Submitted:
09 January 2026
Posted:
12 January 2026
You are already at the latest version
Abstract
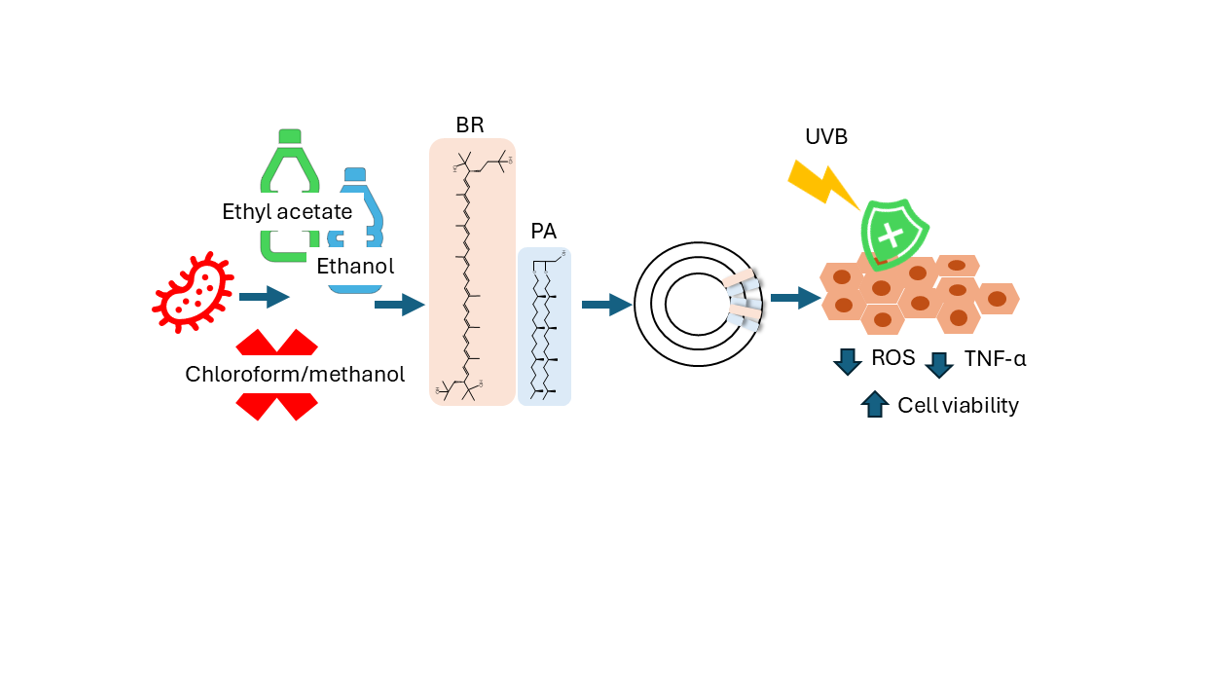
Archaea lipids are a source of new biomaterials for pharmaceutical and nanomedical applications; however, their classic extraction method relies on chloroform and methanol, toxic solvents that conflict with green chemistry principles. In this paper we explore the performance of an eco-friendly method for the extraction of total lipids from the haloarchaea Halorubrum tebenquichense. Using the bio-solvents ethyl acetate and ethanol in a two-step procedure, a fraction of total lipids (135 ± 41 mg phospholipids and 1.1 ± 0.4 mg bacterioruberin (BR) / 100 g cell paste) was obtained containing the same composition as that resulting from extraction with the classical solvents as confirmed by Electrospray Ionization Mass Spectrometry, although with lower phospholipid content, thus with a higher proportion of bacterioruberin. The extracted lipids were subsequently utilized for preparation of archaeosomes, which were characterized by uniform size distribution (406 ± 137 nm, 0.63 ± 0.13 polydispersity index), colloidal stability, and negative ζ potential (-38.2 ± 5.4 mV). The photoprotective potential of these archaeosomes was for the first time determined in human keratinocyte (HaCaT) cells exposed to UVB irradiation (270 mJ/cm2). Treatment with archaeosomes significantly (p< 0.05) enhanced cell viability (from ~43 to ~80 %), reduced intracellular ROS generation and proinflammatory cytokine release (TNF-α) and mitigated UVB-induced apoptosis compared to untreated controls, indicating effective cytoprotection. This study demonstrates that ethyl acetate–ethanol-based extraction offers an alternative for archaeal lipid recovery and highlights the potential of archaeosomes as natural photoprotective agents for skincare applications.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Archaea Growth
2.3. Lipids Extraction by Classical Bligh and Dyer Method
2.4. Lipids Extraction by with Bio-Solvents
2.5. Phospholipids, Proteins and Sugar Quantification
2.6. BR Quantification
2.7. Electrospray Ionization Mass Spectrometry (ESI-MS)
2.8. Antioxidant Activity
2.9. Preparation of Archaeosomes
2.10. Characterization of Archaeosomes
2.11. Cells Line
2.12. Cytotoxicity of BS-TA-ARC
2.13. Uptake of BS-TA-ARC
2.14. UVB Irradiation and Cell Viability Assay
2.15. Photoprotection of BS-TA-ARC
