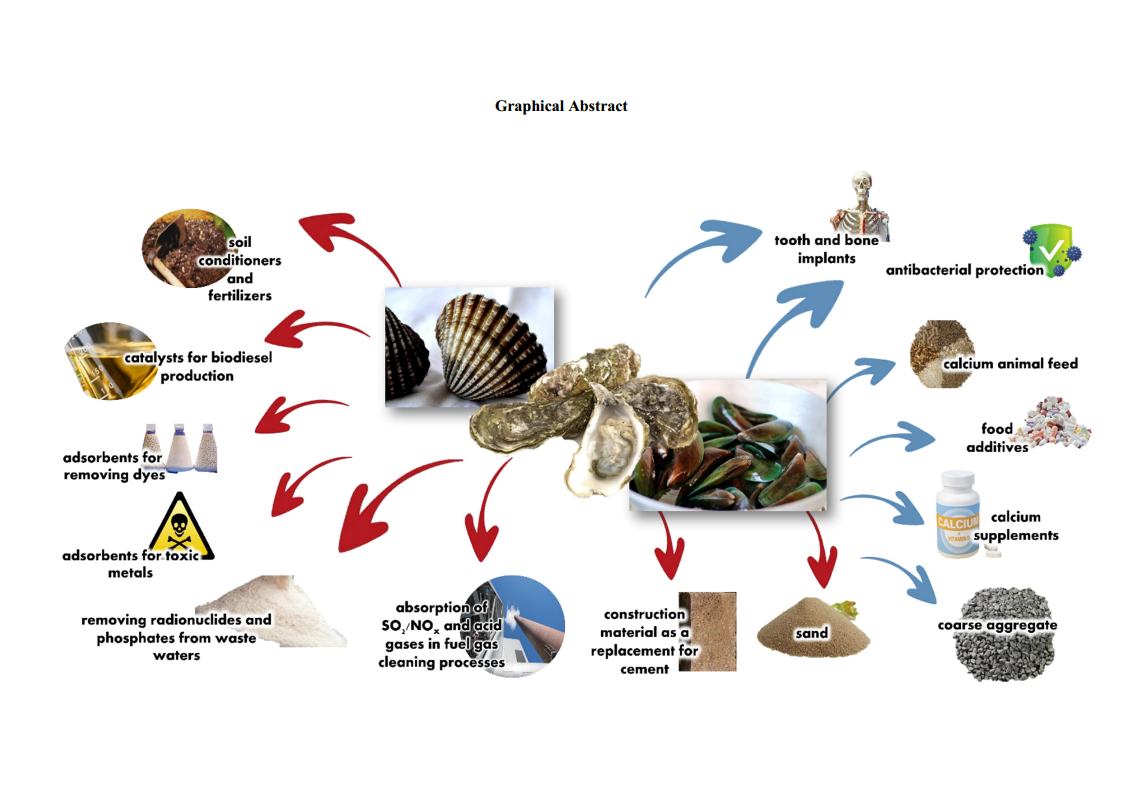
The search for sustainable resources remains a subject of global interest and the conversion of the abundantly available bivalve shell wastes to advanced materials is an intriguing method. By grinding, each shell of bivalves (cockle, mussel, and oyster) was transformed to the same crystal type of calcite phase of CaCO3, revealed by FTIR and XRD results. Each individual shell powder was reacted with H3PO4 and H2O to prepare Ca(H2PO4)2•H2O giving an anorthic crystal structure. The mixture of each shell powder and its produced Ca(H2PO4)2•H2O was heated at 900 °C for 3 h, giving rhombohedral crystal -Ca3(PO4)2 powder. FTIR and XRD results of the CaCO3, Ca(H2PO4)2•H2O, and Ca3(PO4)2 prepared from each shell powder are quite similar showing no impurities. Thermal behaviors of CaCO3 and Ca(H2PO4)2•H2O produced from each shell are slightly different. Particle sizes and morphologies of all products are significantly different, affected by the kind of shells used. Overall, the bivalve shell wastes were successfully converted to CaCO3, Ca(H2PO4)2•H2O, and Ca3(PO4)2 by a simple, rapid, environmentally benign, and cost-effective approach, which can be a huge potential in many industries providing both economic and ecological benefits according to the Bio-Circular-Green Economy (BCG) model.