Submitted:
20 January 2026
Posted:
20 January 2026
You are already at the latest version
Abstract
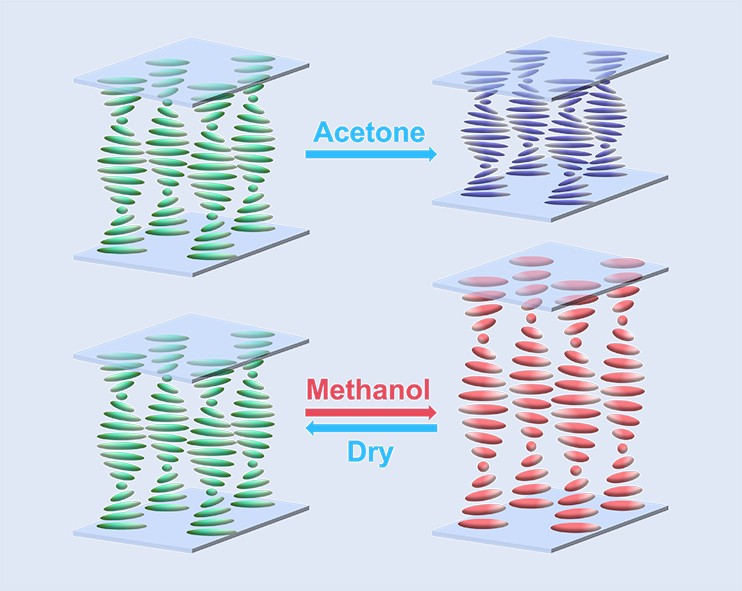
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Instruments
2.2. Preparation of the CLCN Films by Changing the CA-Epoxy Concentration
2.3. Preparation of the CLCN Films by Terminating Cationic Polymerization with Methanol After Different 365-nm LED Lamp Irradiation Times
2.4. Preparation of the CLCN Film with the QR Code of “Epoxy Resin”
2.5. Preparation of the CLCN Film with the QR Code of “LC”
2.6. Preparation of the Dye-Doped CLCN Pattern
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bisoyi, H. K.; Bunning, T. J.; Li, Q. Stimuli-Driven Control of the Helical Axis of Self-Organized Soft Helical Superstructures. Adv. Mater. 2018, 30, 1706512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; de Haan, L. T.; Debije, M. G.; Schenning, A. P. H. J. Liquid Crystal-Based Structural Color Actuators. Light-Sci. Appl. 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Boott, C. E.; MacLachlan, M. J. Understanding the Self-Assembly of Cellulose Nanocrystals—Toward Chiral Photonic Materials. Adv. Mater. 2020, 32, 1905876. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Q. Light-Driven Chiral Molecular Switches or Motors in Liquid Crystals. Adv. Mater. 2012, 24, 1926–1945. [Google Scholar] [CrossRef]
- Mitov, M. Cholesteric Liquid Crystals with a Broad Light Reflection Band. Adv. Mater. 2012, 24, 6260–6276. [Google Scholar] [CrossRef]
- Lan, R.; Shen, W.; Yao, W.; Chen, J.; Chen, X.; Yang, H. Bioinspired Humidity-Responsive Liquid Crystalline Materials: From Adaptive Soft Actuators to Visualized Sensors and Detectors Mater. Horiz. 2023, 10, 2824–2844. [Google Scholar] [CrossRef]
- Ko, H.; Kim, M.; Wi, Y.; Rim, M.; Lim, S.-I.; Koo, J.; Kang, D.-G.; Jeong, K.-U. Chiroptical Smart Paints: Polymerization of Helical Structures in Cholesteric Liquid Crystal Films. J. Chem. Educ. 2021, 98, 2649–2654. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Chen, Y.; Liu, Y. High Humidity Responsive Cholesteric Liquid Crystal Polymer Film for Anti-Counterfeiting Encryption Applications. Opt. Mater. 2025, 168, 117461. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, P.; Zhao, J.; Yang, Y.; Li, H. Circularly Polarized Room-Temperature Phosphorescence from Dye-Doped Cholesteric Liquid Crystalline Polymer Networks. J. Phys. Chem. Lett. 2025, 16, 725–730. [Google Scholar] [CrossRef]
- Guan, P.; Shi, F.; Xu, C.; Yang, Y.; Li, H. Tunable Circularly Polarized Afterglow from Cholesteric Liquid Crystalline Polymer Networks. J. Phys. Chem. Lett. 2025, 16, 10142–10148. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Shen, C.; Huang, R.; Song, C.; Li, Z.; Hu, W.; Lan, R.; Zhang, L.; Yang, H. Freestanding Helical Nanostructured Chiro-Photonic Crystal Film and Anticounterfeiting Label Enabled by a Cholesterol-Grafted Light-Driven Molecular Motor. Small Methods 2022, 6, 2200269. [Google Scholar] [CrossRef] [PubMed]
