Submitted:
05 August 2025
Posted:
06 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Case Presentation
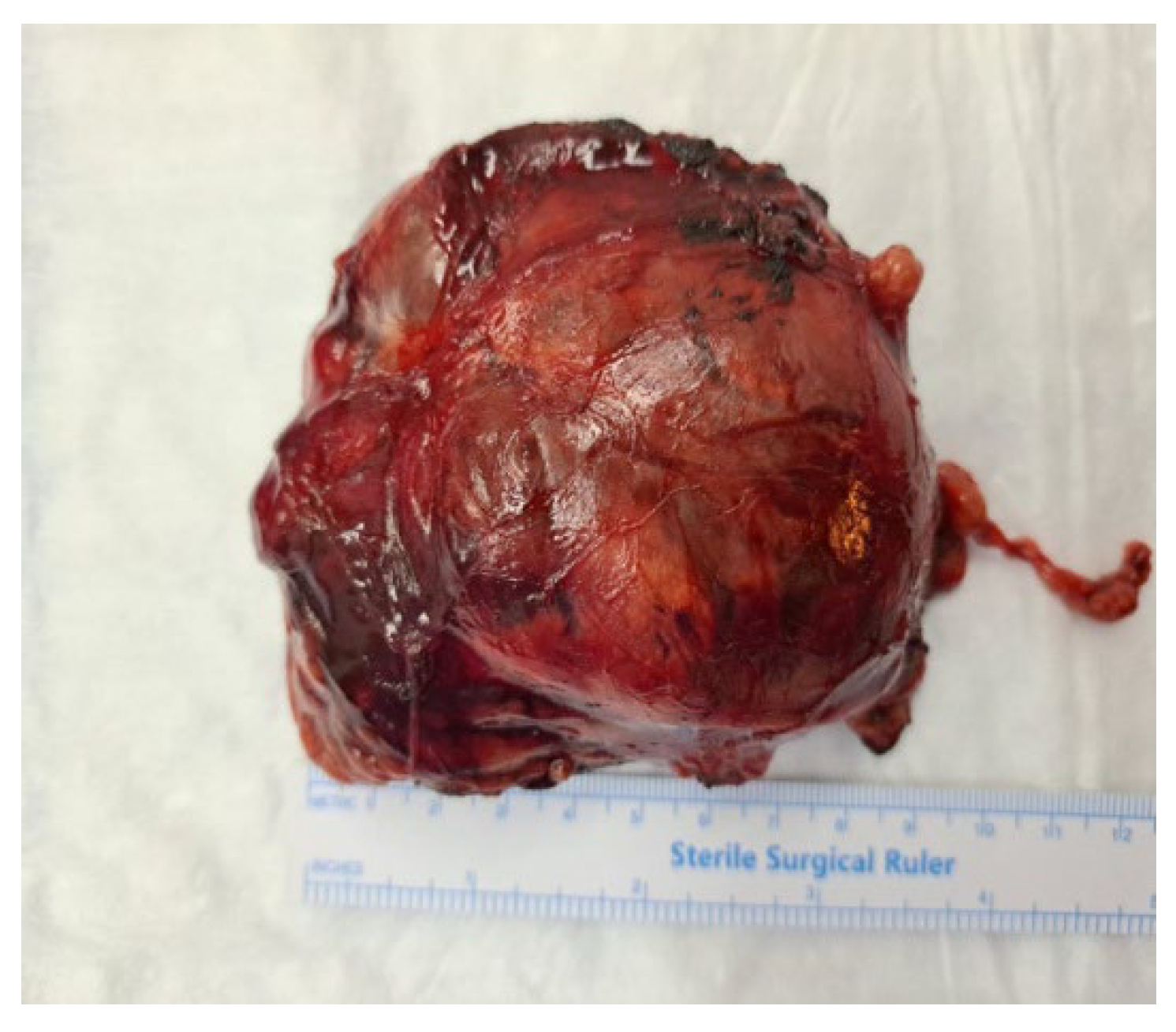
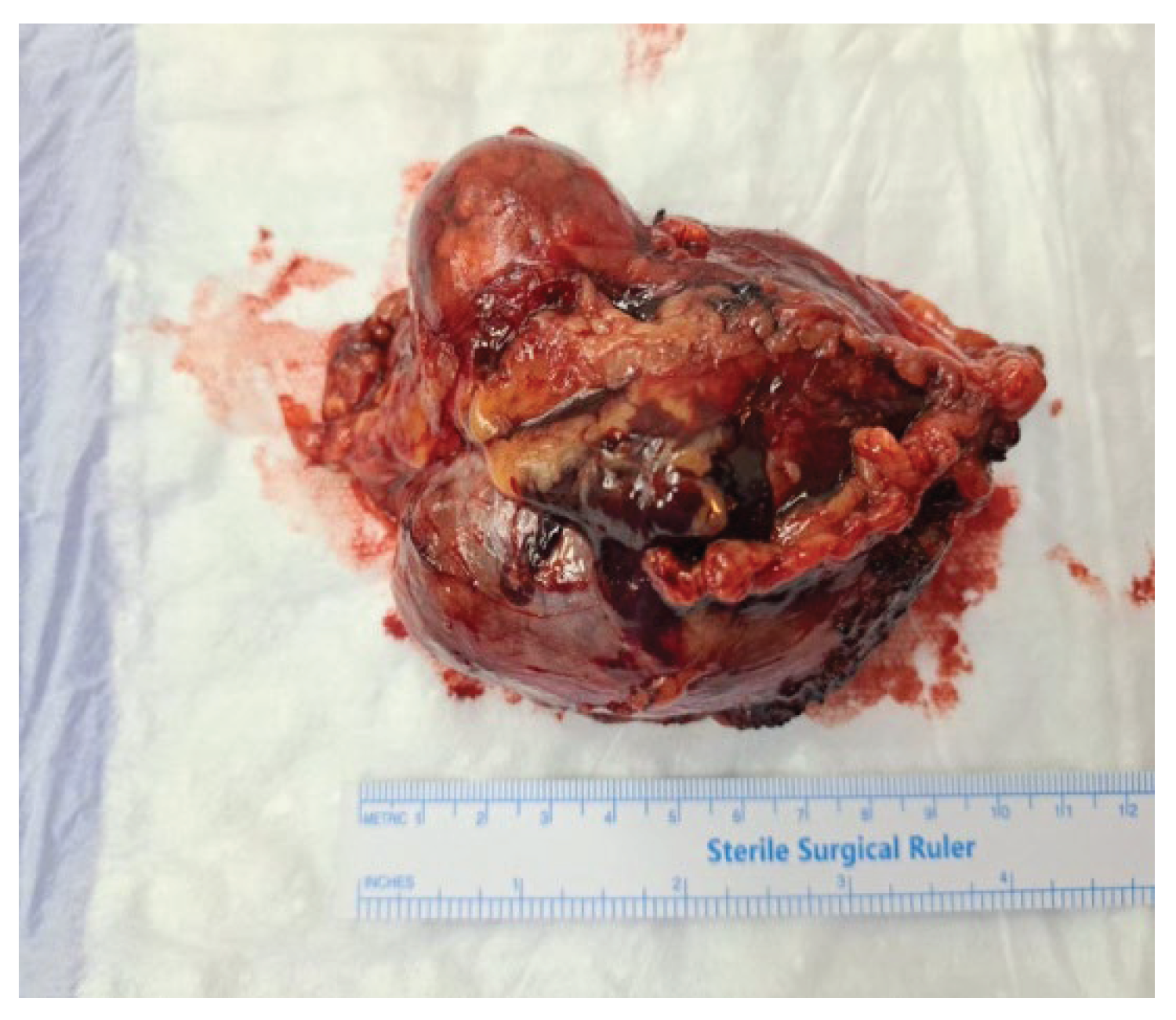
4. Discussion
5. Conclusions
Author Contributions
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ES-ACCs | Estrogen-Secreting Adrenocortical Carcinoma |
| 17-OHP | 17-alpha hydroxyprogesterone |
| DHEA-S | Dehydroepiandrosterone Sulfate |
| ACTH | Adrenocorticotropic Hormone |
| FATs | Feminizing adrenocortical tumors |
|
ACCs MEN1 IHC FSH |
Adrenocortical carcinomas Multiple endocrine neoplasia type 1 Immunohistochemistry Follicle stimulating hormone |
| 11-DOC | 11-deoxycorticosterone |
| LH | Luteinizing hormone |
References
- Fancellu, A. Pinna, and A. Porcu, “Feminizing Adrenocortical Carcinoma with Distant Metastases: Can Surgery Be Considered?,” Clin Pract, vol. 4, no. 2, p. 651, Jul. 2014. [CrossRef]
- M. T. Kidd, N. J. Karlin, and C. B. Cook, “Feminizing Adrenal Neoplasms: Case Presentations and Review of the Literature,” Journal of Clinical Oncology, vol. 29, no. 6, pp. e127–e130, Feb. 2011. [CrossRef]
- J. LaFemina and M. F. Brennan, “Adrenocortical carcinoma: Past, present, and future,” J Surg Oncol, vol. 106, no. 5, pp. 586–594, Oct. 2012. [CrossRef]
- J. M. Rich, V. Duddalwar, P. M. Cheng, M. Aron, and S. Daneshmand, “Feminizing Adrenocortical Tumor with Multiple Recurrences: A Case Report,” Case Rep Oncol, vol. 16, no. 1, pp. 1033–1040, Sep. 2023. [CrossRef]
- S. Moreno, M. Guillermo, M. Decoulx, D. Dewailly, R. Bresson, and Ch. Proye, “Feminizing adreno-cortical carcinomas in male adults. A dire prognosis,” Ann Endocrinol (Paris), vol. 67, no. 1, pp. 32–38, Mar. 2006. [CrossRef]
- F. Chentli, I. Bekkaye, and S. Azzoug, “Feminizing adrenocortical tumors: Literature review,” Indian J Endocrinol Metab, vol. 19, no. 3, p. 332, 2015. [CrossRef]
- D. Vurallı et al., “Feminizing Adrenocortical Tumors as a Rare Etiology of Isosexual/Contrasexual Pseudopuberty,” J Clin Res Pediatr Endocrinol, vol. 14, no. 1, pp. 17–28, Mar. 2022. [CrossRef]
