1. Introduction
Nitrogen oxides (NO
x) are a major component of atmospheric pollution, primarily emanating from energy use, industrial production, and transportation-activities [
1]. NO
x contributes to a range of ecological and environmental issues, including acid rain, photochemical smog, and the exacerbation of the greenhouse effect. Moreover, it poses a significant threat to human health by triggering respiratory diseases. Therefore, the removal of NO
x from the atmosphere is imperative for preserving a clean environment and safeguarding public health [
2,
3].
Among the various DeNO
x technologies, selective catalytic reduction (SCR) has garnered significant research interest. This is due to its ability to facilitate the chemical reaction between NO
x in exhaust gases and a reducing agent, in the presence of a catalyst, converting NO
x into environmentally harmless N
2 [
4]. In particular, methane selective catalytic reduction (CH
4-SCR) technology utilizes methane (CH
4) as a reductant. This method has several advantages, including the abundant availability, cost-effectiveness, and ease of sourcing methane, which suggests a promising future for CH
4-SCR technology in widespread application [
5].
Catalysts constitute a key element of CH
4-SCR DeNO
x technology. In recent years, extensive research has been conducted on zeolite-supported catalysts featuring twelve-membered or five-membered rings, which are pivotal in various catalytic processes [
6]. However, it has been discovered that these catalysts are prone to framework collapse under aqueous conditions, which significantly compromises their structural integrity and catalytic performance [
7]. Identifying a novel zeolite support is pivotal for addressing the issue of catalyst deactivation induced by steam poisoning.
Kwak et al. [
8] have reported that the copper-based zeolite Cu-SSZ-13, characterized by a CHA-type framework, demonstrates commendable catalytic efficacy in NH
3-SCR fields. However, its performance is compromised following thermal treatments exceeding 850 °C, which necessitates a reduction in its high-temperature hydrothermal stability. Consequently, there is an imperative to not only augment the thermal resilience of Cu-SSZ-13 but also to innovate and introduce novel catalytic supports with enhanced robustness. In this vein, a variety of newly synthesized copper-impregnated zeolites with small pores (non-CHA type) have been subjected to NH
3-SCR performance assessments. Studies have documented that Cu-LTA [
9,
10], Cu-KFI [
11], and Cu-AEI [
12,
13] exhibit promising catalytic profiles. Notably, the Cu-SSZ-39 zeolite, which incorporates an AEI-type structure and features cage-like channels with expanded dimensions (3.8×3.8Å), has been highlighted for its exceptional hydrothermal stability and commendable NH
3-SCR catalytic activity [
14,
15,
16]. Fickel et al. [
17] have observed that Cu-SSZ-39 outperforms mesoporous and microporous zeolites in terms of both catalytic activity and hydrothermal stability during NH
3-SCR reactions. Du [
18] has conducted an in-depth investigation into the eight-membered ring channel architecture of Cu-SSZ-39 zeolite, revealing its superior activity under hydrothermal conditions when juxtaposed with Cu-SSZ-13. Remarkably, it sustains its denitration proficiency even after enduring hydrothermal aging at 850 °C for a duration of 16 h. The underlying rationale for this enhanced performance is attributed to the more convoluted channel configuration of SSZ-39 zeolite in contrast to its SSZ-13 counterpart, which effectively mitigates the leaching of aluminum elements and the aggregation of copper species induced by hydrothermal aging. Despite the advancements in CH
4-SCR technology, there is a notable scarcity of research pertaining to catalysts loaded with SSZ-39 zeolites. This observation underscores the potential for further investigation and development in this area.
Based on this, the objective of this research is to synthesize an innovative In/H-SSZ-39 catalyst and to optimize the conditions for its preparation, specifically focusing on the concentration of In ions concentration and the calcination temperature. The optimization will be guided by the evaluation of the catalytic performance in CH4-SCR denitration processes. Subsequently, the catalytic activity of the optimized In/H-SSZ-39 catalyst will be assessed under a variety of reaction conditions. These conditions encompass the concentration of O2, the CH4 to NO ratio, the Gas Hourly Space Velocity (GHSV), and the concentration of water vapor.
2. Experimental
2.1. Catalyst Preparation
In this work, the liquid-phase ion-exchange method was used to synthesize the In/H-SSZ-39 catalysts in the following steps:
The synthesis began with the preparation of a 0.066 M In(NO3)3·4H2O solution from 1 g of indium nitrate in 100 mL of deionized water. Subsequently, 3 g of SSZ-39 zeolites with a Si/Al ratio of 16 were introduced into the solution, which was then mixed thoroughly and magnetically stirred in a constant temperature water bath at 85 °C for 8 h. Post-stirring, the solution underwent centrifugal washing with deionized water five times. The resulting solid was dried in an oven at 80 °C for 12 h before being calcined in a muffle furnace at 500 °C under an air atmosphere for 3 h. The final catalyst was pressed, ground, and sieved through a 40-60 mesh sieve before being stored in a sample tube for subsequent activity evaluation.
In the optimization phase, variations were introduced to the synthesis process to fine-tune the catalyst’s properties. For the optimization of indium content, different amounts of indium nitrate (1 g, 1.5 g, 2 g, and 2.5 g) were dissolved in 100 mL of deionized water during the initial step. Additionally, to determine the optimal calcination temperature, the catalysts were subjected to calcination at temperatures (450 °C, 500 °C, 550 °C, and 600 °C) in the final step.
2.2. Catalytic Activity Measurements
The activity evaluation experiments in this study [
19,
20] was conducted using the programmed temperature-raising surface reaction (TPSR) experiments. The reaction gas components and their respective concentrations were specified as follows: NO (400 ppm), CH
4 (600 ppm), O
2 (10 vol.%), and water vapor (6 vol.%). Ar was utilized as a diluent gas to maintain a total flow rate of the mixed gases at 100 mL/min, with the GHSV controlled at 21000 h
-1. The mixed reaction gas was passed into the quartz reaction tube at room temperature. Once NO
x reached adsorption equilibrium, the programmed temperature increase was initiated to perform the catalytic reaction. The temperature of the reactor was ramped up from 100 °C to 675 °C at a rate of 5 °C/min
-1. The contents of CH
4, CO, CO
2, NO
x, NO, NO
2 and N
2O were detected separately.
The catalytic activity of the catalyst for CH
4-SCR was assessed using the following parameters: NO
x removal efficiency (η), CH
4 conversion (γ), CH
4 selectivity (α), calculated using Equations (1)–(3), respectively. Here, c(NO
x)
in represents the initial concentration of NO
x, c(NO
x)
out is the concentration of NO
x post-reaction; c(CH
4)
in is the initial concentration of CH
4, c(CH
4)
out is the concentration of CH
4 after the reaction, and c(N
2)
out denotes the concentration of N
2 after the reaction.
3. Results and Discussion
3.1. Effect of Preparation Conditions
3.1.1. Si/Al Ratio
The Si/Al ratio is a determinant factor in modulating the acidity of catalysts, which significantly influences catalytic performance [
21]. In this section, molecular sieves with Si/Al ratios of 16 and 4.9 were utilized as supports to fabricate In/H-SSZ-39 catalysts. The synthesis was standardized with an ion-exchange solution concentration of 0.066 M and a calcination temperature of 500 °C. The catalytic performance of the resultant catalysts was assessed for CH
4-SCR DeNO
x under standardized reaction conditions: 400 ppm NO, 600 ppm CH
4, 10% O
2, 6% H
2O, and GHSV of 21000 h
-1.