- Kragt, A. J. J.; Zuurbier, N. C. M.; Broer, D. J.; Schenning, A. P. H. J. Temperature-Responsive, Multicolor-Changing Photonic Polymers. ACS Appl. Mater. Interfaces 2019, 11, 28172–28179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Liu, J.; Ren, Y.; Chen, J.; Hu, W.; Yang, H. Simultaneous or Independent Programming and Reconfiguration of Structural and Fluorescent Information in Multi-Stimuli-Responsive Liquid Crystalline Polymer Film. Chem. Eng. J. 2025, 505, 159583. [Google Scholar] [CrossRef]
- Kragt, A. J. J.; Hoekstra, D. C.; Stallinga, S.; Broer, D. J.; Schenning, A. P. H. J. 3D Helix Engineering in Chiral Photonic Materials. Adv. Mater. 2019, 31, 1903120. [Google Scholar] [CrossRef]
- Zhang, P.; Kragt, A. J. J.; Schenning, A. P. H. J.; de Haan, L. T.; Zhou, G. An Easily Coatable Temperature Responsive Cholesteric Liquid Crystal Oligomer for Making Structural Colour Patterns. J. Mater. Chem. C 2018, 6, 7184–7187. [Google Scholar] [CrossRef]
- Feng, W.; Pal, A.; Wang, T.; Ren, Z.; Yan, Y.; Lu, Y.; Yang, H.; Sitti, M. Cholesteric Liquid Crystal Polymeric Coatings for Colorful Artificial Muscles and Motile Humidity Sensor Skin Integrated with Magnetic Composites. Adv. Funct. Mater. 2023, 33, 2300731. [Google Scholar] [CrossRef]
- Moirangthem, M.; Arts, R.; Merkx, M.; Schenning, A. P. H. J. An Optical Sensor Based on a Photonic Polymer Film to Detect Calcium in Serum. Adv. Funct. Mater. 2016, 26, 1154–1160. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, X.; Schenning, A. P. H. J.; Zhou, G.; de Haan, L. T. A Patterned Mechanochromic Photonic Polymer for Reversible Image Reveal. Adv. Mater. Interfaces 2020, 7, 1901878. [Google Scholar] [CrossRef]
- Brannum, M. T.; Steele, A. M.; Venetos, M. C.; Korley, L. T. J.; Wnek, G. E.; White, T. J. Light Control with Liquid Crystalline Elastomers. Adv. Opt. Mater. 2019, 7, 1801683. [Google Scholar] [CrossRef]
- Herzer, N.; Guneysu, H.; Davies, D. J. D.; Yildirim, D.; Vaccaro, A. R.; Broer, D. J.; Bastiaansen, C. W. M.; Schenning, A. P. H. J. Printable Optical Sensors Based on H-Bonded Supramolecular Cholesteric Liquid Crystal Networks. J. Am. Chem. Soc. 2012, 134, 7608–7611. [Google Scholar] [CrossRef]
- Chang, C.-K.; Bastiaansen, C. W. M.; Broer, D. J.; Kuo, H. L. Discrimination of Alcohol Molecules Using Hydrogen-Bridged Cholesteric Polymer Networks. Macromolecules 2012, 45, 4550–4555. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, D.; Liu, X.; Liu, Y.; Liu, S.; Miao, P.; Shi, Y.; Sun, W. Optical Fiber Sensor Based on a Cholesteric Liquid Crystal Film for Mixed Voc Sensing. Opt. Express 2020, 28, 31872–31881. [Google Scholar] [CrossRef] [PubMed]
- McConney, M. E.; Tondiglia, V. P.; Hurtubise, J. M.; Natarajan, L. V.; White, T. J.; Bunning, T. J. Thermally Induced, Multicolored Hyper-Reflective Cholesteric Liquid Crystals. Adv. Mater. 2011, 23, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Kragt, A. J. J.; Broer, D. J.; Schenning, A. P. H. J. Easily Processable and Programmable Responsive Semi-Interpenetrating Liquid Crystalline Polymer Network Coatings with Changing Reflectivities and Surface Topographies. Adv. Funct. Mater. 2018, 28, 1704756. [Google Scholar] [CrossRef]
