1. Introduction
Iron-containing ZSM-5 zeolites (Fe-ZSM-5) have garnered significant attention as catalysts for the selective catalytic reduction (SCR) of NO
x (including NO and NO
₂) with ammonia or hydrocarbons, as well as for N
₂O decomposition/reduction [
1,
2,
3,
4]. These catalysts exhibit high resistance to SO
₂ and H
₂O poisoning under SCR conditions [
1,
5,
6,
7,
8]. Furthermore, Fe-ZSM-5 catalysts typically operate within a broad temperature window, selectively produce N
₂ and H
₂O without significant N
₂O byproduct formation, and show low activity for the undesirable oxidation of SO
₂ to SO
₃ [
9].
Conventional preparation methods for Fe-ZSM-5 include ion exchange and wet impregnation [
10,
11,
12,
13]. However, these methods often suffer from poor reproducibility and can lead to the formation of large iron oxide particles upon calcination. Chemical vapor deposition (CVD) using gaseous FeCl
₃ has been shown to be an efficient and reproducible technique for achieving high dispersion of extra-framework iron at the exchange sites of the ZSM-5 lattice [
12]. Crucially, the active species in these catalysts are typically formed only after high-temperature treatment of the as-synthesized materials [
11,
15]. Alternatively, hydrothermal synthesis offers a direct route for incorporating iron into the zeolite framework. For instance, the group of J.B. Taboada [
15] demonstrated the synthesis of ZSM-5 zeolites enriched with the ⁵⁷Fe isotope, where iron was present as well-distributed Fe³⁺ ions within the framework.
Despite extensive research, the nature of the active sites in Fe-ZSM-5 catalysts remains a topic of debate. Conventionally prepared Fe-ZSM-5 catalysts often require operating temperatures above 400 °C to achieve high activity, with maximum NOx conversions typically around 90%. However, the influence of iron content introduced via direct hydrothermal synthesis on SCR activity has been less explored. This work aims to systematically investigate the effect of the Fe/Si ratio in catalysts synthesized in-situ by hydrothermal method. The primary objective is to modulate the acidity of the Fe-ZSM-5 catalysts to enhance their low-temperature SCR activity, lower the operating temperature, and widen the effective temperature window.
2. Experimental
2.1. Catalyst Preparation
A series of Fe-ZSM-5 catalysts were produced hydrothermally. Zeolites were synthesized using solution of NaAlO2, TEOS, Fe (NO3)3·9H2O and NaOH. Tetrapropyl ammonium bromide was used as template and mobilizing agent. The starting mixtures were formed using the following procedures:
n Na2O: nSiO2: n (NaAlO2+Fe (NO3)3): n TPABr: nH2O=12: 70: 1: 45: 7000.
NaAlO2 was first dissolved in water to get solution A, into which NaOH solution was added. TEOS, TPABr and Fe (NO3)3·9H2O were added to deionized water, and solution were added to above solution slowly while stirring.
The reagent mixture was prepared by intensive stirring of the initial reagents for 5 h. And then the resulting solution aged 12 h.
Crystallization was carried out at a constant temperature of 170℃ in a steel rotational autoclave for a period of 24 h. After that, the solid phase was filtered from the mother solution, washed several times with distilled water until it had a pH of 7. It was dried at a temperature of 100℃ and calcined at 550℃ for a period of 6 h to remove template and other impurities. Using the procedure, different zeolites with Fe: Al=0:1, 1:2, 1:1, 3:1 and 1:0 for Si/ (Fe+Al) =35 were synthesized.
2.2. Catalyst Characterization and Activity Measurements
X-ray diffraction measurement was performed on a Bruker D8 Advance X-ray diffraction using Cu Kα radiation in the 2θ interval 5-50°.
The scanning electron microscope image was obtained on LEO-1530VP scanning electron microscope. SEM images were recorded on samples with deposited Au for improved conductivity.
The BET surface areas (SBET) and pore volumes (Vpore) of the Fe-ZSM-5 catalyst were determined by surface area and pore porosimetry analyzer (V-Sorbet 2008S). The sample was in vacuum at 150℃ prior for 8 hs to measurement. And then the sample was measured by using N2 adsorption at -196℃.
X-ray photoelectron spectroscopy analysis was performed in ESCA PHI 5000C System. Al/Mg radiation (14.0kV, 250W) was used to excite photoelectrons.
Temperature-programmed desorption (NH₃-TPD and NO-TPD) measurements were performed using a Chembet PULSAR TPR/TPD fully automated dynamic chemisorption analyzer manufactured by Quantachrome Instruments (USA). The NH3-TPD (NO-TPD) experiment was performed by the following procedure: 150 mg of calcined sample was loaded into a quartz tube reactor, pre-treated by heating in a flowing stream of argon, heated from room temperature to 60℃ at 10℃·min-1 and stayed at 60℃ for 1 h. This was done to remove water and desorb unwanted impurities. After that, the argon flow was switched to a flow of NH3/N2 1.94 vol. % or (NO/N2 1.00vol. %) for about 1 h until the signal was steady. The catalyst was saturated with NH3 (NO). Prior to NH3 (NO) desorption measurement, the sample was swept by a flow of dry argon (70mL·min-1) for 1 h to remove physically adsorbed NH3 (NO). Desorption was carried out at a heating rate of 15℃·min-1 from 60℃ to 700℃ in a He flow (70mL ·min-1).
The SCR activity measurement was carried out in a fixed-bed. A sample of 1g Fe-ZSM-5 catalyst (crushed to powder and sieved to 20-40 mesh) was loaded in a fixed-bed. The reaction temperature was controlled by programmable temperature controller. The total flow rate was 100mL·min-1 (ambient conditions), and the gas hourly space velocity (GHSV) was 6000mL3·gcat-1·h-1. The typical reactant gas composition was as follows: the balanced gas N2, 450ppm NO, 450ppm NO2, 1000ppm NH3 and 6% O2. The concentration of NO and NO2 was continually monitored by the coal gas analyzer (Germany ECOM-JZKN). The NO and NO2 conversion was obtained from the difference in the NOx concentrations before and after the SCR reaction,the formula of the NOx conversion is: SO2 and H2O resistance experiment was conducted in the same fixed-bed. A reaction mixture containing 450ppm NO, 450ppm NO2, 1000ppm NH3 , 600ppm SO2 (5%H2O) and 6%O2 balanced with N2 at a flow rate 100mL·min-1 (ambient conditions) was used.
3. Results and Discussion
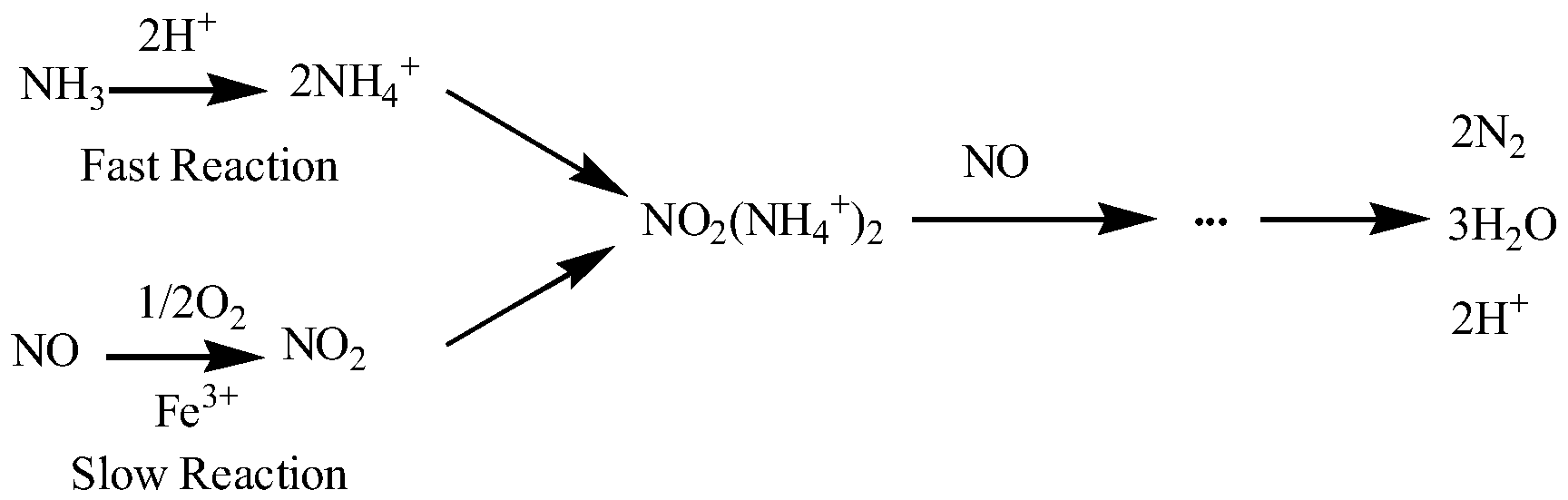
Previously based on the conclusions of in situ FTIR studies, Long and Yang [
16] has came up with the reaction mechanism of NOx SCR with ammonia over Fe-ZSM-5 catalysts shown in
Figure 1. The SCR reaction needs two kinds of sites: the Brönsted acid sites for ammonia adsorption and the metal ion sites (i.e., Fe
3+ ions) for NO oxidation to NO
2, a possible reaction mechanism is proposed for NO reduction involving NO
2 and NO
2(NH
4+)
2 as intermediates. According to this mechanism, first and rate determining step for the SCR reaction is the oxidation of NO to NO
2 on Fe
3+ sites by O
2, while gaseous NH
3 molecules are adsorbed quickly onto the Brönsted sites to form NH
4+ ions. Subsequently, each NO
2 molecule reacts with two adjacent NH
4+ ions to form the active complex, NO
2(NH
4+)
2. The small pore size in ZSM-5 facilitates the formation of this complex, which produce N
2 and H
2O.
