1. Introduction
Docosahexaenoic acid (DHA) is a nutritionally important omega-3 fatty acid that supports visual and neural function, helps regulate inflammatory and oxidative stress responses, and gives rise to bioactive lipid mediators essential for cellular health [
1]. Because human metabolic pathways exhibit limited capacity to convert α-linolenic acid to DHA, adequate intake must be achieved primarily through direct consumption of preformed DHA [
2]. As global interest in DHA-enriched functional foods continues to expand [
3], ensuring the stability of this fatty acid during processing and storage has become an increasingly critical challenge. While marine fish oils have historically supplied much of the dietary DHA, their use is constrained by sustainability and contamination concerns [
4]. Moreover, as plant-based diets become increasingly popular, fish-derived DHA sources are less suitable, making algal oil a sustainable and concentrated alternative that delivers DHA without relying on higher-trophic marine species [
5].
Nevertheless, the high degree of unsaturation in DHA makes it highly susceptible to oxidative degradation, particularly in dispersed systems [
6,
7]. Oxidative degradation of DHA not only diminishes its nutritional value but also accelerates the formation of volatile oxidation products responsible for unpleasant sensory characteristics, thereby limiting its incorporation into emulsified food matrices [
8]. To overcome these barriers, nanoemulsion technology has been recommended as a delivery platform for DHA [
9]. However, the high interfacial area characteristic of nanoemulsions can paradoxically intensify lipid oxidation, especially in the presence of transition metal ions [
10]. This susceptibility highlights the importance of employing antioxidant strategies to effectively protect DHA in nanoemulsion systems.
In food systems, oxidative deterioration of unsaturated fatty acids is commonly mitigated using antioxidants that quench free radicals and agents that limit the catalytic activity of transition metals [
11]. Metal chelators, often classified as secondary antioxidants, limit oxidative reactions by binding pro-oxidant transition metals and stabilizing their redox state, thereby restricting metal-mediated hydroperoxide breakdown and reducing interactions between metals and lipid substrates [
12]. Their protective effects may arise through several mechanisms, including altering the redox behavior of metal ions and restricting their physical access to lipid substrates [
13]. Synthetic chelating additives are commonly used in food formulations, with EDTA being particularly effective due to its strong metal-binding capacity and high efficacy at low concentrations and under acidic conditions [
14]. However, increasing demand for clean-label ingredients has raised concerns regarding the use of synthetic chelators such as EDTA [
15]. Moreover, the effectiveness of metal chelation is highly dependent on the characteristics of the food system itself. In emulsified systems, where the oil-water interface promotes close contact between pro-oxidant metals and lipid hydroperoxides, metal-catalyzed oxidation proceeds more rapidly than in low-moisture foods [
14], indicating the need to carefully tailor chelating strategies to emulsion-based delivery systems such as DHA nanoemulsions.
Marine macroalgae have emerged as promising resources for functional food applications due to their rich and diverse composition of bioactive constituents [
16]. These organisms provide a complex matrix of proteins, sulfated polysaccharides, and phenolic compounds that can contribute to both nutritional value and physicochemical functionality in food systems [
17,
18]. A growing body of research indicates that such components can interact at oil-water interfaces and enhance the oxidative stability of emulsified omega-3 lipids, including DHA, through multiple mechanisms such as antioxidant activity and interfacial stabilization [
19,
20,
21,
22]. Among red macroalgae,
Palmaria palmata has attracted increasing interest as a sustainable source of food-grade bioactives, owing to its wide availability and established history of consumption [
23]. Its use as a food ingredient is permitted under European Union regulations, supporting its suitability for incorporation into edible formulations [
24]. Previous studies from our group have shown that extracts derived from this species using enzymatic [
24], chemical [
25], or combined extraction approaches [
26,
27] contain bioactive compounds with pronounced
in vitro antioxidant capacities. However, antioxidant performance under simplified assay conditions does not necessarily translate to effectiveness in complex food matrices [
28]. Consequently, evaluating the functionality of these seaweed extracts within real food-relevant systems, such as DHA-loaded nanoemulsions, is essential to determine their practical potential for oxidative stabilization.
Considering the susceptibility of DHA-rich nanoemulsions to metal-catalyzed oxidation and the growing interest in natural alternatives to synthetic chelators, this study investigated the metal-chelating and antioxidant potential of seaweed-derived extracts. Extracts were produced using a sequential enzymatic and alkaline extraction strategy with the use of different proteases and were initially screened for their Fe²⁺-chelating capacity
in vitro. Comprehensive characterization was performed to relate chelating performance to compositional attributes, including protein content, degree of hydrolysis, amino acid composition, and total phenolic content. An extract exhibiting superior chelating activity was subsequently selected for incorporation into DHA-enriched nanoemulsions. Because the effectiveness of metal chelators is highly dependent on both the food matrix and the applied concentration [
14], the selected extract was evaluated at three different concentrations and compared with a nanoemulsion without added antioxidant and a formulation containing EDTA as a synthetic reference. Interfacial tension measurements were conducted to assess the surface activity of the extracts at these concentrations, and confocal laser scanning microscopy (CLSM) was employed to visualize the microstructure of the resulting nanoemulsions. The physical stability of the nanoemulsions was assessed through measurements of droplet size, zeta potential, and viscosity, while oxidative stability was monitored during three weeks of storage under iron-accelerated conditions by tracking primary oxidation (peroxide value and tocopherol depletion) and secondary oxidation through measurement of formation of volatile oxidation products. This approach allows a comprehensive evaluation of the potential of seaweed extracts as natural, marine-derived metal chelators for stabilizing DHA nanoemulsions.
2. Materials and Methods
2.1. Macroalgal Biomass
P. palmata was harvested from the Faroe Islands during late autumn to early winter 2023 and obtained from a commercial supplier (Dansk Tang, Nykøbing Sj., Denmark). The biomass was freeze-dried (ScanVac CoolSafe, LaboGene A/S, Allerød, Denmark) to reduce moisture content, then milled to 0.5-1.0 cm particle size using a KN 295 Knifetec™ laboratory mill (Foss A/S, Hillerød, Denmark). The resulting powder was stored at -20 °C in sealed plastic bags protected from light until further use.
2.2. Chemicals, Enzymes, and Oil
Alcalase® (2.4 amino acid units, AU-A/g), Flavourzyme® (500-1000 leucine aminopeptidase units, LAPU/g), and Formea® Prime (140 kilo mannitol units per gram, KMTU/g) were generously provided by Novenesis A/S (formerly Novozymes A/S, Bagsværd, Denmark). DHA-rich microalgal oil derived from Schizochytrium sp. was kindly supplied by Polaris (Quimper, France) and contained and 57.0 ± 0.3 % DHA (C22:6). The oil also comprised α-, β-, γ-, and δ-tocopherols at 133 ± 0.9, 20 ± 0.3, 697 ± 2.9, and 302 ± 6.6 μg g⁻¹, respectively. All solvents employed in analytical procedures were of HPLC grade (Lab-Scan, Dublin, Ireland), and amino acid standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Disodium ethylenediaminetetraacetate (EDTA) was obtained from Sigma-Aldrich (Steinheim, Germany). Additional reagents including ammonium bicarbonate, sodium acetate, imidazole, Tween® 20, sodium hydroxide, sodium azide (NaN3), tocopherol standards (α, β, γ, δ), ammonium thiocyanate, barium dichloride (BaCl2·2H2O), iron chloride (FeCl3·6H2O), 96% ethanol, hydrogen peroxide, and volatile standards were also sourced from Sigma-Aldrich. All other chemicals were purchased from Merck (Darmstadt, Germany). High-purity water used in all experiments was produced at DTU Food using a Milli-Q® Advantage A10 deionization system (Millipore Corporation, Billerica, MA, USA).
2.3. Extraction Method for Macroalgal Biomass
Extracts were obtained from P. palmata biomass using a sequential enzymatic and alkaline extraction procedure, employing different combinations of commercial proteolytic enzymes. In brief, 4 g of freeze-dried biomass was rehydrated in distilled water at a 1:20 w/v ratio and incubated at 50 °C for 1 h. Since the initial pH of the biomass-water mixture was suitable for enzymatic activity (approximately 6-8), no pH adjustment was needed. Enzymatic extraction was carried out with three proteases including Alcalase®, Flavourzyme®, or Formea® Prime applied either individually at 2% of the protein content (dry weight basis) or in binary combinations, with each enzyme added at 1% of the protein content (dry weight basis). The extractions were conducted in a shaking water bath (SW 22, Julabo GmbH, Seelbach, Germany) at 50 °C and 80 rpm for 14 h. Enzyme activity was terminated by heating the mixtures to 95 °C for 20 min. The resulting suspensions were filtered through a 1 mm mesh filter, and the liquid enzymatic extracts were collected in blue-capped bottles and stored at 4 °C. The remaining solids from each enzymatic treatment were subjected to a three-step alkaline extraction using 80 mL of a solution containing 1 g L⁻¹ N-acetyl-L-cysteine (NAC) and 4 g L⁻¹ sodium hydroxide (NaOH). Each extraction was performed on an orbital shaker at 130 rpm at room temperature for 1.5 h, with fresh alkaline solution used for each step. Solid residues were transferred between rounds, and all three alkaline extracts were pooled with the corresponding enzymatic extract. For simplicity, the combined enzymatic/alkaline extracts were coded based on the enzyme(s) used: Ac (Alcalase®), Fz (Flavourzyme®), Fp (Formea® Prime), Ac-Fz (Alcalase® + Flavourzyme®), Ac-Fp (Alcalase® + Formea®), Fz-Fp (Flavourzyme® + Formea®), and NoE (no enzyme). The pH of each pooled extract was adjusted to 8.5-9.0 to ensure complete solubilization of proteins, peptides, and amino acids. All extracts and residual solids were pre-frozen at -20 °C for 2 h, followed by -80 °C for 24 h, before freeze-drying (LaboGene A/S, Allerød, Denmark). The resulting powders were stored in sealed zip-lock bags at -80 °C until further analysis. All extractions were performed in duplicate. Mass balances were calculated by accurately weighing all samples using an analytical balance with 0.01 g readability.
2.4. Characterization of Extracts
2.4.1. Protein Content and Recovery
The total protein in the enzymatic and alkaline extracts was quantified by determining total nitrogen using the Dumas combustion method with a Vario EL Cube analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany). Protein concentrations were calculated by multiplying the measured nitrogen content by a conversion factor of 5.0 [
16]. Protein recovery in the extracts was determined using the following equation:
where
and
denote the mass and protein content of the extract, and
and
correspond to the mass and protein content of the biomass, respectively.
2.4.2. Degree of Hydrolysis (DH)
The extent of protein hydrolysis in the extracts was evaluated using the o-phthaldialdehyde (OPA) assay. The OPA reagent was prepared according to the protocol described in [
24]. Extracts were diluted to achieve protein concentrations ranging from 0.05% to 0.25% and subsequently mixed with the OPA reagent in a microplate format. Absorbance was measured at 340 nm, and sample concentrations were determined using a calibration curve generated with L-serine. Serine equivalents were calculated as follows:
where
and
denote the absorbance of the extract and blank, respectively, and the slope and intercept were obtained from the L-serine standard curve. The degree of hydrolysis was then calculated using:
where
represents the serine equivalents of the extract (mg Ser/mL) and
is the protein content expressed as a percentage. All measurements were performed in duplicate.
2.4.3. Total Phenolic Content (TPC)
The total phenolic content of the extracts was measured using the Folin-Ciocalteu method [
29]. In this assay, extracts were mixed with the Folin-Ciocalteu reagent and allowed to react at room temperature for 5 min, followed by the addition of 6% sodium bicarbonate. The reaction mixtures were incubated for 60 min at ambient temperature, after which absorbance was recorded at 725 nm using a UV-visible spectrophotometer (Shimadzu UV mini 1240, Duisburg, Germany). TPC was expressed as micrograms of gallic acid equivalents (µg GAE) per mL of extract and calculated using the following equation:
where
is the measured absorbance of the sample, and
and
are the slope (0.007) and intercept (0.021) of the gallic acid calibration curve, respectively.
2.4.4. Amino Acid Profile
Approximately 30 mg of each sample was subjected to hydrolysis using 6 M HCl at 110 °C for 18 h. The hydrolyzed samples were filtered through 0.22 µm cellulose acetate syringe filters into 4 mL vials, and 100 µL portions were transferred to fresh vials. The pH of the aliquots was adjusted by sequentially adding 1.5 mL of 0.2 M KOH and 1.6 mL of 100 mM ammonium acetate buffer (pH 3.1, adjusted with formic acid), achieving a final dilution factor of 32. Amino acid profiles were analyzed using liquid chromatography–mass spectrometry (LC-MS) on an Agilent 1260 Infinity II Series system coupled to a Quadrupole 6120 MS with an ESI source (Agilent Technologies, Santa Clara, CA, USA). Separation was carried out on a BioZen 2.6 µm Glycan column (100 × 2.1 mm, Phenomenex, Torrance, CA, USA) at 40 °C, with a flow rate of 0.5 mL/min, injection volume of 1 µL, and a total run time of 16 min. A gradient elution was employed using mobile phase A (10 mM ammonium formate in acetonitrile) and mobile phase B (10 mM ammonium formate in Milli-Q water) as follows: 0-2 min, 0–5% B; 2-7 min, 5-20% B; 7-8 min, 20-80% B; 12.1 min, 0% B; 12.1-16 min, 0% B. Quantification was performed using MassHunter Quantitative Analysis v7.0 (Agilent Technologies) with calibration curves prepared from a mixture of 17 amino acids at five different concentrations. It should be noted that during hydrolysis, glutamine and asparagine were converted into glutamic acid and aspartic acid, respectively, whereas tryptophan and cysteine were degraded and therefore could not be detected.