- M. Lerario, A. Moraitis, and G. D. Hammer, “Genetics and epigenetics of adrenocortical tumors,” Mol Cell Endocrinol, vol. 386, no. 1–2, pp. 67–84, Apr. 2014. [CrossRef]
- Allolio and M. Fassnacht, “Adrenocortical Carcinoma: Clinical Update,” J Clin Endocrinol Metab, vol. 91, no. 6, pp. 2027–2037, Jun. 2006. [CrossRef]
- F. Chentli, I. Bekkaye, and S. Azzoug, “Feminizing adrenocortical tumors: Literature review,” Indian J Endocrinol Metab, vol. 19, no. 3, p. 332, 2015. [CrossRef]
- Phornphutkul et al., “Aromatase P450 Expression in a Feminizing Adrenal Adenoma Presenting as Isosexual Precocious Puberty,” J Clin Endocrinol Metab, vol. 86, no. 2, pp. 649–652, Feb. 2001. [CrossRef]
- F. Chentli, F. Chabour, D. Bouchibane, and N. Nouar, “Feminizing Adrenocortical Carcinoma Without Gynecomastia,” Oman Med J, vol. 32, no. 4, pp. 349–351, Jul. 2017. [CrossRef]
- N. FUKAI et al., “A Case of Estrogen-secreting Adrenocortical Carcinoma with Subclinical Cushing’s Syndrome,” Endocr J, vol. 53, no. 2, pp. 237–245, 2006. [CrossRef]
- E. B. Visconti, R. W. Peters, A. Cangir, G. L. Zorn, and S. Fisher, “Unusual case of adrenal cortical carcinoma in a female infant.,” Arch Dis Child, vol. 53, no. 4, pp. 342–344, Apr. 1978. [CrossRef]
- P.-H. Savoie et al., “RETRACTED: Recommandations françaises du Comité de Cancérologie de l’AFU — Actualisation 2018—2020 : tumeur de la surrénale,” Progrès en Urologie, vol. 28, no. 12, pp. S175–S193, Nov. 2018. [CrossRef]
- Lanigan, R. G. Choa, and J. Evans, “A feminizing adrenocortical carcinoma presenting with gynaecomastia,” Postgrad Med J, vol. 69, no. 812, pp. 481–483, Jun. 1993. [CrossRef]
- J. M. Rich, V. Duddalwar, P. M. Cheng, M. Aron, and S. Daneshmand, “Feminizing Adrenocortical Tumor with Multiple Recurrences: A Case Report,” Case Rep Oncol, vol. 16, no. 1, pp. 1033–1040, Sep. 2023. [CrossRef]
- M. Terzolo et al., “Adjuvant Mitotane Treatment for Adrenocortical Carcinoma,” New England Journal of Medicine, vol. 356, no. 23, pp. 2372–2380, Jun. 2007. [CrossRef]
- M. Terzolo et al., “Adjuvant mitotane versus surveillance in low-grade, localised adrenocortical carcinoma (ADIUVO): an international, multicentre, open-label, randomised, phase 3 trial and observational study,” Lancet Diabetes Endocrinol, vol. 11, no. 10, pp. 720–730, Oct. 2023. [CrossRef]