3.1. X-Ray Diffraction and Specific Surface Area
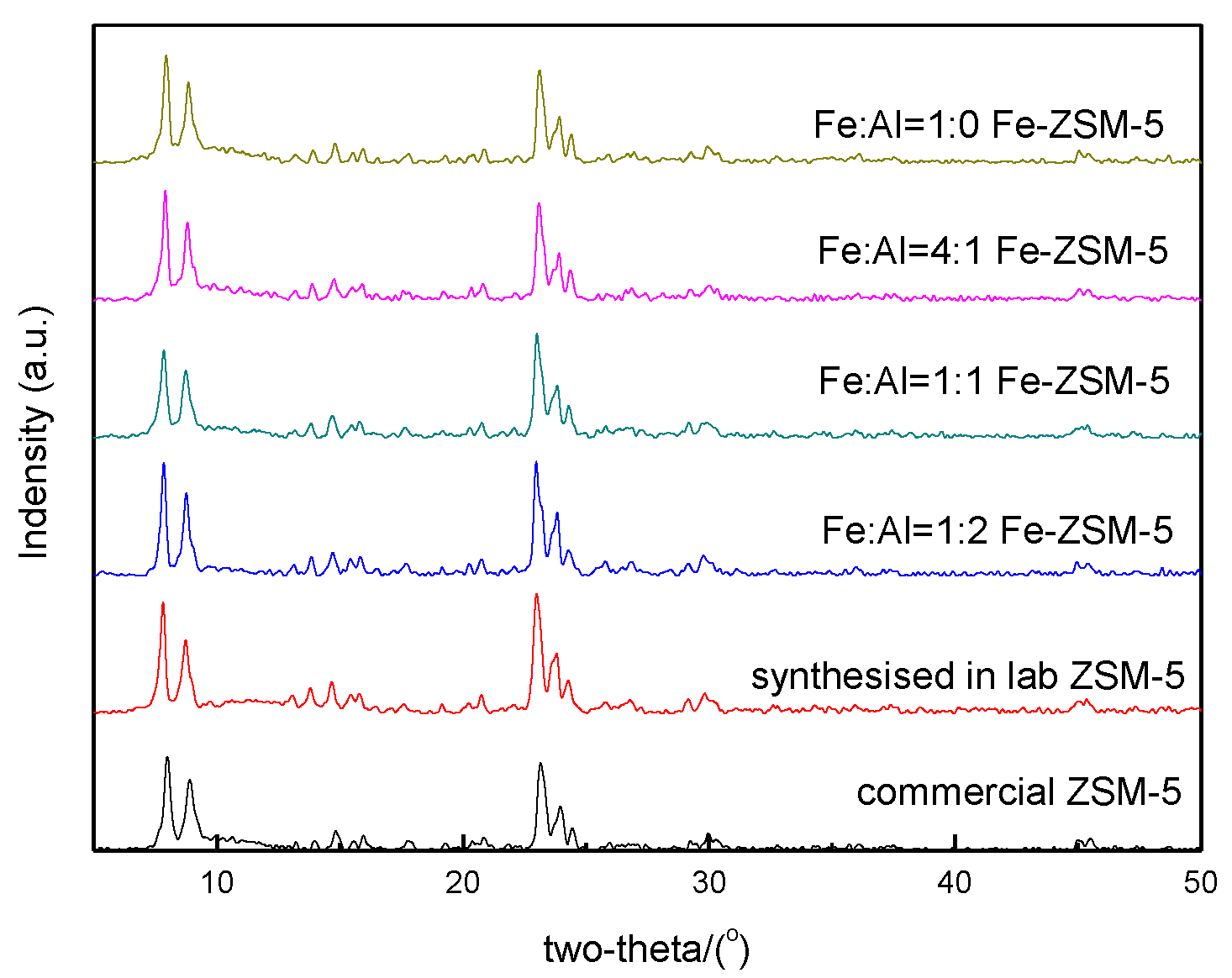
XRD patterns of different iron content ZSM-5 catalysts are very similar (
Figure 2). These patterns were obtained after the zeolite catalyst synthesis and subsequent combustion of organic template. The same peaks appeared at 7.8°, 8.8°, 23.1°, 26.6° and 29.2°. All the Fe-ZSM-5 exhibited the typical lines of MFI framework, showed that all have a ZSM-5 structure with a high degree of crystallinity after Fe loading. All samples do not show any evidence of extra framework crystalline compounds or long-range amorphization of the zeolite catalyst.
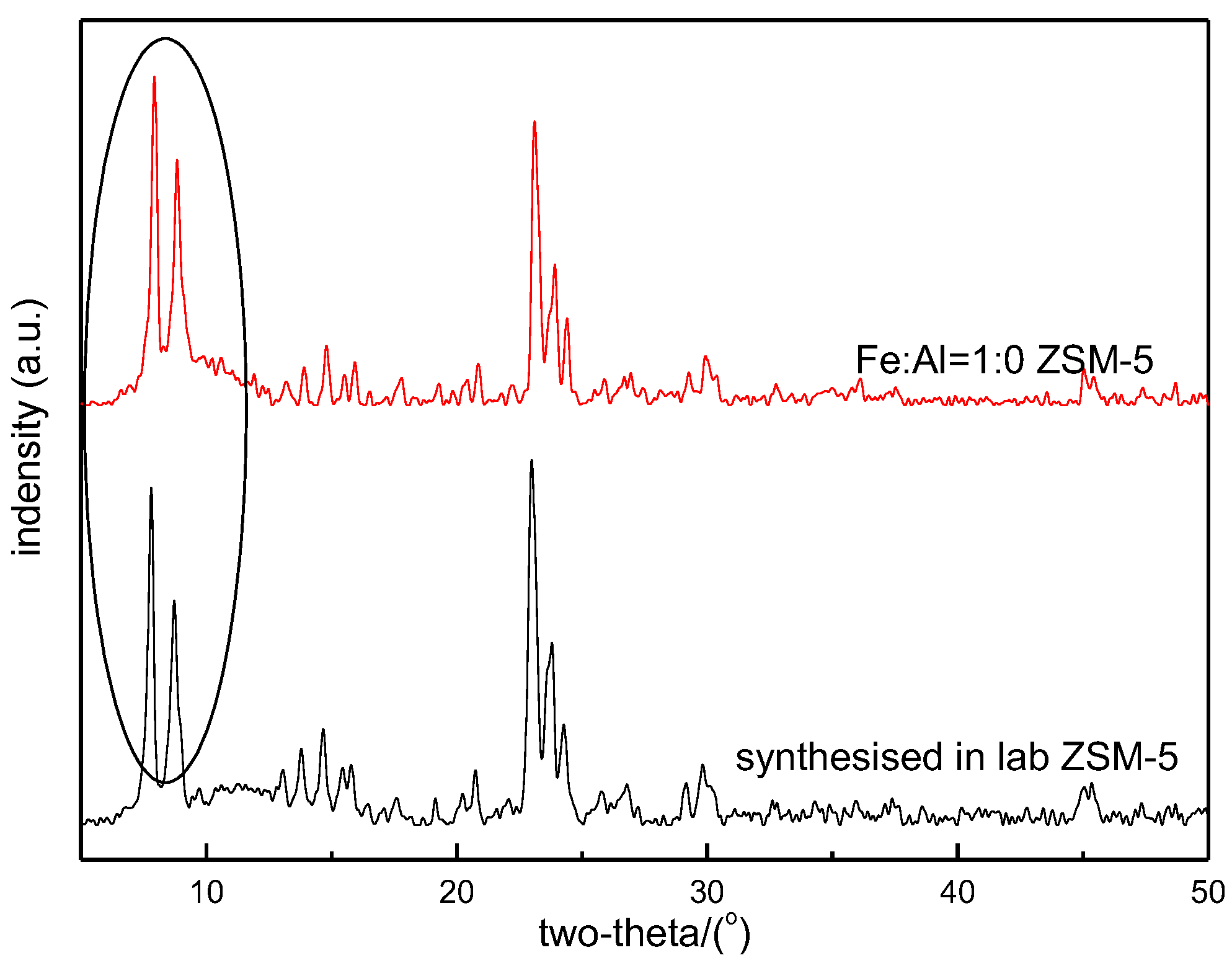
We also observed that for samples with varying iron content, the diffractograms exhibit Bragg peaks around 7.8° and 8.8° 2θ (see inset in
Figure 3), when the Fe: Al=1:0 ZSM-5 catalyst comparing with the Fe: Al=0:1 ZSM-5 catalyst, respectively indicates expansion of the matrix and suggests that iron was incorporated into the framework. We analyze that the radius of Fe
3+ (0.067nm) is bigger than Al
3+ (0.057nm), leading cell parameter a
0 to become larger. These slight differences in the XRD pattern are attributed to ZSM-5 crystals with varying amounts of iron, which causes the unit cells to vary in size (the length of Fe-O bond (1.86±0.1Å)) [
16] is larger than that of Al-O bond (1.73 Å) and Si-O bond (1.63 Å) [
17] and thus might induce small shifts of the Bragg peaks.
From the results of Fe: Al=1:1 Fe-ZSM-5 catalyst specific surface area test, we conclude that the BET specific surface area is 338m2·g-1, Langmuir specific surface area is 446.04930 m2·g-1. The diameter of pore is 0.58nm, which is typical for microporous materials.