2.4.5. Fe2+ Chelating Ability
The ability of the extracts to chelate Fe²⁺ ions was assessed using a modified version of the method described by [
30], adapted for microplate measurements. In brief, 100 µL of each extract solution was combined with 110 µL of distilled water and 20 µL of 2 mM FeCl
2. After 3 min, 20 µL of 5 mM ferrozine was added to initiate complex formation, and the mixture was thoroughly homogenized. The samples were then incubated at room temperature for 10 min, followed by absorbance measurement at 562 nm. Distilled water served as the blank, while control samples were prepared without Fe
2+ and ferrozine. All measurements were performed in triplicate, and 0.06 mM EDTA was used as a positive control. The percentage of Fe
2+ chelation was calculated using the following equation:
where
,
and
correspond to the absorbances of the extract, control, and blank, respectively. The IC
50 values were determined from linear regression of the dose-response curves.
2.4.6. Dynamic Interfacial Tension (IFT)
The interfacial properties of the extract with the highest metal chelating activity were evaluated by monitoring IFT at the oil-water interface. Solutions of this extract were prepared in 10 mM sodium acetate-imidazole buffer (pH 7) at three different concentrations corresponding to those used in the emulsion study. Pendant drops were formed on MCT oil and imaged every 2 s over a 30 min period using an automated drop tensiometer (OCA25, DataPhysics Instruments GmbH, Filderstadt, Germany) at 25 °C. IFT values were calculated using the Young-Laplace equation [
31], and the temporal changes were analyzed to assess adsorption kinetics. For comparison, reference measurements were performed with buffer alone and Milli-Q water.
2.5. Preparation of Nanoemulsions
Oil-in-water nanoemulsions were prepared to evaluate the metal-chelating effects of the seaweed extracts. Only the extract exhibiting the highest Fe2+-chelating activity was used, tested at three concentrations based on its IC50: 0.61 mg/mL (half IC50), 1.22 mg/mL (IC50), and 2.44 mg/mL (twice IC50). Aqueous solutions of the extract were prepared in 10 mM sodium acetate-10 mM imidazole buffer (pH 7). EDTA (0.025 mg/mL) served as positive control, and a solution without any antioxidant was used as a negative control. Nanoemulsions (220 g) were formulated with 5 wt% DHA-rich algal oil and 1 wt% Tween® 20. Pre-emulsification was carried out using a high-shear mixer (Ultraturrax, Ystral, Germany) at 16000 rpm for 3 min, during which the oil phase was gradually added. Final homogenization was performed with a microfluidizer (M110L, Microfluidics, USA) equipped with a ceramic interaction chamber (CIXC, F20Y, 75 µm) at 9000 psi for three passes. Sodium azide (0.05 wt%) and 50 μM FeSO4 were added as preservatives and iron-induced oxidation accelerator, respectively. The nanoemulsions were labeled as follows: E1 (negative control, no antioxidant), E2 (EDTA, positive control), E3 (extract at 0.61 mg/mL, 0.5× IC50), E4 (extract at 1.22 mg/mL, IC50), and E5 (extract at 2.44 mg/mL, 2× IC50). Nanoemulsions were stored at room temperature (19-20 °C) in the dark for up to 8 days. Samples for lipid oxidation analysis were collected on days 0, 2, 4, 6, and 8, transferred into amber bottles, flushed with nitrogen, and stored at -40 °C until further use.
2.6. Characterization of Nanoemulsions
2.6.1. Droplet Size Distribution
The droplet size distribution of the nanoemulsions was assessed on days 1 and 8 using a laser diffraction system (Mastersizer 2000, Malvern Instruments Ltd., Worcestershire, UK). Prior to analysis, samples were diluted with circulating water at 3,000 rpm until an obscuration of 12-15% was achieved. Refractive indices of 1.469 and 1.330 were applied for the oil and aqueous phases, respectively. Particle size was reported as the surface-area mean diameter (D3,2) and volume mean diameter (D4,3). All measurements were performed in duplicate.
2.6.2. Zeta Potential
On the second day, the zeta potential of the nanoemulsion droplets was determined using a Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). For each measurement, 0.032 g of the emulsion was precisely weighed and diluted with 40 g of distilled water, followed by thorough mixing with a vortex mixer. The prepared samples were then placed into disposable folded capillary cells (DTS-1070, Malvern Instruments Ltd., UK). Each analysis was carried out in duplicate at 25 °C, covering a zeta potential range from -100 to +50 mV, with 100 runs per measurement.
2.6.3. Apparent Viscosity
The apparent viscosity of the samples was assessed using a stress-controlled rheometer (Stresstech, Reologica Instruments AB, Lund, Sweden) equipped with a CC25 bob-and-cup configuration. Measurements were conducted at 25 °C over a shear stress range of 0.0025-2 Pa. Viscosity values were recorded at a shear rate of 100 s-1 and reported in centipoise (cP).
2.6.4. CLSM
CLSM was utilized to examine the microstructure of nanoemulsions. Samples were handled carefully to preserve droplet morphology and prevent aggregation or disruption. Oil and protein droplets were stained simultaneously using Nile Red and Nile Blue, respectively. Both dyes were added to the samples at the same time and incubated for 5 minutes at room temperature prior to imaging to allow adequate staining. Nile Red was used for lipid staining and selectively labeled neutral lipid-rich oil droplets, while Nile Blue was used to label protein-containing structures. No washing steps were performed after dye addition to avoid perturbation of droplet structure and spatial organization. Fluorescence imaging was performed using a Zeiss LSM 900 Airyscan confocal laser scanning microscope, operated with ZEN Blue Edition software. Images were acquired using a 60× oil-immersion objective. Nile Red fluorescence was excited using a 488 nm laser line, and emission was collected at 509 nm, producing green fluorescence corresponding to lipid droplets. Nile Blue fluorescence was excited at 627 nm, and emission was collected at 623 nm, producing red fluorescence associated with protein-rich droplets. For all experiments, the detector gain was set to 650 V and kept constant across all samples. Post-acquisition processing was limited to linear adjustments of brightness and contrast applied uniformly across all images. No non-linear processing or artificial signal enhancement was performed.
2.6.5. Peroxide Value (PV)
Lipids were extracted from the emulsions in duplicate using a modified Bligh and Dyer method [
32], which minimized the amount of chloroform/methanol (1:1, w/w) required. The peroxide value of the lipid extracts was determined in duplicate using the ferrothiocyanate colorimetric assay, with absorbance measured at 500 nm. While the original method described by Shantha and Decker [
33] uses cumene hydroperoxide for calibration and expresses PV as mmol peroxide/kg lipid, FeCl
3 was used in the present study to generate the standard curve, and PV was expressed as meq peroxide/kg lipid.
2.6.6. Tocopherol Depletion
Tocopherol concentrations in the lipid extracts were analyzed using normal-phase high-performance liquid chromatography (HPLC) following AOCS Official Method Ce 8-89 [
34]. The analyses were performed on an Agilent 1100 series HPLC system under isocratic conditions with a mobile phase of heptane and 2-propanol (100:0.4, v/v) at a flow rate of 1.0 mL/min and an injection volume of 20 µL. Separation was achieved using a Waters Spherisorb silica column (3 µm, 4.6 mm × 150 mm) coupled with a guard column (5 µm, 4.6 mm × 10 mm). Fluorescence detection was set at 290 nm for excitation and 330 nm for emission. Approximately 2 g of each extract was dissolved in 1 mL of heptane prior to injection. Tocopherols were quantified using external standards with single-point calibration. All measurements were performed in duplicate, and results were expressed as micrograms of individual tocopherols per gram of oil.
2.6.7. Volatile Secondary Oxidation Compounds
Volatile secondary oxidation compounds were quantified using an automated dynamic headspace sampler (Gerstel GmbH & Co. KG, Germany) coupled with gas chromatography-mass spectrometry (GC–MS) (Agilent 6890 N/5973, USA) according to the method described by Thomsen et al. [
35] Approximately 1 g of sample was added with 4-methyl-1-pentanol (30 µg/g) as an internal standard, mixed thoroughly, and incubated at 60 °C for 4 minutes with intermittent agitation. Headspace volatiles were purged with nitrogen (50 mL/min for 20 minutes), captured on Tenax GR 300 sorbent tubes, dried, and thermally desorbed into the GC system. Separation was achieved on a DB-1701 capillary column (30 m × 0.25 mm ID, 0.5 µm film thickness) using a temperature program from 35 °C to 240 °C in stepwise increments. Mass spectrometric detection was performed in electron ionization mode (70 eV) scanning m/z 30–250. Compound identification relied on the Wiley 138K spectral library, and quantification was based on 6-point calibration curves (1-250 μg/mL) prepared from authentic external standards under identical conditions. The compounds quantified included 2-ethylfuran, 4-methyl-1-pentanol, 1-penten-3-one, 1-penten-3-ol, hexanal, and (
E,E)-2,4-heptadienal.. Each emulsion was analyzed in triplicate.
2.7. Statistical Analysis
Statistical analyses were performed using one-way Analysis of Variance (ANOVA), and differences among means were evaluated with Bonferroni’s post hoc test. Paired t-tests were applied to compare droplet size between Day 1 and Day 8 for each emulsion. All computations were carried out in OriginPro 2023 (OriginLab Corporation, Northampton, MA, USA). A significance level of p < 0.05 was adopted for all tests.
3. Results and Discussion
3.1. Characteristics of Macroalgal Extracts
3.1.1. Protein Content and Recovery and DH
Table 1 presents the protein content and recovery of extracts and solid residues, as well as the DH for all seven treatments. Alcalase
® (Ac) achieved the highest protein content in extracts (10.11 ± 0.15%), significantly surpassing all other treatments (p < 0.05), confirming its superior ability to solubilize seaweed proteins into the water-soluble fraction [
24]. Consistently, the protein content of solid residues after Alcalase
® treatment was among the lowest (14.17 ± 0.79%), indicating more extensive protein removal from the seaweed matrix. Protein recovery in extracts followed a similar trend: Alcalase
® alone yielded ~95%, significantly higher than all other treatments (p < 0.05). Dual-enzyme combinations (Ac-Fz and Ac-Fp) resulted in recovery of approximately 81-82%, which was lower than that obtained with Alcalase
® alone (94.99%), but still higher than single Flavourzyme
® or Formea
® Prime treatments (circa 61–70%). The lower recovery in the dual-enzyme treatments is likely due to the reduced concentration of each enzyme (1% each) compared to 2% when Alcalase
® was used alone, suggesting that 2% Alcalase
® is required to achieve optimal protein recovery. In contrast, NoE (no enzyme) resulted in the lowest recovery (53.65%), highlighting the critical role of enzymatic hydrolysis in protein extraction.
Regarding DH, the highest value was observed for Ac-Fz (45.76 ± 8.36%), followed by Fz-Fp (34.50 ± 4.07%) and Alcalase
® alone (30.90 ± 3.22%). These results indicate that combining Alcalase
® and Flavourzyme
® significantly enhances hydrolysis compared to single-enzyme treatments (p < 0.05). However, protein recovery with this combination did not exceed that obtained with Alcalase
® alone. This is likely because, although Flavourzyme
® efficiently cleaves peptides into shorter chains and increases DH, shorter peptides and free amino acids may be lost during extraction, limiting total protein recovery. Similar trends were observed in our previous studies, where extensive hydrolysis was achieved with Flavourzyme
®-containing treatments despite lower protein content in the extracts [
24]. Formea
® Prime alone exhibited the lowest DH (22.91 ± 4.31%), consistent with its classification as a mild protease (manufacturer’s data). The strong hydrolytic performance of Alcalase
® and Flavourzyme
® can be attributed to their broad substrate specificity and multiple catalytic sites, which facilitate extensive cleavage of peptide bonds [
36,
37]. Overall, Alcalase
® demonstrated superior protein solubilization and recovery, while dual-enzyme systems, particularly Ac-Fz, achieved the highest degree of hydrolysis.