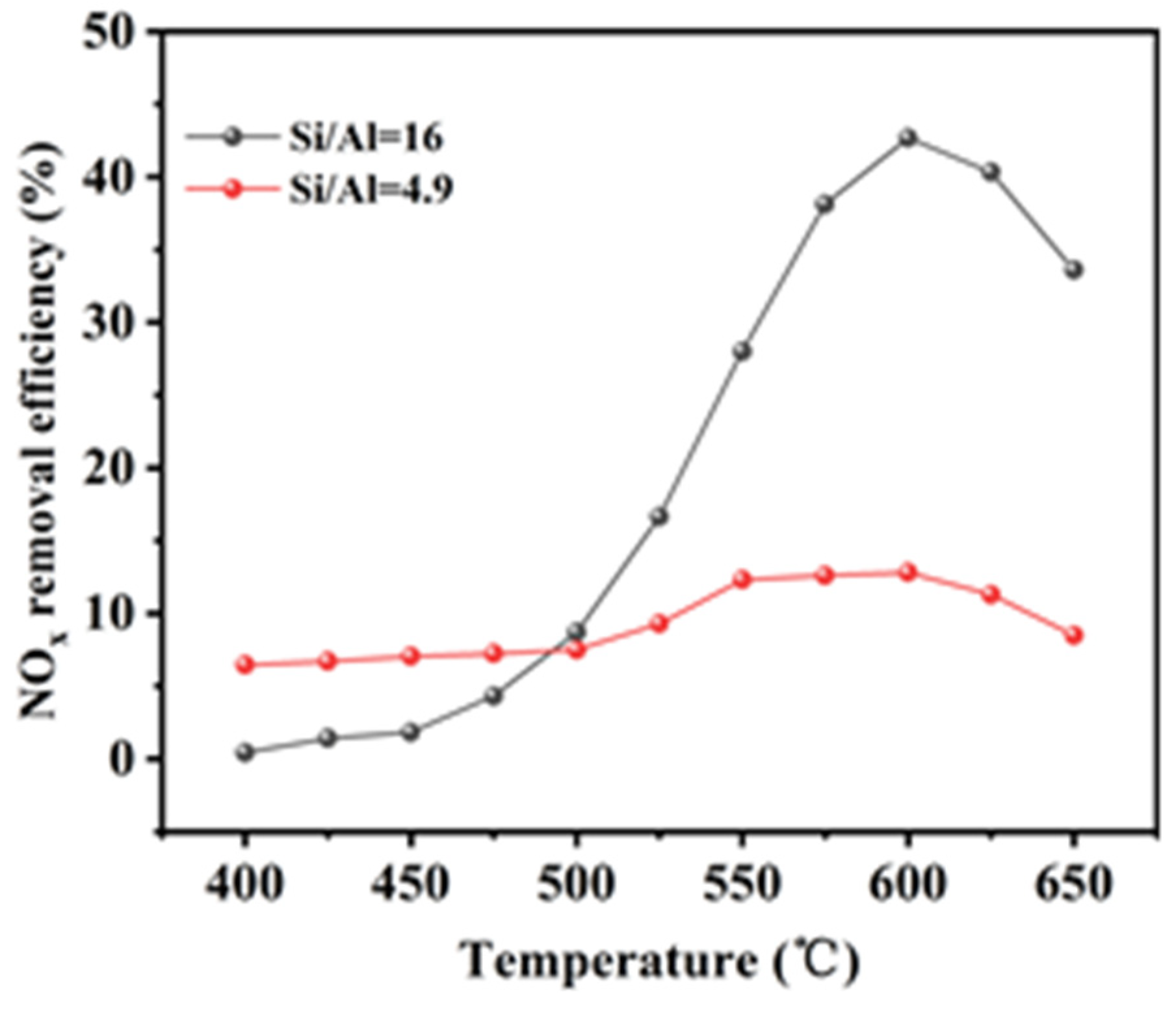
Analysis of
Figure 1 revealed that the In/H-SSZ-39 catalyst with a Si/Al ratio of 16 exhibited a NO
x removal efficiency of 42.1%, contrasting with the 15.0% DeNO
x efficiency of the catalyst with a Si/Al ratio of 4.9. This disparity underscored the substantial impact of the Si/Al ratio on catalytic activity. A catalyst with a low Si/Al ratio was characterized by reduced acidity, which correlated with a diminished number of active sites, thereby adversely affecting the CH
4-SCR reaction [
22]. Consequently, the In/H-SSZ-39 catalyst with a Si/Al ratio of 16 was selected for further optimization of the synthesis conditions.
3.1.2. Effect of Calcination Temperature
The In/H-SSZ-39 catalysts were synthesized and their catalytic performance was evaluated under varying calcination temperatures (450 °C, 500 °C, 550 °C, 600 °C), with the ion-exchange solution concentration maintained at 0.066 M. The experimental conditions were standardized as follows: 400 ppm NO, 600 ppm CH
4, 10% O
2, 6% H
2O, and GHSV of 23600 h
-1. The results are depicted in
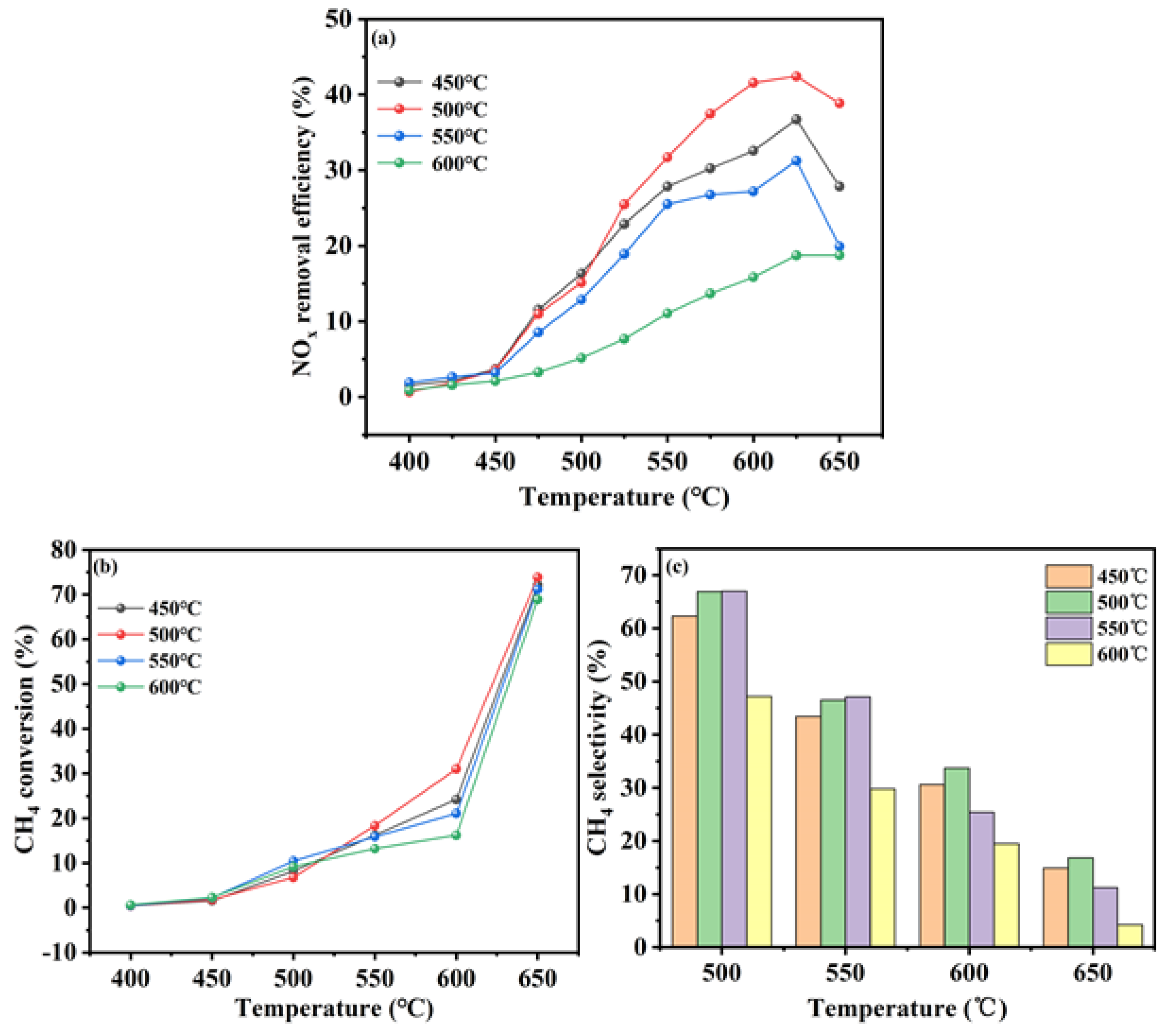
Figure 2a–c.