3.2. Scanning Electron Microscope and X-Ray Photoelectron Spectroscopy
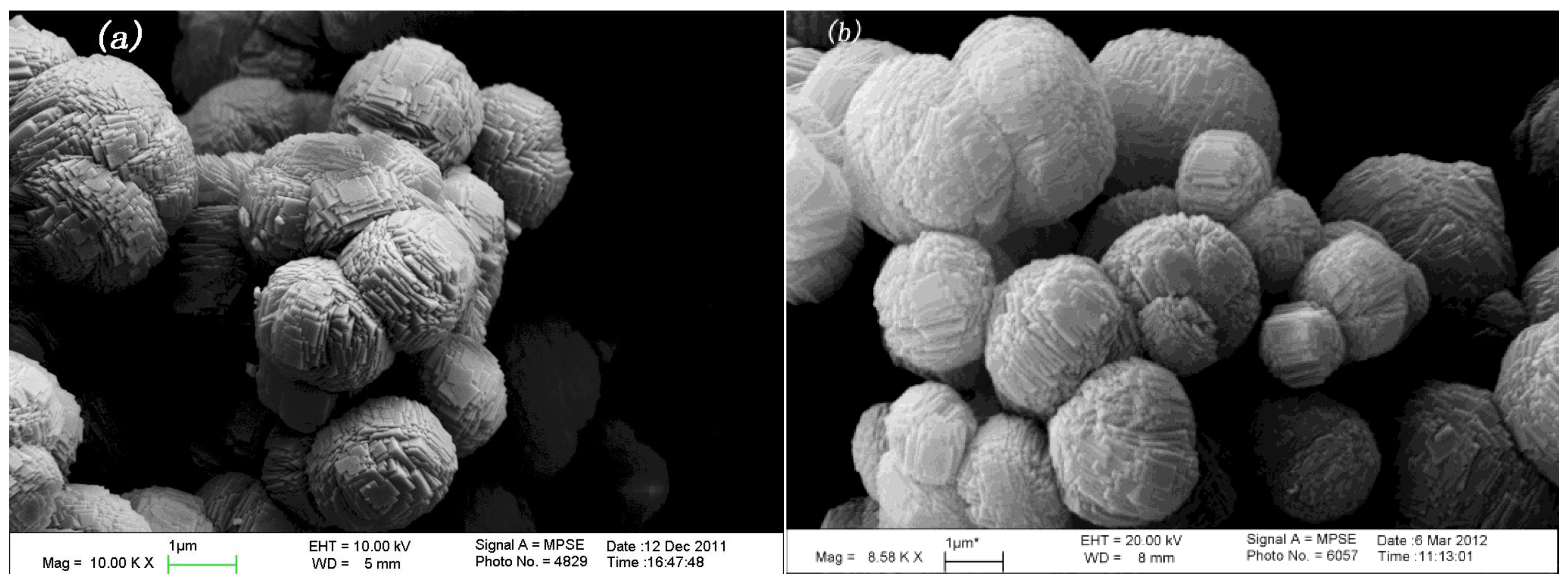
In
Figure 4, it is show that the zeolite catalyst has formed nanoparticles with an average size between 1 and 3 μm which are named nanocrystalline. Besides, the surface of nanoparticles is very smooth, and there are no Fe
2O
3 particles in the surface of the zeolite catalyst.
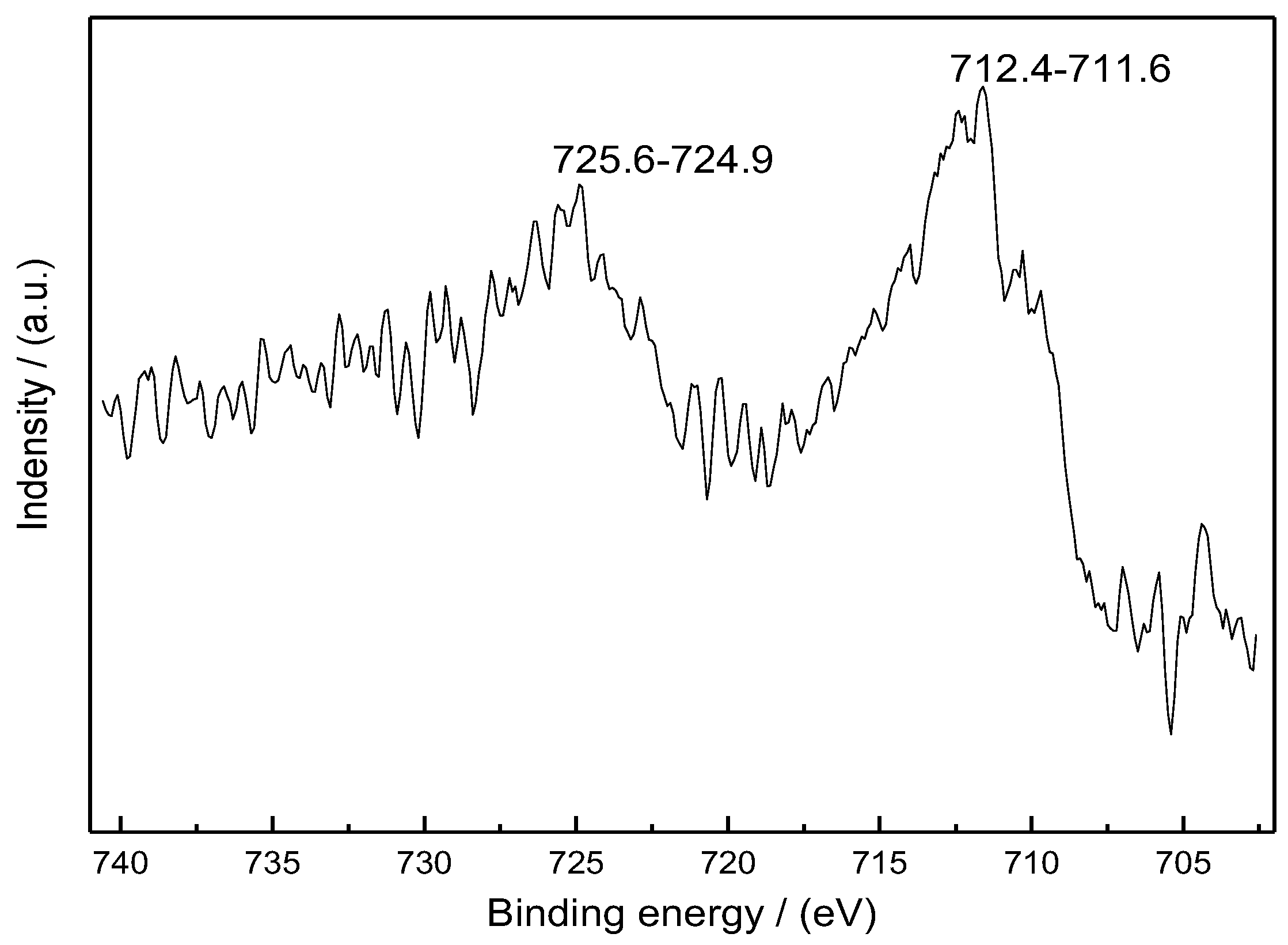
On the basis of XPS spectra (
Figure 5) measured on Fe-ZSM-5 (Fe: Al=1:1, prepared by hydrothermal method), it is found that the binding energies of Fe2p
3/2 photoelectron are between 711.6eV and 712.4eV. The value are higher than those for bulk α-Fe
2O
3 (710.5 [
18], 711.2 eV [
19]) and Fe
3O
4 (710.5 [
20], 711.2 eV [
18]). This may indicate a strong interaction between iron and zeolite, and in fact, the binding energies of 711.6 and 712.1-711.4 eV are reported for iron incorporated in Fe-ZSM-5 [
20] and Fe
2O
3-SiO
2 [
21].
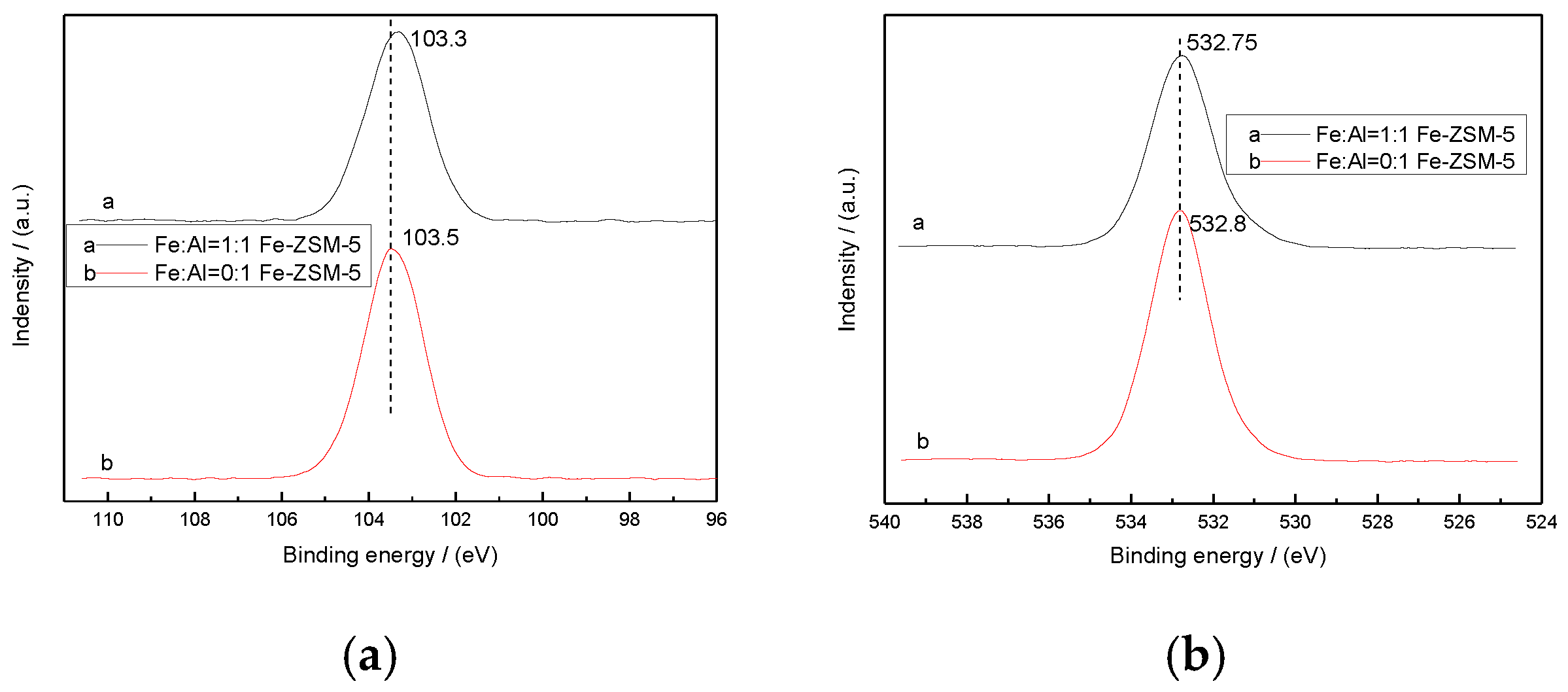
In
Figure 6 (1) and (2), they show Si2p and O1s spectra obtained over Fe: Al=0:1 and 1:1 Fe-ZSM-5. The main peaks of Si2p and O1s over Fe: Al=1:1 Fe-ZSM-5 are both slightly smaller than that for Fe: Al=0:1 Fe-ZSM-5. It may indicate that Fe was incorporated into the framework and formed Fe-O-Si. The electronegativity of Fe is smaller than Al, so that the main peak shifts to low binding energy. These findings are supported by the X-ray powder diffraction and scanning electron microscope.
