Submitted:
19 June 2024
Posted:
21 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Structure and Composition Characterization of Electrocatalysts
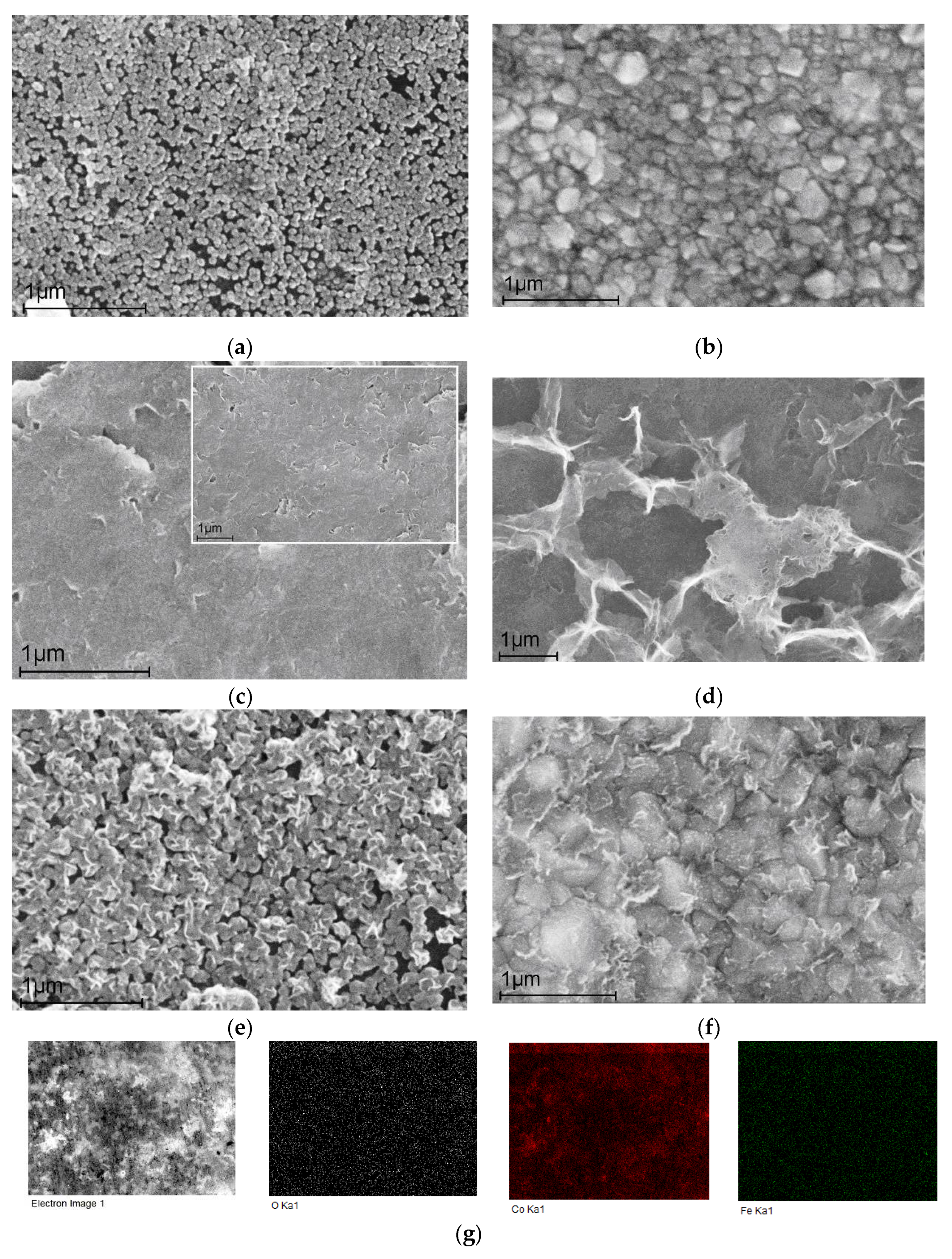
2.1.1. SEM analysis