Figure 2a illustrated the catalytic performance of the In/H-SSZ-39 catalysts prepared at different calcination temperatures. It was observed that the catalytic activity initially increased with temperature and then declined. The In/H-SSZ-39 catalyst calcined at 500 °C demonstrated the highest DeNO
x efficiency at 42.1%. The catalyst prepared at 450 °C exhibited the next highest efficiency, with a NO
x removal efficiency of 37.6%. In contrast, the catalyst calcined at 600 °C showed markedly reduced activity, with the performance not surpassing 20%.
Figure 2b presents the CH
4 conversion of the In/H-SSZ-39 catalysts. The conversion of CH
4 increased progressively with temperature, peaking at 500 °C. This suggested that a greater proportion of CH
4 participates in the activation reaction at this temperature, thereby enhancing the catalytic reduction of NO
x and the overall DeNO
x efficiency.
Figure 2c examined the CH
4 selectivity of the In/H-SSZ-39 catalysts across different calcination temperatures. The selectivity initially rised and then falled with increasing temperature, with the optimal at 500 °C. This trend was attributed to the increased likelihood of the methane combustion side reaction at higher temperatures, which reduces CH
4 selectivity. Notably, at 600 °C, CH
4 selectivity correlated positively with catalytic performance, with the highest selectivity observed for the catalyst calcined at 500 °C.
3.1.3. Effect of In Ions Concentration
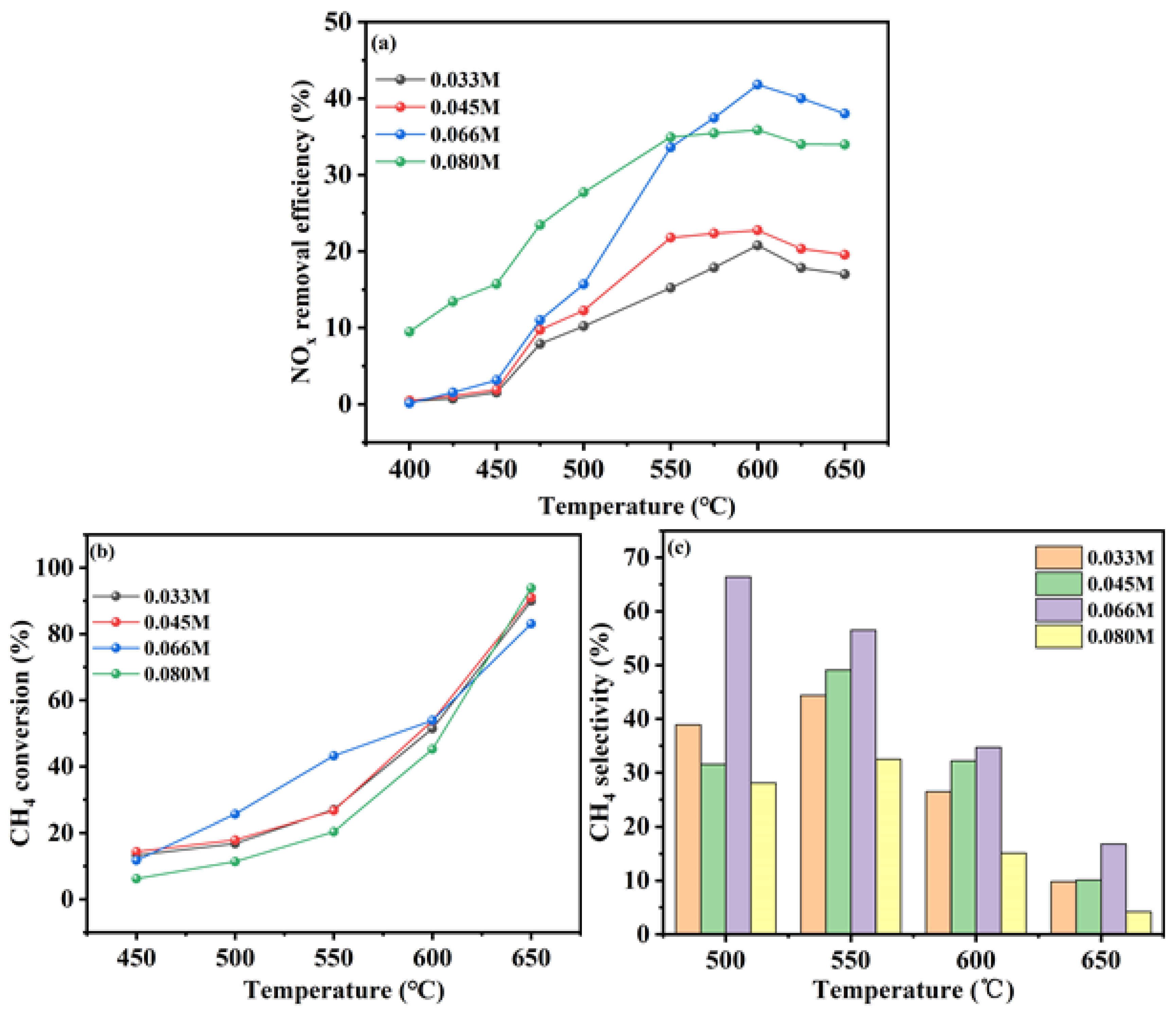
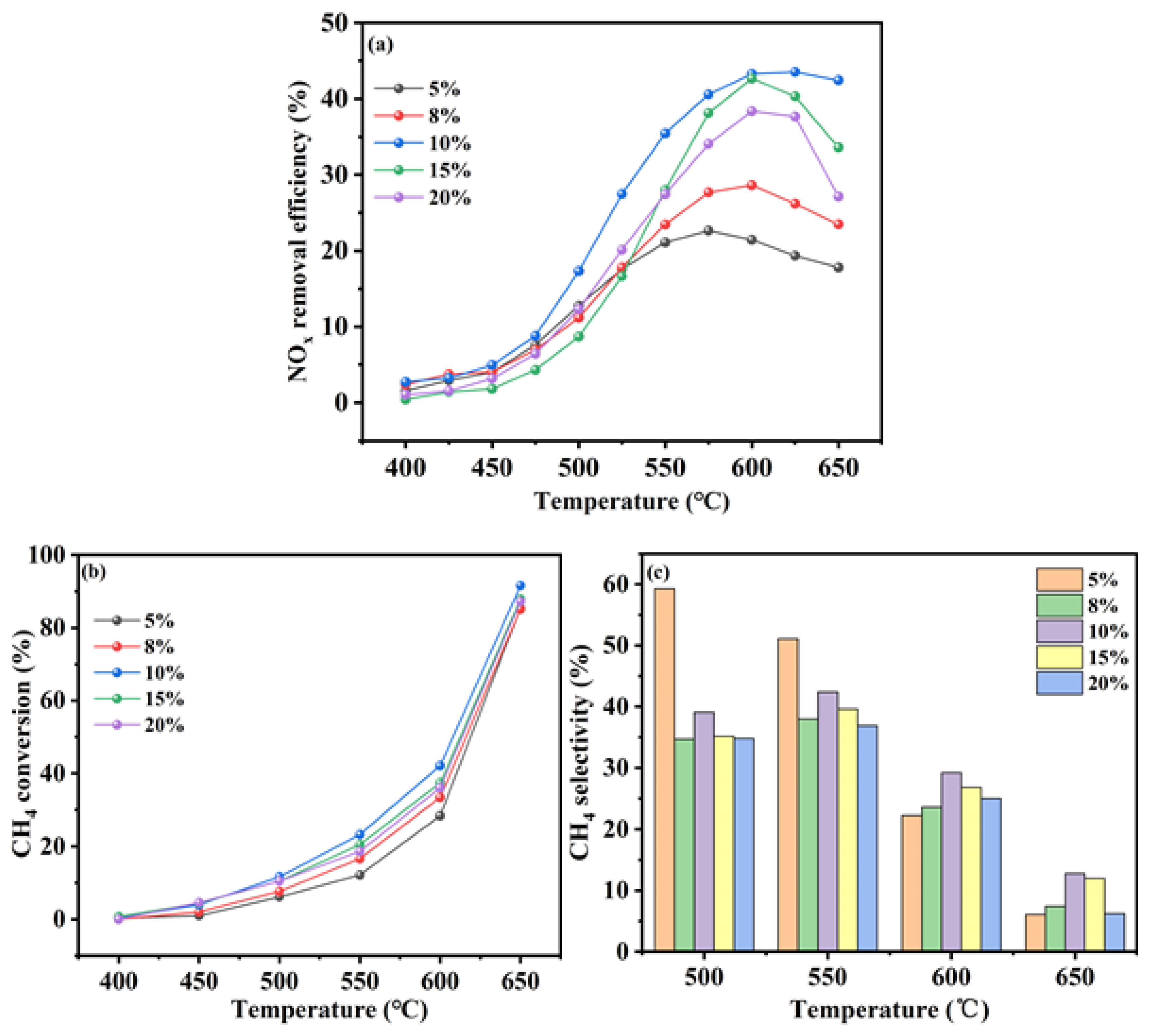
The loading of indium (In) on the catalysts was systematically varied by employing different concentrations of In ions in the ion-exchange solution. The In/H-SSZ-39 catalysts were synthesized using ion-exchange solutions with In concentrations of 0.033 M, 0.045 M, 0.066 M, and 0.08 M, each followed by a calcination step at 500 °C. The catalytic performance of the synthesized catalysts was evaluated under NO concentration of 400 ppm, CH4 concentration of 600 ppm, O2 concentration of 10%, H2O content of 6%, and GHSV of 21000 h-1.