Iron in the framework sites is essentially in tetrahedral oxygen coordination. On the other hand, iron in the extra framework sites is probably present as iron oxide clusters, which are likely to occupy the octahedral sites such as those observed in the bulk phases of α-FeOOH (goethite) and α-Fe
2O
3 (hematite). Moreover, the XPS of the catalysts, exhibited no other little peaks expect Fe
3+ ions in
Figure 5. This indicates that all iron must be in the framework position since the Fe
3+ ions in the framework are hard to reduce, in agreement with previous studies [
15].
3.3. Temperature Programmed Desorption of Ammonia (NH3-TPD)
Acidity and pore structure of ZSM-5 catalysts are main factors affecting the catalytic properties. It is concluded that the introduction of iron will not damage the skeleton structure of molecular sieve from the previous results of XRD characterization, and the pore structure of the zeolite catalyst does not change. In order to investigate the introduction of iron effect on the zeolite catalyst acidity, the NH3-TPD measurements were performed for catalysts of different iron content.
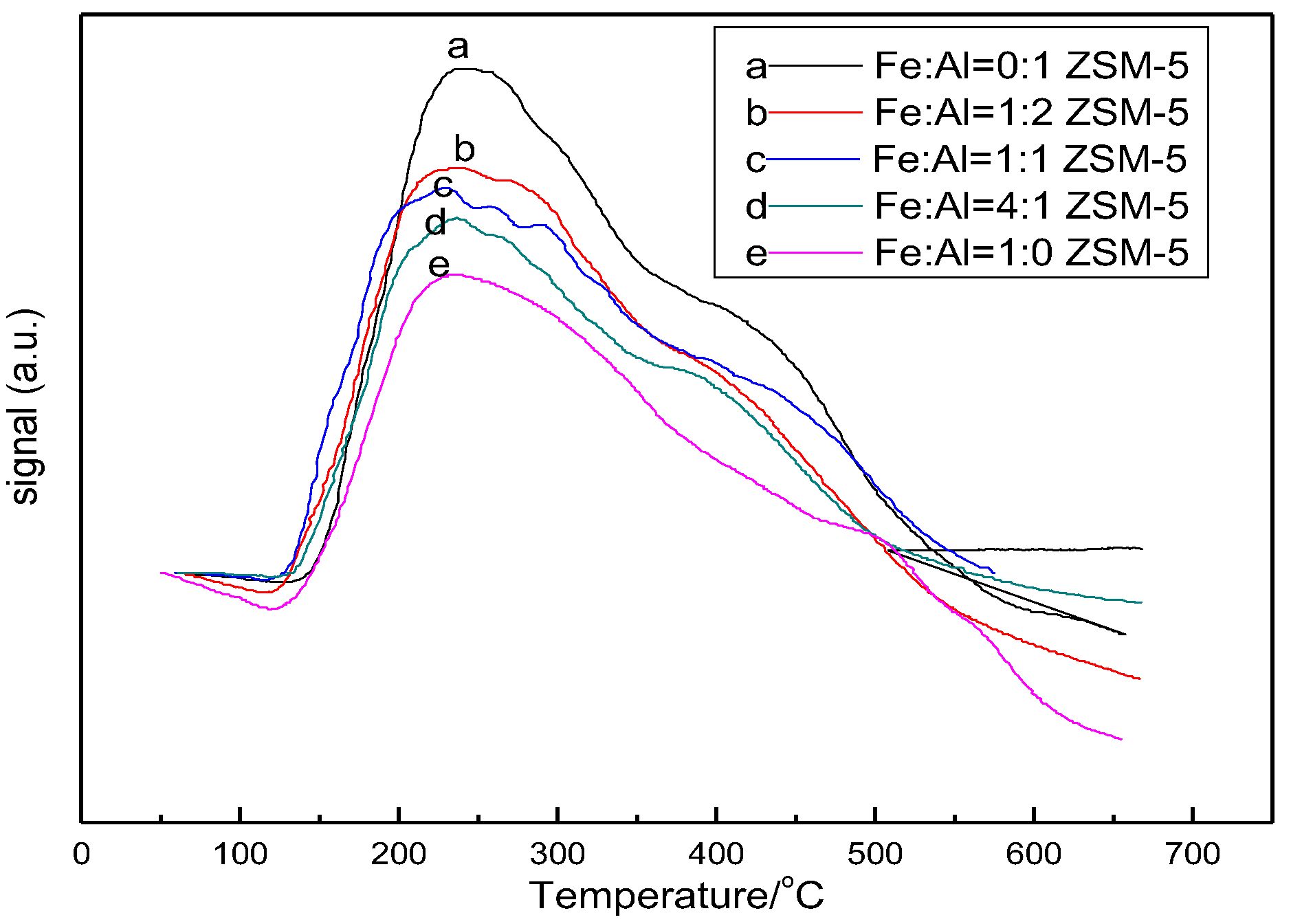
According to the results of ammonia-TPD (
Figure 7), introduction of iron leads to the decrease of the total amount of desorbed ammonia in all cases. For Fe-ZSM-5 catalysts, the intensity of the peak is lower than that of Fe: Al=0:1 Fe-ZSM-5, because the Brönsted acid protons were substituted by Fe
3+. At the same time, the total amount of acidity decreases slightly when the Fe/Al ratios increases. It indicates that Al adsorbed ammonia more easily than Fe, so Al adsorbed ammonia firstly and then Fe followed.