2.1.2. The Electrocatalysts Used in the Study
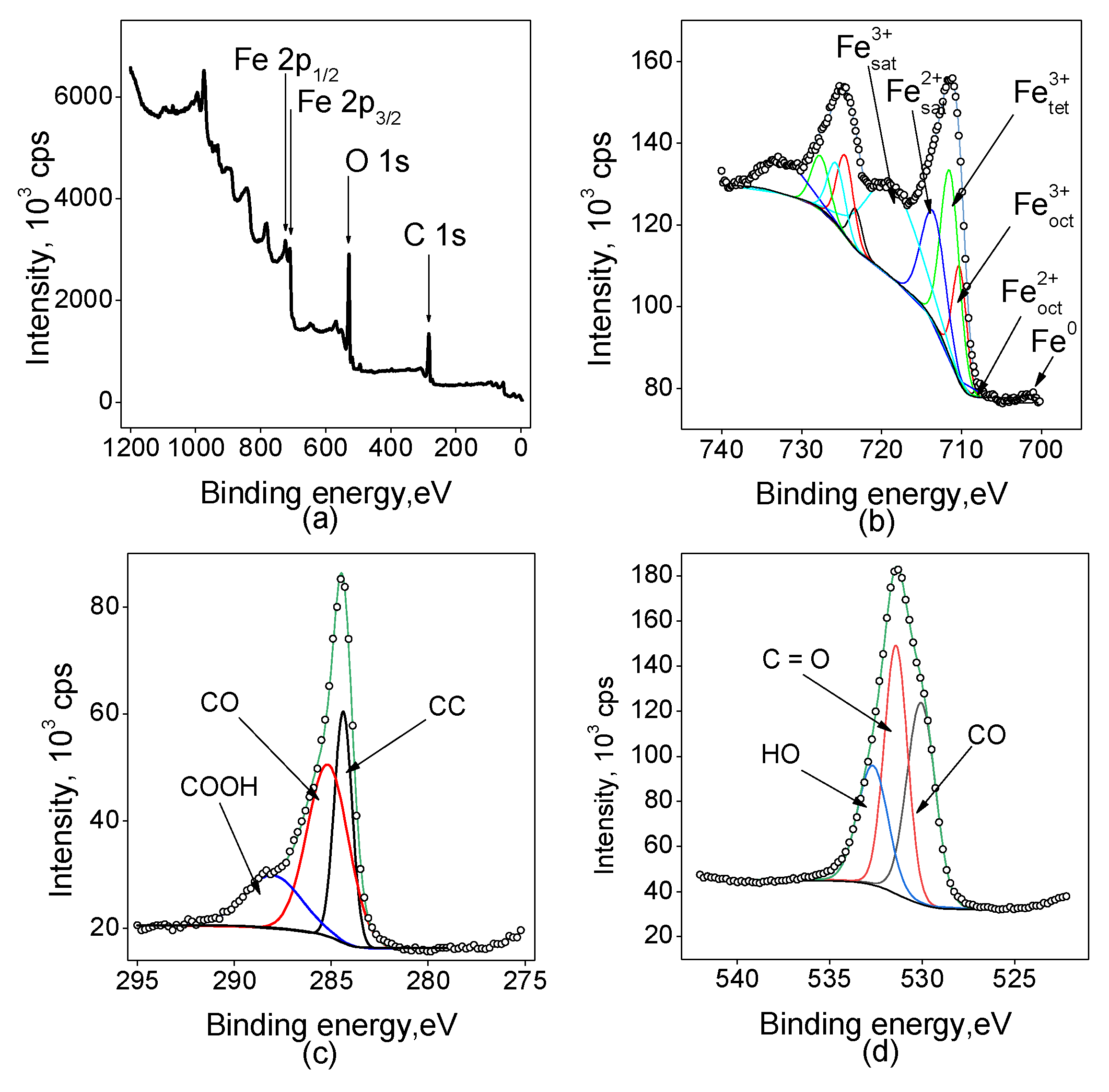
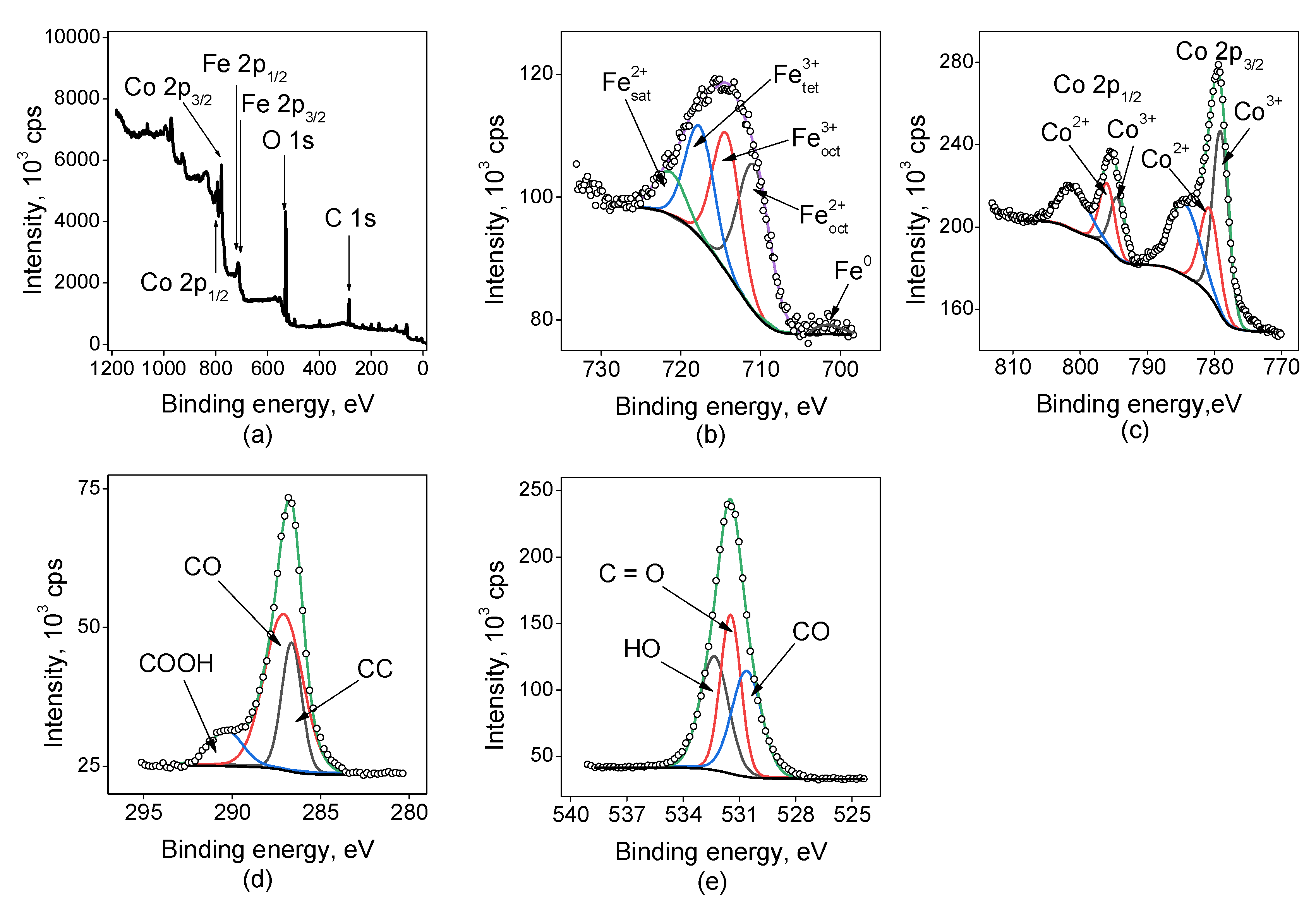