Figure 3a illustrated the catalytic performance of the In/H-SSZ-39 catalysts as a function of the In ion concentration. The catalytic activity was observed to increase with increasing In concentration, reaching a peak, and then declining. Specifically, at an In concentration of 0.045 M, the In/H-SSZ-39 catalyst exhibited a maximum NO
x removal efficiency of 21.4%. This rate peaked at 42.1% for the catalyst prepared with a 0.066 M solution at 605 °C, beyond which the DeNO
x efficiency decreased to 35.1% at an In concentration of 0.08 M. These findings suggested that the In loading on the catalyst becomes saturated at an ion-exchange solution concentration of 0.066 M, with further increases in concentration leading to a decrease in catalytic activity.
Figure 3b presented the CH
4 conversion of the In/H-SSZ-39 catalysts under various In ion concentration conditions. When the In ion concentration was 0.066 M, the In/H-SSZ-39 catalyst demonstrated superior CH
4 conversion at lower temperatures compared to other formulations, with minimal differences observed above 600 °C. At this temperature, CH
4 conversions were consistent across all catalysts at approximately 45%, except for the catalyst prepared with an 0.08 M solution, which showed a slightly lower conversion rate of 40%.
Figure 3c shows the CH
4 selectivity of the In/H-SSZ-39 catalysts. At a reaction temperature of 500 °C, When the In ion concentration was 0.066 M, the In/H-SSZ-39 catalyst displayed the highest CH
4 selectivity, followed by the 0.045 M In ion concentration. As the reaction temperature increased, the CH
4 selectivity of the catalysts decreased, likely due to the onset of methane combustion side reactions.
In summary, the In/H-SSZ-39 catalyst prepared with an In ion concentration of 0.066 M and calcined at 500 °C exhibited the highest NOx removal efficiency under hydrothermal conditions.