- News Medical, “Study shows not all adrenal carcinoma patients need mitotane after surgery,” News-Medical.net.
- J. Sykes, J. L. Ellis, L. Bukavina, C. A. Koch, S. Wei, and A. Kutikov, “Estradiol-secreting adrenal oncocytoma in a 31-year old male,” Urol Case Rep, vol. 44, p. 102138, Sep. 2022. [CrossRef]
- M. Hatano et al., “Feminizing Adrenocortical Carcinoma with Distinct Histopathological Findings,” Internal Medicine, vol. 55, no. 22, pp. 3301–3307, 2016. [CrossRef]
- Fadi Ibrahim, “MALE GYNECOMASTIA: A RARE CASE OF FEMINIZING ADRENAL CORTICAL CARCINOMA,” Journal of Hospital Medicine 2018; April 8-11; Orlando, Fla., Jun. 2018.
- T. Takeuchi et al., “Adrenocortical carcinoma characterized by gynecomastia: A case report.,” Clin Pediatr Endocrinol, vol. 27, no. 1, pp. 9–18, 2018. [CrossRef]
- Y. Jeong et al., “Estrogen-secreting adrenocortical carcinoma,” Yeungnam Univ J Med, vol. 36, no. 1, pp. 54–58, Jan. 2019. [CrossRef]
- H. C. De, E. Philipse, and B. C. De, “An adrenal tumor with gynecomastia,” Endocrine Abstracts, Oct. 2019. [CrossRef]
- S. M. Gibbons, N. Jassam, A. Abbas, K. Stuart, A. Fairhurst, and J. H. Barth, “Gynaecomastia caused by a feminizing adrenal tumour,” Annals of Clinical Biochemistry: International Journal of Laboratory Medicine, vol. 57, no. 1, pp. 99–101, Jan. 2020. [CrossRef]
- C. Vogt et al., “Feminizing adrenal tumor identified by plasma steroid profiling,” Endocrinol Diabetes Metab Case Rep, vol. 2021, Nov. 2021. [CrossRef]
- J. Saini, P. Navin, M. Rivera, and I. Bancos, “Gynecomastia in a Man With Adrenal Mass,” JCEM Case Reports, vol. 2, no. 1, Dec. 2023. [CrossRef]
- M. Abir, “Bilateral gynecomastia revealing adrenocorticaloma,” Endocrine Abstracts, May 2025. [CrossRef]
| Laboratory findings | Preoperative | One month postoperative | Three month postoperative | Normal ranges |
| ACTH | 2,6 | 61,8 | 114 | 7.2 - 63.3 pg/ml |
| Morning cortisol | 12,1 | 10,1 | 19,9 | 4,2-19,6 ug/dl |
| DHEAS | - | 128,4 | 85 - 690 uUI/ml | |
| Estradiol | 90,1 | 33,7 | 24,2 | 29.8 - 33.1 pg/ml |
