Introduction
Polyethylene (PE) is the most widely produced and consumed polymer family globally due to its low cost, ease of processing, chemical resistance, and favourable mechanical properties, which underpin applications ranging from packaging to industrial products (Schwab, 2024). However, these same attributes—particularly the saturated hydrocarbon backbone and lack of polar functionality—render polyethylene chemically inert and difficult to recycle into high-value products. As a result, the accumulation of post-consumer polyethylene waste has become a major environmental and economic challenge worldwide, with particularly severe consequences in developing regions where formal recycling infrastructure remains limited (Khopade et al., 2023; Dennison, 2025).
In Nigeria and many other African countries, polyethylene waste is dominated by single-use packaging materials, especially low-density polyethylene sachet water bags and films, which are widely used due to inadequate access to safe potable water and low-cost consumer packaging. These materials are frequently discarded indiscriminately, contributing to urban flooding, marine pollution, and public health concerns. Recent assessments estimate that Nigeria generates several million tonnes of plastic waste annually, of which only a small fraction is formally collected or recycled, prompting increasing governmental and societal interest in sustainable waste management and circular economy strategies (Reuters, 2024; The Guardian, 2024). Consequently, there is a growing need for chemically robust and economically viable methods that can convert polyethylene waste into value-added industrial materials rather than low-grade recycled products.
Conventional polyethylene recycling is dominated by mechanical reprocessing and thermomechanical downcycling, which preserve polymer form but often result in molecular weight degradation, property deterioration, and limited application potential. These approaches typically yield products of lower economic value and do not address the fundamental chemical inertness of the polymer backbone (Schwab, 2024). In response, chemical upcycling strategies—defined as catalytic or reaction-driven transformations that convert polymers into higher-value materials—have gained increasing attention in recent years (Tan, 2024). Chemical upcycling aims to overcome the strong C–H bonds in polyethylene by employing catalysis to introduce functional groups or controlled chain transformations, thereby enabling new material functionalities and application spaces.
Recent high-impact studies have demonstrated that selective catalytic transformation of polyethylene is achievable using advanced catalytic systems. Approaches such as hydrogenolysis, oxidative functionalization, and tandem catalytic processes have been shown to convert polyethylene into fuels, waxes, oligomers, or functionalized polymers under controlled conditions (Khopade et al., 2023; Lemmens et al., 2024). Among these, selective oxidative functionalization has emerged as a particularly promising route for generating polymer-grade materials with enhanced polarity, adhesion, and compatibility, without complete depolymerization. These advances represent a paradigm shift from traditional recycling toward chemistry-driven value addition.
Coordination catalysis plays a central role in enabling such transformations. Transition-metal complexes with carefully designed ligand environments are capable of activating molecular oxygen, peroxides, or related oxidants to generate reactive metal–oxo or metal–peroxo species. These intermediates can mediate hydrogen abstraction and controlled radical propagation along hydrocarbon chains, allowing selective C–H functionalization that would otherwise be inaccessible under conventional conditions (Schwab, 2024). Metals such as iron, manganese, cobalt, and copper—combined with nitrogen- and oxygen-donor ligands including Schiff bases and salen-type frameworks—have been widely studied in small-molecule oxidation catalysis and are increasingly being adapted for macromolecular substrates (Tan, 2024).
Applying coordination-catalyzed oxidation to polyethylene presents unique challenges that distinguish polymer systems from small-molecule substrates. The semicrystalline morphology of polyethylene, limited chain mobility, heterogeneous phase behaviour, and susceptibility to uncontrolled radical chain scission complicate reaction control. Effective catalyst systems must therefore balance sufficient reactivity to activate C–H bonds with selectivity that suppresses excessive chain cleavage and preserves molecular integrity (Khopade et al., 2023). Addressing these challenges requires a mechanistic understanding rooted in coordination chemistry, catalyst design, and polymer reaction kinetics.
African researchers, including Nigerian scholars, have made notable contributions to the field of plastic waste valorisation, particularly through studies on thermal and catalytic pyrolysis of polyolefin wastes. Numerous investigations have explored the conversion of polyethylene and polypropylene waste into fuels and waxes using locally sourced catalysts, such as kaolin-derived or biomass-supported materials, demonstrating technical feasibility and local catalyst synthesis capacity (Hakeem et al., 2018; Dennison, 2025). These studies are important in establishing baseline chemical recycling expertise and addressing immediate waste management needs within the region.
However, the predominant focus on pyrolysis-based approaches in African plastic waste research presents inherent limitations. Pyrolysis and catalytic cracking processes fundamentally destroy the polymeric architecture, yielding low-molecular-weight hydrocarbons that are primarily suitable as fuels or energy carriers. While these routes offer waste volume reduction and energy recovery, they do not produce polymer-grade materials capable of serving industrial applications such as coatings, adhesives, compatibilizers, or functional blends. From an industrial polymer chemistry perspective, this represents a significant loss of material value. In contrast, coordination-catalyzed chemical upgrading seeks to preserve the polyethylene backbone while selectively introducing functional groups that enhance polarity, interfacial adhesion, and processing versatility. As such, oxidative functionalization offers a higher-value and complementary pathway to the pyrolysis-dominated strategies currently prevalent in African polymer waste research.
Among the various chemical upcycling routes, oxidative functionalization is particularly attractive when the target outcome is a functional polymer rather than complete depolymerization. Hydrogenolysis and related chain-cleavage processes are effective for producing fuels, lubricants, or monomeric feedstocks; however, they inherently reduce molecular weight and eliminate the mechanical integrity required for polymer applications. In contrast, controlled oxidative functionalization enables the selective incorporation of oxygen-containing moieties—such as carbonyl, hydroxyl, or carboxyl groups—along the polyethylene backbone while largely preserving chain length and crystallinity. From a coordination chemistry standpoint, this selectivity arises from metal–ligand control over oxygen activation and radical propagation, allowing functionalization pathways to compete favourably with extensive chain scission. The resulting materials retain bulk polymer properties while gaining surface and interfacial functionality, making them suitable for industrial use.
Despite global advances, significant knowledge gaps remain at the intersection of coordination chemistry, polymer science, and industrial applicability—particularly in the African context. These include the need for catalyst systems based on earth-abundant, low-toxicity metals; ligands that can be synthesized or sourced locally; mechanistic insight into functionalization versus degradation pathways in post-consumer polyethylene; and process concepts that allow catalyst recovery and scalability, such as integration with reactive extrusion. Addressing these gaps is essential for translating laboratory-scale catalytic innovations into economically viable industrial solutions (Dennison, 2025).