3.1.2. TPC
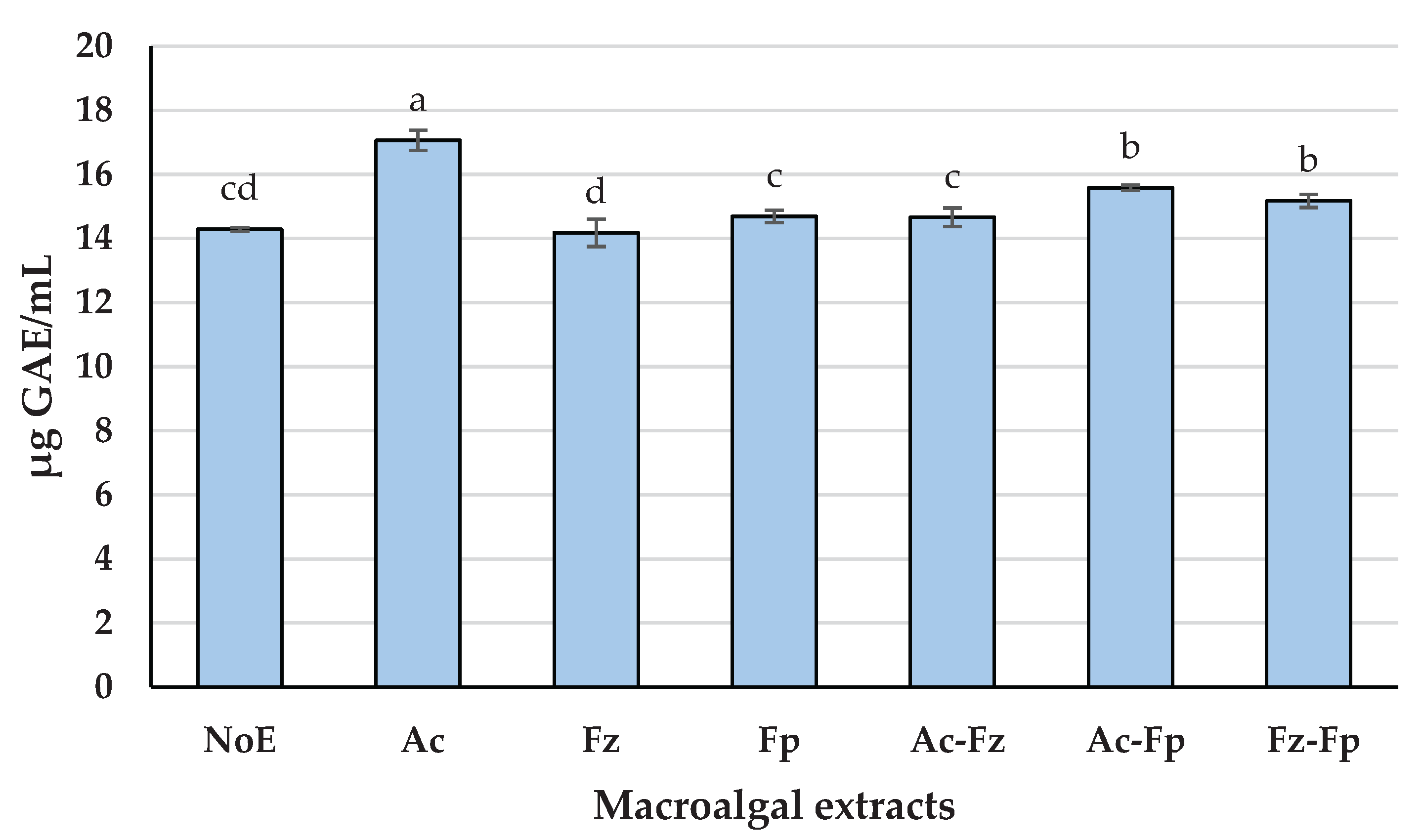
The total phenolic content of macroalgal extracts obtained through sequential enzymatic and alkaline extraction is shown in
Figure 1. Significant differences were observed among treatments (p < 0.05). Ac exhibited the highest TPC (≈17 µg GAE/mL), clearly outperforming all other treatments. This indicates that Alcalase
® is particularly effective in promoting the release of phenolic compounds from the seaweed matrix when combined with alkaline extraction. Two combined-enzyme treatments, Ac-Fp and Fz-Fp, resulted in intermediate TPC values (≈15–15.6 µg GAE/mL), significantly lower than Ac but higher than the remaining treatments (p < 0.05). These results suggest that adding Formea
® Prime to Alcalase
® or Flavourzyme
® provides some benefit, although not as pronounced as using Alcalase
® alone. Fp and Ac-Fz yielded TPC values around 14.7 µg GAE/mL, which were significantly lower than Ac and the two combination treatments mentioned above (p < 0.05). The control (NoE) and Fz showed the lowest TPC (≈14.2 µg GAE/mL), indicating minimal improvement compared to untreated samples. The superior performance of Alcalase
® may be attributed to its broad endoprotease activity, which facilitates extensive protein and cell wall disruption, thereby exposing phenolic compounds bound to structural polysaccharides. This observation is consistent with previous reports highlighting the strong role of Alcalase
® in enhancing TPC in macroalgal extracts [
38]. The subsequent alkaline extraction likely enhances solubilization of these compounds into the aqueous phase, as previously reported [
26]. Since most macroalgal polyphenols are associated with cell wall components, their release largely depends on the extent of cell wall degradation [
39], which appears to be more effectively achieved when Alcalase
® is used as the enzymatic treatment.
3.1.3. Amino acid Composition
Table 2 summarizes the amino acid composition of extracts obtained from seven enzymatic treatments: NoE (no enzyme), Ac (Alcalase®), Fz (Flavourzyme®), Fp (Formea® Prime), Ac-Fz (Alcalase® + Flavourzyme®), Ac-Fp (Alcalase® + Formea® Prime), and Fz-Fp (Flavourzyme® + Formea® Prime), each followed by alkaline extraction. Except for phenylalanine, histidine, and cystine, most amino acids were significantly higher in Ac compared to all other treatments (p < 0.05), confirming the strong hydrolytic efficiency of Alcalase
® in releasing amino acids into the water-soluble fraction. This trend was particularly evident for leucine, isoleucine, valine, and alanine, which are key contributors to nutritional quality and flavor development. The total amino acid (TAA) content was highest in Fz-Fp (111.77 mg/g), followed closely by Ac-Fz and Ac (102.33 and 100.19 mg/g, respectively), indicating that combining proteases can enhance overall protein solubilization. However, Alcalase
® alone still provided a competitive yield compared to dual-enzyme systems. Notably, the order of TAA content does not fully match the protein contents measured by the Dumas method, which showed higher values for Alcalase
® extracts. This discrepancy could be due to several factors, for instance loss of tryptophan or other labile amino acids during hydrolysis, or the release of non-protein nitrogen-containing compounds that are detected by the Dumas method but not counted in TAA analysis.Essential amino acids (EAA) followed a similar pattern, with Ac showing the highest EAA/TAA ratio (0.33), suggesting a superior nutritional profile compared to other treatments. This aligns with previous findings that Alcalase
® preferentially liberates essential residues [
24].
Methionine levels were significantly higher in Ac (2.06 mg/g) than in other treatments (p < 0.05), which is nutritionally relevant since methionine and histidine are limiting essential amino acids in macroalgal proteins [
16]. The elevated methionine content in Alcalase
®-treated extracts reinforces its potential for improving amino acid balance in seaweed-derived ingredients. A striking observation was the cystine distribution. While Alcalase
®-treated extracts, either alone or in combination with other two proteases, contained maximum ~16 mg/g cystine, treatments involving Flavourzyme
® or Formea
® Prime exhibited extremely high cystine levels (68-84 mg/g). This discrepancy is unexpected given the uniform biomass source. One plausible explanation is that Alcalase
®’s broad specificity and aggressive cleavage pattern released more overall protein but retained disulfide-rich regions in insoluble fragments, whereas Flavourzyme
® and Formea
® Prime generated smaller peptides enriched in cystine that remained soluble. The exact mechanism behind this phenomenon warrants further investigation, as it may involve enzyme-specific cleavage preferences and peptide solubility dynamics. Overall, Alcalase
® demonstrated superior efficiency in liberating essential amino acids and improving the EAA/TAA ratio, while dual-enzyme treatments maximized total amino acid recovery. These findings highlight the importance of enzyme selection and combination strategies for tailoring the nutritional and functional properties of macroalgal extracts.
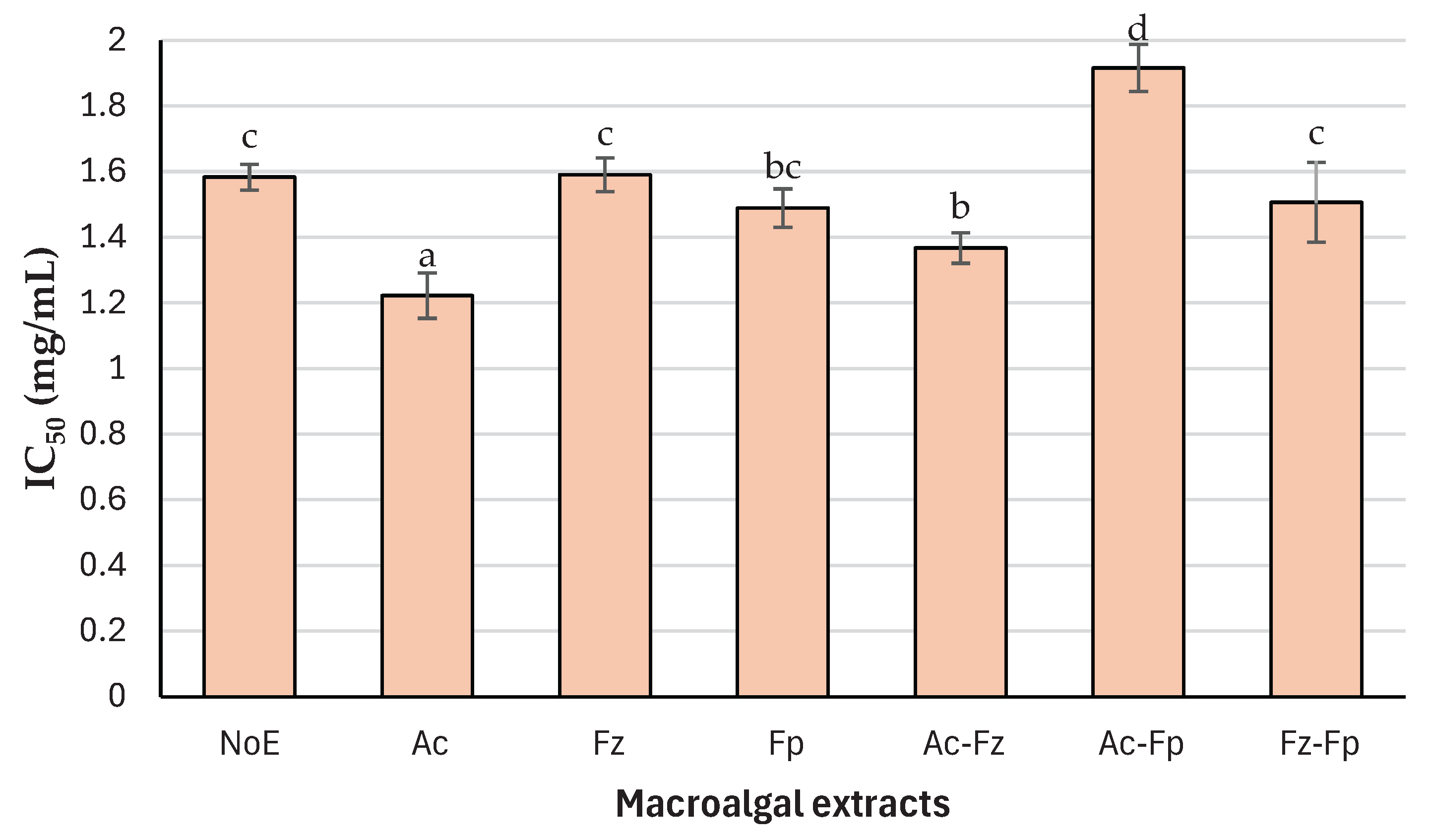
3.1.4. Metal Chelating Ability
The Fe
2+ chelating capacity of the macroalgal extracts, expressed as IC
50 values, revealed clear differences among treatments (
Figure 2). Ac demonstrated the strongest chelating ability, with an IC
50 of 1.22 mg/mL, significantly lower than all other treatments (p < 0.05). This indicates that Alcalase
® hydrolysis, combined with alkaline extraction, produces components highly effective in binding ferrous ions. At the opposite end, Ac-Fp exhibited the weakest chelating activity (IC₅₀ ≈ 1.92 mg/mL), suggesting that adding Formea
® Prime to Alcalase
® may not enhance, and could even hinder, the formation of chelating-active molecules under the tested conditions. Intermediate IC
50 values were observed for Fz, NoE, and Fz-Fp (≈1.50-1.59 mg/mL), while Fp and Ac-Fz achieved slightly better performance (≈1.36–1.49 mg/mL), though still significantly less effective than Ac (p < 0.05). The superior chelating activity of Alcalase
® may be linked to its ability to generate peptides enriched in acidic amino acids such as glutamic acid (
Table 2), which are known to form stable complexes with Fe²⁺ ions [
40]. Additionally, Alcalase
® treatment likely promotes the release of phenolic compounds (
Figure 1), which can contribute to metal ion chelation when combined with alkaline extraction [
41]. The synergy between peptides and phenolics may explain the enhanced activity observed for Ac, whereas treatments lacking this combination showed weaker performance. Considering the significantly better metal chelating properties of Ac (p < 0.05), this extract was selected for testing in DHA oil-in-water nanoemulsion.