| Testosterone, total | - | 3,1 | 2.59 - 8.16 ng/ml | |
| Androstendione | - | 1,68 | 2,05 | 0,50 - 3,50 ng/ml |
| LH | - | 1,2 | 1.24 - 8.62 mIU/ml | |
| FSH | - | 2,72 | 1.27 - 19.26 mIU/ml | |
| Plasmatic Metanephrines | N | 18,8 | 25,7 | < 100 pg/ml |
| Plasmatic Normetanephrines | N | 22,5 | 70,5 | < 216 pg/ml |
| Aldosterone | N | 5,74 | 11,3 | 1.76 - 23.2 ng/dl |
| Renine | N | 13,11 | 36,19 | 2.8-39.9 uUI/ml |
| Author | Year | Age | Clinical presentation | Tumour size&side | Hormonal profile preoperative | Treatment | Outcome | Follow-up |
| Sykes J et al. [21] | 2015 | 31 | Gynecomastia, infertility | 9 cm, right adrenal | ↑Estradiol (83 pg/mL); ↑ DHEAS (502 μg/dL); ↓FSH (0.8 mlU/mL) | Right modified Makuuchi incision-open adrenalectomy | Hormone normalization after 2 weeks postoperative Normal gonadotrophines at 5 months follow up |
Endocrine labs every 6 months, and CT scans every 6–12 months for at least 5 years |
| Hatano M. et al. [22] | 2016 | 60 | Gynecomastia, right hyponcondriac pain, low libido | 16x11x14 cm, right adrenal | ↑Estradiol (284 pg/mL); ↑ DHEAS (560 μg/dL); ↓FSH (0.2 mlU/mL) and LH (0.1 mIU/mL); ↓ free testosterone <0.6 pg/ml | Right adrenalectomy p0T2N0M0, Weiss score 7, ki 67 18% |
Hormone normalization -locoregional lymph nodes and peritoneum metastases |
No adjuvant therapy until 11 months Initiation of Mitotane after discover of locoregional lymph nodes |
| Ibrahim F et al. [23] | 2018 | 55 | Gynecomastia, testicular hypotrophy, pleuritic left chest pain, hypertension | 6.3 cm, right adrenal | ↑Estradiol 134 pg/mL; ↓testosterone 109 ng/dL; ↓ FSH and LH | Laparoscopic right adrenalectomy | ? | ? |
| Takeuchi et al. [24] | 2018 | 4 | Gynecomastia, acute growth spurt | 8 cm, right adrenal | ↑ Estradiol 28.1 pg/mL;N testosterone (0.82 ng/mL); ↓ LH (<0.1 mIU/mL); ↓ FSH (0.13 mIU/mL); ↑DHEAS 1950 ng/ml, ↑ androstenedione 4.6 ng/ml |
Initial- combined chemoterapy of ARAR0332 with mitotane 120-150 mg/d pre-operative Surgical resection Weiss score 7 |
Gynecomastia disappeared after 1 year post-operative Normal growth velocity after tumor resection |