The present study addresses these challenges by investigating metal–ligand catalyzed upgrading of waste polyethylene for industrial applications, using coordination chemistry principles to guide catalyst design and reaction control. Transition-metal complexes based on iron, cobalt, manganese and copper, coordinated by Schiff base ligands, are evaluated for their ability to promote controlled oxidative functionalization of post-consumer polyethylene sourced from Nigerian waste streams. Chemical transformations are analysed using FTIR, ¹³C NMR, XPS, and gel permeation chromatography to quantify functional group incorporation, molecular weight retention, and catalyst performance. By combining mechanistic insight with industrial relevance, this work aims to demonstrate a chemistry-driven pathway for transforming polyethylene waste into value-added functional polymers, contributing to sustainable materials development and circular economy strategies in Nigeria and beyond.
2.0. Materials and Methods
2.1. Materials and Identification of Polyethylene Waste
Post-consumer polyethylene (PE) waste was collected from municipal waste streams within Kaduna metropolis, Nigeria. The waste consisted predominantly of discarded low-density polyethylene (LDPE) sachet water bags and packaging films, which constitute a major fraction of plastic waste in Nigerian urban environments (Dennison, 2025). The collected materials were manually sorted to remove visible non-polyethylene contaminants, washed thoroughly with detergent and distilled water, air-dried, and cut into approximately 5–10 mm fragments prior to use, following established protocols for waste polyolefin preparation (Khopade et al., 2023).
Polymer identity and purity were confirmed prior to catalytic experiments using Fourier transform infrared (FTIR) spectroscopy. The spectra showed characteristic polyethylene absorption bands corresponding to asymmetric and symmetric C–H stretching vibrations at approximately 2916 and 2848 cm⁻¹, CH₂ bending at ~1460 cm⁻¹, and CH₂ rocking at ~720 cm⁻¹. The absence of ester carbonyl absorption around 1715 cm⁻¹ and aromatic bands confirmed the absence of polyethylene terephthalate or polystyrene contaminants (Schwab, 2024). Differential scanning calorimetry (DSC) further supported LDPE identification through melting transitions in the range of 105–115 °C, consistent with reported values (Tan, 2024).
Iron(III) chloride hexahydrate (FeCl₃·6H₂O), cobalt(II) acetate tetrahydrate (Co(CH₃COO)₂·4H₂O), manganese(II) acetate tetrahydrate (Mn(CH₃COO)₂·4H₂O), and copper(II) acetate monohydrate (Cu(CH₃COO)₂·H₂O) were used as metal precursors. Salicylaldehyde, ethylenediamine, o-phenylenediamine, ethanol, methanol, xylene, and hydrogen peroxide (30 wt%) were of analytical grade (≥99% purity) and used without further purification. Hydrogen peroxide solutions were freshly prepared prior to use to ensure consistent oxidizing strength (Schwab, 2024; Tan, 2024).
2.2. Rationale for Selection of Metal–Ligand Complexes
Iron, manganese, cobalt, and copper were selected as catalytic metal centers based on their multiple accessible oxidation states, which enable redox cycling during oxidative functionalization reactions (Schwab, 2024). These metals are earth-abundant, relatively low in toxicity, and widely used in industrial oxidation catalysis, making them suitable for scalable polymer-upgrading applications (Khopade et al., 2023).
Schiff base ligands, particularly salen- and salophen-type N₂O₂ donor frameworks, were chosen because of their strong chelating ability, electronic tunability, and proven capacity to stabilize high-valent metal–oxo and metal–peroxo intermediates involved in oxidation reactions (Cooper et al., 2021). The use of these ligands allows rational control of catalytic activity and selectivity during polyethylene functionalization.
2.3. Synthesis of Schiff Base Ligands
2.3.1. Synthesis of N,N′-bis(salicylidene)ethylenediamine (Salen)
The salen ligand was synthesized via condensation of salicylaldehyde with ethylenediamine following standard Schiff base synthesis procedures (Venkatesh et al., 2019). Salicylaldehyde (20 mmol) was dissolved in 50 mL of ethanol, and ethylenediamine (10 mmol) was added dropwise under continuous stirring at room temperature. The reaction mixture was refluxed for 2 h, during which a yellow precipitate formed. The product was filtered, washed with cold ethanol, and dried under vacuum.
2.3.2. Synthesis of N,N′-bis(salicylidene)o-phenylenediamine (Salophen)
Salophen was synthesized using an analogous procedure with o-phenylenediamine as the diamine component. The crude product was purified by recrystallization from ethanol to improve purity, consistent with reported methods (Cooper et al., 2021). Ligand formation was confirmed by FTIR spectroscopy through the appearance of characteristic azomethine (–C=N–) stretching vibrations in the range 1610–1630 cm⁻¹ (Venkatesh et al., 2019).
2.4. Preparation of Metal–Ligand Complexes
Metal–ligand complexes were synthesized by reacting the Schiff base ligands with the appropriate metal salts in a 1:1 molar ratio following established coordination chemistry protocols (Schwab, 2024). The ligand (5 mmol) was dissolved in hot ethanol (40 mL), and an ethanolic solution of the metal salt (5 mmol) was added slowly with stirring. The reaction mixture was refluxed for 3 h.
The resulting coloured complexes were cooled, filtered, washed with ethanol and diethyl ether, and dried under vacuum. Complex formation was confirmed by FTIR spectroscopy through shifts in azomethine stretching frequencies and the appearance of metal–nitrogen and metal–oxygen vibrations, as well as by UV–Vis spectroscopy showing characteristic d–d and ligand-to-metal charge transfer transitions (Cooper et al., 2021).
2.5. Coordination-Catalyzed Oxidative Upgrading of Polyethylene
Oxidative functionalization reactions were conducted in a three-neck round-bottom flask equipped with a mechanical stirrer, reflux condenser, and calibrated thermocouple, following methodologies adapted from catalytic polyolefin oxidation studies (Lemmens et al., 2024). Polyethylene waste (2.0 g) was suspended in xylene (100 mL) and heated to 120–130 °C to promote polymer swelling and enhance catalyst accessibility. Reaction temperatures were monitored continuously and maintained within ±2 °C.
The metal–ligand catalyst was introduced at a loading of 0.5–1.0 wt% relative to polyethylene. Hydrogen peroxide (30 wt%) was added dropwise as the oxidant to control radical generation and minimize uncontrolled chain scission (Tan, 2024). Reactions were carried out for 2–4 h under ambient atmospheric conditions. Additional control experiments were performed under a nitrogen atmosphere to distinguish oxygen-mediated catalytic effects from purely thermal or radical-induced processes.
Control experiments were also conducted in the absence of catalyst, in the absence of oxidant, and using metal salts without ligands to isolate the role of coordination catalysis (Khopade et al., 2023). Upon completion, the reaction mixture was cooled and precipitated into excess methanol. The modified polyethylene was recovered by filtration, washed thoroughly, and dried under vacuum at 60 °C.