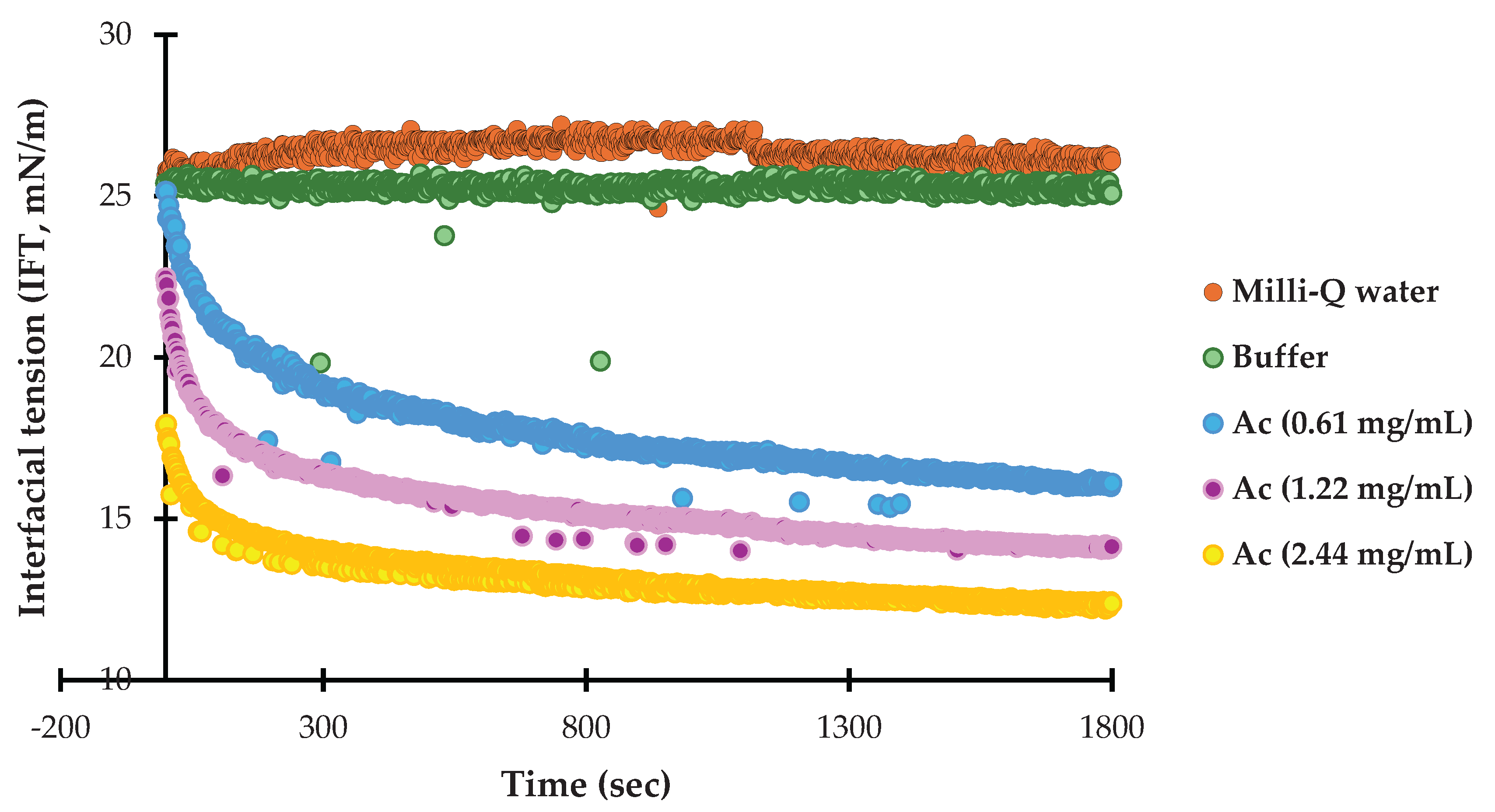
3.1.5. IFT
The dynamic interfacial tension at the oil-water interface was monitored for Milli-Q water, buffer solution, and Alcalase
® extract (Ac) at three concentrations corresponding to those tested in nanoemulsion formulations (
Section 2.5) (
Figure 3). As expected, the IFT of Milli-Q water and buffer remained nearly constant over time (≈26 and 25 mN/m, respectively), confirming the absence of surface-active components in these systems. In contrast, the presence of Ac caused a pronounced reduction in IFT, and the extent of this reduction was strongly concentration dependent. At the lowest concentration (0.61 mg/mL), the IFT decreased from ~25 mN/m to ~19 mN/m within the first few minutes, whereas at 1.22 mg/mL and 2.44 mg/mL, the final IFT values reached ~14 and ~12 mN/m, respectively. This rapid decline followed by stabilization indicates favorable adsorption kinetics, suggesting that peptides and other surface-active molecules in the extract quickly migrate and rearrange at the oil-water interface [
42]. This dose-dependent effect highlights the role of molecular composition and availability of amphiphilic peptides in interfacial activity. Alcalase
® hydrolysis likely generates peptides with hydrophobic and hydrophilic domains, enabling them to act as natural emulsifiers. Additionally, co-extracted polysaccharides and phenolic compounds may contribute to interfacial behavior, as previously reported for seaweed-derived extracts [
43]. The ability of Ac extract to significantly lower IFT suggests its potential to improve emulsion stability; however, the final IFT values remain higher than those typically achieved with synthetic surfactants (lower than 5 mN/m) [
44], indicating that additional stabilizing strategies may still be required for commercial applications.
3.2. Physical Stability of Nanoemulsions
The particle charge (Zeta potential) values of the nanoemulsions are reported in
Table 3. On Day 1, zeta potential ranged from -17.25 ± 0.49 mV in E4 to -18.95 ± 0.21 mV in E2, with no significant differences among the samples (p > 0.05). The relatively small variation in zeta potential suggests that the addition of Ac at different concentrations did not appreciably alter the surface charge of the droplets. This is likely because the extract components either did not adsorb to the oil-water interface or carried insufficient charge to influence droplet potential. The main contributor to the overall surface charge of the emulsifier-coated oil droplets could be Tween
® 20. Although Tween
® 20 is a nonionic surfactant, previous studies have shown that oil droplets coated by it can exhibit a measurable negative charge, attributed to the presence of surface-active anionic impurities in the surfactant and/or oil phase (such as free fatty acids or phospholipids) [
45]. Even small amounts of these substances can impart an appreciable surface charge. Overall, the moderate negative charge on the droplets should contribute to good colloidal stability by generating electrostatic repulsion between oil droplets [
46], complemented by steric repulsion from the hydrophilic head groups of Tween
® 20 [
47].
The surface-weighted (D
3,2) and volume-weighted (D
4,3) mean diameters of the oil droplets were measured at Day 1 and Day 8 (
Table 3). At Day 1, D
3,2 values ranged from 77.65 nm in E5 to 80.25 nm in E3, with significant differences among samples (p < 0.05). Similarly, D
4,3 values ranged from 199.0 nm in E4 to 215.0 nm in E2 and E3. After 8 days of storage, droplet sizes remained relatively stable, with only minor changes observed. These results indicate that the nanoemulsions exhibited good physical stability during storage. The slight differences in droplet size among samples are unlikely to be due to interfacial competition, as the Ac extract components were probably not sufficiently amphiphilic to displace Tween
® 20 from the oil-water interface. Instead, the variations may reflect minor differences in formulation or measurement variability. Similar trends were observed for D
4,3 values, which also showed minimal changes over time. These findings are consistent with the excellent emulsifying properties of Tween
® 20, which rapidly adsorbs to oil-water interfaces during homogenization and forms a protective coating around droplets [
48], which inhibits flocculation and coalescence by generating strong steric and electrostatic repulsive forces.
Consistent with the behavior typically observed in dilute emulsion systems, all formulations demonstrated non-Newtonian flow characteristics, exhibiting shear-thinning as their apparent viscosity decreased with increasing shear rate (data not shown) [
49]. The apparent viscosity of the nanoemulsions at 100 s
-1 ranged from 1.34 to 1.47 cP on Day 1 and 1.16 to 1.41 cP on Day 8, with no significant differences among samples (p > 0.05). All emulsions exhibited low viscosity typical of dilute systems, and the slight decrease observed during storage is unlikely to be caused by droplet aggregation, given the stable size profiles. Overall, the combination of moderate negative zeta potential, small and stable droplet sizes, and low viscosity indicates that the DHA nanoemulsions were physically stable throughout storage. Incorporation of Ac extract at different concentrations did not compromise these properties, supporting its suitability for subsequent oxidative stability evaluations.
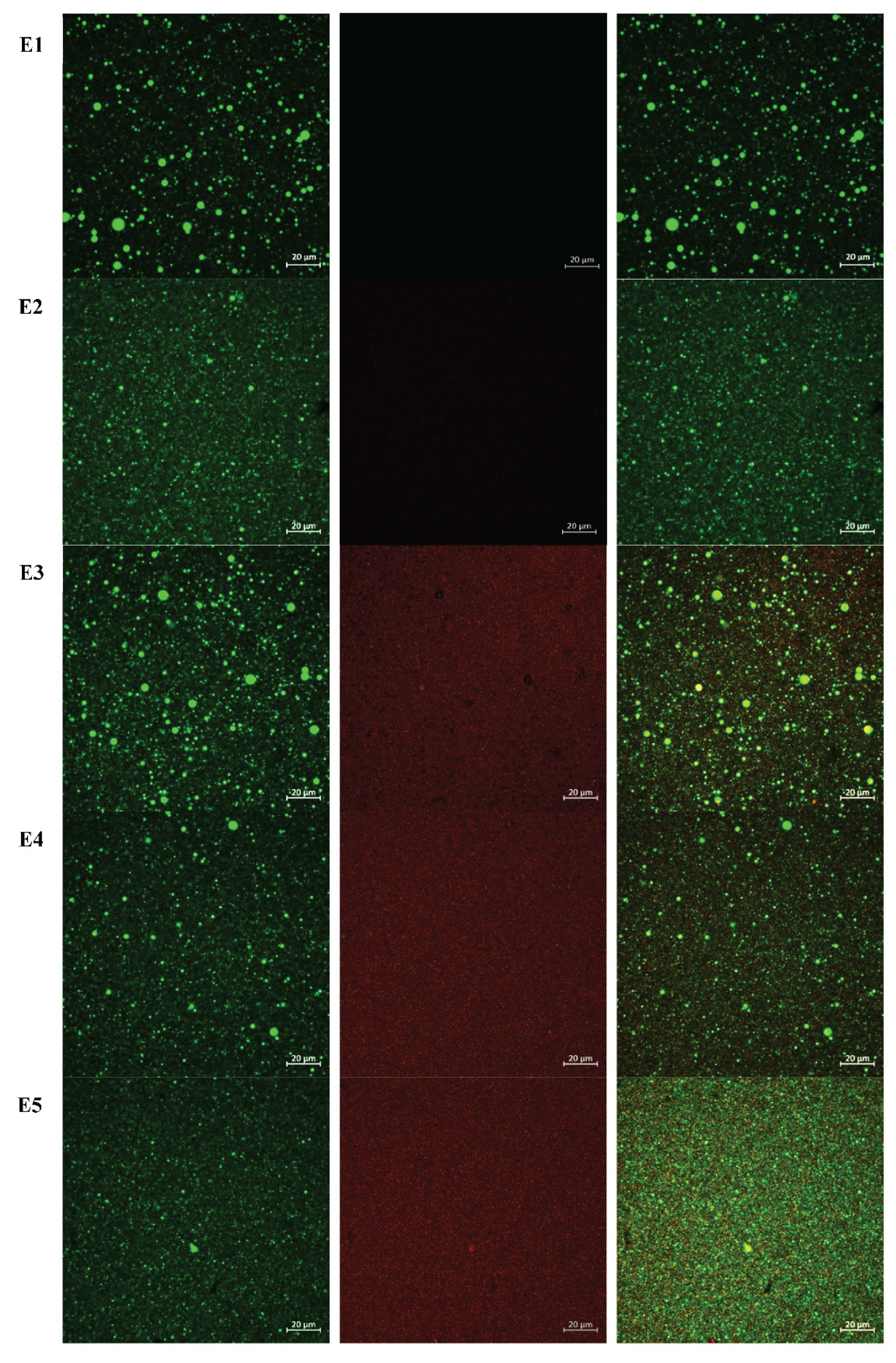
3.3. Microstructure of Nanoemulsions
The microstructure of DHA oil-in-water nanoemulsions was examined using CLSM, as shown in
Figure 4. The CLSM images reveal clear differences in interfacial coverage and droplet organization among the samples, which correspond to the presence and concentration of Ac extract. All nanoemulsions exhibit uniform droplet distribution and no evidence of flocculation or coalescence, supporting the droplet size and stability data presented in Table 2. Nanoemulsions containing Ac extract (E3-E5) displayed increasing red fluorescence intensity at the droplet interface with rising extract concentration. At the lowest Ac level (E3), partial coverage was observed, with red fluorescence appearing discontinuous around some droplets, suggesting limited adsorption of extract components at the oil-water interface. At intermediate concentration (E4), interfacial labeling was more pronounced and continuous, indicating improved coverage. At the highest concentration (E5), dense and uniform red fluorescence surrounded the droplets, confirming substantial adsorption of extract-derived proteins or other amphiphilic molecules. This progressive increase in interfacial labeling aligns with the dose-dependent incorporation of Ac extract. Overall, CLSM analysis confirms that Ac extract adsorbs at the interface in a concentration-dependent manner. This interfacial association may play a role in antioxidant performance without compromising physical stability, supporting the suitability of Ac for further oxidative stability evaluation.