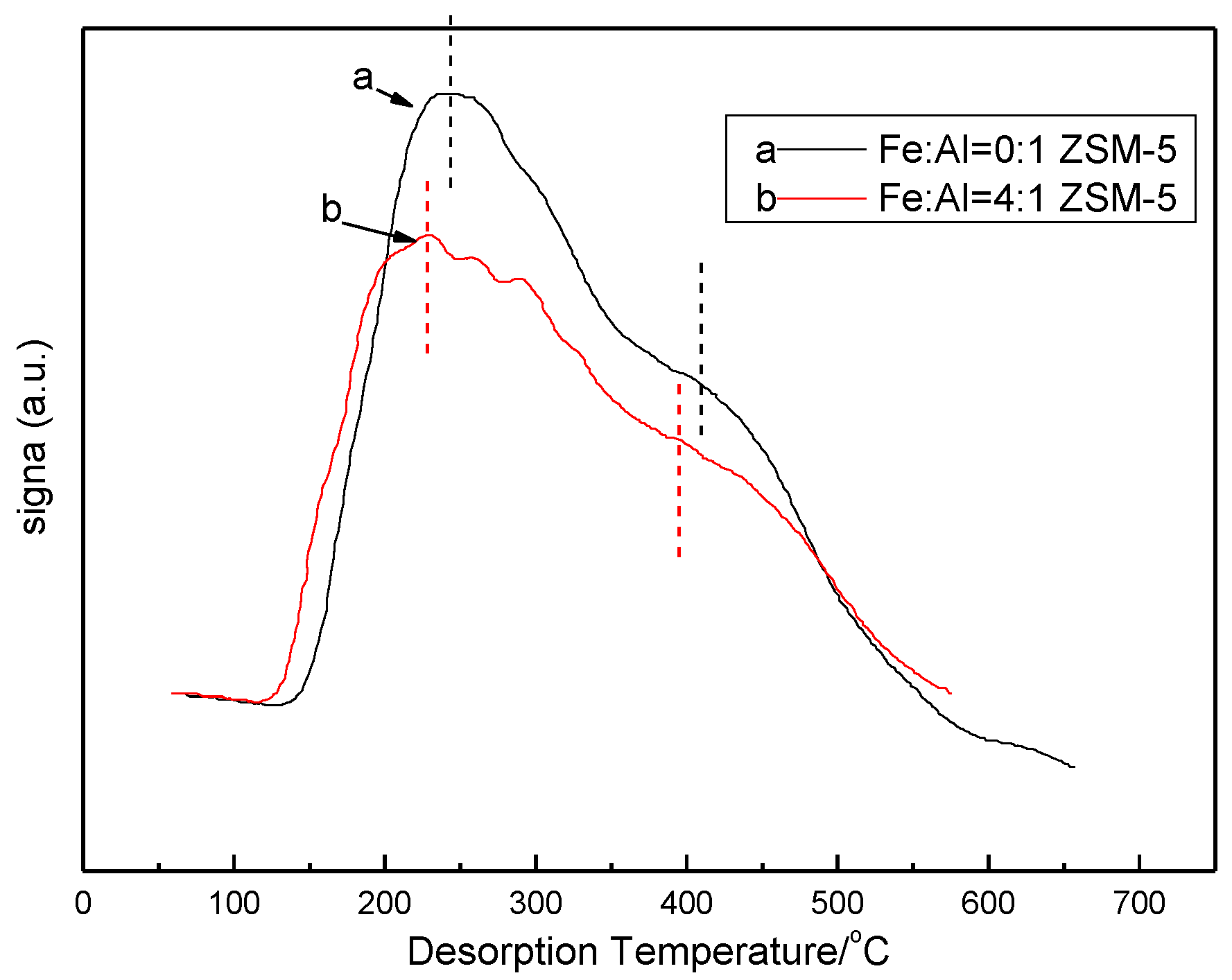
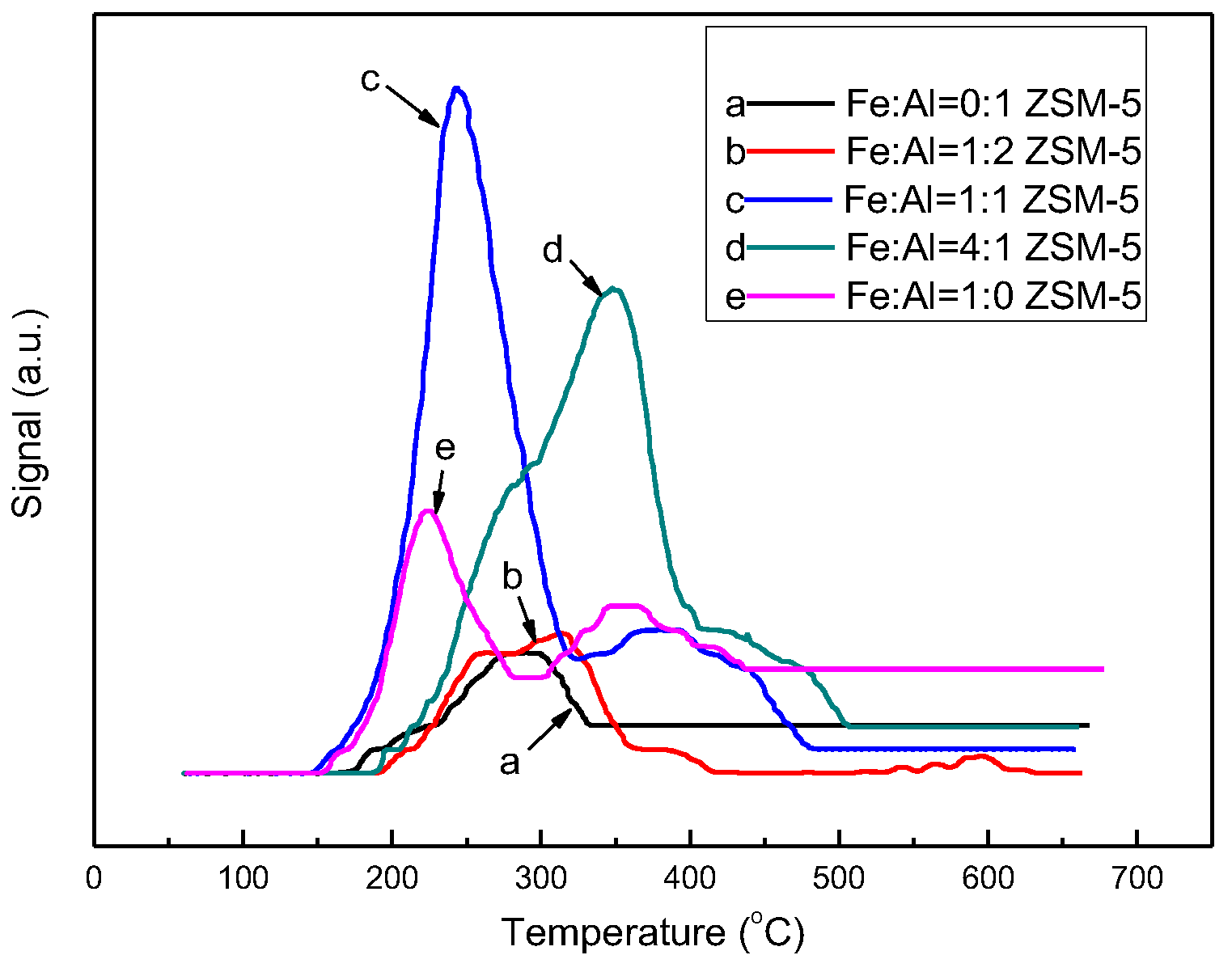
The
Figure 8 shows the comparison of Fe: Al=4:1 and Fe: Al=0:1 Fe-ZSM-5 catalyst, two different desorption peaks. The peak around 240℃ is due to desorption of weakly bound NH
3 followed by desorption around 400℃ due to strongly bound NH
3. The strongly bound NH
3 is arising from Fe-ZSM-5 unoccupied Brønsted hydroxyl groups and strong Lewis iron sites. The significant decrease in NH
3 desorption in the Fe: Al=4:1 Fe-ZSM-5 catalyst implies that Fe
3+ is associated with ZSM-5 structure. It also shows that the center of the acidity sites shifts to the direction of low temperature when iron is introduced.
3.4. Temperature Programmed Desorption of Nitric Oxides (NO-TPD)
In
Figure 9, the amount of NO adsorption of Fe: Al=1:1 zeolite catalyst is the largest among the different iron content zeolite catalysts. The result indicates that the Fe: Al=1:1 Fe-ZSM-5 catalyst adsorbed more NO than other different iron content between 250℃ and 350℃. As shown before, NH