2.1.3. X-Ray Photoelectron Spectroscopy (XPS)
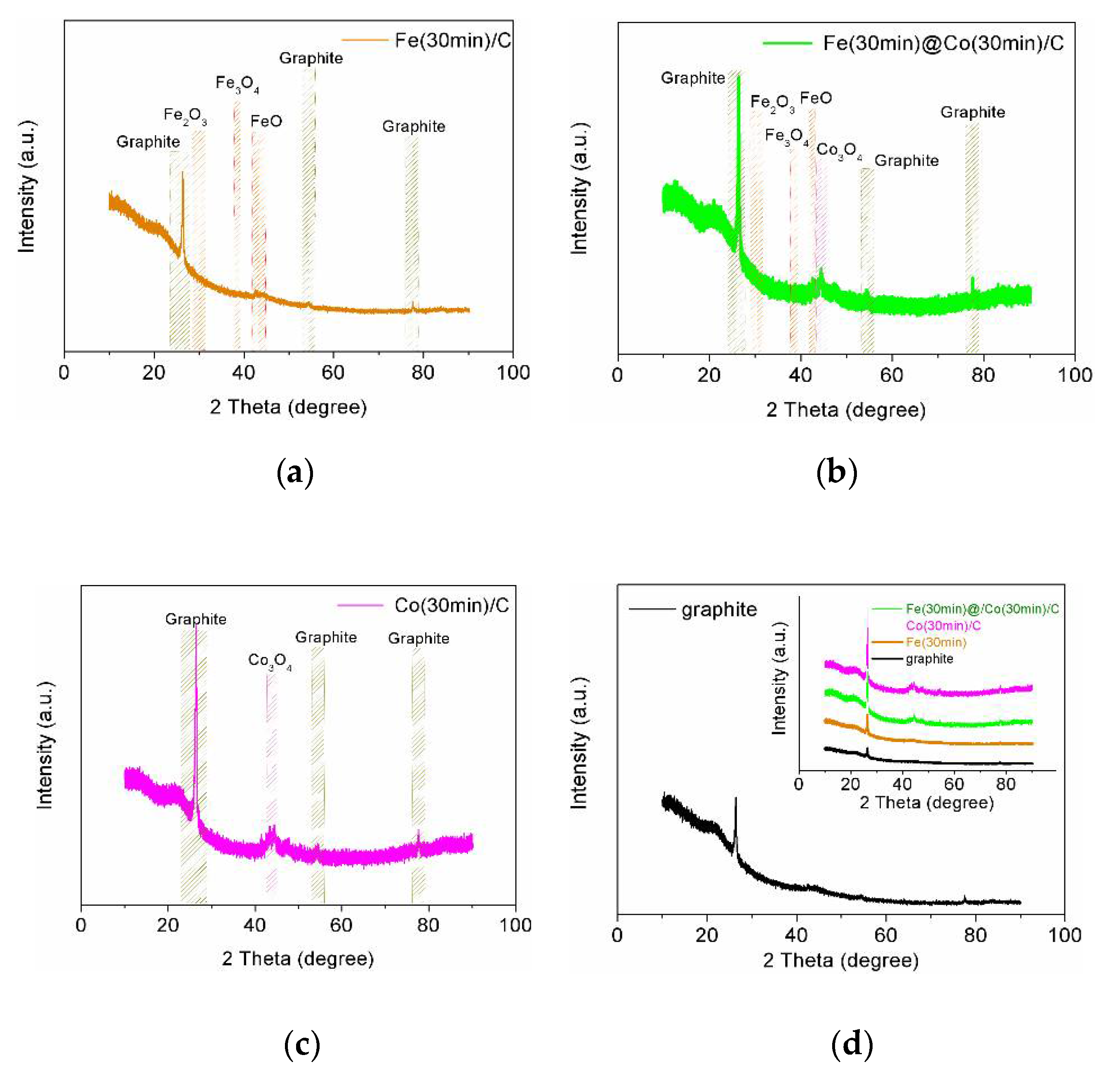