3.2. Effect of Reaction Conditions
3.2.1. CH4/NO Ratio
The CH
4-SCR process primarily removes NO
x through the reaction of methane, nitric oxide, and oxygen to form carbon dioxide, nitrogen, and water, which are non-polluting substances. The principal reaction is represented by the equation [
23]:
The ratio of methane to nitrogen is pivotal to the reaction’s effectiveness and indicative of methane utilization efficiency across different CH
4/NO ratios. Experiments were conducted under the following conditions: a calcination temperature of 500 °C, an oxygen concentration of 10%, a water vapor concentration of 6%, an airspeed of 21000 h
-1, with argon as the equilibrium gas, and a NO concentration of 400 ppm. The CH
4/NO ratio was varied by adjusting the methane concentration to ratios of 1, 1.5, and 2. The results are illustrated in
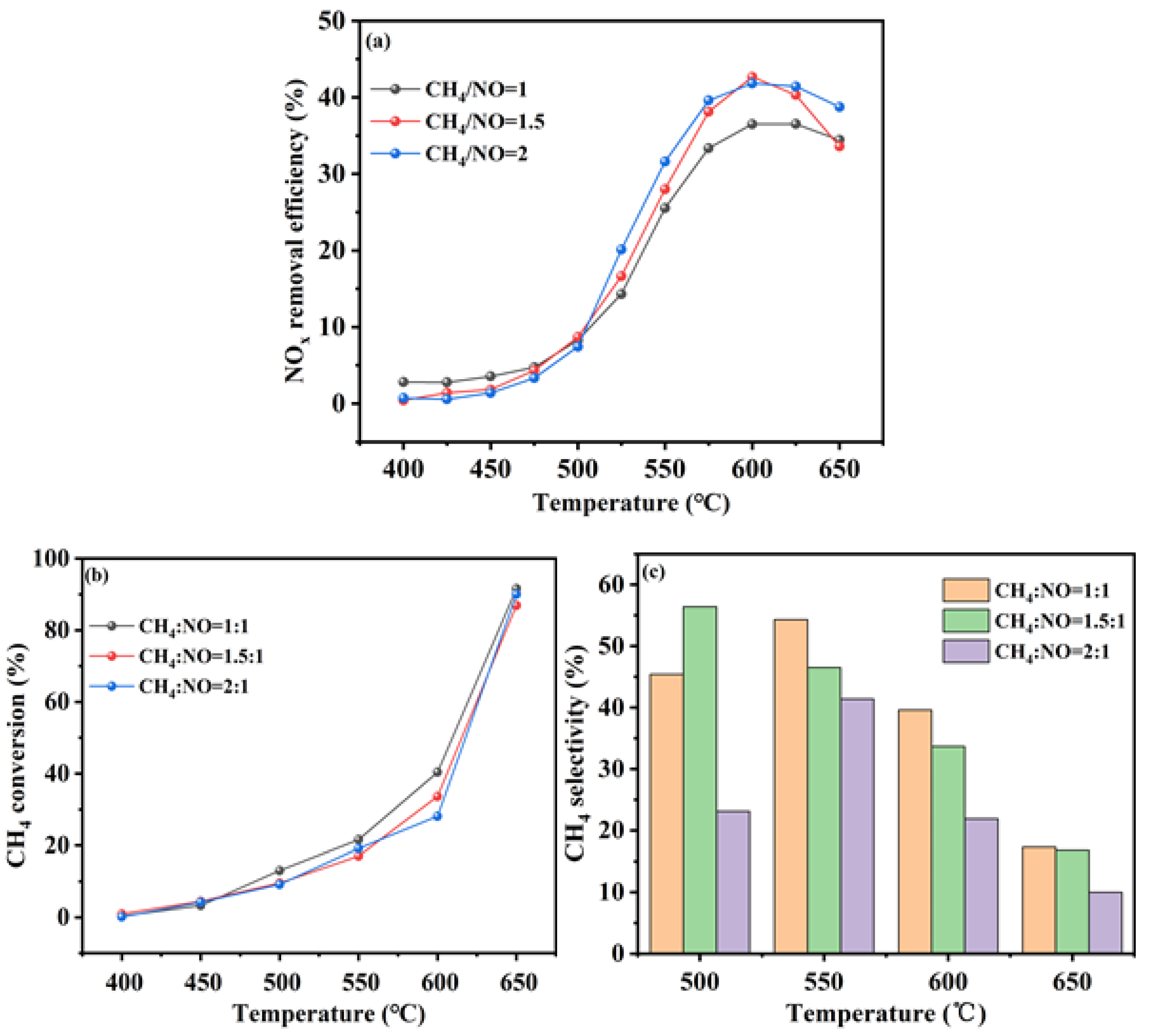
Figure 4.
Figure 4a presented the NO
x removal efficiency of the In/H-SSZ-39 catalysts for varying CH
4 to NO
x concentration ratios. The DeNO
x efficiency was observed to increase with the CH
4/NO ratio, particularly at temperatures above 500 °C. At CH
4/NO ratios of 1.5 and 2, the catalyst exhibited optimal performance at 600 °C, achieving the highest NO
x removal efficiency of 42.1%. At a CH
4/NO ratio of 1, the catalyst’s NO
x removal efficiency peaked at 33.0%. From a cost-effectiveness perspective, a CH
4/NO ratio of 1.5 emerged as the most favorable reaction condition.
Figure 4b,c depicted the CH
4 conversion and selectivity of the In/H-SSZ-39 catalyst under hydrothermal conditions for different CH
4 to NO
x concentration ratios, respectively.
Figure 4b indicated that the CH
4 conversion declined as the CH
4 to NO
x concentration increased. A lower CH
4 to NO
x ratio resulted in a higher CH
4 conversion, with the In/H-SSZ-39 catalyst at a CH
4/NO ratio of 1.5 showing a 28.1% CH
4 conversion at 600 °C.
Figure 4c demonstrated that CH
4 selectivity initially increased with temperature and then decreased. The highest CH
4 selectivity of 57.3% was recorded at a reaction temperature of 500 °C for a CH
4/NO
x ratio of 1.5. At 600 °C, CH
4 selectivity was positively correlated with catalytic performance, with the catalyst at a CH
4/NO ratio of 1.5 displaying a CH
4 selectivity of 35%. Consequently, subsequent experiments were conducted based on the 1.5:1 CH
4/NO ratio for further catalyst modification studies.
3.2.2. O2 Concentration
Oxygen (O
2) plays a critical role as a reactant in the DeNO
x process. An insufficient concentration of O
2 may lead to incomplete reactions between methane and NO
x, while an excess can result in excessive combustion of methane. Therefore, determining the optimal O
2 concentration was crucial for maximizing the catalyst’s performance. This section reports on the DeNO
x performance of the In/H-SSZ-39 catalyst at varying O
2 concentrations, while keeping other experimental parameters constant. These parameters include the optimized preparation conditions (calcination temperature of 500 °C, In concentration of 0.066 M) and reaction conditions (400 ppm NO, 600 ppm CH
4, 6% water vapor, argon as the equilibrium gas). Oxygen concentrations tested were 5%, 8%, 10%, 15%, and 20%. The results are presented in
Figure 5.
Figure 5a illustrated the NO
x removal efficiency of the In/H-SSZ-39 catalyst under hydrothermal conditions at different O
2 concentrations. The NO
x removal efficiency initially increases with O
2 concentration, reaching a peak, and then declines. Notably, the efficiency rose significantly from 5% to 10% O
2, with the highest NO
x removal efficiency of 23.1% achieved at 575 °C under 5% O
2, and 42.1% at 600 °C under 10% O
2. At O
2 concentrations of 15% and 20%, the NO
x removal efficiency decreased, although the decline was less pronounced at 15%, indicating the catalyst’s robustness against oxygen enrichment.
Figure 5b depicted the CH
4 conversion of the In/H-SSZ-39 catalyst under hydrothermal conditions at various O
2 concentrations. CH
4 conversions increased with temperature, with minimal differences observed between the concentrations tested. At 600°C, the CH
4 conversion was 40.0% at 10% O
2 and approximately 33.0% at both 15% and 20% O
2. These results suggest that the In/H-SSZ-39 catalyst exhibits its best hydrothermal DeNO
x performance under an O
2 concentration of 10%.