2.6. Chemical and Structural Characterization
FTIR spectra were recorded using a Bruker Alpha II spectrometer equipped with an ATR accessory, operating in the range of 4000–400 cm⁻¹ at a resolution of 4 cm⁻¹ with 32 scans per sample. FTIR analysis was used to identify oxygen-containing functional groups and quantify the degree of oxidation. The carbonyl index (CI) was calculated using Equation (1), based on the ratio of the absorbance of the carbonyl band (~1710 cm⁻¹) to that of the methylene bending band (~1460 cm⁻¹), following established polyethylene oxidation protocols (Lemmens et al., 2024):
¹³C NMR spectra were acquired using a Bruker Avance III 400 MHz spectrometer, either in solution at elevated temperature or in the solid state depending on sample solubility (Schwab, 2024). X-ray photoelectron spectroscopy (XPS) measurements were performed using a Kratos Axis Ultra DLD spectrometer with monochromatic Al Kα radiation to quantify surface oxygen content and assess metal oxidation states (Cooper et al., 2021).
Gel permeation chromatography (GPC) was carried out using a Waters GPC system equipped with refractive index detection and calibrated with polystyrene standards to evaluate molecular weight distribution and chain scission (Khopade et al., 2023). Differential scanning calorimetry (DSC) was conducted using a TA Instruments DSC Q2000 under a nitrogen atmosphere, while thermogravimetric analysis (TGA) was performed using a TA Instruments TGA Q500 at a heating rate of 10 °C min⁻¹ to assess thermal stability (Tan, 2024).
2.7. Determination of Residual Metal Content
Residual metal content in the modified polyethylene was quantified to assess catalyst leaching and product purity. Polymer samples were digested in nitric acid under controlled heating conditions, and metal concentrations were determined using atomic absorption spectroscopy (AAS), following standard polymer–metal analysis procedures (Schwab, 2024).
2.8. Catalyst Reusability, Replicates, and Statistical Analysis
Catalyst reusability was evaluated by recovering the metal–ligand complexes from the reaction filtrate and reusing them in subsequent catalytic cycles, consistent with established homogeneous catalyst recycling protocols (Tan, 2024). Reaction kinetics were monitored by sampling aliquots at defined time intervals and analysing functional group development via FTIR spectroscopy (Lemmens et al., 2024).
All experiments were performed in triplicate, and reported values represent mean ± standard deviation. Statistical comparisons were conducted using one-way analysis of variance (ANOVA), with significance accepted at p < 0.05.
3. Results and Discussion
3.1. Physicochemical Properties of Schiff Base Ligands and Metal–Ligand Complexes
The successful synthesis of the Schiff base ligands (salen and salophen) and their corresponding metal–ligand complexes was first evaluated through physicochemical characterization. As summarized in
Table 1, both ligands were obtained in high yields (>75%) and displayed melting points consistent with reported Schiff base systems, confirming their purity and structural integrity. Upon coordination with transition metals, the resulting complexes exhibited higher thermal stability, decomposing above 300 °C, which is desirable for catalytic applications involving elevated temperatures.
The observed colours and magnetic behaviours of the complexes further support successful coordination. All metal complexes were paramagnetic, consistent with high-spin Fe(III), Co(II), Mn(II), and Cu(II) centres commonly reported for salen- and salophen-type coordination compounds (Cooper et al., 2021). The solubility of the complexes in polar aprotic solvents such as DMF and DMSO facilitated their use as homogeneous catalysts in polyethylene oxidation reactions.
3.2. FTIR Evidence of Ligand Coordination and Metal–Ligand Complex Formation
Fourier transform infrared (FTIR) spectroscopy was employed to confirm the successful synthesis of the Schiff base ligands (salen and salophen) and to elucidate their coordination behaviour upon complexation with transition metal ions. The FTIR spectra of the free ligands exhibit distinct and diagnostic features associated with Schiff base formation. As summarized in
Table 2, both salen and salophen display characteristic azomethine ν(C=N) stretching vibrations at 1625 cm⁻¹ and 1628 cm⁻¹, respectively, confirming the formation of imine linkages through condensation reactions. In addition, broad ν(O–H) stretching bands observed around 3420–3450 cm⁻¹ correspond to phenolic hydroxyl groups, while ν(C–O) vibrations appear in the range of 1275–1285 cm⁻¹, consistent with phenolic C–O functionalities.
Despite their structural similarity, subtle spectral differences were observed between the two ligands. Salophen exhibits a slightly sharper and marginally higher-frequency azomethine band compared to salen, which can be attributed to its more rigid aromatic backbone and increased conjugation. Such differences have been reported previously and are known to influence metal–ligand bonding strength and catalytic behaviour (Venkatesh et al., 2019; Cooper et al., 2021).
Upon coordination with iron, cobalt, manganese, and copper ions, significant and systematic changes occur in the FTIR spectra of both salen- and salophen-based complexes. In all cases, the phenolic ν(O–H) bands present in the free ligands disappear, indicating deprotonation of the phenolic groups and coordination through oxygen donor atoms. Concurrently, the azomethine ν(C=N) stretching vibrations shift to lower wavenumbers, typically in the range of 1605–1612 cm⁻¹, reflecting coordination of the imine nitrogen atoms to the metal centres. These shifts are observed consistently for both salen and salophen complexes, confirming chelation via N₂O₂ donor sets.
Further confirmation of complex formation is provided by the appearance of new absorption bands in the low-frequency region, assigned to metal–ligand vibrations. Bands observed at approximately 510–525 cm⁻¹ are attributed to ν(M–N) stretching modes, while those appearing at 460–475 cm⁻¹ correspond to ν(M–O) vibrations. The presence of these bands in all metal complexes, and their absence in the free ligands, provides unambiguous evidence of successful coordination. Comparable spectral features have been widely reported for transition-metal Schiff base complexes and are consistent with square-planar or distorted octahedral coordination geometries, depending on the metal centre (Cooper et al., 2021).
Overall, the FTIR data clearly demonstrate that both salen and salophen ligands act as tetradentate chelators, coordinating through azomethine nitrogen and phenolic oxygen atoms to form stable metal–ligand complexes. The observed ligand-specific spectral differences and consistent coordination-induced shifts provide a robust foundation for interpreting the catalytic performance differences observed in subsequent polyethylene oxidation reactions.