2.1.4. X-Ray Diffraction Analysis
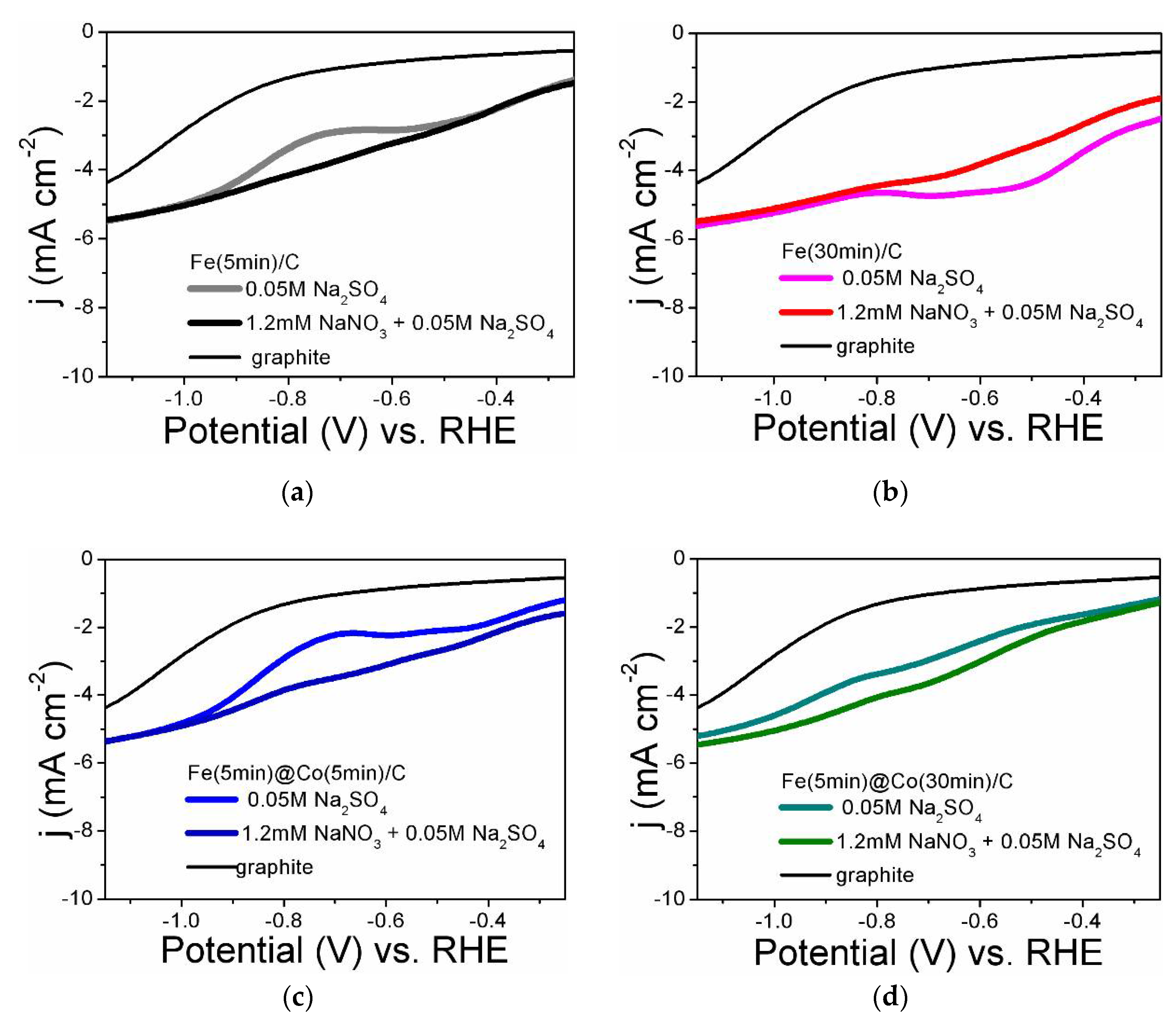
2.2. Linear Voltammetry Researches