- Miao, Z.; Chen, X.; Zhang, Y.; Wang, D.; Wang, L. Bistable Cholesteric Liquid Crystal Films with Excellent Electro-Optical Performance and Spacing Stability for Reflective Displays. ACS Appl. Polym. Mater. 2023, 5, 476–484. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N. J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic Photopolymerization of Bio-Renewable Epoxidized Monomers. Prog. Org. Coat. 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Abad, M.; Martínez-Bueno, A.; Concellón, A. Shaping Liquid Crystal Polymer Networks: From Molecular Design and Processing to Multifunctional Materials. Adv. Mater. Technol. 2025, e01236. [Google Scholar] [CrossRef]
- Fu, C.; Li, D.; Liu, C.; Zhang, Y.; Zhang, J.; Cheng, Y. Chiral Co-Assembled Liquid Crystal Polymer Network Enabled by In-Situ Photopolymerization for High-Performance CP-OLEDs. Angew. Chem. Int. Ed. 2025, 64, e202512257. [Google Scholar] [CrossRef]
- Park, J.; Yu, T.; Inagaki, T.; Akagi, K. Helical Network Polymers Exhibiting Circularly Polarized Luminescence with Thermal Stability. Synthesis via Photo-Cross-Link Polymerizations of Methacrylate Derivatives in a Chiral Nematic Liquid Crystal. Macromolecules 2015, 48, 1930–1940. [Google Scholar] [CrossRef]
- Pu, Y.; Wen, X.; Gu, H.; Zhu, H.; Yuan, M.; Huang, J.; Zhan, Q.; Jiang, X.-F.; Polavarapu, L.; Hu, X.; Zhou, G. Upconversion Circularly Polarized Luminescence with Dissymmetry Factor up to 1.80 from Flexible Perovskite-Liquid Crystal Membranes. Chem. Eng. J. 2025, 512, 162515. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Valenzuela, C.; Zhang, X.; Wang, L.; Feng, W. Mechanochromic, Shape-Programmable and Self-Healable Cholesteric Liquid Crystal Elastomers Enabled by Dynamic Covalent Boronic Ester Bonds. Angew. Chem. Int. Ed. 2022, 61, e202116219. [Google Scholar]
- Yi, Y.; Yang, W.; Li, Y.; Liu, W.; Yang, Y. Colorful Patterns Prepared Using Alicyclic Epoxy-Based Cholesteric Liquid Crystal Inks for Decoration. Liq. Cryst. 2025, 52, 434–444. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Li, Y.; Liu, W.; Li, H.; Yang, Y. Colorfully Patterned Epoxy Resin Films with a Cholesteric Structure Prepared through a Photopolymerization Approach. ACS Appl. Polym. Mater. 2023, 5, 193–200. [Google Scholar] [CrossRef]
- Guo, Y.; Yi, Y.; Wu, L.; Liu, W.; Li, Y.; Yang, Y. A Structural Colored Epoxy Resin Sensor for the Discrimination of Methanol and Ethanol. Chemistry 2025, 7, 122. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Guo, Y.; Yang, Z.; Li, H.; Liu, H. Paper-Structure Inspired Multiresponsive Hydrogels with Solvent-Induced Reversible Information Recording, Self-Encryption, and Multidecryption. Adv. Funct. Mater. 2022, 32, 2201009. [Google Scholar] [CrossRef]
- Sang, J.; Lv, Z.; Li, T.; Chen, F.; Li, H.; Yang, B.; Sun, J.; Shang, J.; Zheng, J. Flexible and Wearable Dual-Channel Liquid Crystal Polymer Film for High-Performance Security Information Encryption. Opt. Express 2025, 33, 25718–25727. [Google Scholar] [CrossRef]
