinical Approach. Endocrinol Metab Clin North Am 2019, 48. [Google Scholar]
- Vimal, R.K.Y.S.K.K.N.K.C.Y.T. Virilizing Adrenal Tumor: Case Report. IOSR Journal of Dental and Medical Sciences 2019, 18, 76–77. [Google Scholar]
- Sciarra, F.; Tosti-Croce, C.; Toscano, V. Androgen-Secreting Adrenal Tumors. Minerva Endocrinol 1995, 20. [Google Scholar]
- Kebebew, E.; Reiff, E.; Duh, Q.Y.; Clark, O.H.; McMillan, A. Extent of Disease at Presentation and Outcome for Adrenocortical Carcinoma: Have We Made Progress? World J Surg 2006, 30. [Google Scholar] [CrossRef]
- Allolio, B.; Fassnacht, M. Clinical Review: Adrenocortical Carcinoma: Clinical Update. Journal of Clinical Endocrinology and Metabolism 2006, 91. [Google Scholar]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr Rev 2014, 35. [Google Scholar] [CrossRef]
- Libé, R.; Huillard, O. Adrenocortical Carcinoma: Diagnosis, Prognostic Classification and Treatment of Localized and Advanced Disease. Cancer Treat Res Commun 2023, 37. [Google Scholar] [CrossRef]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of Adrenal Incidentalomas: European Society of Endocrinology Clinical Practice Guideline in Collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol 2016, 175. [Google Scholar] [CrossRef]
- Low, G.; Sahi, K. Clinical and Imaging Overview of Functional Adrenal Neoplasms. International Journal of Urology 2012, 19. [Google Scholar] [CrossRef]
- Huang, B.L.; Liu, Q.; Teng, Y.Y.; Peng, S.Q.; Liu, Z.; Li, M.L.; Liang, J.Y.; Zhang, Y.; Wang, M. Global Trends and Current Status in Pheochromocytoma: A Bibliometric Analysis of Publications in the Last 20 Years. Front Endocrinol (Lausanne) 2023, 14. [Google Scholar] [CrossRef]
- Conzo, G.; Pasquali, D.; Colantuoni, V.; Circelli, L.; Tartaglia, E.; Gambardella, C.; Napolitano, S.; Mauriello, C.; Avenia, N.; Santini, L.; et al. Current Concepts of Pheochromocytoma. International Journal of Surgery 2014, 12. [Google Scholar] [CrossRef]
- Kleihues, P.; Sobin, L.H. World Health Organization Classification of Tumors. Cancer 2000, 88. [Google Scholar] [CrossRef]
- DeLellis, RA. , Lloyd RV., Heitz PU., E.C. WHO Classification of Tumors Pathology and Genetics of Tumors of Endocrine Organs.
- Juan Rosai, R.Y.O.G.K.R.V.L. 2017.
- Ebbehoj, A.; Kaur, R.J.; Li, D.; Singh, S.; Zhang, C.; Atkinson, E.J.; Achenbach, S.J.; Rocca, W.A.; Khosla, S.; Bancos, I. SAT-176 Epidemiology of Adrenal Tumors: A Population Based Study of 1287 Patients. J Endocr Soc 2020, 4. [Google Scholar] [CrossRef]
- Zahir, S.T.; Aalipour, E.; Barand, P.; Kaboodsaz, M. Clinicopathological Features of Adrenal Tumors: A Ten-Year Study in Yazd, Iran. Asian Pacific Journal of Cancer Prevention 2015, 16. [Google Scholar] [CrossRef]
- Al Subhi, A.R.; Boyle, V.; Elston, M.S. Systematic Review: Incidence of Pheochromocytoma and Paraganglioma over 70 Years. J Endocr Soc 2022, 6. [Google Scholar] [CrossRef] [PubMed]
- Ebbehoj, A.; Stochholm, K.; Jacobsen, S.F.; Trolle, C.; Jepsen, P.; Robaczyk, M.G.; Rasmussen, Å.K.; Feldt-Rasmussen, U.; Thomsen, R.W.; Søndergaard, E.; et al. Incidence and Clinical Presentation of Pheochromocytoma and Sympathetic Paraganglioma: A Population-Based Study. Journal of Clinical Endocrinology and Metabolism 2021, 106. [Google Scholar] [CrossRef]
- Sharma, E.; Dahal, S.; Sharma, P.; Bhandari, A.; Gupta, V.; Amgai, B.; Dahal, S. The Characteristics and Trends in Adrenocortical Carcinoma: A United States Population Based Study. J Clin Med Res 2018, 10. [Google Scholar] [CrossRef]
- Kerkhofs, T.M.A.; Verhoeven, R.H.A.; Van Der Zwan, J.M.; Dieleman, J.; Kerstens, M.N.; Links, T.P.; Van De Poll-Franse, L. V.; Haak, H.R. Adrenocortical Carcinoma: A Population-Based Study on Incidence and Survival in the Netherlands since 1993. Eur J Cancer 2013, 49. [Google Scholar] [CrossRef]