Figure 5c showed the CH
4 selectivity of the catalyst under hydrothermal conditions across different O
2 concentrations. Between 400 °C and 550 °C, a lower O
2 concentration of 5% offered better CH
4 selectivity, possibly due to high O
2 concentrations promoting non-selective oxidation of CH
4. At 600 °C, CH
4 selectivity was comparable across all tested O
2 concentrations, with values of 30.5% at 10% O
2 and 27.4% at 15% O
2 concentration.
In conclusion, subsequent experiments will be conducted based on the optimal reaction condition of an O2 concentration of 10% for further catalyst modification studies.
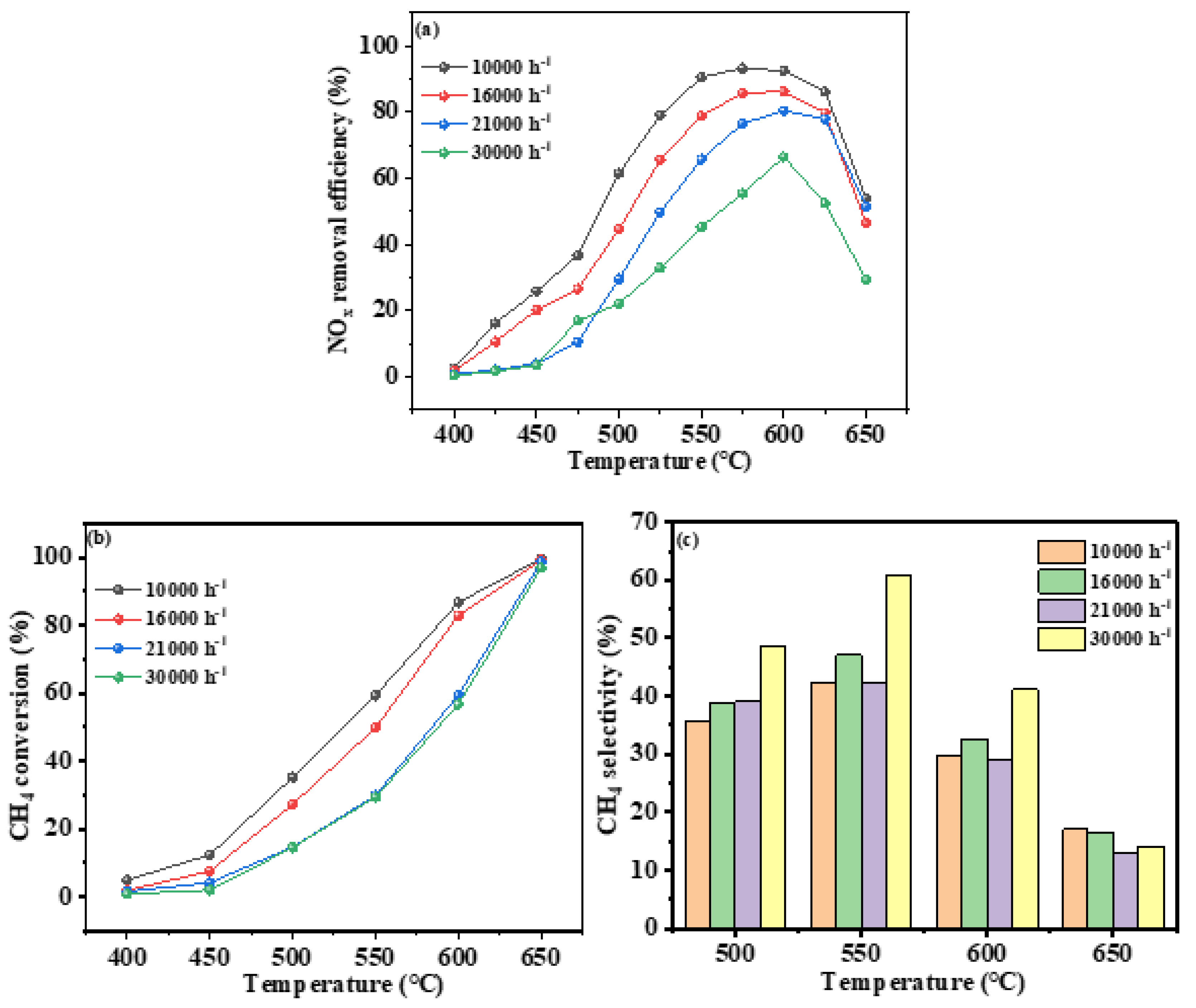
3.2.3. Gaseous Hourly Space Velocity
Gaseous hourly space velocity is a critical parameter in the industrial application of catalysts, as it affects the catalyst’s ability to maintain high catalytic performance at high flow rates. This study investigates the impact of airspeed on the performance of In/H-SSZ-39 catalysts under hydrothermal conditions, with other experimental conditions held constant. These include a NO concentration of 400 ppm, a CH
4/NO ratio of 1.5, a H
2O concentration of 6%, and an O
2 concentration of 10%. GHSV tested were 10000 h
-1, 16000 h
-1, 21000 h
-1, and 30000 h
-1. The experimental results are depicted in
Figure 6.
Figure 6a shows the CH
4-SCR NO
x removal efficiency of the In/H-SSZ-39 catalyst at various airspeeds and temperatures. The results indicated that lower GHSV correspond to higher NO
x removal efficiency, lower optimal activity temperatures, and broader activity windows. At an airspeed of 10000 h
-1, the widest catalyst temperature window was observed, with the highest NO
x removal efficiency of 59.0% achieved at 590 °C. At an airspeed of 16000 h
-1, the highest NO
x removal efficiency was 49.3% at 600 °C. The efficiencies were 41.9% and 39.5% at GHSV of 21000 h
-1 and 30000 h
-1, respectively.
Figure 6b,c presented the CH
4 conversion and selectivity of the In/H-SSZ-39 catalyst at different GHSV under hydrothermal conditions.
Figure 6b demonstrates that CH
4 conversion generally increases with decreasing airspeed, approaching 100% at 650 °C for all airspeeds except 30000 h
-1.
Figure 6c revealed that while low GHSV enhance NO
x removal efficiency and CH
4 conversion, they also reduced CH
4 selectivity, particularly in the temperature range of 500 °C to 600 °C. This suggested that at high temperatures, low airspeed does not improved CH
4 selectivity but instead promoted the selective oxidation of CH
4, thereby enhancing the DeNO
x activity of the catalyst.