3.4. Oxidative stability of Nanoemulsion
3.4.1. PV
The oxidative stability of DHA-rich oil-in-water nanoemulsions was assessed by monitoring PV during 8 days of storage under FeSO
4-induced accelerated oxidation conditions (
Table 4). The use of FeSO
4 promoted metal-catalyzed initiation reactions, thereby providing a stringent system for discriminating antioxidant efficacy in a highly unsaturated (approximately 57% DHA) nanoemulsion matrix. At Day 0, the negative control (E1) exhibited a significantly higher PV than all other formulations (p < 0.05) (PV of the oil was 0.18 meq/kg), indicating extensive hydroperoxide formation during emulsification when no antioxidant protection was present. This pronounced susceptibility can be attributed to the combined effects of high interfacial area and iron-catalyzed radical generation, which rapidly consumed endogenous tocopherols and initiated uncontrolled lipid oxidation. Similar behavior has been reported in omega-3-rich nanoemulsions subjected to prooxidant conditions, where iron ions markedly accelerate primary oxidation processes [
50]. Throughout storage, E1 showed a sharp and continuous increase in PV, reaching values exceeding 350 meq/kg oil by Day 8, which were significantly higher than those of all other treatments at each corresponding time point (p < 0.05). It should be noted that PV values above ~60 meq/kg approach the upper limit of reliable measurement for the ferric thiocyanate assay, and values above this threshold should be interpreted with caution. This severe oxidation was accompanied by rapid depletion of α- and γ-tocopherols (
Table 4), consistent with the sequential consumption of tocopherols under intense oxidative stress [
51]. In contrast, the EDTA-containing nanoemulsion (E2) exhibited significantly lower PVs than E1 and Ac-treated samples across the entire storage period (p < 0.05). The strong protective effect of EDTA under FeSO
4-accelerated conditions highlights the dominant role of iron-catalyzed reactions in this system. By chelating ferrous ions, EDTA effectively suppressed hydroperoxide formation and slowed tocopherol depletion, confirming that metal chelation is a highly effective strategy for stabilizing DHA-rich nanoemulsions in the presence of transition metals. Nanoemulsions containing Ac extract (E3-E5) showed significantly improved oxidative stability compared to the negative control (p < 0.05 at all time points beyond Day 0), demonstrating that the extract conferred measurable protection even under iron-induced oxidative stress. However, PVs in these samples remained significantly higher than in E2 (p < 0.05), indicating that Ac extract could not fully counteract FeSO
4-driven oxidation. This suggests that the antioxidant mechanisms of Ac extract were less effective against metal-catalyzed initiation than those of EDTA. Among the extract-treated formulations, a concentration-dependent trend emerged during later storage stages. Although PV increased in all Ac-containing nanoemulsions, E4 and particularly E5 tended to exhibit lower PVs than E3 at Day 8, although these differences were not significant (p > 0.05).
3.4.2. Tocopherol Consumption
Changes in endogenous tocopherol content (α-, γ-, and δ-tocopherols) were monitored in the DHA-rich oil-in-water nanoemulsions during storage under FeSO₄-accelerated oxidation conditions (
Table 4). The initial tocopherol profile reflected that of the Schizochytrium sp. oil used in this study, which was naturally rich in γ- and δ-tocopherols, with lower levels of α-tocopherol. β-Tocopherol was not detected in any of the nanoemulsions at any storage time. This observation is consistent with the very low β-tocopherol content of the parent microalgal oil and has been reported previously for algal omega-3 oils used in emulsified systems [
20]. Therefore, subsequent discussion focuses on α-, γ-, and δ-tocopherols. At Day 0, measurable amounts of α- and γ-tocopherols were detected in all antioxidant-containing nanoemulsions, whereas these tocopherols were either absent or present at markedly lower levels in the negative control (E1), indicating rapid consumption during emulsification when no antioxidant protection was provided. Except for δ-tocopherol, tocopherol contents in E1 remained negligible throughout storage, consistent with severe oxidative stress promoted by ferrous ions and the absence of added antioxidants. In contrast, the EDTA-containing nanoemulsion (E2) preserved tocopherols to a significantly greater extent than all other treatments during storage (p < 0.05). Only minor decreases in γ- and δ-tocopherol contents were observed over time, while α-tocopherol levels remained stable. This behavior highlights the effectiveness of EDTA in limiting iron-catalyzed initiation reactions, thereby reducing the demand on endogenous tocopherols as chain-breaking antioxidants. Similar protective effects of metal chelators on tocopherol preservation have been reported previously in omega-3-rich emulsified systems [
52].
Nanoemulsions containing Ac extract (E3–E5) exhibited intermediate tocopherol stability between E1 and E2. Tocopherol depletion was significantly slower than in the negative control (p < 0.05) but more pronounced than in the EDTA-stabilized nanoemulsion. Across storage, α-tocopherol was the first to decline, followed by γ- and then δ-tocopherol, consistent with the established sequential depletion order of tocopherols during lipid oxidation [
51]. Interestingly, this pattern differed in the EDTA-containing emulsion, where only δ-tocopherol declined while α- and γ-tocopherol remained largely unchanged. This behavior may be attributed to the strong metal-chelating activity of EDTA, which effectively suppressed iron-catalyzed oxidation and preserved the more reactive α- and γ-tocopherols. δ-Tocopherol, being less reactive, may have continued to participate in slower radical-trapping reactions or undergo degradation through alternative pathways, highlighting the influence of metal chelation on tocopherol oxidation dynamics. Overall, the observed pattern confirms that endogenous tocopherols actively participated in scavenging radicals generated under iron-induced oxidative stress. Increasing the concentration of Ac extract influenced tocopherol dynamics. Higher extract levels generally resulted in faster depletion of α- and γ-tocopherols compared with E3, particularly at later storage times, although differences among extract-treated samples were not always statistically significant (p > 0.05). This accelerated consumption may suggest that tocopherols were more actively engaged in redox processes in the presence of higher extract concentrations, potentially due to interactions between extract constituents and endogenous antioxidants. Despite the enhanced tocopherol depletion observed at higher extract concentrations, δ-tocopherol remained relatively stable across all Ac-treated nanoemulsions, reflecting its lower reactivity and its role as a later-stage antioxidant. The persistence of δ-tocopherol may contribute to residual oxidative protection once more reactive tocopherols are exhausted. Overall, these results demonstrate that Ac extract partially protected endogenous tocopherols compared with the negative control but was less effective than EDTA. The findings further indicate that extract concentration influences tocopherol consumption patterns, highlighting the complex and concentration-dependent interactions between natural extracts and endogenous antioxidants in omega-3 emulsified systems.
3.4.3. Volatile Secondary Oxidation Products
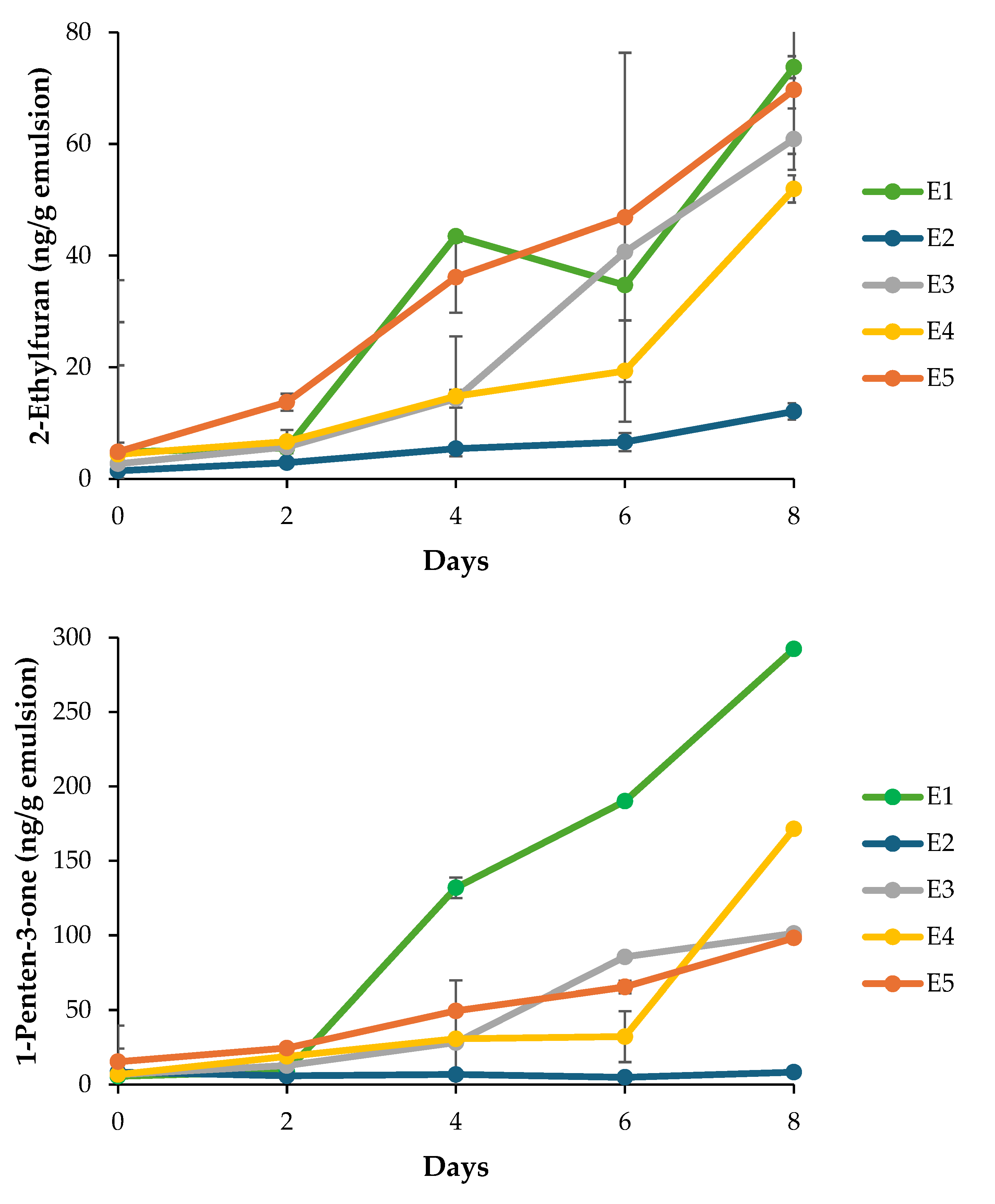
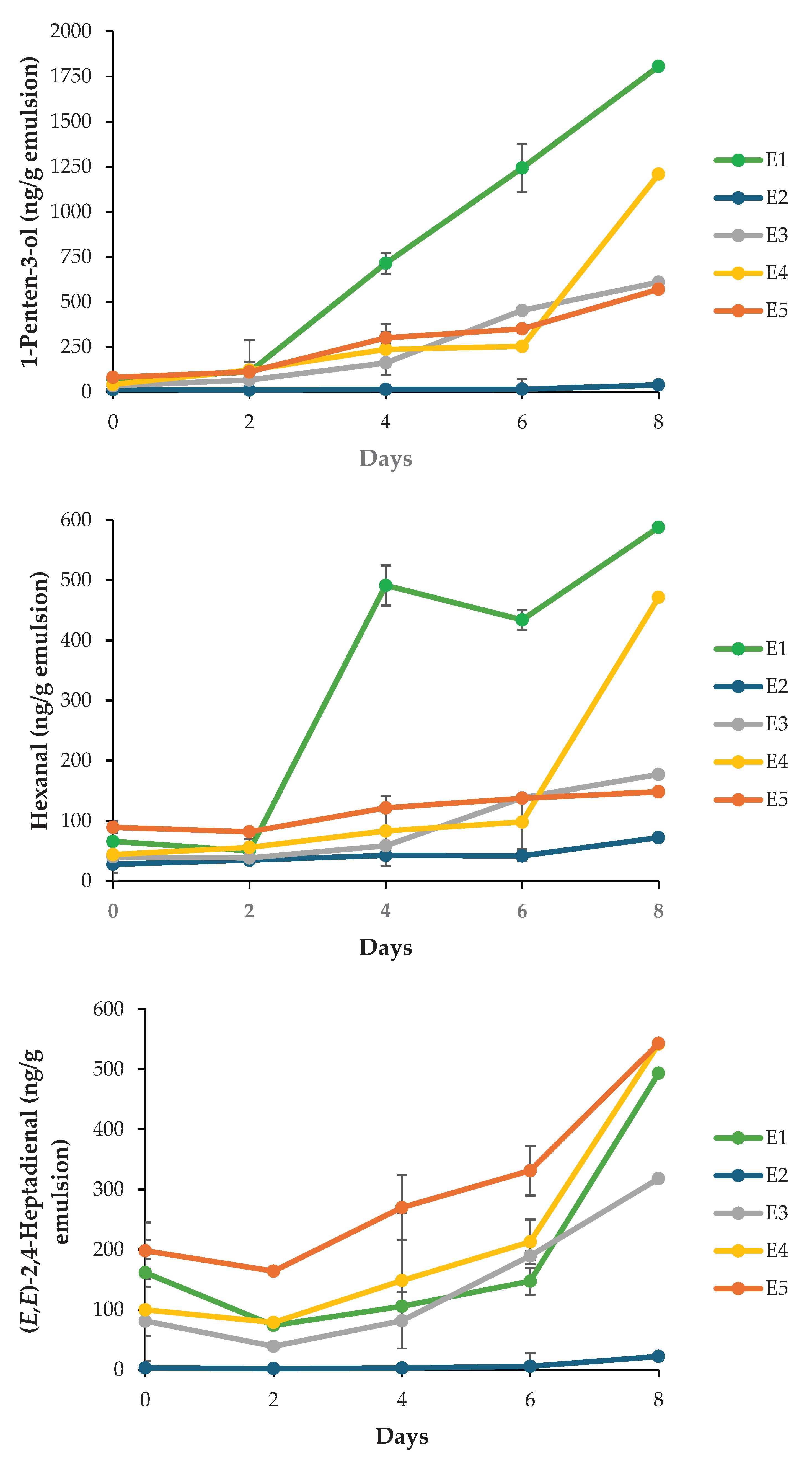
The formation of volatile secondary oxidation products provides a sensitive indicator of lipid degradation beyond hydroperoxide accumulation. In the present study, five key volatiles, i.e. 2-ethylfuran, 1-penten-3-ol, 1-penten-3-one, hexanal, and (
E,E)-2,4-heptadienal, were monitored during FeSO
4-accelerated storage (
Figure 5). These compounds are well-established markers of omega-3 PUFA oxidation and arise predominantly from the cleavage of hydroperoxides formed on DHA and EPA [
20,
51]. Across all monitored volatiles, the negative control (E1) exhibited the most pronounced increases, confirming extensive secondary oxidation in the absence of antioxidant protection. For instance, 1-penten-3-ol concentrations reached approximately 1750 ng/g by Day 8 in E1, whereas emulsions containing EDTA (E2) maintained levels below 250 ng/g throughout storage. This marked contrast indicates the dominant role of iron-catalyzed radical initiation and highlights the effectiveness of metal chelation in suppressing both primary and secondary oxidation pathways. Nanoemulsions containing Ac extract (E3-E5) displayed intermediate behavior. Volatile concentrations were significantly lower than those in E1 (p < 0.05) but consistently higher than in E2, indicating partial protection under strong prooxidant conditions. A concentration-dependent trend was observed for several volatiles, for instance, E5 exhibited lower levels of 1-penten-3-one, 1-penten-3-ol and hexanal compared to E3 and E4 towards the end of the storage period, suggesting that higher extract concentrations confer improved oxidative stability. However, differences among extract-treated samples were not always statistically significant (p > 0.05), reflecting the complex and multifactorial nature of antioxidant performance in emulsified systems. This was particularly the case for 2-ethylfuran which exhibited large standard deviations. The reduction in volatile formation with increasing extract concentration, except for (
E,E)-2,4-heptadienal, correlates with CLSM observations of interfacial structure (
Figure 4). CLSM images revealed progressively enhanced adsorption of Ac extract components at the oil-water interface, ranging from partial coverage in E3 to dense and uniform interfacial labeling in E5. Such interfacial accumulation likely forms a physical barrier that limits prooxidant access while localizing antioxidant molecules at the primary sites of radical initiation [
53]. This interfacial stabilization provides a plausible explanation for the improved suppression of volatile formation observed in E5 relative to E3 and E4, despite identical oxidative stress conditions. Nevertheless, the persistence of measurable volatile formation in Ac-treated emulsions compared to EDTA-stabilized systems indicates that interfacial antioxidant mechanisms alone are insufficient to fully counteract metal-driven oxidation. This limitation is further supported by tocopherol depletion patterns, in which higher extract concentrations were associated with accelerated α- and γ-tocopherol consumption (
Table 4), possibly reflecting synergistic or competing redox interactions. Collectively, these findings suggest that while interfacial adsorption enhances antioxidant efficacy, further optimization of extract concentration and formulation strategies may be required to achieve protection levels comparable to conventional antioxidants.