2.16. Determination of TNF-α
2.17. Reactive Oxygen Species (ROS) Assay
2.18. Apoptosis and Necrosis
2.19. Statistics
3. Results and Discussion
3.1. Total Archaeolipid Extraction and Analysis
3.2. Structural Characterization of BS-TA-ARC
3.3. Cellular Toxicity and Uptake of AM-TA-ARC
3.4. Impact of UVB Irradiation on Cell Viability
3.5. Photoprotective Activity of AM-TA-ARC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morilla, M.J.; Romero, E.L. Ether lipids from archaea in nano-drug delivery. Int J Pharm. 2023, 634, 122632.
- Jain, S.; Caforio, A.; Driessen, A.J. Biosynthesis of archaeal membrane ether lipids. Front Microbiol. 2014, 5, 641.
- Villanueva, L.; Schouten, S.; Damsté, J.S.S. Phylogenomic analysis of lipid biosynthetic genes of Archaea shed light on the lipid divide. Environ Microbiol. 2017, 19, 54–69.
- Caforio, A.; Driessen, A.J.M. Archaeal phospholipids: structural properties and biosynthesis. Biochim Biophys Acta Mol Cell Biol Lipids 2017, 1862, 1325–1339.
- Matsumi, R.; Atomi, H.; Driessen, A.J.; van der Oost, J. Isoprenoid biosynthesis in Archaea--biochemical and evolutionary implications. Res Microbiol. 2011, 162(1), 39-52.
- Chong, P.L.G.; Chang, A.; Yu, A.; Mammedova, A. Vesicular and planar membranes of archaeal lipids: unusual physical properties and biomedical applications. Int J Mol Sci. 2022, 23, 7616.
- Lizama, C.; Romero-Parra, J.; Andrade, D.; Riveros, F.; Bórquez, J.; Ahmed, S.; Venegas-Salas, L.; Cabalín, C.; Simirgiotis, M.J. Analysis of Carotenoids in Haloarchaea Species from Atacama Saline Lakes by High Resolution UHPLC-Q-Orbitrap-Mass Spectrometry: Antioxidant Potential and Biological Effect on Cell Viability. Antioxidants 2021, 10, 1230.
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J Microbiol Biotechnol. 2012, 28(4), 1781-90.
- Shahmohammadi, H.R.; Asgarani, E.; Terato, H.; Saito, T.; Ohyama, Y.; Gekko, K.; Yamamoto, O.; Ide, H. Protective Roles of Bacterioruberin and Intracellular KCl in the Resistance of Halobacterium Salinarium against DNA-Damaging Agents. J. Radiat. Res. 1998, 39, 251–262.
- Fong, N.J.; Burgess, M.L.; Barrow, K.D.; Glenn, D.R. Carotenoid accumulation in the psychrotrophic bacterium Arthrobacter agilis in response to thermal and salt stress. Appl Microbiol Biotechnol. 2001, 56(5–6), 750–6.
- Martínez-Espinosa, R.M. Bacterioruberin (C50 carotenoid): nutritional and biomedical potential. Nutrients 2025, 17, 3899.