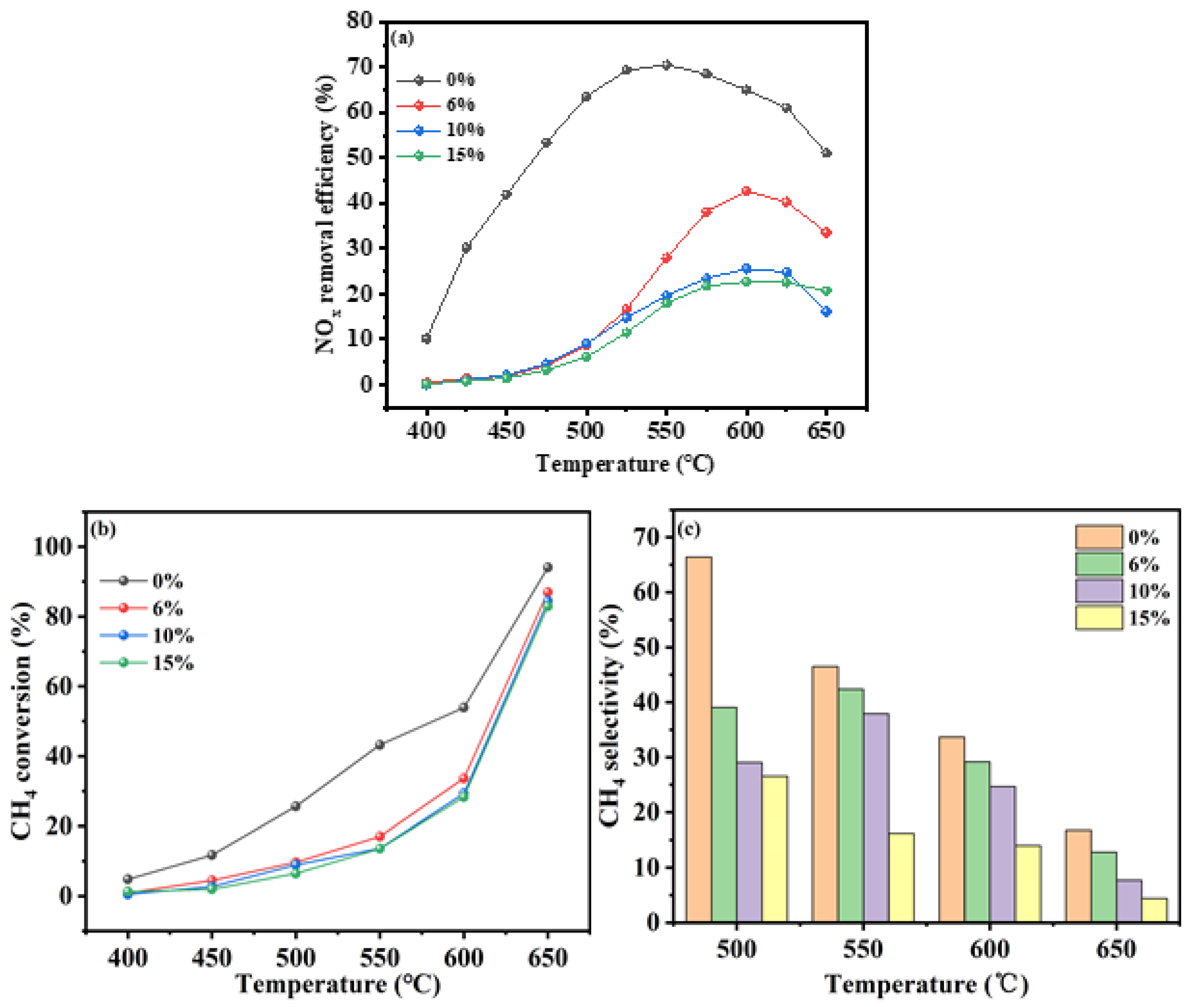
3.2.4. Water Vapor
The ability of a catalyst to tolerate various concentrations of water vapor, particularly at high levels, is a crucial aspect of its performance, especially in industrial applications where hydrothermal conditions are common. This section presented experiments that assess the In/H-SSZ-39 catalyst’s tolerance to water vapor while maintaining all other conditions constant: an O
2 of 10%, a CH
4/NO ratio of 1.5, and a NO
x concentration of 400 ppm. The water vapor concentrations tested were 0%, 5%, 10%, and 15%. The outcomes of these experiments are detailed in
Figure 7.
Figure 7 illustrated the NO
x removal efficiency of the In/H-SSZ-39 catalyst under hydrothermal conditions at varying water vapor levels. In the absence of water vapor, the catalyst demonstrates a broad activity temperature window, achieving over 70.0% NO
x removal at 550 °C. However, at a water vapor concentration of 5%, the efficiency at the same temperature drops to below 35.0%, indicating a significant impact of water vapor on the catalyst’s DeNO
x performance. As water vapor concentration further increases, the negative effect intensifies, leading to a progressive decline in the DeNO
x performance. At a 10% water vapor concentration, the NO
x removal efficiency was reduced to only 19.2%.
Figure 7b depicted the CH
4 conversion of the In/H-SSZ-39 catalyst for the CH
4-SCR reaction across different water contents and reaction temperatures. The data indicated that methane conversion increases with temperature, with the highest rates occurring between 400-650 °C in the absence of water, suggesting that water inhibited methane activation. At a reaction temperature of 600 °C, the methane conversion with 6% water content was 28.0%.
Figure 7c presented the CH
4 selectivity of the In/H-SSZ-39 catalyst at different water contents. Similar to conversion rates, CH
4 selectivity peaks when the reaction system was devoid of water, indicating that water vapor adversely affects both the conversion and selectivity of CH
4.
In summary, the experiments demonstrated that water vapor content significantly influenced the catalytic activity of the In/H-SSZ-39 catalyst. In corroboration with the literature [
24,
25,
26], the catalyst’s activity was also attenuated under conditions of high water vapor content, suggesting that water might occupy the active sites, thereby impeding the oxidation of NO and activation of CH
4, which consequently leaded to a decrease in catalytic performance. Future work could focus on the enhancement of the SSZ-39 supported catalyst’s activity through bimetallic modification to increase the number of active sites, presenting a potential avenue to improve the catalytic efficacy of the SSZ-39 zeolite under hydrothermal conditions.
4. Conclusion and Perspectives
In this paper, a novel In-H-SSZ-39 catalyst supported on a small-pore zeolite with an eight-membered ring framework was synthesized. The preparation conditions of the catalysts were optimized, and the effects of their reaction conditions were investigated, obtaining the following conclusions:
(1) The In/H-SSZ-39 catalyst demonstrated a peak catalytic activity of 42.1% at 605 °C, under the optimized synthesis conditions where the indium ion concentration was 0.066 M and the calcination temperature was maintained at 500 °C. These findings were obtained under reaction conditions comprising a CH4 concentration of 600 ppm, a NO concentration of 400 ppm, an O2 concentration of 10 vol%, and a Gas Hourly Space Velocity (GHSV) of 21000 h-1.
(2) The In/H-SSZ-39 catalyst displayed a negative correlation between GHSV and NO removal efficiency, achieving 59.0% NO removal efficiency at 580 °C with a GHSV of 10000 h-1. This underscores the necessity for GHSV optimization to enhance the catalyst’s performance in NOx reduction applications.
(3) The water content presented a considerable effect on In/H-SSZ-39 catalyst and elevated H2O concentration reduced the catalytic performance. This decline in catalytic performance is predominantly attributed to the occupancy of active sites by water molecules, which subsequently hinders the oxidation of NO and the activation of CH4, culminating in the observed deactivation of the catalyst. Future research endeavors could be directed towards augmenting the intrinsic activity of the SSZ-39 zeolite-supported catalyst by employing bimetallic doping strategies, which may effectively amplify the availability of active sites. This approach holds promise as a viable strategy to bolster the catalytic potency of SSZ-39 under the demanding hydrothermal conditions prevalent in practical applications.