4. Conclusions
This study demonstrated that extracts from Palmaria palmata, obtained through sequential enzymatic–alkaline extraction, exhibit strong Fe²⁺-chelating activity and can improve oxidative stability of DHA-rich nanoemulsions without compromising physical properties. The selected extract reduced hydroperoxide formation, delayed tocopherol depletion, and lowered volatile oxidation markers in a concentration-dependent manner, while confocal microscopy confirmed interfacial adsorption of bioactive components. Although these natural extracts offer promising clean-label functionality, EDTA remained more effective under severe prooxidant conditions. Future work should focus on optimizing extract composition and delivery strategies to enhance antioxidant performance and fully replace synthetic chelators in functional food applications.
Author Contributions
Conceptualization, S.G and C.J.; methodology, S.G and C.J.; validation, S.G and C.J.; formal analysis, S.G.; investigation, S.G., B.S.Y., M.H., S.H.H., A,S., S.K. and C.J.; resources, S.G. and C.J.; data curation, S.G., B.S.Y., M.H., S.H.H., A,S., S.K. and C.J.; writing—original draft preparation, S.G. and M.H.; writing—review and editing, S.G. and C.J.; visualization, S.G. and S.H.H.; supervision, C.J.; project administration, S.G. and C.J.; funding acquisition, S.G. and C.J. All authors have read and agreed to the published version of the manuscript.
Funding
The project is funded by the European Union under the Horizon Europe grant. The project is part of DOEP, a Marie Skłodowska-Curie Actions (MSCA) European Postdoctoral Fellowship Project under grant No. 101106112.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data acquired in this study can be obtained on request.
Acknowledgments
The project is funded by the European Union under the Horizon Europe grant. The project is part of DOEP, a Marie Skłodowska-Curie Actions (MSCA) European Postdoctoral Fellowship Project under grant No. 101106112. The authors also wish to acknowledge Jessie Klang Lorentzen and Jannie Felskov Agersten for technical assistance in the laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zeppieri, M.; Gagliano, C.; D’Esposito, F.; Musa, M.; Gattazzo, I.; Zanella, M.S.; Rossi, F.B.; Galan, A.; Babighian, S. Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA): A Targeted Antioxidant Strategy to Counter Oxidative Stress in Retinopathy. Antioxidants 2025, Vol. 14, Page 6 2024, 14, 6. [CrossRef]
- Zhu, P.; Fan, L.; Yan, X.; Li, J. Advances of α-Linolenic Acid: Sources, Extraction, Biological Activity and Its Carrier. Trends Food Sci Technol 2024, 152, 104676. [CrossRef]
- Zhou, Y.; Tian, S.; Qian, L.; Jiang, S.; Tang, Y.; Han, T. DHA-Enriched Phosphatidylserine Ameliorates Non-Alcoholic Fatty Liver Disease and Intestinal Dysbacteriosis in Mice Induced by a High-Fat Diet. Food Funct 2021, 12, 4021–4033. [CrossRef]
- Jiang, W.; Zhang, Y.; Julian McClements, D.; Liu, F.; Liu, X. Impact of Pea Protein-Inulin Conjugates Prepared via the Maillard Reaction Using a Combination of Ultrasound and PH-Shift Treatments on Physical and Oxidative Stability of Algae Oil Emulsions. Food Research International 2022, 156, 111161. [CrossRef]
- Czerniel, J.; Gostyńska-Stawna, A.; Urbaniak, N.; Sommerfeld-Klatta, K.; Stawny, M. Harnessing Algae Oil as a Sustainable DHA Source for Parenteral Nutrition in Vegan Patients. Scientific Reports 2025 15:1 2025, 15, 18548-. [CrossRef]
- Van Nguyen, L.; Shahidi, F. Fatty Acid Distribution and Oxidative Stability of DHA/EPA-Enriched Structured Lipids From Virgin Coconut Oil. JAOCS, Journal of the American Oil Chemists’ Society 2025, 102, 1439–1452. doi:10.1002/AOCS.70002;WEBSITE:WEBSITE:AOCS;JOURNAL:JOURNAL:15589331D;WGROUP:STRING:PUBLICATION. [CrossRef]
- Jala, R.C.R.; Zhang, H.; Yang, M.; Guo, R.; Li, S.; Xu, X.; Yang, D.; Xu, X. Encapsulation of DHA Oils for Better Bioavailability: A Review from the Practical Aspect. JAOCS, Journal of the American Oil Chemists’ Society 2025. [CrossRef]
- Hajfathalian, M.; Jorjani, S.; Ghelichi, S. Characterization of Fish Sausage Manufactured with Combination of Sunflower Oil and Fish Oil Stabilized with Fish Roe Protein Hydrolysates. J Food Sci Technol 2020, 57, 1439–1448. [CrossRef]
- Mubeena, S.A.; Preetha, R. Ultrasound-Assisted Microalgal Docosahexaenoic Acid (DHA) Nanoemulsion Preparation Using Casein, Chitosan, and Pectin as Emulsifiers for Enhanced Oxidative Stability and Shelf Life for Food Fortification. Sustainable Food Technology 2025. [CrossRef]
- Zeinalzadegan, M.; Nejadmansouri, M.; Golmakani, M.T.; Mesbahi, G.R.; McClements, D.J.; Hosseini, S.M.H. Higher Oxidative Stability of Alpha-Linolenic Acid Than Linoleic Acid in Nanoemulsions: A Comparison Between Bulk Flaxseed Oil and Its O/W Nanoemulsions. Food Biophysics 2021 16:2 2021, 16, 203–213. [CrossRef]
- Aslam, A.; Schroën, K. Lipid Oxidation in Food Emulsions: A Review Dedicated to the Role of the Interfacial Area. Curr Opin Food Sci 2023, 51, 101009. [CrossRef]
- Guiotto, E.N.; Julio, L.M.; Ixtaina, V.Y. Natural Antioxidants in the Preservation of Omega-3-Rich Oils: Applications in Bulk, Emulsified, and Microencapsulated Chia, Flaxseed, and Sacha Inchi Oils. Phytochemistry Reviews 2025 24:6 2025, 24, 6139–6167. [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, Vol. 10, Page 132 2022, 10, 132. [CrossRef]
- Bayram, I.; Decker, E.A. Underlying Mechanisms of Synergistic Antioxidant Interactions during Lipid Oxidation. Trends Food Sci Technol 2023, 133, 219–230. [CrossRef]
- Hennebelle, M.; Villeneuve, P.; Durand, E.; Lecomte, J.; van Duynhoven, J.; Meynier, A.; Yesiltas, B.; Jacobsen, C.; Berton-Carabin, C. Lipid Oxidation in Emulsions: New Insights from the Past Two Decades. Prog Lipid Res 2024, 94, 101275. [CrossRef]
- Ghelichi, S.; Jacobsen, C. Seaweed Proteins: Properties, Extraction, Challenges, and Prospects. J Food Sci 2025, 90, e70418. [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Marine Drugs 2022, Vol. 20, Page 141 2022, 20, 141. [CrossRef]
- Ghelichi, S.; Hajfathalian, M.; Helalat, S.H.; Svensson, B.; Jacobsen, C. Purification, Identification, and In Silico Analysis of Anti-Obesity and Antidiabetic Peptides from the Red Seaweed Palmaria Palmata. Mar Drugs 2025, 23, 392. [CrossRef]
- Ghelichi, S.; Shokrollahi Yancheshmeh, B.; Hong Lin, M.K.T.; Jacobsen, C. Fabrication and Characterization of High Internal Phase Pickering Emulsions (HIPPEs) of ω-3 Algal Oil Stabilized with Seaweed-Derived Particles. Food Chem 2026, 498, 147073. [CrossRef]
- Ghelichi, S.; McClements, D.J.; Sørensen, A.D.M.; Jacobsen, C. Omega-3 Microalgal Oil-in-Water Nanoemulsions Stabilized by Red Seaweed Extracts: Physical and Oxidative Stability. Algal Res 2026, 93, 104461. [CrossRef]
- Gunathilake, T.; Akanbi, T.O.; Wang, B.; Barrow, C.J. Antioxidant Benefits of Ecklonia Radiata Algae Phenolic Extracts in Food Grade Omega-3 Delivery Systems. Future Foods 2024, 10, 100406. [CrossRef]
- Hermund, D.B.; Anagnostara, I.; Hou, X.; Mikkelsen, M.D.; Rhein-Knudsen, N.; Bjerre, A.B.; Meyer, A.S.; Jacobsen, C. Physical and Oxidative Stability of N-3 Delivery Emulsions Added Seaweed-Based Polysaccharide Extracts from Nordic Brown Algae Saccharina Latissima. J Am Oil Chem Soc 2022, 99, 239–251. [CrossRef]
- Stévant, P.; Schmedes, P.S.; Le Gall, L.; Wegeberg, S.; Dumay, J.; Rebours, C. Concise Review of the Red Macroalga Dulse, Palmaria Palmata (L.) Weber & Mohr. Journal of Applied Phycology 2023 35:2 2023, 35, 523–550. [CrossRef]
- Ghelichi, S.; Sørensen, A.-D.M.; Hajfathalian, M.; Holdt, S.L.; Jacobsen, C. Screening Enzymatic Extracts of Palmaria Palmata Based on Composition and Bioactivity. Algal Res 2025, 89, 104048. [CrossRef]
- Ghelichi, S.; Sørensen, A.-D.M.; Náthia-Neves, G.; Jacobsen, C. PH-Dependent Extraction of Antioxidant Peptides from Red Seaweed Palmaria Palmata: A Sequential Approach. Mar Drugs 2024, 22, 413. [CrossRef]
- Ghelichi, S.; Sørensen, A.-D.M.; Hajfathalian, M.; Jacobsen, C. Effect of Post-Extraction Ultrasonication on Compositional Features and Antioxidant Activities of Enzymatic/Alkaline Extracts of Palmaria Palmata. Mar Drugs 2024, 22. [CrossRef]
- Ghelichi, S.; Hajfathalian, M.; Falcione, S.; Jacobsen, C. Antioxidant and Anti-Obesity Properties of Acidic and Alkaline Seaweed Extracts Adjusted to Different PH Levels. Mar Drugs 2025, 23, 35. [CrossRef]
- Le, T.H. Tracking Lipid Oxidation and Antioxidant Activity in Foods Using Calorimetry: Methods, Mechanisms, and Applications. European Journal of Lipid Science and Technology 2025, e70093. [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am J Enol Vitic 1965, 16, 144–158. [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch Biochem Biophys 1994, 315, 161–169. [CrossRef]
- Yesiltas, B.; Sørensen, A.-D.M.; García-Moreno, P.J.; Anankanbil, S.; Guo, Z.; Jacobsen, C. Modified Phosphatidylcholine with Different Alkyl Chain Length and Covalently Attached Caffeic Acid Affects the Physical and Oxidative Stability of Omega-3 Delivery 70% Oil-in-Water Emulsions. Food Chem 2019, 289, 490–499. [CrossRef]
- Bligh, E.G.; Dyer, W.J. A RAPID METHOD OF TOTAL LIPID EXTRACTION AND PURIFICATION. Can J Biochem Physiol 1959, 37, 911–917. [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, Sensitive, Iron-Based Spectrophotometric Methods for Determination of Peroxide Values of Food Lipids. J AOAC Int 1994, 77, 421–424. [CrossRef]
- AOCS Official Method Ce 8-89 Determination of Tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC 1998.