Followed by administration of GPOH-MET97 therapy and mitotane *mitotane was discontinued after 6 months post-operative Postoperative- replacement therapy with hydrocortisone and mineralocorticoid No relapse after 2 years |
| Jeong Y et al. [25] | 2019 | 53 | Gynecomastia, abdominal discomfort, right-sided flank pain | 21×15.3×12 cm, right retroperitoneum | ↑Estradiol (820 pg/mL); ↑ DHEAS (578 μg/dL); ↓FSH (1.07 mlU/mL); ↓ACTH (4.2pg/mL), inappropriately normal cortisol (16,3 ug/dl), ↑ free cortisol/24 h urine 134 ug/day |
Open adrenalectomy with partial liver resection pT2N0M0, stage 2, Weiss score 6 IHC: positive staining for inhibin α, MART-1, and calretinin and a Ki67 proliferation index of 20% |
Estradiol 70.32 pg/ml; the gynecomastia had almost disappeared at 3 month postoperative Without any local recurrence or metastasis at 21 months after diagnosis and treatment, and the gynecomastia has almost resolved |
Additional adjuvant radiation therapy. Postoperative- replacement therapy with hydrocortisone |
| C De Herdt et al.[26] | 2019 | 42 | Gynecomastia, icterus | 5.1 cm, right adrenal | ↑Estradiol 44 ng/l; hypogonadotropic hypogonadism | Open right adrenalectomy pT3L0V0Pn0R0, stage 3, Weiss score 4 |
Estradiol 9 ng/l; almost complete recovery of the gonadotropic axis after 2 weeks postoperative -disappearance of gynecomastia |
Adjuvant treatment with mitotane |
| Gibbons S et al. [27] | 2020 | 52 | Gynaecomastia, low libido, erectile dysfunction | 8 x 8 cm, right adrenal | ↑ Estradiol 932 pmol/L;↓ testosterone (0.7 nmol/L); N LH (1.2 IU/L); ↓ FSH (<0.1 IU/L) | Right open adrenalectomy pT3 Nx Ki-67 60% |
Hormone normalization -disappearance of gynecomastia |
Adjuvant treatment with mitotane for two years in addition to bi-annual CT scans |
| Vogt E et al. [28] | 2021 | 58 | Gynaecomastia, low libido, | 6.5 cm × 5.2 cm, left adrenal | ↑ Estradiol (208 pmol/L); ↑ 11-deoxyxortisol (23.5 nmol/L), ↑ DHEAS (10.6 µmol/L), ↑ androstenedione (18.1 nmol/L), cortisol overproduction, low normal FSH and LH | Transabdominal laparoscopic Weiss score of 7 IHC: inhibin, synaptophysin, CD31 (PECAM-1) and aromatase (CYP19A1) Ki 67 5% |