- He, Q.; Zhao, Q.; Zhang, L. Long-Chain Crosslinker-Induced Patterning on an Elastic Polymer Film for Robust and Reversible Information Encryption/Decryption. Mater. Horiz. 2025, 12, 2360–2368. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Z.; Zhang, Q.; Cheng, R.; Yin, D.; Ge, F.; Zhang, Q. Carbon Black-Containing Cholesteric Liquid Crystal Elastomers Enabling Multi-Level Information Encryption and Multimode Actuation. Adv. Funct. Mater. 2025, e17597. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Z.; Lu, Y.; Yang, D.; Li, Y.; Zhao, Y.; He, Z.; Luan, Y. Cholesterol Liquid Crystals with Thermoelectric Stimulation for Multi-Decryption Condition-Dependent Digital Information Encryption and Visible Light Camouflage. Chem. Eng. J. 2025, 525, 170212. [Google Scholar] [CrossRef]
- Michaudel, Q.; Kottisch, V.; Fors, B. P. Cationic Polymerization: From Photoinitiation to Photocontrol. Angew. Chem. Int. Ed. 2017, 56, 9670–9679. [Google Scholar] [CrossRef] [PubMed]
- Pierau, L.; Elian, C.; Akimoto, J.; Ito, Y.; Caillol, S.; Versace, D.-L. Bio-Sourced Monomers and Cationic Photopolymerization–the Green Combination Towards Eco-Friendly and Non-Toxic Materials. Prog. Polym. Sci. 2022, 127, 101517. [Google Scholar] [CrossRef]
- Park, S.; Kilgallon, L. J.; Yang, Z.; Ryu, D. Y.; Ryu, C. Y. Molecular Origin of the Induction Period in Photoinitiated Cationic Polymerization of Epoxies and Oxetanes. Macromolecules 2019, 52, 1158–1165. [Google Scholar] [CrossRef]
- Kepkow, M.; Heinz, M.; Strehmel, B.; Strehmel, V. Synthesis and Photoinitiated Cationic Polymerization of Epoxy Monomers Derived from Oleic Acid Comprising One to Three Epoxy Groups. Sustainable Chem. Pharm. 2024, 39, 101588. [Google Scholar] [CrossRef]
- Liao, F.-Q.; Chen, Y.-C. Siloxane-Based Epoxy Coatings through Cationic Photopolymerization for Corrosion Protection. Prog. Org. Coat. 2023, 174, 107235. [Google Scholar] [CrossRef]
- Jellali, R.; Campistron, I.; Laguerre, A.; Lecamp, L.; Pasetto, P.; Bunel, C.; Mouget, J.-L.; Pilard, J.-F. Synthesis and Crosslinking Kinetic Study of Epoxidized and Acrylated/Epoxidized Oligoisoprenes: Comparison between Cationic and Radical Photopolymerization. J. Appl. Polym. Sci. 2013, 128, 2489–2497. [Google Scholar] [CrossRef]
- Ficek, B. A.; Thiesen, A. M.; Scranton, A. B. Cationic Photopolymerizations of Thick Polymer Systems: Active Center Lifetime and Mobility. Eur. Polym. J. 2008, 44, 98–105. [Google Scholar] [CrossRef]
- Frison, T.; Irlweg, A.; Ergül, Ö.; Totev, D. I.; Tuinier, R.; Esteves, A. C. C. Electron Beam-Induced Cationic Polymerization Drives Structure and Properties of Epoxy Coatings. Prog. Org. Coat. 2025, 200, 109017. [Google Scholar] [CrossRef]
- Kaalberg, S.; Jessop, J. L. P. Enhancing Cationic Ring-Opening Photopolymerization of Cycloaliphatic Epoxides Via Dark Cure and Oxetanes. J. Polym. Sci. A Polym. Chem. 2018, 56, 1436–1445. [Google Scholar] [CrossRef]