- Thomsen, B.R.; Yesiltas, B.; Sørensen, A.D.M.; Hermund, D.B.; Glastrup, J.; Jacobsen, C. Comparison of Three Methods for Extraction of Volatile Lipid Oxidation Products from Food Matrices for GC–MS Analysis. JAOCS, Journal of the American Oil Chemists’ Society 2016, 93, 929–942. [CrossRef]
- Di Filippo, G.; Melchior, S.; Plazzotta, S.; Calligaris, S.; Innocente, N. Effect of Enzymatic Hydrolysis with Alcalase or Protamex on Technological and Antioxidant Properties of Whey Protein Hydrolysates. Food Research International 2024, 188, 114499. [CrossRef]
- Wen, R.; Yan, X.; Kong, F.; Chen, F.; Zang, Y.; Sun, C.; Yu, Q. Structural and Functional Modifications of Porphyra Yezoensis Proteins via Ultrasound-Assisted Enzymolysis: Insights into Taste Enhancement and Mechanisms. Food Chem 2025, 492, 145537. [CrossRef]
- Nova, P.; Cunha, S.A.; Costa-Pinto, A.R.; Gomes, A.M. Chemical and Antioxidant Properties of Solvent and Enzyme-Assisted Extracts of Fucus Vesiculosus and Porphyra Dioica. Marine Drugs 2024, Vol. 22, Page 319 2024, 22, 319. [CrossRef]
- Lee, Z.J.; Xie, C.; Ng, K.; Suleria, H.A.R. Unraveling the Bioactive Interplay: Seaweed Polysaccharide, Polyphenol and Their Gut Modulation Effect. Crit Rev Food Sci Nutr 2025, 65, 382–405. [CrossRef]
- Li, J.; Lin, S.; Chen, S.; Li, Z.; Hu, X. Collagen-Derived Fe2+-Chelating Peptide: Key Amino Acids for Fe2+ Chelating and Mechanisms for Enhancing Cellular Fe2+ Bioavailability. Food Chem 2025, 493, 145847. [CrossRef]
- Gunathilake, T.; Akanbi, T.O.; Suleria, H.A.R.; Nalder, T.D.; Francis, D.S.; Barrow, C.J. Seaweed Phenolics as Natural Antioxidants, Aquafeed Additives, Veterinary Treatments and Cross-Linkers for Microencapsulation. Marine Drugs 2022, Vol. 20, Page 445 2022, 20, 445. [CrossRef]
- Yesiltas, B.; Gregersen, S.; Lægsgaard, L.; Brinch, M.L.; Olsen, T.H.; Marcatili, P.; Overgaard, M.T.; Hansen, E.B.; Jacobsen, C.; García-Moreno, P.J. Emulsifier Peptides Derived from Seaweed, Methanotrophic Bacteria, and Potato Proteins Identified by Quantitative Proteomics and Bioinformatics. Food Chem 2021, 362, 130217. [CrossRef]
- Melanie, H.; Taarji, N.; Zhao, Y.; Khalid, N.; Neves, M.A.; Kobayashi, I.; Tuwo, A.; Nakajima, M. Formulation and Characterisation of O/W Emulsions Stabilised with Modified Seaweed Polysaccharides. Int J Food Sci Technol 2019, 55, 211–221. [CrossRef]
- Najimi, S.; Nowrouzi, I.; Khaksar Manshad, A.; Mohammadi, A.H. Experimental Study of the Performances of Commercial Surfactants in Reducing Interfacial Tension and Wettability Alteration in the Process of Chemical Water Injection into Carbonate Reservoirs. Journal of Petroleum Exploration and Production Technology 2019 10:4 2019, 10, 1551–1563. [CrossRef]
- Cho, H.T.; Salvia-Trujillo, L.; Kim, J.; Park, Y.; Xiao, H.; McClements, D.J. Droplet Size and Composition of Nutraceutical Nanoemulsions Influences Bioavailability of Long Chain Fatty Acids and Coenzyme Q10. Food Chem 2014, 156, 117–122. [CrossRef]
- Abbas, A.; Zhang, C.; Hussain, S.; Li, Y.; Gao, R.; Li, J.; Liu, X.; Zhang, M.; Xu, S. A Robust Switchable Oil-In-Water Emulsion Stabilized by Electrostatic Repulsions between Surfactant and Similarly Charged Carbon Dots. Small 2023, 19, 2206621. [CrossRef]
- Yusuf, H.H.; Roddick, F.; Pramanik, B.K. Sodium Alginate – Tween 20 Dispersant for Sustainable in-Sewer Control of Fats, Oils, and Grease (FOG) Accumulation. Water Res 2026, 290, 125124. [CrossRef]
- Varona, E.; García-Moreno, P.J.; Gregersen Echers, S.; Olsen, T.H.; Marcatili, P.; Guardiola, F.; Overgaard, M.T.; Hansen, E.B.; Jacobsen, C.; Yesiltas, B. Antioxidant Peptides from Alternative Sources Reduce Lipid Oxidation in 5% Fish Oil-in-Water Emulsions (PH 4) and Fish Oil-Enriched Mayonnaise. Food Chem 2023, 426, 136498. [CrossRef]
- Ghelichi, S.; Sørensen, A.D.M.; García-Moreno, P.J.; Hajfathalian, M.; Jacobsen, C. Physical and Oxidative Stability of Fish Oil-in-Water Emulsions Fortified with Enzymatic Hydrolysates from Common Carp (Cyprinus Carpio) Roe. Food Chem 2017, 237, 1048–1057. [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Mikkelsen, R.K.; Echers, S.G.; Hansen, D.K.; Greve-Poulsen, M.; Hyldig, G.; Hansen, E.B.; Jacobsen, C. Physical and Oxidative Stability of Emulsions Stabilized with Fractionated Potato Protein Hydrolysates Obtained from Starch Production Side Stream. Antioxidants 2023, 12, 1622. [CrossRef]
- Irankunda, R.; Bjørlie, M.; Yesiltas, B.; Muhr, L.; Canabady-Rochelle, L.; Jacobsen, C. Evaluation of Primary and Secondary Oxidation Products in Fish Oil-in-Water Emulsions: Effect of Metal-Complexing Peptides and Protein Hydrolysates. Food Chem 2024, 439, 138042. [CrossRef]
- Bjørlie, M.; Hartmann, J.C.; Rasmussen, L.H.; Yesiltas, B.; Sørensen, A.D.M.; Gregersen Echers, S.; Jacobsen, C. Screening for Metal-Chelating Activity in Potato Protein Hydrolysates Using Surface Plasmon Resonance and Peptidomics. Antioxidants 2024, 13, 346. [CrossRef]
- Salvia-Trujillo, L.; McClements, D.J.; Martín-Belloso, O. Nanoemulsion Design for the Delivery of Omega-3 Fatty Acids: Formation, Oxidative Stability, and Digestibility. Omega-3 Delivery Systems: Production, Physical Characterization and Oxidative Stability 2021, 295–319. [CrossRef]
Figure 1.
Total phenolic compounds (TPC) of macroalgal extracts obtained through sequential enzymatic and alkaline procedure. Data were expressed as mean ± standard deviation (n = 2, representing independent experimental replicates).Different superscript letters indicate significant differences among treatments (p<0.05). NoE (no enzyme), Ac (Alcalase®), Fz (Flavourzyme®), Fp (Formea® Prime), Ac-Fz (Alcalase® + Flavourzyme®), Ac-Fp (Alcalase® + Formea® Prime), and Fz-Fp (Flavourzyme® + Formea® Prime), each followed by alkaline extraction.
Figure 1.
Total phenolic compounds (TPC) of macroalgal extracts obtained through sequential enzymatic and alkaline procedure. Data were expressed as mean ± standard deviation (n = 2, representing independent experimental replicates).Different superscript letters indicate significant differences among treatments (p<0.05). NoE (no enzyme), Ac (Alcalase®), Fz (Flavourzyme®), Fp (Formea® Prime), Ac-Fz (Alcalase® + Flavourzyme®), Ac-Fp (Alcalase® + Formea® Prime), and Fz-Fp (Flavourzyme® + Formea® Prime), each followed by alkaline extraction.
Figure 2.
In vitro metal ion chelating activities of macroalgal extracts obtained through sequential enzymatic and alkaline procedure. Data were expressed as mean ± standard deviation (n = 6, representing measurements from two independent extracts, each analyzed in triplicate). Different superscript letters indicate significant differences among treatments (p<0.05). NoE (no enzyme), Ac (Alcalase®), Fz (Flavourzyme®), Fp (Formea® Prime), Ac-Fz (Alcalase® + Flavourzyme®), Ac-Fp (Alcalase® + Formea® Prime), and Fz-Fp (Flavourzyme® + Formea® Prime), each followed by alkaline extraction.
Figure 2.
In vitro metal ion chelating activities of macroalgal extracts obtained through sequential enzymatic and alkaline procedure. Data were expressed as mean ± standard deviation (n = 6, representing measurements from two independent extracts, each analyzed in triplicate). Different superscript letters indicate significant differences among treatments (p<0.05). NoE (no enzyme), Ac (Alcalase®), Fz (Flavourzyme®), Fp (Formea® Prime), Ac-Fz (Alcalase® + Flavourzyme®), Ac-Fp (Alcalase® + Formea® Prime), and Fz-Fp (Flavourzyme® + Formea® Prime), each followed by alkaline extraction.
Figure 3.
Dynamic interfacial tension (mN/m) between MCT oil and Milli-Q water, sodium acetate-imidazole buffer, and Alcalase®/alkaline macroalgal extract at three different concentrations.
Figure 3.
Dynamic interfacial tension (mN/m) between MCT oil and Milli-Q water, sodium acetate-imidazole buffer, and Alcalase®/alkaline macroalgal extract at three different concentrations.
Figure 4.
CLSM of DHA oil-in-water nanoemulsions. Oil was stained with Nile Red and Ac was labeled with Nile Blue. Oil droplets were stained with Nile Red (green) and Ac extract with Nile Blue (red). Panels show oil only (left), extract only (middle; absent in E1 and E2), and combined staining (right). E1 (negative control, no antioxidant), E2 (EDTA, positive control), E3 (Ac at 0.61 mg/mL, 0.5× IC50), E4 (Ac at 1.22 mg/mL, IC50), and E5 (Ac at 2.44 mg/mL, 2× IC50).
Figure 4.
CLSM of DHA oil-in-water nanoemulsions. Oil was stained with Nile Red and Ac was labeled with Nile Blue. Oil droplets were stained with Nile Red (green) and Ac extract with Nile Blue (red). Panels show oil only (left), extract only (middle; absent in E1 and E2), and combined staining (right). E1 (negative control, no antioxidant), E2 (EDTA, positive control), E3 (Ac at 0.61 mg/mL, 0.5× IC50), E4 (Ac at 1.22 mg/mL, IC50), and E5 (Ac at 2.44 mg/mL, 2× IC50).
Figure 5.
Secondary oxidation volatile compounds of DHA oil-in-water nanoemulsions (n = 3) in an 8-day storage: E1 (negative control, no antioxidant), E2 (EDTA, positive control), E3 (Ac at 0.61 mg/mL, 0.5× IC50), E4 (Ac at 1.22 mg/mL, IC50), and E5 (Ac at 2.44 mg/mL, 2× IC50).
Figure 5.
Secondary oxidation volatile compounds of DHA oil-in-water nanoemulsions (n = 3) in an 8-day storage: E1 (negative control, no antioxidant), E2 (EDTA, positive control), E3 (Ac at 0.61 mg/mL, 0.5× IC50), E4 (Ac at 1.22 mg/mL, IC50), and E5 (Ac at 2.44 mg/mL, 2× IC50).
Table 1.
Protein content (% dry matter), protein recovery (%), and degree of hydrolysis (DH, %), of macroalgal extracts obtained through sequential enzymatic and alkaline procedure.
Table 1.