Hormone normalization Gynecomastia had not improved significantly 1 year after surgery, and therefore liposuction and perioareolar incision was planned. |
Adjuvant therapy with the adrenolytic drug mitotane was started 8 weeks after resection, |
| Rich J et al.[4] | 2023 | 35 | Gynecomastia, low libido, RUQ pain | 18×8.5×14.5 cm, right adrenal | ↓FSH (<0.1 mIU/mL), ↓ LH(<0.1 mIU/mL), ↓ testosterone (37 ng/dL), ↑ estradiol (181 pg/mL), normal cortisol (8.0–14.5 μg/dL), ↓ ACTH (1 pg/mL) | Open right adrenalectomy |
Hormone normalization after 2 months postoperative - gynecomastia decreased to near baseline, and libido/erections returned to normal |
Local recurence: a new 1 cm nodule in the right adrenalectomy bed -plans includehemotherapy with mitotane or etoposide/doxorubicin/cisplatin |
| Saini J et al. [29] | 2023 | 65 | Gynecomastia | 4.3 cm, right adrenal, with metastatic lessions after 5 years | ↑ Estradiol 72 pg/mL; ↑ Estrone 345 pg/mL; ↑ progesterone 0.59 ng/mL; ↓ total testosterone 157 ng/dL; ↑ 11 DOC 204 ng/dL; ↑ renine plasma activity 204 ng/dL |
Initial- right laparoscopic adrenalectomy locally Reintervention-debulking surgery with the removal of multiple metastatic lesions Weiss score 4 |
Redeveloped gynecomastia after 5 years After reintervention: Hormone normalization after 3 months postoperative - gynecomastia improved |
Adjuvant therapy with mitotne plus replacement therapy with hydrocortisone - progressive disease at 3 the month-follow up - cytotoxic chemotherapy with etoposide, doxorubicin, and cisplatin was initiated, and mitotane was discontinued in an attempt to reduce the overall toxicity |
| Abir M et al. [30] | 2025 | 57 | Gynecomastia, weight loss, decreased libido, abdominal pain | large left adrenal tumor mass | Hyperestrogenism, increase in the precursors of adrenal androgens 17OHP, androstendione, DHEAS | A biopsy of the adrenal mass performed externally came back in favor of a left adrenocorticaloma with positive immunohistochemistry, synaptophysin and Melan A | Patient died following a state of hemorrhagic shock | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