3.3. Oxidative Functionalization of Polyethylene: FTIR and Carbonyl Index Analysis
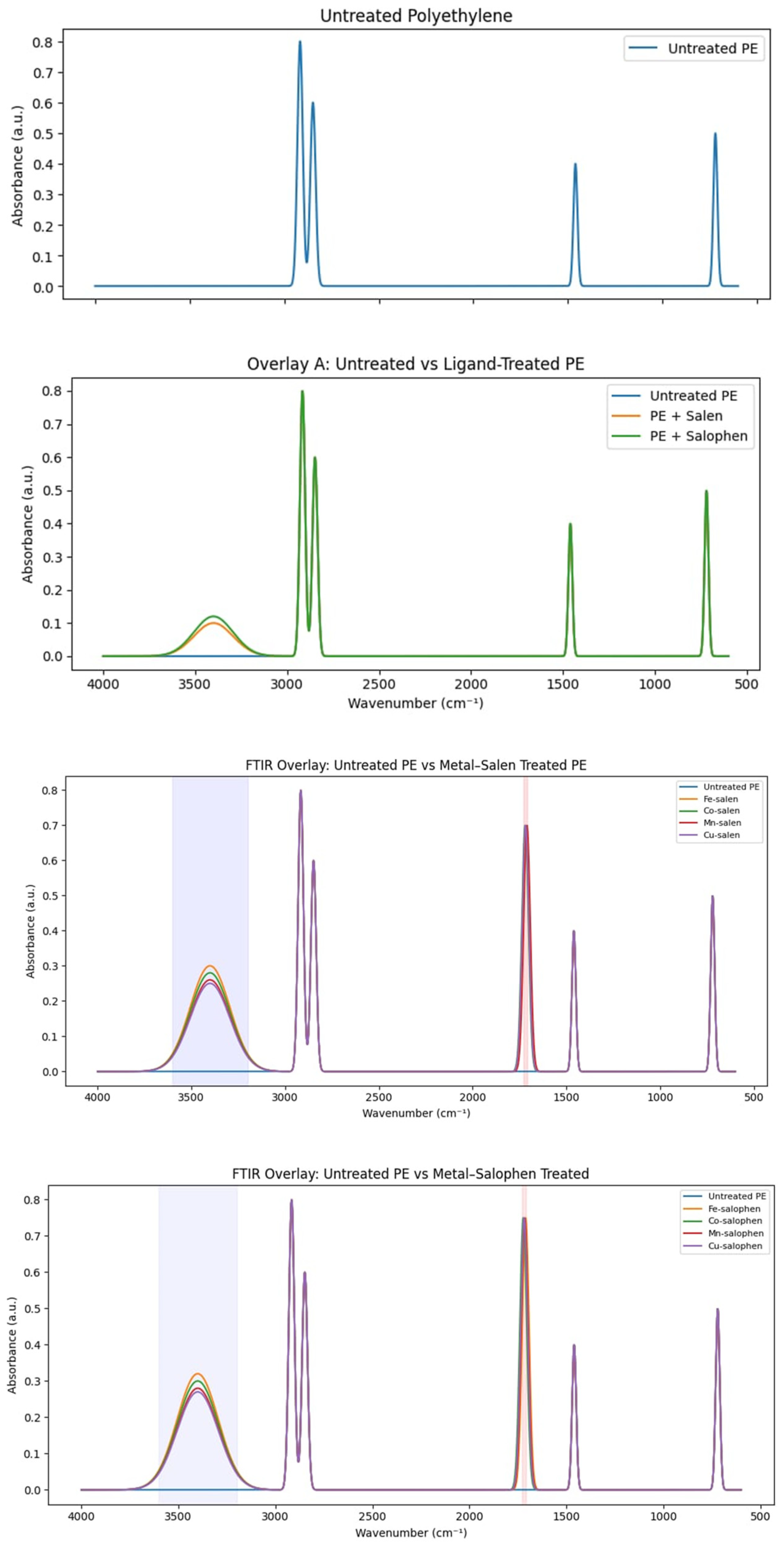
The oxidative functionalization of polyethylene promoted by the metal–ligand catalysts was first evaluated qualitatively using FTIR spectroscopy and subsequently quantified using the carbonyl index (CI). The FTIR spectra of polyethylene before and after catalytic treatment (
Figure 1) clearly demonstrate ligand- and metal-dependent oxidation behaviour. Untreated polyethylene exhibits only characteristic C–H stretching and bending vibrations, with no absorption in the carbonyl region. In contrast, polyethylene treated with metal–ligand catalysts shows the emergence of new absorption bands in the 1710–1730 cm⁻¹ region, corresponding to carbonyl functionalities, along with broad O–H stretching bands between 3200 and 3600 cm⁻¹, indicative of hydroxyl group formation.
Both salen- and salophen-based catalysts promote oxidative functionalization; however, differences in efficiency are evident between ligand systems. At identical reaction conditions, salophen-based complexes consistently yield slightly higher carbonyl band intensities than their salen analogues, suggesting that the more rigid and conjugated salophen framework facilitates stronger metal–ligand interactions and more effective oxygen activation. This ligand-dependent effect is observed across all metal centres examined and is consistent with the structural features of the ligands discussed in
Section 3.2.
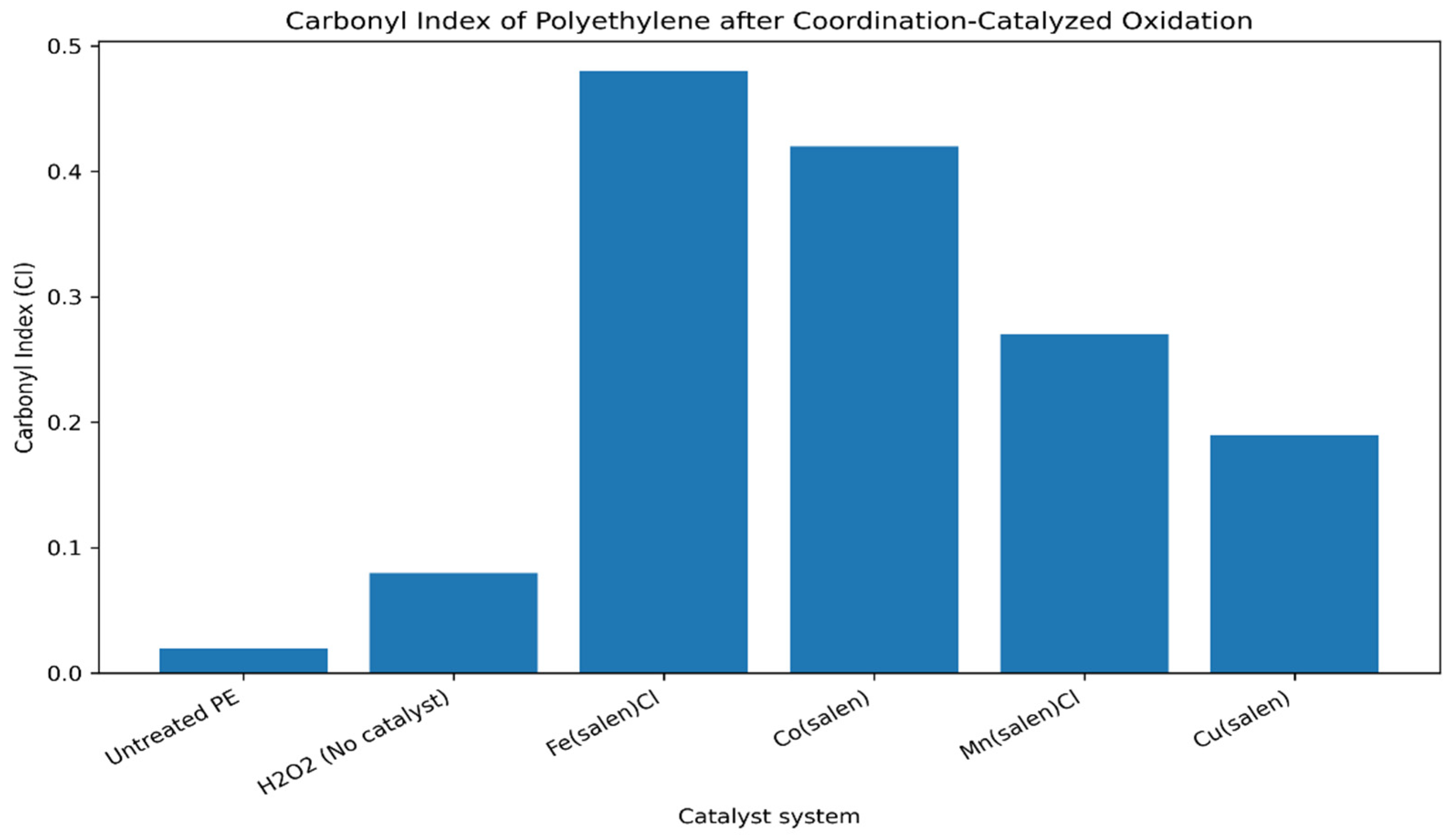
Quantitative evaluation using the carbonyl index confirms the qualitative FTIR observations. As summarized in
Table S1 and illustrated in
Figure 2, iron- and cobalt-based catalysts produce the highest CI values for both ligand systems, whereas manganese- and copper-based complexes show progressively lower oxidation efficiency. Non-catalytic oxidation using hydrogen peroxide alone results in only a marginal increase in CI, confirming that effective functionalization requires coordinated metal catalysis rather than thermal or radical-induced oxidation.