- Malik, M. S.; Schlögl, S.; Wolfahrt, M.; Sangermano, M. Review on UV-Induced Cationic Frontal Polymerization of Epoxy Monomers. Polymers 2020, 12, 2146. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Irska, I.; Gziut, K.; Ossowicz-Rupniewska, P. Synthesis of Epoxy Methacrylate Resin and Coatings Preparation by Cationic and Radical Photocrosslinking. Molecules 2021, 26, 7663. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J. V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Škola, O.; Jašúrek, B.; Veselý, D.; Němec, P. Mechanical Properties of Polymer Layers Fabricated Via Hybrid Free Radical-Cationic Polymerization of Acrylate, Epoxide, and Oxetane Binders. Prog. Org. Coat. 2016, 101, 279–287. [Google Scholar]
- Hartwig, A.; Koschek, K.; Lühring, A.; Schorsch, O. Cationic Polymerization of a Cycloaliphatic Diepoxide with Latent Initiators in the Presence of Structurally Different Diols. Polymer 2003, 44, 2853–2858. [Google Scholar] [CrossRef]
- Dillman, B.; Jessop, J. L. P. Chain Transfer Agents in Cationic Photopolymerization of a Bis-Cycloaliphatic Epoxide Monomer: Kinetic and Physical Property Effects. J. Polym. Sci. A Polym. Chem. 2013, 51, 2058–2067. [Google Scholar]
- Li, S.; He, Y.; Nie, J. Photopolymerization of Hybrid Monomer 3-(1-Propenyl)Oxypropyl Acrylate. J. Photochem. Photobiol. A Chem. 2007, 191, 25–31. [Google Scholar]
- Olsson, R. T.; Bair, H. E.; Kuck, V.; Hale, A. Acceleration of the Cationic Polymerization of an Epoxy with Hexanediol. J. Therm. Anal. Calorim. 2004, 76, 367–377. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Wu, L.; Liu, W.; Li, Y.; Yang, Y. Control the Structure of the Polyacrylate/Epoxy Resin Film through Photopolymerisation. Liq. Cryst. 2024, 51, 223–232. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, Z.; Jiang, Y.; He, B.; Du, M.; Lu, P.; Hong, Y.; Kwok, H. S.; Qin, A.; Qiu, H.; Zhao, Z.; Tang, B. Z. From Tetraphenylethene to Tetranaphthylethene: Structural Evolution in Aie Luminogen Continues. Chem. Commun. 2013, 49, 2491–2493. [Google Scholar] [CrossRef]
- Shustova, N. B.; McCarthy, B. D.; Dincă, M. Turn-On Fluorescence in Tetraphenylethylene-Based Metal–Organic Frameworks: An Alternative to Aggregation-Induced Emission. J. Am. Chem. Soc. 2011, 133, 20126–20129. [Google Scholar] [CrossRef]
- Dong, Y.; Lam, J. W. Y.; Qin, A.; Liu, J.; Li, Z.; Tang, B. Z.; Sun, J.; Kwok, H. S. Aggregation-Induced Emissions of Tetraphenylethene Derivatives and Their Utilities as Chemical Vapor Sensors and in Organic Light-Emitting Diodes. Appl. Phys. Lett. 2007, 91, 011111. [Google Scholar] [CrossRef]
- Iasilli, G.; Battisti, A.; Tantussi, F.; Fuso, F.; Allegrini, M.; Ruggeri, G.; Pucci, A. Aggregation-Induced Emission of Tetraphenylethylene in Styrene-Based Polymers. Macromol. Chem. Phys. 2014, 215, 499–506. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Lu, X.; Liu, W.; Wang, Y.; Li, H.; Yang, Y. Tetraphenylethene-Decorated Difluoroboron β-Diketonates with Terminal Chiral α-Phenylethylamine: Aggregation-Induced Emission, Circularly Polarized Luminescence and Mechanofluochromism. Dyes Pigm. 2021, 192, 109396. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