- Pedersen, J.; Jarløv, A.E.; Rasmussen, Å.K.; Stochholm, K. Incidence, Treatment, and Survival of Adrenocortical Carcinoma in Denmark 2003-2019. J Endocr Soc 2024, 8. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomedica 2020, 91. [Google Scholar]
- Zhang, Y.N.; Chen, Y.; Wang, Y.; Li, F.; Pender, M.; Wang, N.; Yan, F.; Ying, X.H.; Tang, S.L.; Fu, C.W. Reduction in Healthcare Services during the COVID-19 Pandemic in China. BMJ Glob Health 2020, 5. [Google Scholar] [CrossRef]
- Pujolar, G.; Oliver-Anglès, A.; Vargas, I.; Vázquez, M.L. Changes in Access to Health Services during the COVID-19 Pandemic: A Scoping Review. Int J Environ Res Public Health 2022, 19. [Google Scholar]
- Berends, A.M.A.; Buitenwerf, E.; de Krijger, R.R.; Veeger, N.J.G.M.; van der Horst-Schrivers, A.N.A.; Links, T.P.; Kerstens, M.N. Incidence of Pheochromocytoma and Sympathetic Paraganglioma in the Netherlands: A Nationwide Study and Systematic Review. Eur J Intern Med 2018, 51. [Google Scholar] [CrossRef]
- Ponzoni, M.; Bachetti, T.; Corrias, M.V.; Brignole, C.; Pastorino, F.; Calarco, E.; Bensa, V.; Giusto, E.; Ceccherini, I.; Perri, P. Recent Advances in the Developmental Origin of Neuroblastoma: An Overview. Journal of Experimental and Clinical Cancer Research 2022, 41. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Nam, J.M.; Co Chien, H.T.; McLaughlin, J.K.; Fraumeni, J.F. Risk Factors for Adrenal Cancer: An Exploratory Study. Int J Cancer 1996, 65. [Google Scholar] [CrossRef]
- Wagnerova, H.; Lazurova, I.; Felsoci, M. Adrenal Metastases. Bratislava Medical Journal 2013, 114. [Google Scholar] [CrossRef]
- Samsel, R.; Cichocki, A.; Roszkowska-Purska, K.; Papierska, L.; Koalasinska-Cwikla, A.; Karpeta, E.; Ostrowski, T.; Nowak, K. Adrenal Metastases-Long-Term Results of Surgical Treatment, Single-Centre Experience. Wspolczesna Onkologia 2020, 24. [Google Scholar] [CrossRef]
- Kälviäinen-Mejía, H.K.; Sancho-Pardo, P.; Miguelena-Bobadilla, J.M.; Casamayor-Franco, M.C.; Dobón-Rascón, M.A. Surgery of the Adrenal Metastases. Cirugia y Cirujanos (English Edition) 2021, 89. [Google Scholar] [CrossRef]
| Patology Type | Number (%) | Age (years) Mean ±SD* |
Age (years) Range |
Women/ Men |
Women age Mean ±SD | Men age Mean ±SD | Laterality Left/Right |
|---|---|---|---|---|---|---|---|
| Cortical tumors | 71 (61.2) | 53.43±11.40 | 20-78 | 46/25 | 51.95±10.79 | 56.16±12.19 | 45/26 |
| Adenoma | 37 (31.9) | 53.68 ±9.40 | 35- 78 | 27/10 | 52.33±9.15 | 57.30±9.54 | 24/13 |
| Adrenal cyst | 8 (6.9) | 51.63 ±13.33 | 26- 66 | 8/0 | 51.63±13.33 | - | 6/2 |
| Myelolipoma | 6 (5.2) | 59.50 ±8.80 | 49- 71 | 5/1 | 57.20±7.56 | 71 | 2/4 |
| Hemangioma | 3 (2.6) | 47.33 ±22.36 | 23- 67 | 0/3 | - | 47.33±22.36 | 2/1 |
| Nodular hyperplasia | 4 (3.4) | 54.00 ±7.35 | 50- 65 | 1/3 | 50 | 55.33±8.38 | 2/2 |
| Cortical carcinoma | 13 (11.2) | 52.31 ±15.12 | 20- 74 | 5/8 | 45.60±16.60 | 57.22±12.13 | 9/4 |
| Medullar tumors | 31 (26.7) | 48.51±20.27 | 3-78 | 14/17 | 52.71±15.07 | 45.05±23.62 | 16/15 |
| Pheochromocytoma | 28 (24.1) | 51.11 ±17.12 | 10- 78 | 14/14 | 52.71±15.07 | 49.50±19.38 | 15/13 |
| Ganglioneuroma | 1 (0.9) | 65.00 | - | 0/1 | - | 65 | 0/1 |
| Ganglioneuroblastoma | 2 (1.7) | 4.00 ±1.41 | 3- 5 | 0/2 | - | 4.00±1.00 | 1/1 |
| Others | 14 (12.1) | 58.21±8.32 | 46-74 | 4/10 | 55.75±7.50 | 59.2±8.80 | 6/8 |
| Metastases | 12 (10.4) | 57.33 ±7.48 | 46- 71 | 4/8 | 55.75±7.50 | 59.88±9.04 | 6/6 |
| Lymphoma | 2 (1.7) | 63.50 ±14.84 | 53- 74 | 0/2 | - | 63.50±14.84 | 0/2 |
| Total | 116 (100) | 52.78 ± 14.21 | 3- 78 | 64/52 | 52.35±11.55 | 53.30± 16.98 | 67/49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