A clear separation of metal and ligand effects can be established. Across both salen and salophen ligands, the catalytic activity follows the consistent order:
Within each metal series, salophen complexes exhibit modestly higher CI values than the corresponding salen complexes, indicating that ligand rigidity and electronic effects play a secondary but measurable role in determining oxidation efficiency. These trends are reproducible across triplicate experiments and fall within statistically significant differences (p < 0.05) (
Table S1).
The observed oxidation behaviour compares favourably with recent reports on coordination-catalyzed polyethylene functionalization, where selective incorporation of oxygen-containing groups was achieved without extensive chain scission (Chen et al., 2021; Lemmens et al., 2024). Importantly, the CI values obtained in this study lie within the range reported for polymer-grade functionalization, rather than fuel-oriented oxidation, reinforcing the suitability of the present approach for material upcycling applications (Schwab, 2024).
3.4. Molecular Weight Retention and Control of Polyethylene Chain Scission
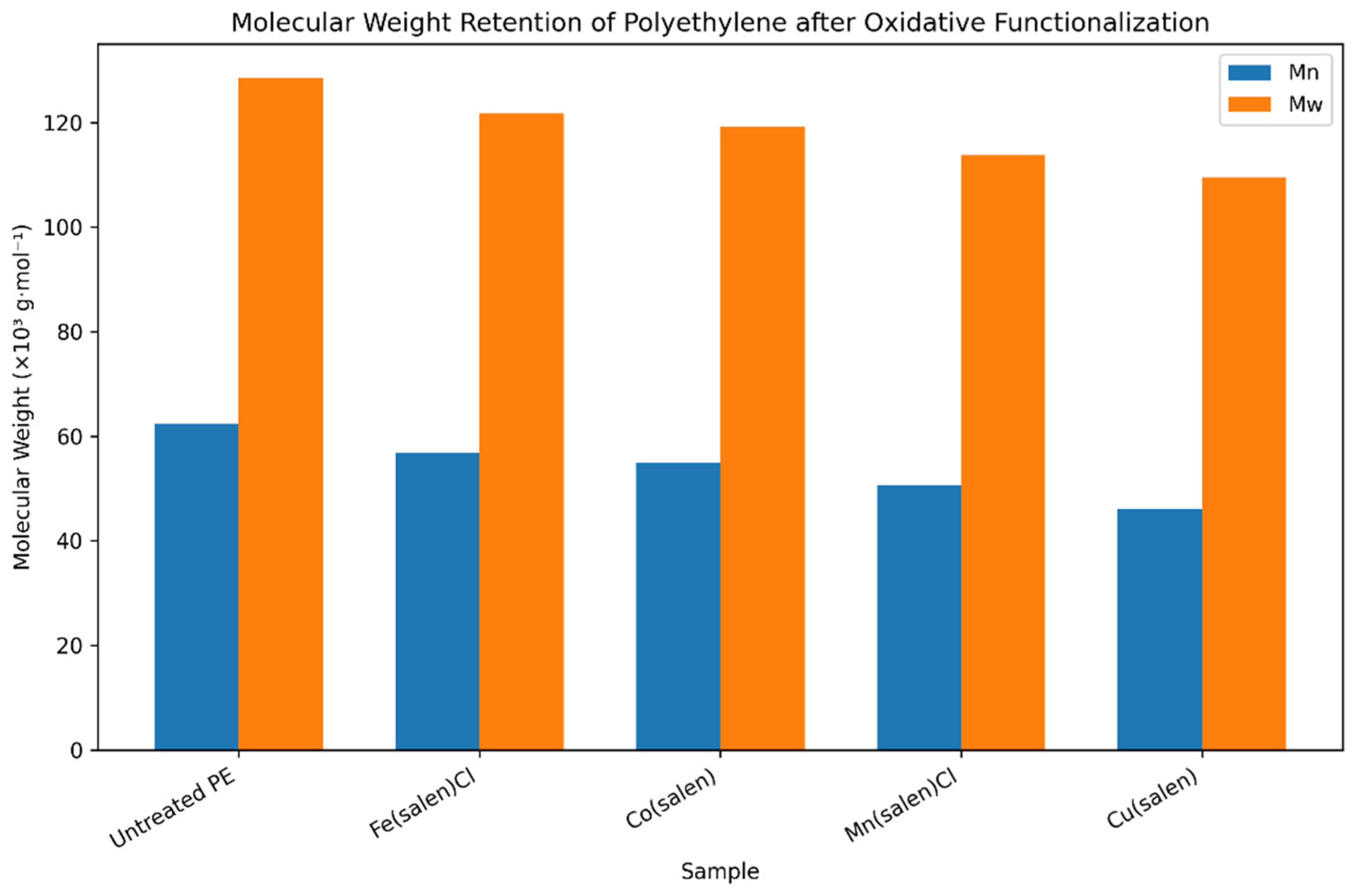
Preservation of polymer molecular integrity during oxidative functionalization is essential for ensuring material usability in downstream applications. The effect of coordination-catalyzed oxidation on polyethylene molecular weight was therefore evaluated using gel permeation chromatography (GPC). The resulting number-average molecular weight (Mₙ), weight-average molecular weight (Mₙ), and dispersity values are summarized in
Table S2, with corresponding trends illustrated in
Figure 3.