Acknowledgments
We express our sincere gratitude for the financial support provided by the Special Project for Sustainable Development Science Technology in Shenzhen (KCXFZ20201221173000001). The Natural Science Foundation of Guangdong (No. 2022A1515011075), the Special Foundation for Sustainable Development Research of Shenzhen (No. KCXST20221021111405012).
Conflicts of interest: The authors declare no competing interests.
References
- Lim, J.; Shin, J.; Ahn, N.; Heo, I.; Hong, S. Selective catalytic reduction of NO with CH4 over cobalt-exchanged cage-based, small-pore zeolites with different framework structures. Appl. Catal. B: Environ. 2020, 267, 118710. [Google Scholar] [CrossRef]
- Jing, G.; Li, J.; Yang, D.; Hao, J. Promotional mechanism of tungstation on selective catalytic reduction of NOx by methane over In/WO3/ZrO2. Appl. Catal. B: Environ. 2009, 91, 123–134. [Google Scholar] [CrossRef]
- Shan, Y.; Sun, Y.; Du, J.; Zhang, Y.; Shi, X.; Yu, Y.; Shan, W.; He, H. Hydrothermal aging alleviates the inhibition effects of NO2 on Cu-SSZ-13 for NH3-SCR. Appl. Catal. B Environ. 2020, 275, 119105. [Google Scholar] [CrossRef]
- Bellmann, A.; Atia, H.; Bentrup, U.; Brueckner, A. Mechanism of the selective reduction of NOx by methane over Co-ZSM-5. Appl. Catal. B: Environ. 2018, 230, 184–193. [Google Scholar] [CrossRef]
- Li, Y.; Armor, J. Selective catalytic reduction of NO, with methane over metal exchanged zeolites. Appl. Catal. B Environ. 1993, 2, 239–256. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, G.; He, J.; Wen, Z.; Li, Z.; Gu, T.; Ding, R.; Zhu, Y.; Zhu, R. Effect of preparation and reaction conditions on the performance of In/H-Beta for selective catalytic reduction of NOx with CH4. Chemosphere 2020, 252, 126458. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, Y.; Wang, Y.; Li, Z.; Nkinahamira, F.; Zhu, R.; Li, C.; Zhang, J.; Sun, S.; Zhu, Y.; Li, H.; Li, C. The poisoning mechanism of H2O/SO2 to In/H-Beta for selective catalytic reduction of NOx with methane. Appl. Catal. A General 2023, 649, 118973. [Google Scholar] [CrossRef]
- Kwak, J.; Tonkyn, R.; Kim, D.; Szanyi, J.; Peden, C. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Jo, D.; Ryu, T.; Park, G. Synthesis of high-silica LTA and UFI zeolites and NH3-SCR performance of their copper-exchanged form. ACS Catal. 2016, 6, 2443–2447. [Google Scholar] [CrossRef]
- Jo, D.; Park, G.; Ryu, T.; Hong, S. Economical synthesis of high-silica LTA zeolites: a step forward in developing a new commercial NH3-SCR catalyst. Appl. Catal. B Environ. 2019, 243, 212–219. [Google Scholar] [CrossRef]
- Kim, J.; Cho, S.; Kim, D. Facile synthesis of KFI-type zeolite and its application to selective catalytic reduction of NOx with NH3. ACS Catal. 2017, 7, 6070–6081. [Google Scholar] [CrossRef]
- Ming, S.; Chen, Z.; Fan, C. The effect of copper loading and silicon content on catalytic activity and hydrothermal stability of Cu-SAPO-18 catalyst for NH3-SCR. Appl. Catal. A: Gen. 2018, 559, 47–56. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, C.; Pang, L.; Ming, M.S.; Guo, W.; Liu, P.; Chen, H.; Li, T. One-pot synthesis of high-performance Cu-SAPO-18 catalyst for NO reduction by NH3-SCR: Influence of silicon content on the catalytic properties of Cu-SAPO-18. Chem. Eng. J. 2018, 348, 608–617. [Google Scholar] [CrossRef]
- Corma, A.; Puche, M.; Rey, F.; Sankar, G.; Teat, S. A zeolite structure (ITQ-13) with three sets of medium-pore crossing channels formed by 9- and 10-Rings. Angew. Chem. Int. Ed. 2003, 42, 1156–1159. [Google Scholar] [CrossRef]
- Kwak, J.; Tran, D.; Burton, S.; Szanyi, J.; Lee, J. C. Peden Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J. Catal. 2012, 287, 203–209. [Google Scholar] [CrossRef]
- Du, J.; Han, S.; Huang, C.; Shan, Y.; Zhang, Y.; Shan, W.; He, H. Comparison of precursors for the synthesis of Cu-SSZ-39 zeolite catalysts for NH3-SCR reaction. Appl. Catal. B Environ. 2023, 338, 123072. [Google Scholar] [CrossRef]
- Fickel, D.; D’Addio, E.; Lauterbach, J. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B: Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Du, J.; Han, S.; Huang, C.; Shan, Y.; Zhang, Y.; Shan, W.; He, H. Comparison of precursors for the synthesis of Cu-SSZ-39 zeolite catalysts for NH3-SCR reaction. Appl. Catal. B Environ. 2023, 338, 123072. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, L.; Wang, Y.; Zhang, J.; Zhu, R.; Li, C.; Hong, M. Amino-acid modulated hierarchical In/H-Beta zeolites for selective catalytic reduction of NO with CH4 in the presence of H2O and SO2. Nanoscale 2022, 14, 5915–5928. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Wang, Y.; Zhu, R.; Sun, S.; Zhang, J.; Li, C.; Hong, M. Desilication tuning of In/Hβ catalysts for sulfur- and steam-resistant CH4-SCR of NO. Catal. Commun. 2023, 175, 106619. [Google Scholar] [CrossRef]
- Campa, C.; Pietrogiacomi, D.; Occhiuzzi, M. The simultaneous selective catalytic reduction of N2O and NOx with CH4 on Co- and Ni-exchanged mordenite. Appl. Catal. B: Environ. 2015, 168–169, 293–302. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. Supported proline and proline-derivatives as recyclable organocatalysts. Chem. Soc. Rev. 2008, 37, 1666–1688. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kennedy, M.; Dlugogorski, Z. Partial oxidation of methane with nitrous oxide forms synthesis gas over cobalt exchanged ZSM-5. Catal. Commun. 2014, 53, 42–46. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Wang, Y.; Li, Z.; Nkinahamira, F.; Zhu, R.; Zhang, J.; Sun, S.; Zhu, Y.; Li, H.; Li, C. The poisoning mechanism of H2O/SO2 to In/H-Beta for selective catalytic reduction of NOx with methane. App. Catal. A Gen. 2023, 649, 118973. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Zhu, R.; Zhang, J.; Ding, R.; Wen, Z.; Zhu, Y.; Zhang, G.; Chen, B. Mechanism of the selective catalytic reduction of NOx with CH4 on In/H-beta. Catal. Sci. Technol. 2021, 11, 5050–5061. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, J.; Gao, L.; Shan, S. Selective catalytic reduction of NO with NH3 and CH4 over zeolite supported indiumcerium bimetallic catalysts for lean-burn natural gas engines. Chem. Eng. J. 2021, 403, 126394. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).