3 is also adsorbed at 250℃, so the adsorbed NO could react with other molecules in the active acid sites.
For molecular sieve-type catalysts, it was reported that both NOx and NH
3 molecules could adsorb on the catalysts [
22,
23].
3.5. SCR Activity Testing
All catalysts used in this test were highly crystalline as determined by XRD and contained no trace impurities. BET showed that the Fe-ZSM-5 catalysts had microporous volumes consistent with the ZSM-5 structure. Iron was incorporated into the framework from the results of XPS, SEM and XRD. In some literature data, NO
2 may dissociate onto the red-ox sites. Meanwhile, monoxide would participate in the fast SCR path (for which a mixture of NO and NO
2 is required) [
24]. The best DeNOx performances could be obtained always with NO
2/NOx ratios close to 0.5 [
25].
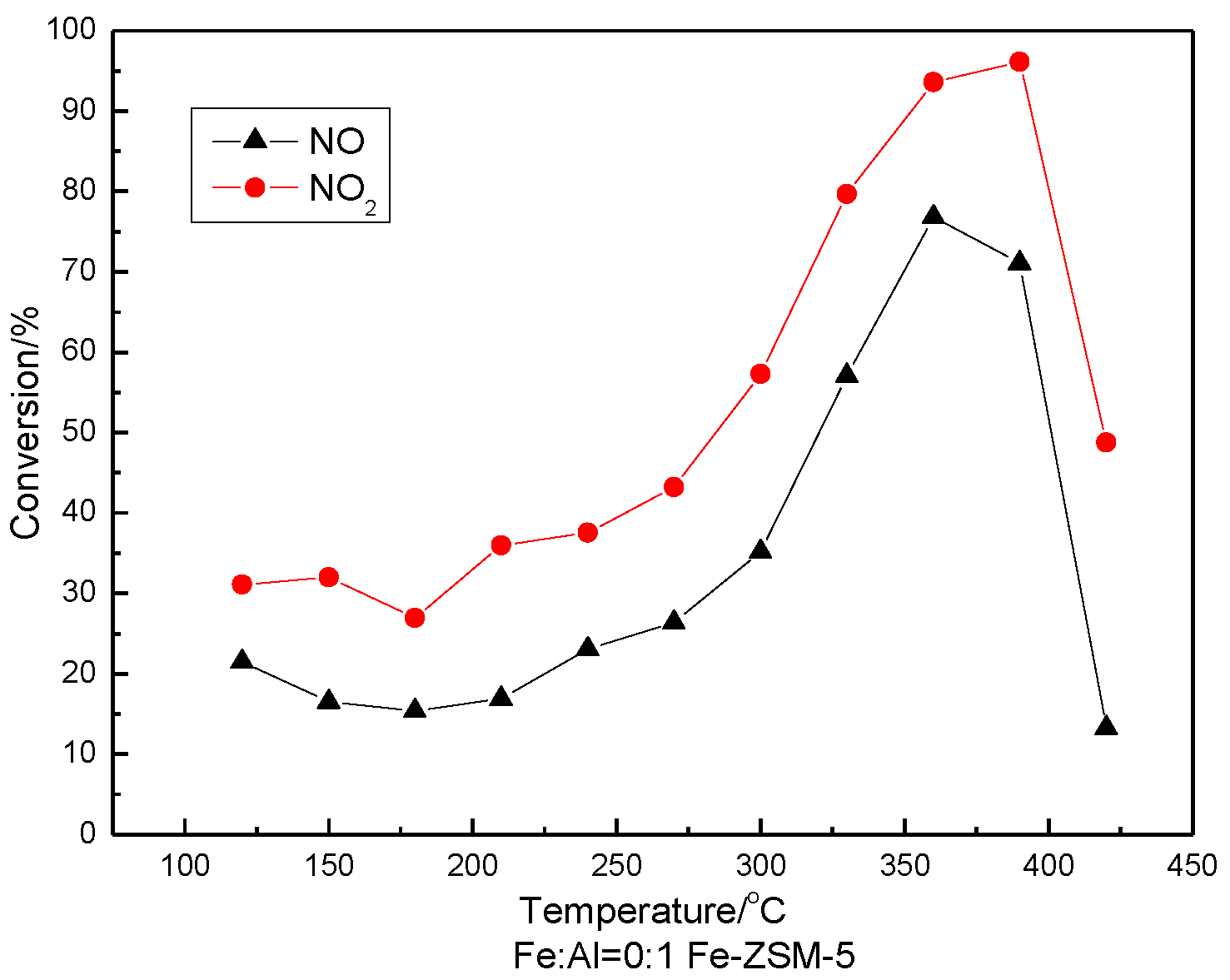
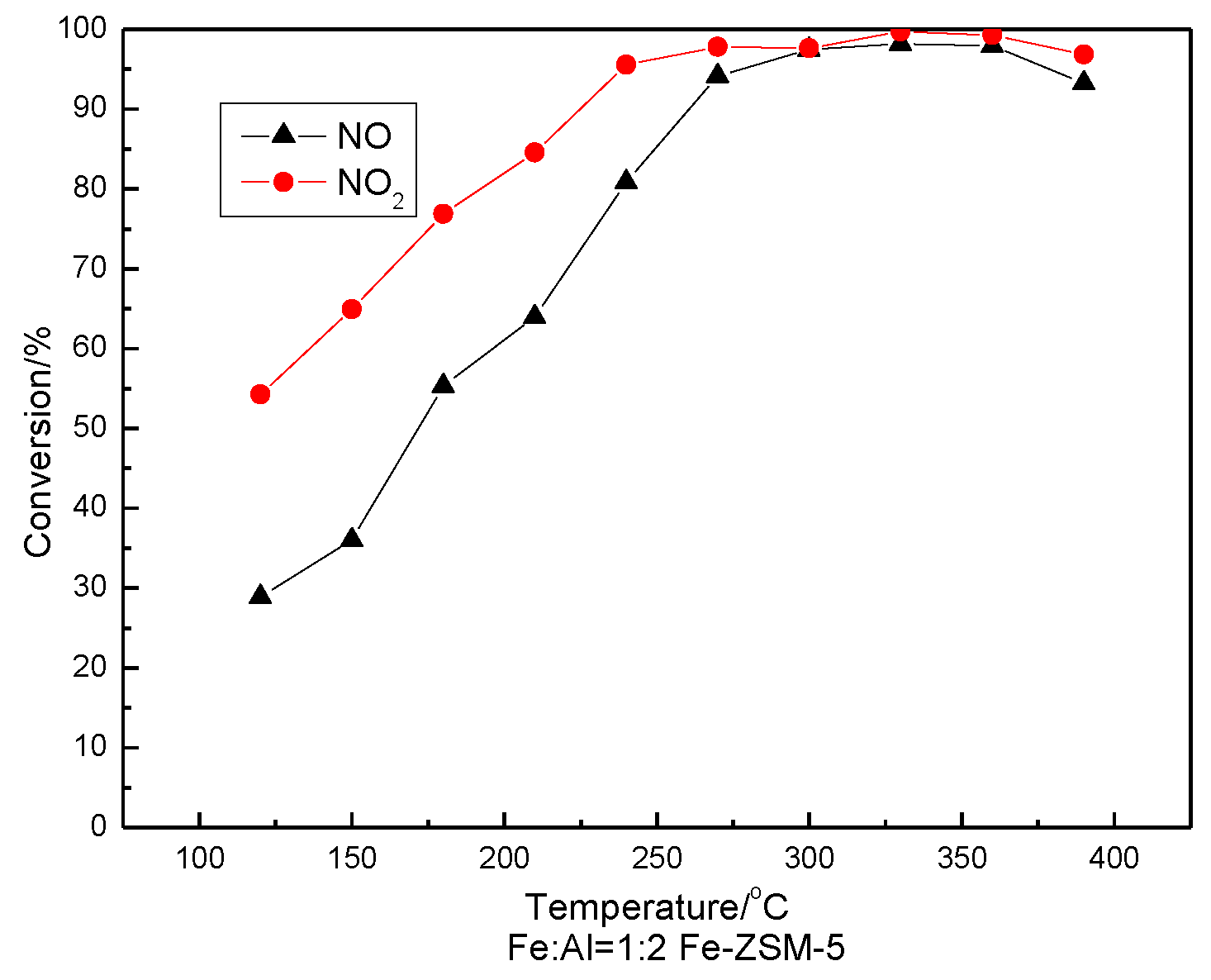
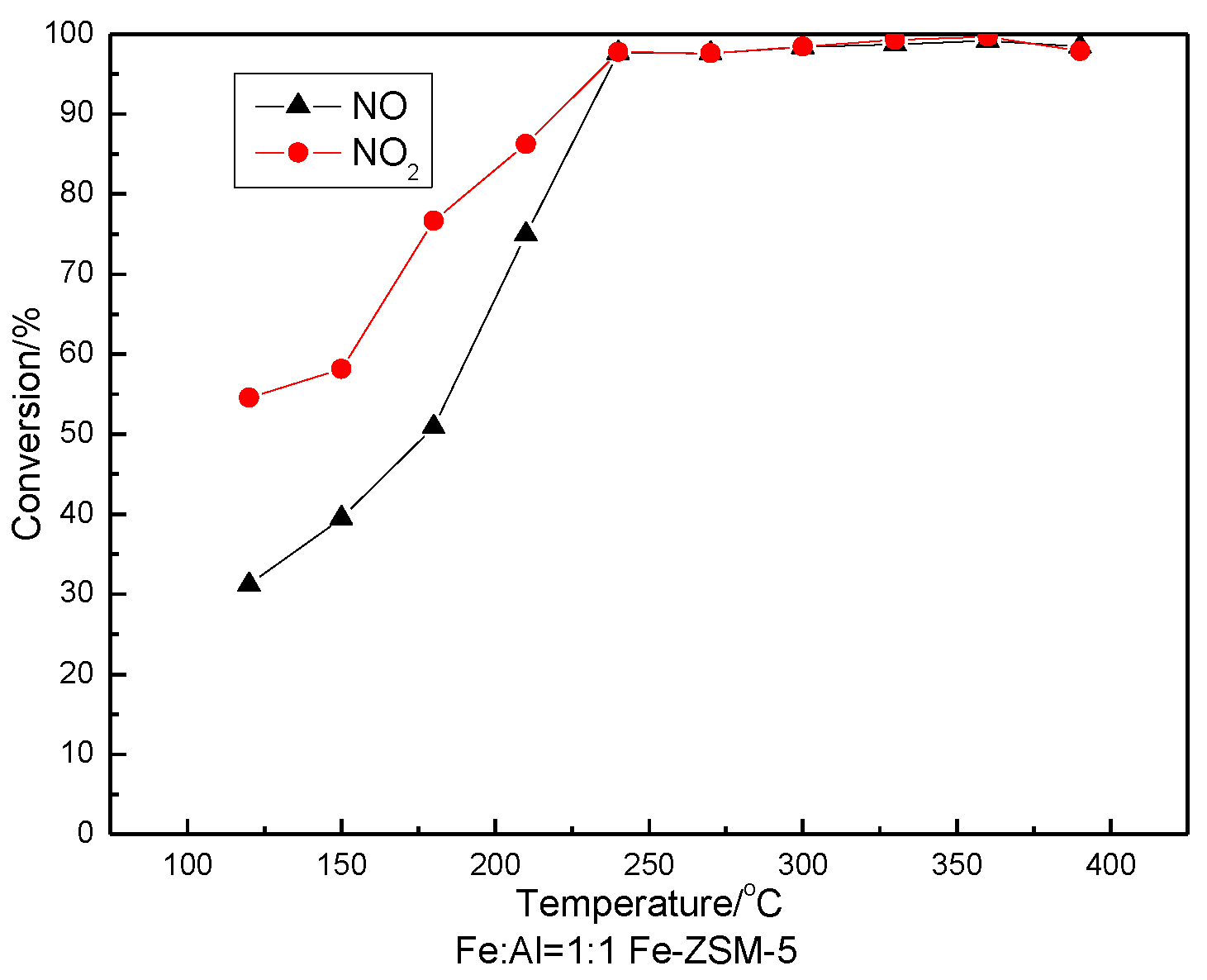
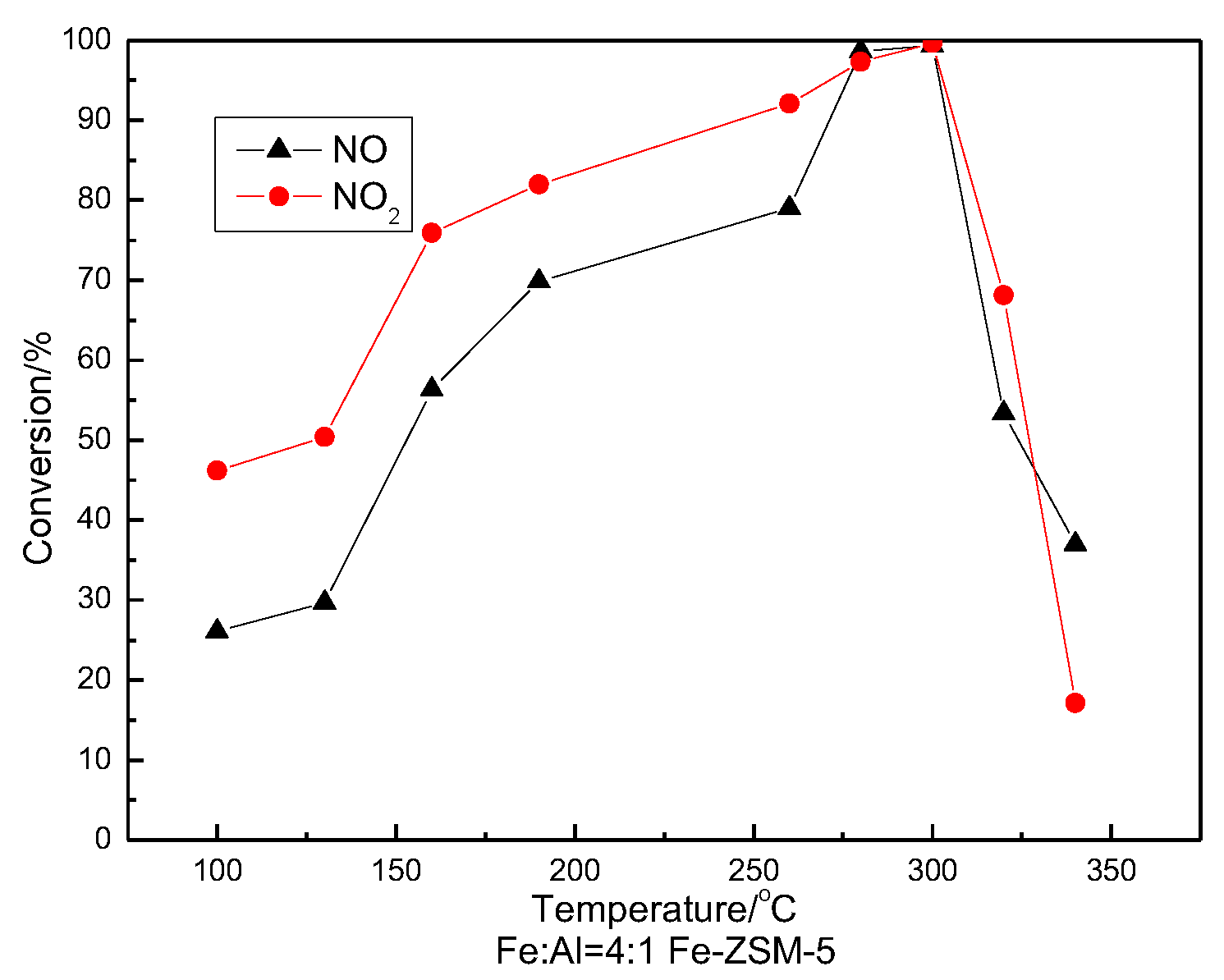
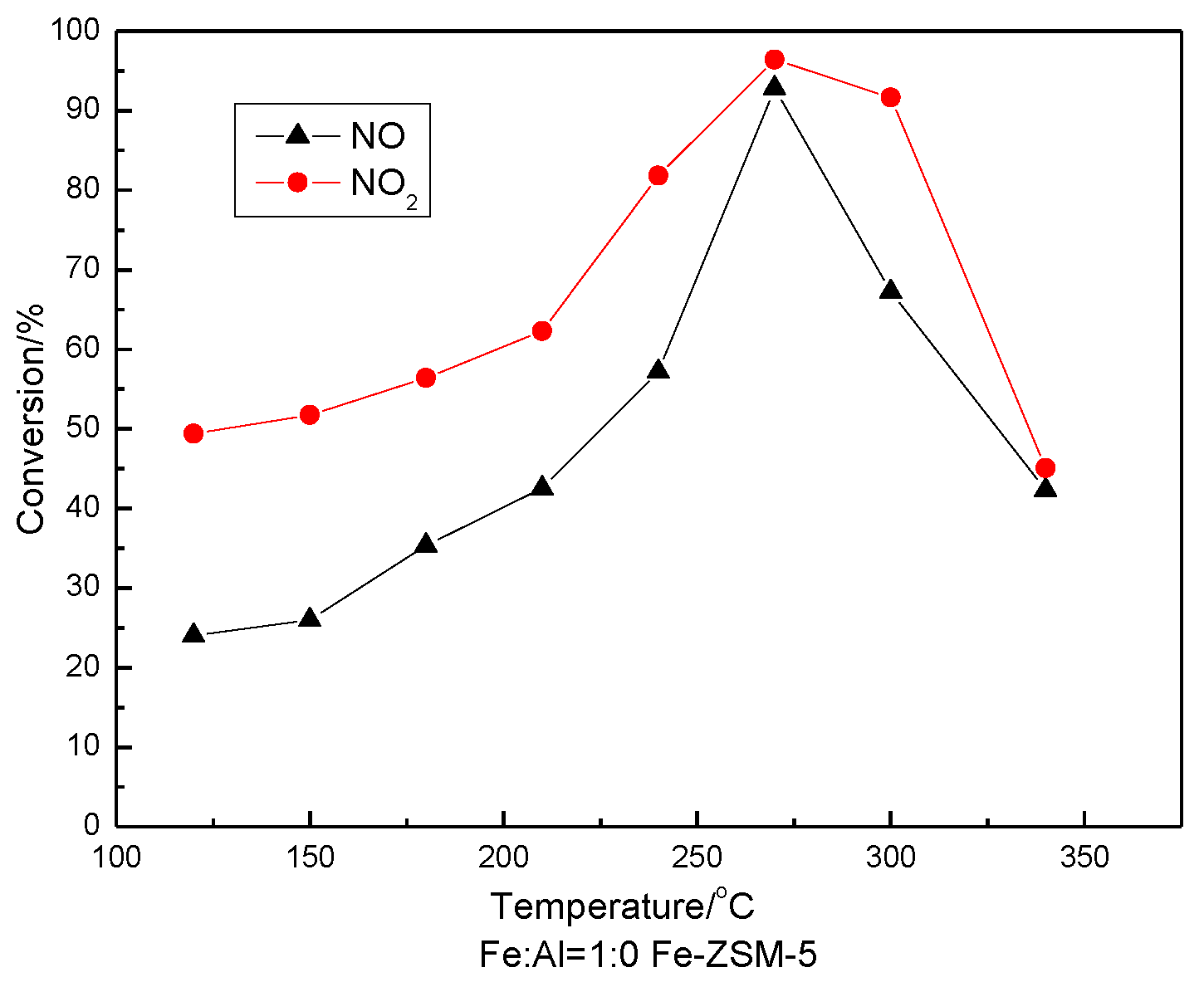