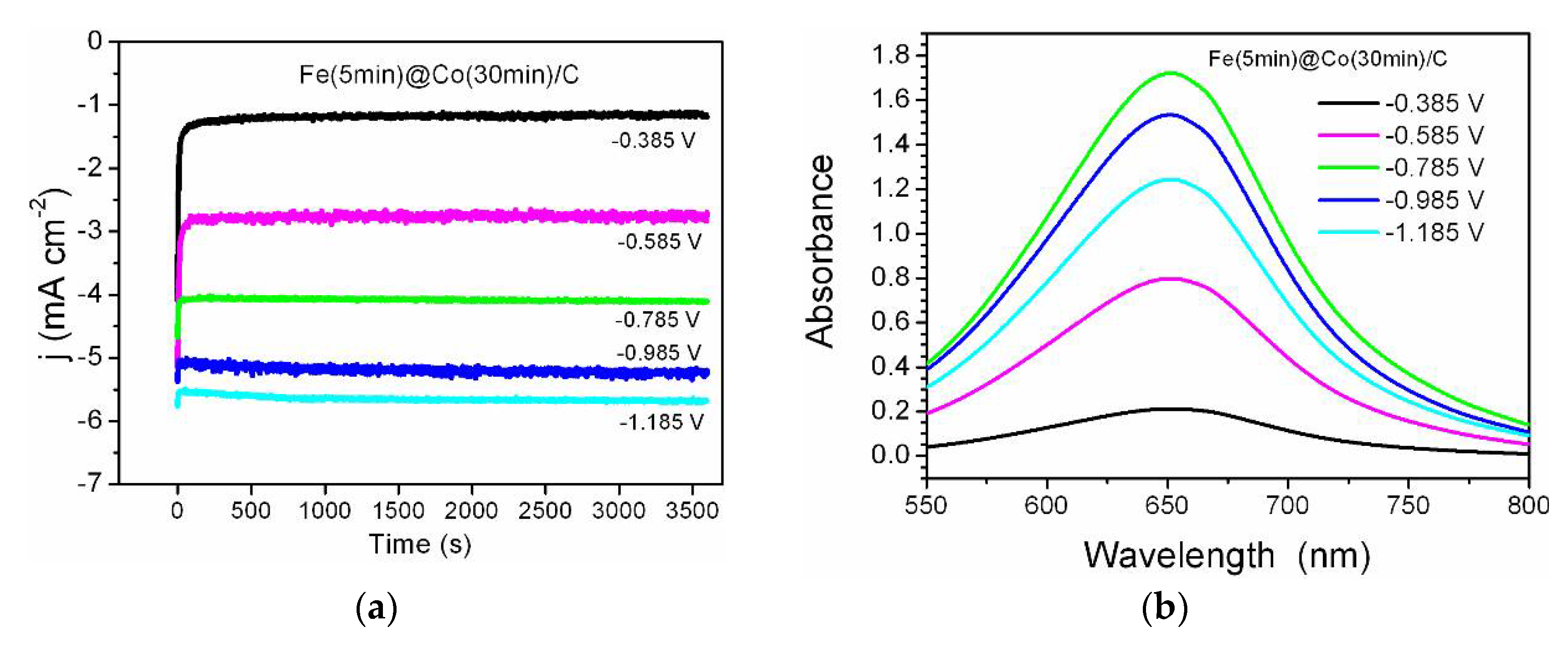
2.3. Chronoamperometric Measurements and Nitrate Conversion