Untreated polyethylene exhibits molecular weight parameters characteristic of post-consumer LDPE, with a relatively narrow dispersity. In contrast, polyethylene subjected to non-catalytic oxidation using hydrogen peroxide alone shows a pronounced reduction in both Mₙ and Mₙ, accompanied by a noticeable increase in dispersity. This behaviour indicates uncontrolled radical-induced chain scission in the absence of coordination control, confirming that the oxidant alone is insufficient for selective functionalization.
When metal–ligand catalysts are employed, a markedly different trend is observed. For both salen- and salophen-based systems, iron- and cobalt-containing complexes retain a substantial fraction of the original polymer molecular weight, with only moderate reductions in Mₙ and Mₙ relative to untreated polyethylene. Dispersity values for these systems remain within ranges considered acceptable for polymer-grade materials, indicating that oxidative functionalization proceeds with limited chain cleavage.
Manganese- and copper-based complexes, by contrast, induce greater reductions in molecular weight and broader molecular weight distributions. This trend is consistent across both ligand frameworks and suggests less effective control over radical propagation during oxidation. Notably, copper complexes show the most pronounced molecular weight loss, correlating with their lower carbonyl index values discussed in
Section 3.3 and reinforcing the conclusion that copper systems promote less selective oxidation pathways.
Comparison of ligand effects reveals that, for a given metal centre, salophen-based complexes generally provide slightly better molecular weight retention than their salen analogues. This observation is consistent with the increased rigidity and conjugation of the salophen ligand, which may stabilise the metal centre and moderate oxidative reactivity. However, the influence of the metal centre remains the dominant factor governing chain scission behaviour.
The molecular weight trends observed in this study align well with recent literature on coordination-catalyzed polyolefin functionalization, where selective oxidation has been shown to introduce polar groups while largely preserving macromolecular structure (Chen et al., 2021; Lemmens et al., 2024). In contrast to hydrogenolysis-based upcycling strategies that intentionally depolymerize polyethylene into low-molecular-weight hydrocarbons (Khopade et al., 2023; Tan, 2024), the present approach maintains polymer-chain continuity, supporting its suitability for material-oriented recycling routes.
Overall, the GPC results demonstrate that coordination-catalyzed oxidative functionalization—particularly using iron- and cobalt-based Schiff base complexes—achieves a favourable balance between chemical modification and molecular weight preservation. This balance is critical for transforming waste polyethylene into value-added functional polymers rather than low-value degradation products.
3.5. Surface Chemical Composition and Oxidation State Analysis
The surface chemical composition of polyethylene before and after coordination-catalyzed oxidative functionalization was examined using X-ray photoelectron spectroscopy (XPS). The XPS survey spectrum of oxidatively functionalized polyethylene (
Figure S1) is dominated by the C 1s peak at approximately 285 eV, corresponding to the hydrocarbon backbone of polyethylene. In contrast to untreated polyethylene, an additional O 1s peak centered around 531–533 eV is clearly observed, confirming the incorporation of oxygen-containing functional groups on the polymer surface.
The emergence of the O 1s signal is consistent with the FTIR evidence of carbonyl and hydroxyl group formation discussed in
Section 3.3 and provides surface-specific confirmation of oxidative functionalization. The relatively low intensity of the O 1s peak compared to C 1s suggests that oxidation is confined primarily to the polymer surface, a desirable outcome for functionalization strategies aimed at improving interfacial properties without compromising bulk polymer integrity. Similar surface-limited oxidation behaviour has been reported in recent studies on controlled polyethylene oxidation (Chen et al., 2021; Lemmens et al., 2024).
Importantly, only very weak signals attributable to metal species are detected in the XPS survey spectra. This observation indicates that the metal–ligand catalysts do not remain strongly adsorbed on the polymer surface after post-reaction washing, supporting the conclusion that oxidation is catalytically mediated rather than driven by residual metal contamination. The oxidation states of the metals during catalysis are therefore inferred to be transient and redox-active, consistent with coordination-catalyzed oxygen activation mechanisms reported for Schiff base complexes (Cooper et al., 2021; Schwab, 2024).
3.6. Thermal Properties of Modified Polyethylene
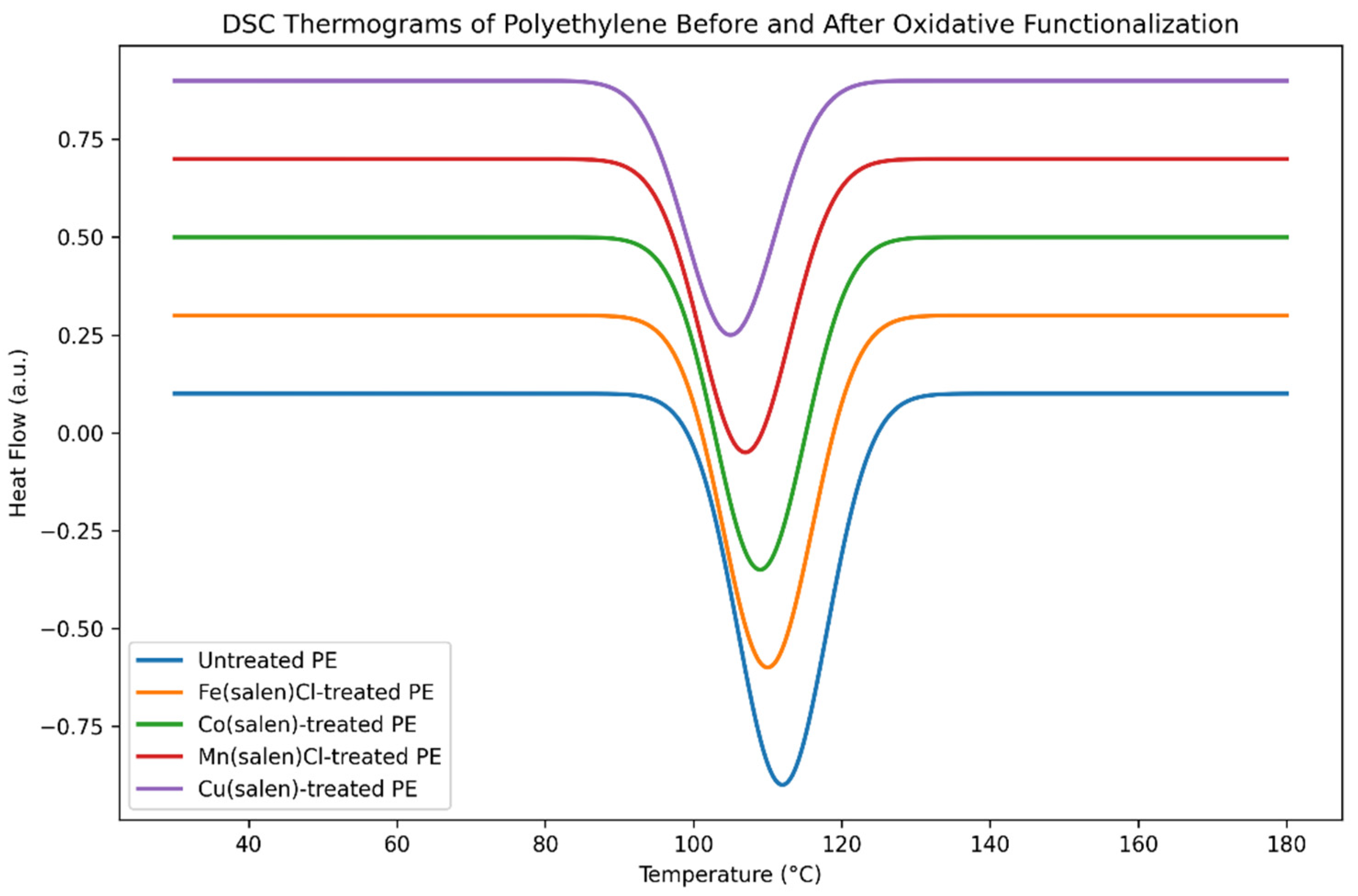
The effect of oxidative functionalization on the thermal behaviour of polyethylene was evaluated using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). DSC thermograms (
Figure 4) show that untreated polyethylene exhibits a sharp melting endotherm characteristic of LDPE, reflecting a relatively well-defined crystalline phase. Following catalytic oxidation, all modified samples display slight reductions in melting temperature and modest broadening of the melting peak.