Protein content (% dry matter), protein recovery (%), and degree of hydrolysis (DH, %), of macroalgal extracts obtained through sequential enzymatic and alkaline procedure.
| Treatment |
Protein in extracts (%) |
Protein in solid residues (%) |
Protein recovery in extracts (%) |
Protein recovery in solid residues (%) |
DH (%) |
| NoE |
6.41 ± 0.16d
|
20.59 ± 1.24ab
|
53.65 ± 0.87e
|
45.97 ± 1.37a
|
24.66 ± 4.84c
|
| Ac |
10.11 ± 0.15a
|
14.17 ± 0.79c
|
94.99 ± 1.40a
|
4.97 ± 1.15e
|
30.90 ± 3.22bc
|
| Fz |
6.70 ± 0.29d
|
21.45 ± 0.91a
|
61.13 ± 1.78d
|
36.61 ± 1.48b
|
28.81 ± 4.45bc
|
| Fp |
7.89 ± 0.29c
|
19.32 ± 1.21ab
|
69.88 ± 3.00c
|
27.20 ± 2.16c
|
22.91 ± 4.31c
|
| Ac-Fz |
9.05 ± 0.06b
|
17.70 ± 0.44b
|
82.13 ± 2.13b
|
15.64 ± 2.18d
|
45.76 ± 8.36a
|
| Ac-Fp |
9.14 ± 0.14b
|
17.79 ± 1.72b
|
81.41 ± 0.93b
|
17.20 ± 2.70d
|
29.06 ± 4.44bc
|
| Fz-Fp |
7.71 ± 0.04c
|
20.82 ± 1.11ab
|
68.81 ± 0.36c
|
30.92 ± 0.38c
|
34.50 ± 4.07b
|
Table 2.
Amino acid composition (mg/g dry matter) of macroalgal extracts obtained through sequential enzymatic and alkaline procedure.
Table 2.
Amino acid composition (mg/g dry matter) of macroalgal extracts obtained through sequential enzymatic and alkaline procedure.
| |
NoE |
Ac |
Fz |
Fp |
Ac-Fz |
Ac-Fp |
Fz-Fp |
| Phenylalanine |
0.74 ± 0.40a
|
2.40 ± 1.57a
|
1.07 ± 0.58a
|
0.74 ± 0.26a
|
1.38 ± 0.25a
|
1.55 ± 0.29a
|
0.86 ± 0.61a
|
| Leucine |
1.63 ± 0.02d
|
6.80 ± 0.15a
|
1.26 ± 0.02d
|
0.66 ± 0.31e
|
3.34 ± 0.04c
|
4.96 ± 0.11b
|
1.37 ± 0.16d
|
| Isoleucine |
0.97 ± 0.08d
|
3.96 ± 0.16a
|
0.81 ± 0.07d
|
0.70 ± 0.05d
|
1.89 ± 0.17c
|
3.12 ± 0.45b
|
0.99 ± 0.41d
|
| Methionine |
0.57 ± 0.09bc
|
2.06 ± 0.05a
|
0.46 ± 0.04c
|
0.46 ± 0.06c
|
0.93 ± 0.10b
|
1.62 ± 0.04a
|
0.68 ± 0.35bc
|
| Tyrosine |
0.80 ± 0.15cd
|
3.06 ± 0.11a
|
0.68 ± 0.17cd
|
0.52 ± 0.19d
|
1.27 ± 0.20bc
|
1.82 ± 0.18b
|
0.85 ± 0.39cd
|
| Proline |
3.05 ± 0.04c
|
5.48 ± 0.08a
|
0.79 ± 0.02d
|
0.61 ± 0.01d
|
3.86 ± 0.05b
|
4.42 ± 0.14b
|
1.09 ± 0.48d
|
| Valine |
1.36 ± 0.09d
|
5.78 ± 0.20a
|
1.06 ± 0.04d
|
0.89 ± 0.04d
|
3.00 ± 0.09c
|
4.36 ± 0.17b
|
1.59 ± 0.73d
|
| Alanine |
3.00 ± 0.25c
|
8.13 ± 0.22a
|
1.58 ± 0.28d
|
1.11 ± 0.07d
|
5.27 ± 0.03b
|
6.06 ± 0.30b
|
2.02 ± 0.83cd
|
| Threonine |
0.81 ± 0.07cd
|
3.75 ± 0.28a
|
1.39 ± 1.03bcd
|
0.58 ± 0.05d
|
2.07 ± 0.28bc
|
2.74 ± 0.04ab
|
1.09 ± 0.48cd
|
| Glycine |
2.85 ± 0.25cd
|
5.76 ± 0.50a
|
1.97 ± 0.24cd
|
1.41 ± 0.24d
|
3.48 ± 1.12bc
|
4.96 ± 0.75ab
|
1.48 ± 0.61d
|
| Serine |
2.07 ± 0.73bc
|
5.40 ± 0.85a
|
1.72 ± 0.37bc
|
1.30 ± 0.83c
|
3.62 ± 0.42ab
|
4.68 ± 0.21a
|
2.27 ± 0.91bc
|
| Arginine |
2.25 ± 0.19b
|
6.51 ± 0.09a
|
2.19 ± 0.03b
|
1.96 ± 0.05b
|
2.97 ± 0.11b
|
6.23 ± 0.24a
|
3.02 ± 1.19b
|
| Histidine |
0.78 ± 0.30a
|
1.23 ± 0.15a
|
0.76 ± 0.30a
|
0.69 ± 0.47a
|
0.60 ± 0.25a
|
1.54 ± 0.23a
|
0.63 ± 0.39a
|
| Lysine |
1.34 ± 0.08b
|
4.00 ± 0.27a
|
1.35 ± 0.21b
|
1.14 ± 0.39b
|
1.99 ± 0.41b
|
4.37 ± 0.51a
|
2.06 ± 1.24b
|
| Glutamic acid |
7.45 ± 0.04d
|
13.94 ± 0.03a
|
2.43 ± 0.21e
|
2.28 ± 0.21e
|
9.80 ± 0.30c
|
12.05 ±0.58b
|
2.98 ± 1.04e
|
| Cystine |
5.98 ± 0.64f
|
7.35 ± 0.99ef
|
78.24 ±2.38b
|
68.92 ±1.88c
|
11.31±1.03de
|
15.47 ±1.22d
|
83.73 ± 1.09a
|
| Aspartic acid |
7.55 ± 0.83bc
|
14.59 ± 1.13a
|
4.58 ± 0.66c
|
4.09 ± 0.60c
|
9.66 ± 1.38b
|
13.73 ±1.33a
|
5.05 ± 2.12c
|
| TAA |
43.20 ±1.59d
|
100.19±2.83ab
|
102.33±1.95ab
|
88.07±1.89b
|
66.42 ±2.68c
|
93.68 ±3.93b
|
111.77±10.86a
|
| EAA |
9.00 ± 0.32d
|
33.03 ± 1.61a
|
8.84 ± 0.87d
|
6.39 ± 0.62d
|
16.47 ±0.69c
|
26.08 ±1.35b
|
10.12 ± 4.23d
|
| EAA/TAA |
0.21 ± 0.01c
|
0.33 ± 0.01a
|
0.09 ± 0.01d
|
0.07 ± 0.01d
|
0.25 ± 0.01b
|
0.28 ± 0.01b
|
0.09 ± 0.03d
|
Table 3.
Zeta potential, droplet size, and apparent viscosity of DHA oil-in-water nanoemulsions.
Table 3.
Zeta potential, droplet size, and apparent viscosity of DHA oil-in-water nanoemulsions.
| |
ζ-Potential (mV) Day 1 |
D3,2 (nm) Day 1 |
D3,2 (nm) Day 8 |
D4,3 (nm) Day 1 |
D4,3 (nm) Day 8 |
Viscosity (cP) Day 1 |
Viscosity (cP) Day 8 |
| E1 |
-17.30 ± 0.42a
|
79.90 ± 0.14a
|
78.15 ± 0.07b
|
205.0 ± 0.0b
|
204.5 ± 0.71b
|
1.34 ± 0.06a
|
1.16 ± 0.05a
|
| E2 |
-18.95 ± 0.21a
|
79.90 ± 0.28a
|
78.55 ± 0.07b
|
215.0 ± 1.41a
|
212.0 ± 0.0a
|
1.40 ± 0.09a
|
1.39 ± 0.09a
|
| E3 |
-17.30 ± 0.14a
|
80.25 ± 0.35a
|
80.40 ± 0.28a
|
214.5 ± 2.12a
|
212.0 ± 1.41a
|
1.44 ± 0.10a
|
1.36 ± 0.06a
|
| E4 |
-17.25 ± 0.49a
|
78.35 ± 0.21b
|
77.05 ± 0.21c
|
199.0 ± 1.41b
|
197.5 ± 0.71c
|
1.47 ± 0.10a
|
1.41 ± 0.01a
|
| E5 |
-18.40 ± 0.57a
|
77.65 ± 0.07b
|
75.85 ± 0.21d
|
201.5 ± 0.71b
|
198.0 ± 0.0c
|
1.43 ± 0.10a
|
1.34 ± 0.05a
|
Table 4.
Peroxide value (PV) and tocopherol content of DHA nanoemulsions during an 8-day storage.
Table 4.
Peroxide value (PV) and tocopherol content of DHA nanoemulsions during an 8-day storage.
| Sample |
Day |
Peroxide value (meq/kg oil) |
α-tocopherol (µg/g emulsion) |
γ-tocopherol (µg/g emulsion) |
δ-tocopherol (µg/g emulsion) |
| E1 |
Day 0 |
99.43 ± 26.45b,w
|
3.27 ± 0.13a,x
|
0.00 ± 0.00a,x
|
25.34 ± 1.00a,x
|
| Day 2 |
118.85 ± 4.68b,y
|
2.98 ± 0.79a,w
|
0.00 ± 0.00a,z
|
20.71 ± 0.78ab,x
|
| Day 4 |
289.44 ± 8.52a,w
|
3.36 ± 0.99a,w
|
0.00 ± 0.00a,z
|
23.32 ± 1.55a,w
|
| Day 6 |
336.93 ± 20.14a,w
|
2.98 ± 0.13a,y
|
0.00 ± 0.00a,z
|
10.21 ± 1.06c,y
|
| Day 8 |
357.15 ± 24.36a,w
|
2.58 ± 0.24a,x
|
0.00 ± 0.00a,z
|
17.48 ± 1.11b,w
|
| E2 |
Day 0 |
21.33 ± 0.43b,xy
|
8.76 ± 0.00b,w
|
37.06 ± 0.14a,w
|
38.54 ± 0.74a,w
|
| Day 2 |
16.62 ± 1.38b,z
|
9.07 ± 0.41ab,w
|
36.01 ± 0.21a,w
|
39.81 ± 2.78a,w
|
| Day 4 |
22.17 ± 1.59b,y
|
10.51 ± 0.22a,w
|
35.75 ± 1.35a,w
|
14.37 ± 0.47b,x
|
| Day 6 |
34.38 ± 2.15a,y
|
10.38 ± 0.02a,w
|
34.16 ± 0.55a,w
|
13.76 ± 0.17b,w
|
| Day 8 |
32.00 ± 1.00a,y
|
10.49 ± 0.52a,w
|
34.27 ± 1.77a,w
|
13.81 ± 0.65b,x
|
| E3 |
Day 0 |
41.67 ± 3.60c,wxy
|
10.20 ± 2.44a,w
|
27.18 ± 7.78a,w
|
11.38 ± 3.07a,y
|
| Day 2 |
113.73 ± 2.74b,y
|
9.53 ± 1.78a,w
|
31.17 ± 0.08a,x
|
13.52 ± 0.19a,y
|
| Day 4 |
126.71 ± 1.74ab,x
|
8.65 ± 1.91a,w
|
30.03 ± 0.21a,x
|
13.49 ± 0.04a,wx
|
| Day 6 |
158.10 ± 28.22ab,x
|
7.68 ± 1.74ab,wx
|
26.12 ± 1.61a,x
|
12.87 ± 0.19a,x
|
| Day 8 |
186.63 ± 5.08a,x
|
0.00 ± 0.00b,x
|
15.08 ± 0.85a,xy
|
11.65 ± 0.28a,xy
|
| E4 |
Day 0 |
11.39 ± 1.70b,y
|
8.27 ± 0.66a,wx
|
31.00 ± 1.17a,w
|
13.39 ± 0.40a,y
|
| Day 2 |
183.92 ± 2.75a,w
|
6.49 ± 0.43ab,w
|
27.88 ± 0.40a,xy
|
12.81 ± 0.00ab,y
|
| Day 4 |
156.38 ± 7.69a,x
|
4.51 ± 1.36ab,w
|
21.66 ± 0.90ab,xy
|
12.31 ± 0.37ab,x
|
| Day 6 |
127.24 ± 37.95a,xy
|
4.95 ± 0.46ab,xy
|
24.10 ± 1.42bc,x
|
12.59 ± 0.01ab,wx
|
| Day 8 |
133.95 ± 8.12a,x
|
3.34 ± 1.50b,x
|
19.62 ± 1.36c,x
|
12.12 ± 0.19b,xy
|
| E5 |
Day 0 |
77.93 ± 10.56a,wx
|
8.73 ± 0.07a,w
|
30.39 ± 0.02a,w
|
13.26 ± 0.11a,y
|
| Day 2 |
152.38 ± 9.94a,x
|
3.75 ± 3.71a,w
|
23.51 ± 0.83b,y
|
11.70 ± 0.40b,y
|
| Day 4 |
134.79 ± 35.92a,x
|
3.27 ± 2.97a,w
|
19.47 ± 0.76c,y
|
11.23 ± 0.13b,x
|
| Day 6 |
153.61 ± 1.31a,x
|
5.33 ± 0.00a,xy
|
19.64 ± 0.31c,y
|
11.20 ± 0.15b,xy
|
| Day 8 |
129.52 ± 20.58a,x
|
0.00 ± 0.00a,x
|
13.49 ± 0.52d,y
|
10.29 ± 0.54b,y
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).