Since the reaction temperature plays an important role in SCR of NOx, it is necessary to seek the optimum temperature window for the prepared Fe-ZSM-5 catalysts. The activity of the catalysts in the series is systematically investigated as shown in
Figure 10,
Figure 11,
Figure 12,
Figure 13 and
Figure 14.
The NOx conversion increases in all cases and passes through maxima as the temperature increased to 390℃. When the Fe: Al=1:1, the activity of the Fe-ZSM-5 catalyst is the most efficient catalyst. The NOx conversion between 240℃ and 390℃ is nearly 100%. Besides, the NOx window is shifted to the direction of lower temperature. The window of the Fe: Al=1:1 ZSM-5 catalyst is the widest (from 240℃ to 390℃), and the conversion is almost 100%. The conversion of NO2 is higher than that of NO over the Fe-ZSM5 catalysts, although a 1:1 mixture of NO and NO2 was fed to the catalyst.
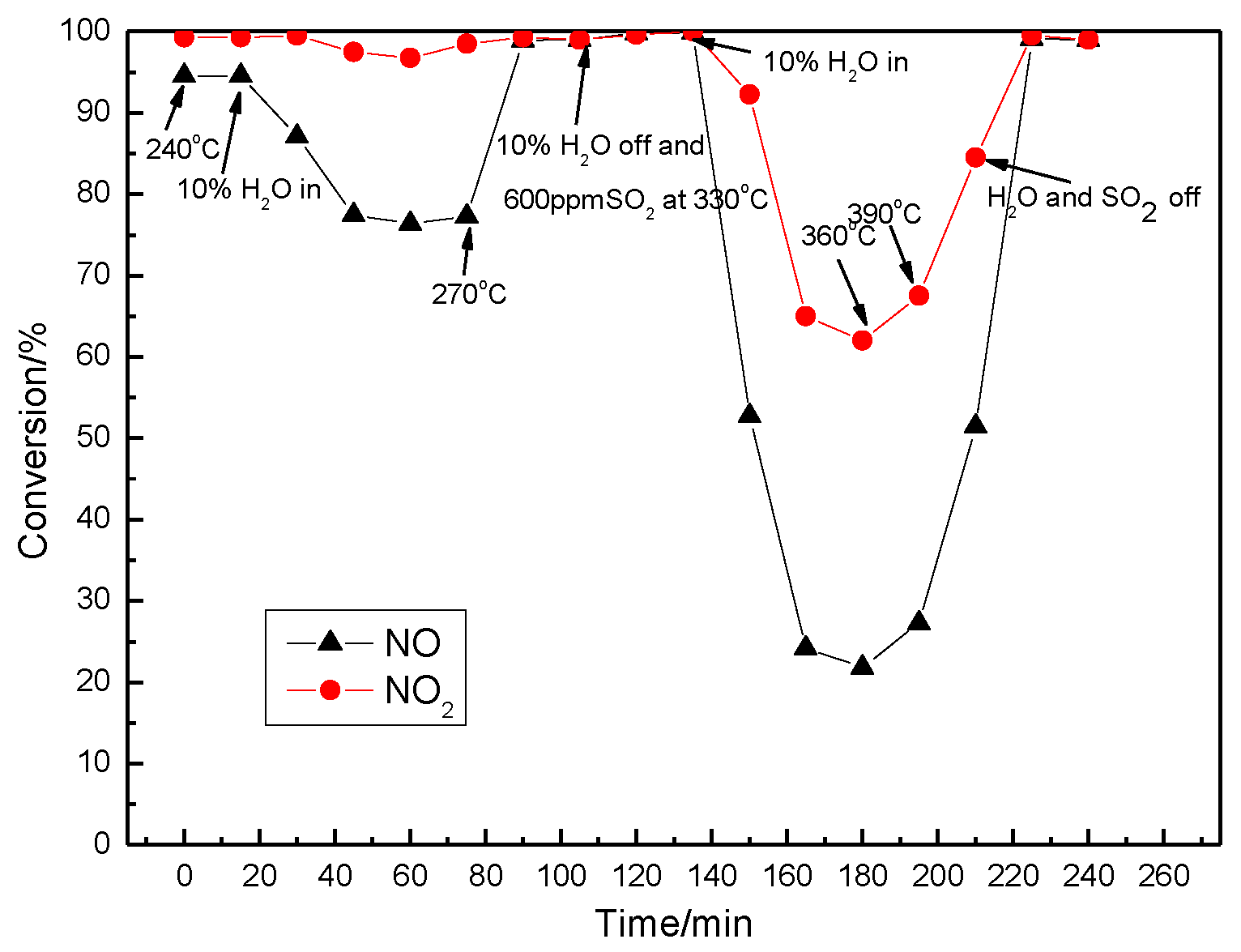
Moreover, we conducted a SO
2 and H
2O resistance experiment for the catalyst. This type of study is essential for initiating the practical use of the Fe-ZSM-5 catalysts for NOx removal with NH
3.