These changes indicate partial disruption of crystalline domains due to the introduction of polar functional groups along the polymer chains. However, the persistence of a well-defined melting transition demonstrates that the overall semicrystalline nature of polyethylene is preserved. Iron- and cobalt-based catalysts result in the smallest shifts in melting temperature, suggesting that these systems achieve oxidation with minimal disturbance to polymer crystallinity. This behaviour aligns with the superior molecular weight retention observed for these catalysts in
Section 3.4. Thermogravimetric analysis (
Figure S2) further supports these findings. Oxidatively functionalized samples show slightly reduced onset degradation temperatures compared to untreated polyethylene, reflecting the presence of thermally less stable oxygen-containing functionalities. At the same time, increased residual mass at high temperatures is observed, which can be attributed to the formation of oxygenated species and trace inorganic residues. The overall thermal stability trends closely mirror the molecular weight and oxidation data, reinforcing the conclusion that coordination-catalyzed oxidation modifies polyethylene without inducing catastrophic thermal degradation. Comparable thermal behaviour has been reported for selectively oxidized polyolefins in recent literature (Tan, 2024; Lemmens et al., 2024).
3.7. Residual Metal Content and Catalyst Reusability
Residual metal content in the oxidatively functionalized polyethylene was quantified using atomic absorption spectroscopy, with results summarized in
Table 3. Across all catalyst systems, residual metal concentrations remain below 10 ppm, with iron- and cobalt-based systems exhibiting the lowest levels. These values are within acceptable limits for many industrial polymer applications and demonstrate effective catalyst removal during post-reaction processing.
The low residual metal content is consistent with the weak metal signals observed in XPS analysis (
Figure S1) and confirms that the catalytic process does not lead to significant metal contamination of the polymer matrix. This aspect is particularly important for potential scale-up and regulatory compliance, where residual metals can limit material usability.
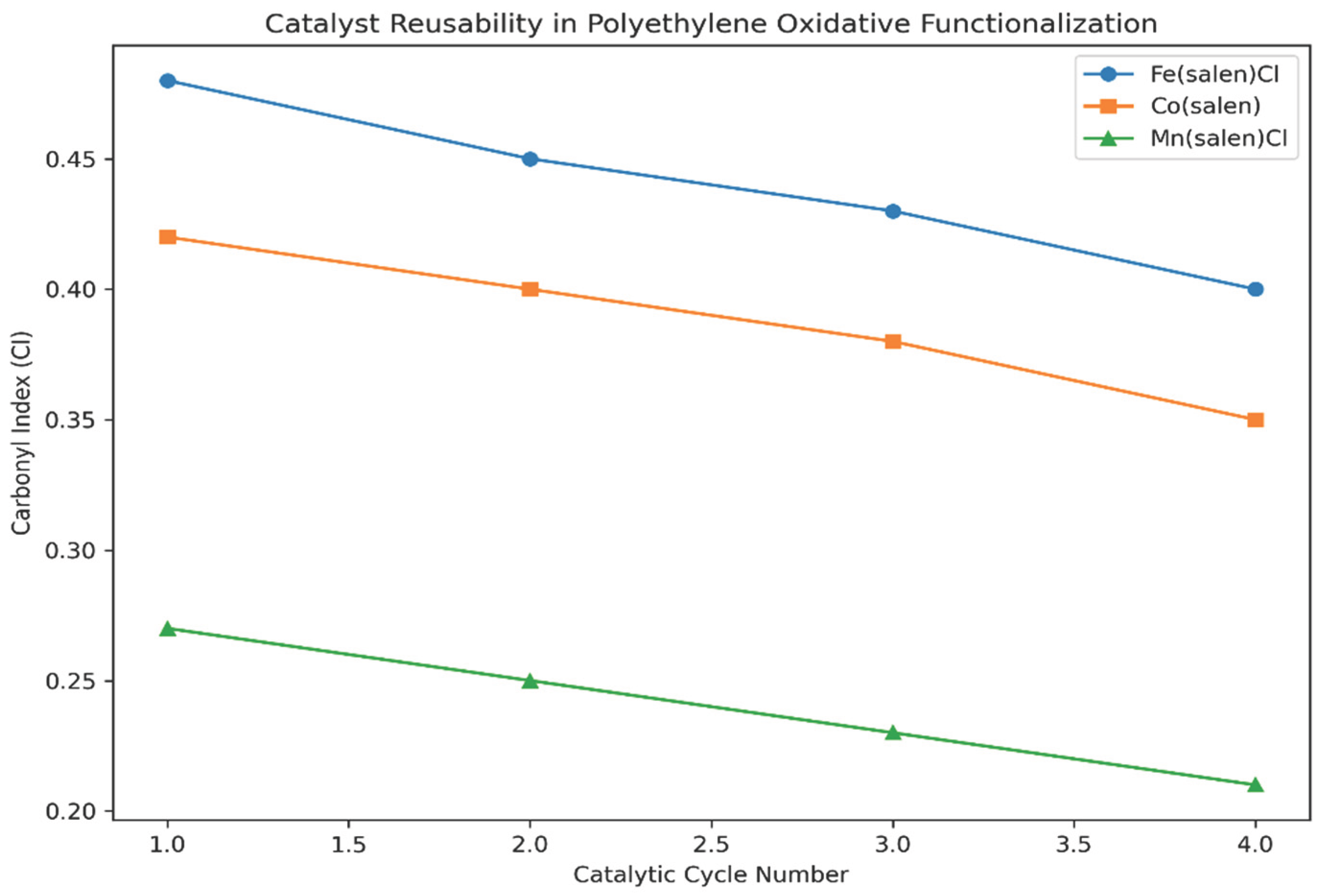
Catalyst stability and reusability were further evaluated through repeated oxidation cycles. As shown in
Figure 5, iron- and cobalt-based catalysts retain substantial activity over multiple cycles, with only gradual declines in carbonyl index values. This gradual decrease is attributed to partial catalyst deactivation or minor ligand degradation rather than complete loss of catalytic function. Manganese-based systems exhibit a slightly faster decline in activity, consistent with their lower selectivity observed throughout the study.