- Ma, Y.; Sun, Z.; Yang, H.; Xie, W.; Song, M.; Zhang, B.; Sui, L. The biosynthesis mechanism of bacterioruberin in halophilic archaea revealed by genome and transcriptome analysis. Appl Environ Microbiol. 2024, 90(7), e0054024.
- Giani, M.; Martínez-Espinosa, R.M. Carotenoids as a protection mechanism against oxidative stress in Haloferax mediterranei. Antioxidants 2020, 9, 1060.
- Caimi, A.T.; Parra, F.; de Farias, M.A.; Portugal, R.V.; Perez, A.P.; Romero, E.L.; Morilla, M.J. Topical vaccination with super-stable ready to use nanovesicles. Colloids Surf B: Biointerfaces 2016, 152, 114-123.
- Altube, M.J.; Selzer, S.M.; de Farias, M.A.; Portugal, R.V.; Morilla, M.J.; Romero, E.L. Surviving nebulization-induced stress: dexamethasone in pH-sensitive archaeosomes. Nanomedicine (Lond) 2016, 11(16), 2103-17.
- Higa, L.H.; Schilrreff, P.; Briski, A.M.; Jerez, H.E.; de Farias, M.A.; Villares Portugal, R.; Romero, E.L.; Morilla, M.J. Bacterioruberin from Haloarchaea plus dexamethasone in ultra-small macrophage-targeted nanoparticles as potential intestinal repairing agent. Colloids Surf B Biointerfaces. 2020, 191, 110961.
- Schilrreff, P.; Simioni, Y.R.; Jerez, H.E.; Caimi, A.T.; de Farias, M.A.; Villares Portugal, R.; Romero, E.L.; Morilla, M.J. Superoxide dismutase in nanoarchaeosomes for targeted delivery to inflammatory macrophages. Colloids Surf B Biointerfaces. 2019, 179, 479-487.
- Charó, N.; Jerez, H.; Tatti, S.; Romero, E.L.; Schattner, M. The Anti-Inflammatory Effect of Nanoarchaeosomes on Human Endothelial Cells. Pharmaceutics 2022, 14, 736.
- Caimi, A.T.; Yasynska, O., Rivas Rojas, P.C.; Romero, E.L.; Morilla, M.J. Improved stability and biological activity of bacterioruberin in nanovesicles. J Drug Deliv Sci Technol 2022, 77, 103896.
- Kates, M. Membrane lipids of archaea. Biochem Soc Trans. 1993, 21, 100–104.
- Angelini, R.; Corral, P.; Mavridou, D.A.I.; Texeira, M., Ventosa, A. Lipidomics of haloarchaea. Appl Environ Microbiol 2012, 78(14), 5353–5363.
- Gonzalez Epelboim, V.R.D.; Lamas, D.G.; Huck-Iriart, C.; Caputo, E.N.; Altube, M.J.; Jerez, H.E.; Simioni, Y.R.; Ghosal, K.; Morilla, M.J.; Higa, L.H.; Romero, E.L. Nebulized Bacterioruberin/Astaxanthin-Loaded Nanovesicles: Antitumoral Activity and Beyond. Int. J. Mol. Sci. 2025, 26, 8607.
- https://www.epa.gov/sites/default/files/2016-09/documents/methanol.pdf (accessed on 20 Dec 2025).
- National Institute for Occupational Safety and Health. (2023). NIOSH pocket guide to chemical hazards: Methanol. Centers for Disease Control and Prevention. https://www.cdc.gov/niosh/npg (accessed on 20 Dec 2025).
- EPA. (2024). Managing hazardous waste solvents. U.S. Environmental Protection Agency. https://www.epa.gov/hw.
- Prat, D.; Wells, A.; Hayler, J.; et al. CHEM21 solvent selection guide. Green Chem. 2016, 18, 288–296.
- Anastas, P.T.; Warner, J.C. Green chemistry: theory and practice. Oxford: Oxford University Press 1998.
- Breil, C.; Abert Vian, M.; Zemb, T.; Kunz, W.; Chemat, F. “Bligh and Dyer” and Folch methods for solid-liquid-liquid extraction of lipids from microorganisms. Comprehension of Solvatation mechanisms and towards substitution with alternative solvents. Int J Mol Sci. 2017, 18(4), 1–21.