Figure 15 shows the evolution of NO and NO
2 conversion over Fe: Al=1:1 Fe-ZSM-5 under different conditions with time on. Adding H
2O and SO
2 individually had almost no influence on NO and NO
2 conversion above 270℃ to Fe: Al=1:1 Fe-ZSM-5. However, the conversion NOx decreased deeply, when H
2O and SO
2 were added at the same time. The above inhibitions could be reduced by increasing reaction temperature and were reversible.
4. Conclusions
A series of Fe-ZSM-5 catalysts with varying Fe/Al ratios were successfully synthesized via a one-pot hydrothermal method. Comprehensive characterization (XRD, XPS, SEM) confirmed the isomorphous substitution of Fe³⁺ ions into the zeolite framework, forming Fe–O–Si linkages. The catalyst with a Fe:Al molar ratio of 1:1demonstrated the highest catalytic activity, achieving nearly 100% NOx conversion within a wide temperature window of 240–390 °C.NH₃-TPD studies revealed that iron incorporation modifies the acidity, reducing the overall acid amount and strength. NO-TPD results correlated with catalytic performance, showing the highest NO adsorption capacity for the Fe:Al=1:1 catalyst. The catalyst also showed good resistance to individual poisoning by H₂O or SO₂, though synergistic inhibition was observed when both were present. This work highlights the effectiveness of hydrothermal synthesis in creating highly active and durable Fe-ZSM-5 catalysts for low-temperature NH₃-SCR of NOx.
Funding
Fund Project: “Research on the Construction of Aviation Emergency Rescue System from the Perspective of Low-altitude Economy” (2025SJYB1676), a 2025 Philosophy and Social Sciences Research Project of Universities in Jiangsu Province.
References
- Shi, X; Wang, Y; Shan, Y; et al. Investigation of the common intermediates over Fe-ZSM-5 in NH3-SCR reaction at low temperature by in situ DRIFTS[J]. Journal of Environmental Sciences 2020, 94(08), 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, K Q; Xia, H A; Hensen, E; van, Santen R; Li, C. Chemistry of N2O decomposition on active sites with different nature: Effect of high-temperature treatment of Fe/ZSM-5 [J]. Journal of Catalysis 2006, 238, 186~195. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J. Active iron sites associated with the reaction mechanism of N2O conversions over steam-actived FeMFI zeolites [J]. Journal of Catalysis 2004, 227, 512~522. [Google Scholar] [CrossRef]
- Schwidder, M; KumarM, S; Klementiev, K; et al. Selective reduction of NO with Fe-ZSM-5 catalysts of low Fe content I. Relations between active site structure and catalytic performance [J]. Journal of Catalysis 2005, 231, 314~330. [Google Scholar] [CrossRef]
- Qi, G; Yang, R T. Ultra-active Fe/ZSM-5 catalyst for selective catalytic reduction of nitric oxide with ammonia [J]. Applied Catalysis. B Environmental 2005, 60, 13~22. [Google Scholar] [CrossRef]
- Yu-bo, ZHANG; Pan, WANG; Dan, YU. Evolution mechanism of active sites for NH3-selective catalytic reduction of NOₓ over Ce/Cu-doped Fe-ZSM-5 catalysts[J]. Journal of Central South University 2022, 29(7), 2239–2252. [Google Scholar]
- Long, R Q; Yang, R T. Selective catalytic oxidation (SCO) of ammonia to nitrogen over Fe-exchanged zeolites [J]. Journal of Catalysis 2001, 201, 145~152. [Google Scholar] [CrossRef]
- Krishna, K; Seijer, G B F; Bleek, C M; van den; et al. Selective catalytic reduction of NO with NH3 over Fe-ZSM-5 catalysts prepared by sublimation of FeCl3 at different temperatures [J]. Catalysis Letter 2003, 86, 121~132. [Google Scholar] [CrossRef]
- Long, R Q; Yang, R T. Temperature-programmed desorption/surface reaction (TPD/TPSR) study of Fe-exchanged ZSM-5 for selective catalytic reduction of nitric oxide by ammonia [J]. Journal of catalysis 1999, 198, 20~28. [Google Scholar] [CrossRef]
- Li, Z; Shen, L; Huang, W; et al. Kinetics of selective catalytic reduction of NO by NH3 on Fe-Mo/ZSM-5 catalyst [J]. Journal of Environmental Science 2007, 19, 1516~1519. [Google Scholar] [CrossRef]
- Zhou, H; Liu, J; Zhang, Y; et al. Hydrothermal aging alleviates hydrocarbon poisoning effects on high-silica Cu-SSZ-16catalysts for NH3-SCR[J]. Journal of Environmental Sciences 2025, 158(12), 13–23. [Google Scholar] [CrossRef] [PubMed]
- Delahay, G; Valade, D; Guzmán-Vargas, A; et al. Selective catalytic reduction of nitric oxide with ammonia on Fe-ZSM-5 catalysts prepared by different methods [J]. Applied Catalysis B: Environmental 2005, 55, 149~155. [Google Scholar] [CrossRef]
- Kustov, A L; Hansen, T W; Kustova, M; et al. Selective catalytic reduction of NO by ammonia using mesoporous Fe-containing HZSM-5 and HZSM-12 zeolite catalysts: an option for automotive applications [J]. Applied Catalysis B: Environmental 2007, 76, 311~319. [Google Scholar] [CrossRef]
- Wang, X; Chi, R; Gu, L; et al. Effect of water vapor on low temperature SCR performances over Cu and Mn-based catalysts:A comparison study[J]. Journal of Rare Earths 2025, 43(08), 1661–1667. [Google Scholar] [CrossRef]
- Taboada, J B; Oveweg, A R; Crajé, M W J; et al. Systematic variation of 57Fe and Al content in isomorphously substituted and characterization [J]. Microporous and Mesoporous Materials 2004, 75, 237~246. [Google Scholar] [CrossRef]
- Long, R Q; Yang, R T. Reaction mechanism of selective catalytic reduction of NO with NH3 over Fe-ZSM-5 catalyst [J]. Journal of Catalysis 2002, 207, 224~231. [Google Scholar] [CrossRef]
- Berlier, G; Spoto, G; Bordiga, S; et al. Evolution of extraframework iron species in Fe silicalite 1. Effect of Fe content, activation temperature, and interaction with redox agents [J]. Journal of Catalysis 2002, 208, 64~82. [Google Scholar]
- Li, H; Ren, W; Xue, H; et al. Significantly enhanced low-temperature activity and SO2/H2O tolerance of Mn-Ce-Ox/TiO2 catalysts prepared by a facile citric acid assisted impregnation method[J]. Journal of Rare Earths 2025, 43(06), 1195–1204. [Google Scholar] [CrossRef]
- Brundle, C R; Chuang, T J; Wandelt, K. Core and valence level photoemission studies of iron-oxide surfaces and oxidation of iron [J]. Surface Science 1997, 68, 459~468. [Google Scholar] [CrossRef]
- Udovic, T J; Dumesic, J A. Preparation and characterization of magnetite surfaces on metallic iron substrates [J]. Journal of Catalysis 1984, 89, 303~313. [Google Scholar] [CrossRef]
- Fabrizioli, P; Bürgi, T; Burgener, M; et al. Synthesis, structural and chemical properties of iron oxide-silica aerogels. Journal of Materials Chemistry 2002, 12, 619~630. [Google Scholar]
- Chen, H Y; Sachtler, W M H. Activity and durability of Fe/ZSM-5 catalysts for lean burn NOx reduction in the presence of water vapor [J]. Catalysis Today 1998, 42, 73~83. [Google Scholar] [CrossRef]
- Long, R Q; Yang, R T. Characterization of Fe-ZSM-5 catalyst for selective catalytic reduction of nitric oxide by ammonia [J]. Journal of Catalysis 2000, 194, 80~90. [Google Scholar] [CrossRef]
- Malpartida, I; Marie, O; Bazin, P; et al. The NO/NOx ratio effect on the NH3-SCR efficiency of a commercial automotive Fe-zeolite catalyst studied by operando IR-MS [J]. Applied Catalysis B: Environmental 2012, 113-114, 52~60. [Google Scholar] [CrossRef]
- Colombo, M; Nova, I; Tronconi, E. A comparative study of the NH3-SCR reaction over a Cu-zeolite and a Fe-zeolite catalyst [J]. Catalysis Today 2010, 151, 223~230. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).