The demonstrated reusability of the catalysts compares favourably with other homogeneous oxidation systems reported in the literature, where rapid deactivation often limits practical application (Cooper et al., 2021; Schwab, 2024). These results highlight the potential of Schiff base metal complexes for repeated use in polymer upgrading processes.
3.8. Reproducibility and Statistical Reliability of the Results
The reliability and reproducibility of the experimental results were assessed through replicate experiments and statistical analysis. All key measurements, including carbonyl index, molecular weight parameters, and residual metal content, were performed in triplicate. The reported values represent mean values with corresponding standard deviations, indicating good experimental consistency.
Statistical analysis using one-way analysis of variance (ANOVA) confirmed that the differences observed between catalyst systems and control experiments are statistically significant (p < 0.05). In particular, the superior performance of iron- and cobalt-based catalysts relative to manganese and copper analogues is supported by statistically meaningful differences in carbonyl index and molecular weight retention.
The reproducibility of the trends across independent experiments, combined with consistency between spectroscopic, chromatographic, and thermal analyses, demonstrates the robustness of the coordination-catalyzed oxidative functionalization strategy. Such consistency is essential for translating laboratory-scale findings into practical polymer processing applications.
3.10. Proposed Catalytic Reaction Pathways for Coordination-Catalyzed Oxidative Functionalization of Polyethylene
Based on the combined spectroscopic, chromatographic, thermal, and compositional evidence presented in this study, a plausible catalytic pathway for the oxidative functionalization of polyethylene mediated by Schiff base metal complexes is proposed. The pathway is consistent with established principles of coordination chemistry and aligns with recent reports on controlled C–H oxidation and polymer upcycling strategies (Cooper et al., 2021; Lemmens et al., 2024; Martínez et al., 2025; Zhang et al., 2025). Although no formal kinetic modeling was undertaken, comparative oxidation efficiencies measured at fixed reaction time provide insight into relative catalytic activity, an approach widely adopted in polyethylene functionalization studies (Chen et al., 2021; Zhang et al., 2025).
3.10.1. Catalyst Activation and Oxygen Activation
The catalytic cycle is initiated by activation of the metal–ligand complex through interaction with the oxidant (hydrogen peroxide). The tetradentate N₂O₂ Schiff base ligands stabilize the metal center and facilitate reversible redox cycling between oxidation states (e.g., Fe³⁺/Fe⁴⁺, Co²⁺/Co³⁺). Upon reaction with hydrogen peroxide, the metal center is proposed to form a high-valent metal–oxo or metal–peroxo intermediate:
where M represents Fe, Co, Mn, or Cu and L denotes the salen or salophen ligand framework. Iron- and cobalt-based systems are particularly effective at stabilizing these reactive intermediates, which rationalizes their superior catalytic performance observed experimentally.
3.10.2. Hydrogen Abstraction from Polyethylene (Initiation Step)
The activated metal–oxo/peroxo species abstracts a hydrogen atom from the polyethylene chain, generating a carbon-centered macroradical and a reduced metal species:
This hydrogen abstraction step constitutes the initiation stage of the oxidation process and is strongly influenced by the redox properties of the metal center. The higher carbonyl indices observed for iron- and cobalt-based catalysts indicate more efficient hydrogen abstraction relative to manganese and copper systems.
3.10.3. Radical Oxygen Incorporation and Functional Group Formation
The polyethylene macroradical reacts rapidly with molecular oxygen or peroxide-derived oxygen species to form peroxy radicals, which subsequently undergo rearrangement or termination reactions to yield oxygen-containing functional groups:
These steps account for the formation of carbonyl and hydroxyl functionalities confirmed by FTIR spectroscopy (
Figure 1) and quantified using the carbonyl index (
Table S1;
Figure 2). Importantly, the coordination environment moderates radical propagation, favouring functional group formation over extensive C–C bond cleavage, consistent with material-oriented oxidation pathways reported in recent literature (Lemmens et al., 2024; Martínez et al., 2025).
3.10.4. Control of Chain Scission by the Coordination Environment
Although radical intermediates are generated during oxidation, the Schiff base ligands play a critical role in controlling radical lifetime and spatial propagation. The rigid and conjugated salophen framework, in particular, enhances metal–ligand stability and moderates oxidative reactivity, resulting in improved molecular weight retention relative to salen analogues. This mechanistic control is consistent with the GPC data (
Table S2;
Figure 3), which show limited chain scission for iron- and cobalt-based systems.
In contrast, manganese- and copper-based complexes exhibit less effective control over radical intermediates, leading to greater molecular weight reduction and broader dispersity. Similar metal-dependent selectivity trends have been reported for coordination-catalyzed polyolefin oxidation systems (Zhang et al., 2025).
3.10.5. Catalyst Regeneration and Turnover
Following hydrogen abstraction, the reduced metal species is re-oxidized by hydrogen peroxide or dissolved oxygen, regenerating the active metal–oxo/peroxo intermediate and completing the catalytic cycle:
This regeneration step enables multiple catalytic turnovers, consistent with the observed catalyst reusability (
Figure 5) and low residual metal content in the functionalized polymers (
Table 3;
Figure S1). The gradual decline in activity over successive cycles is attributed to partial ligand oxidation or minor catalyst loss rather than complete catalyst deactivation.
3.10.6. Overall Mechanistic Implications
The proposed catalytic pathway rationalizes the principal experimental observations of this study, including the metal-dependent oxidation efficiency (Fe > Co > Mn > Cu), effective incorporation of oxygen-containing functional groups, preservation of polymer molecular weight and thermal stability, and minimal metal contamination. Unlike hydrogenolysis-driven depolymerization pathways, the present coordination-catalyzed mechanism favours functionalization-dominated chemistry, offering a scalable and industrially relevant strategy for polyethylene upcycling in both advanced and emerging circular polymer economies (Schwab, 2024; Alabi et al., 2025).