- Mussagy, C.; Santos-Ebinuma, V.C.; Kurnia, K.A.; Dias, A.C.R.V.; Carvalho, P.; Coutinho, J.A.P.; Pereira, J.F.B. Integrative platform for the selective recovery of intracellular carotenoids and lipids from Rhodotorula glutinis CCT-2186 yeast using mixtures of bio-based solvents. Green Chem 2020, 23.
- Marques, F.; Pinho, M.; Guerra, I.M.S.; Conde, T.A.; Silva, J.; Cardoso, H.; Martins, M.; Abreu, M.H.; Cerqueira, M.A.; Domingues, M.R. Unlocking functional lipid ingredients from algae by foodgrade biosolvents and ultrasound-assisted extraction for nutritional applications. LWT 2024, 200, 116136.
- Popescu, M.; Iancu, P.; Plesu, V.; Todasca, M.C.; Isopencu, G.O.; Bildea, C.S. Valuable Natural Antioxidant Products Recovered from Tomatoes by Green Extraction. Molecules 2022, 27, 4191.
- Prasad, W.; Wani, A.D.; Khamrui, K.; Hussain, S.A.; Khetra, Y. Green solvents, potential alternatives for petroleum based products in food processing industries. Clean. Chem. Eng. 2022, 3, 100052.
- Viñas-Ospino, A.; López-Malo, D.; Esteve, M.J.; Frígola, A.; Blesa, J. Green Solvents: Emerging Alternatives for Carotenoid Extraction from Fruit and Vegetable By-Products. Foods 2023, 12, 863.
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ Int. 2024, 185, 108535.
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging - review of in vitro studies. J Cosmet Dermatol. 2021, 20(11), 3427-3431.
- Tanveer, M.A.; Rashid, H.; Tasduq, S.A. Molecular basis of skin photoaging and therapeutic interventions by plant-derived natural product ingredients: A comprehensive review. Heliyon 2023, 9(3), e13580.
- Budzianowska, A.; Banaś, K.; Budzianowski, J.; Kikowska, M.; Antioxidants to Defend Healthy and Youthful Skin—Current Trends and Future Directions in Cosmetology. Applied Sciences 2025, 15(5), 2571.
- Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431.
- Catanzaro, E.; Bishayee, A.; Fimognari, C. On a Beam of Light: Photoprotective Activities of the Marine Carotenoids Astaxanthin and Fucoxanthin in Suppression of Inflammation and Cancer. Mar. Drugs 2020, 18, 544.
- Ma, Y.; Li, C.; Su, W.; Sun, Z.; Gao, S.; Xie, W.; Zhang, B.; Sui, L. Carotenoids in Skin Photoaging: Unveiling Protective Effects, Molecular Insights, and Safety and Bioavailability Frontiers. Antioxidants 2025, 14(5), 577.
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV–Vis/H2O2) Degradation of Carotenoids: Kinetics and Molecular End Products. Chemosphere 2022, 286, 131697.
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017, 46(14), 4218-4244.
- Lima, S.G.M.; Freire, M.C.L.C.; Oliveira, V.dS.; Solisio, C.; Converti, A.; de Lima, Á.A.N. Astaxanthin Delivery Systems for Skin Application: A Review. Mar. Drugs 2021, 19, 511.
- Villalaín, J. Location and dynamics of astaxanthin in the membrane. Biochim Biophys Acta (BBA)-Biomembranes. 2025, 270, 105512. [CrossRef]
- Flegler, A.; Lipski, A. The C₅₀ carotenoid bacterioruberin regulates membrane fluidity in pink-pigmented Arthrobacter species. Arch Microbiol 2021, 204(1), 70.
- Ihara, K.; Watanabe, S.; Tamura, T. Haloarcula argentinensis sp. nov. and Haloarcula mukohataei sp. nov., two new extremely halophilic archaea collected in Argentina. Int J Syst Bacteriol. 1997, 47(1), 73-7.
- Bottcher, C.J.F.; Van Gent, C.M.; Pries, C.A. Rapid and Sensitive Sub-Micro Phosphorus Determination. Anal. Chim. Acta 1961, 24, 203–204.
- Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A., Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350– 356.
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids. Basel: Birkhäuser, 2004.
- Jimenez-Escrig, A.; Jimenez-Jimenez, I.; Sanchez-Moreno, C.; Saura-Calixto, F. Evaluation of Free Radical Scavenging of Dietary Carotenoids by the Stable Radical 2,2-Diphenyl-1-Picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690.
- Wang, Y.; Eilertsen, K.E.; Elvevoll, E.O.; Walquist, M.J. Assessing the efficiency of ethyl acetate for lipid extraction as an alternative to the Folch method. J Am Oil Chem Soc. 2025, 102, 871–883.
- Naziri, D.; Hamidi, M.; Hassanzadeh, S.; Tarhriz, V.; Zanjani, B.M.; Nazemyieh, H.; Hejazi, M.A.; Hejazi, M.S. Analysis of Carotenoid Production by Halorubrum Sp. TBZ126; an Extremely Halophilic Archeon from Urmia Lake. Adv. Pharm. Bull. 2013, 4, 61.
- Gonzalez, R.O.; Higa, L.H.; Cutrullis, R.A.; Bilen, M.; Morelli, I.; Roncaglia, D.I.; Corral, R.S.; Morilla, M.J.; Petray, P.B.; Romero, E.L. Archaeosomes made of Halorubrum tebenquichense total polar lipids: a new source of adjuvancy. BMC Biotechnol. 2009, 9, 71.
- Gag, O.; Dinu, Ș.; Manea, H.; Marcovici, I.; Pînzaru, I.; Popovici, R.; Crăiniceanu, Z.; Gyori, Z; Iovănescu, G.; Chiriac, S. UVA/UVB Irradiation Exerts a Distinct Phototoxic Effect on Human Keratinocytes Compared to Human Malignant Melanoma Cells. Life (Basel) 2023, 13(5), 1144.
- Ávila-Román, J.; Gómez- Villegas, P.; de Carvalho, C.C.C.R.; Vigara, J.; Motilva, V.; León, R.; Talero, E. Up-Regulation of the Nrf2/HO-1 Antioxidant Pathway in Macrophages by an Extract from a New Halophilic Archaea Isolated in Odiel Saltworks. Antioxidants 2023, 12, 1080.
- Yuan, X.; Li, H.; Lee, J.S.; Lee, D.H. Role of Mitochondrial Dysfunction in UV-Induced Photoaging and Skin Cancers. Exp Dermatol 2025, 34, e70114.
- Garcia-Mouronte, E.; Pérez-González, L.A.; Naharro-Rodriguez, J.; Fernández Guarino, M. Understanding Active Photoprotection: DNA-Repair Enzymes and Antioxidants. Life (Basel) 2024, 4(7), 822.
- Mohan, M.; Taneja, T.K.; Sahdev, S.; Mohareer, K.; Begum, R.; Athar, M.; Sah, N.K.; Hasnain, S.E. Antioxidants prevent UV-induced apoptosis by inhibiting mitochondrial cytochrome c release and caspase activation in Spodoptera frugiperda (Sf9) cells. Cell Biol Int. 2003, 27(6), 483-90.
- Baburina, Y.; Lomovsky, A.; Lomovskaya, Y.; Sotnikov, R.; Sotnikova, L.; Krestinina, O. Mitochondrial Protection by Astaxanthin Reduces Toxicity Caused by H2O2 and Doxorubicin in Human Cardiomyocytes. Cells 2025, 14(22), 1772. [CrossRef]










| Formulation | PL (mg/ml) | Z Average (nm ± SD) |
Pdi ± SD | BR/PL (µg/mg ± SD) |
ζ Potential (mV ± SD) |
FA | GP |
|---|---|---|---|---|---|---|---|
| BD-TA-ARC | 18.3 ± 1.6 | 297 ± 74.2 | 0.57 ± 0.13 | 6.7 ± 0.9 | - 41.6 ± 5 | 0.27 ± 0.04 | -0.29 ± 0.07 |
| BS-TA-ARC | 18.9 ± 3.2 | 406 ± 137 | 0.63 ± 0.13 | 8.2 ± 1.0 | - 38.2 ± 5.4 | 0.29 ± 0.05* | -0.12 ± 0.02** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