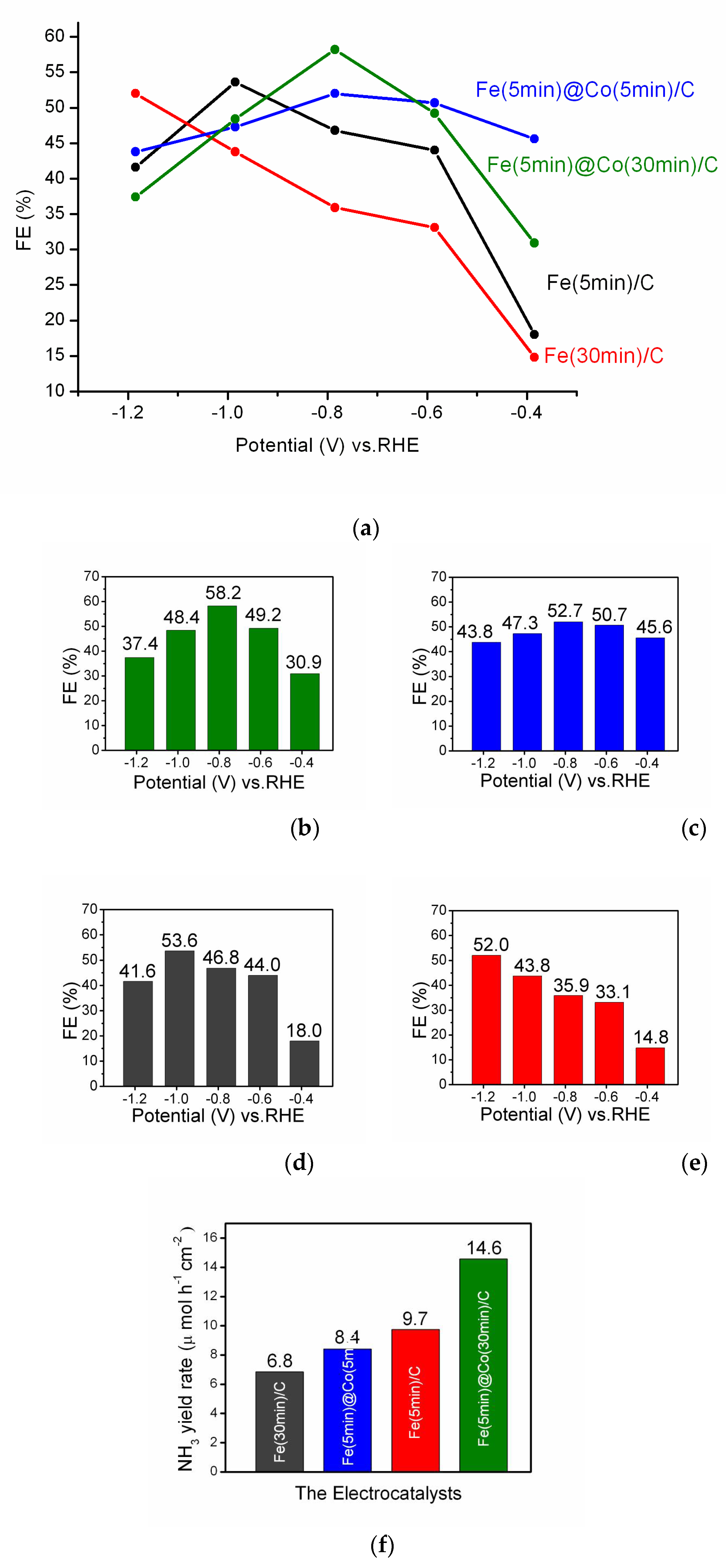
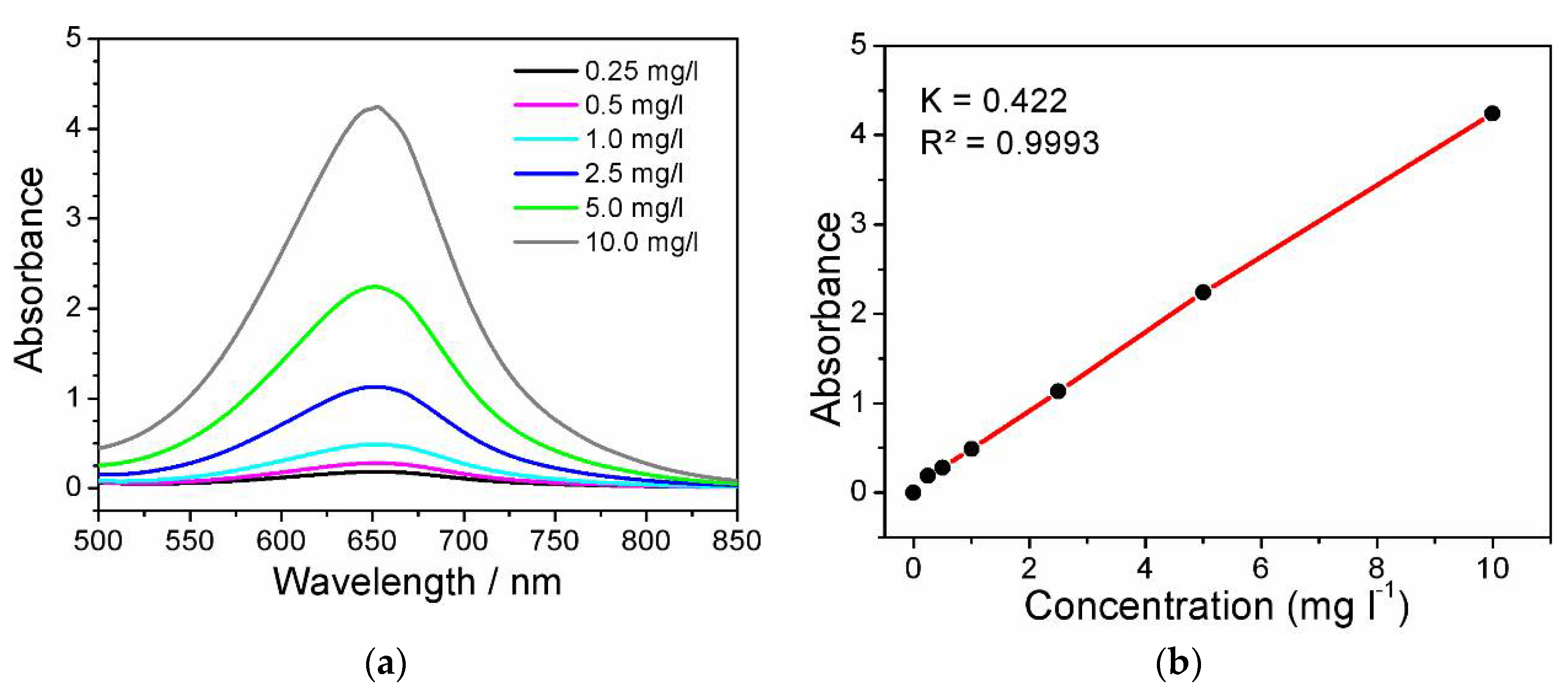
2.4. Analysis of Faradaic Efficiency
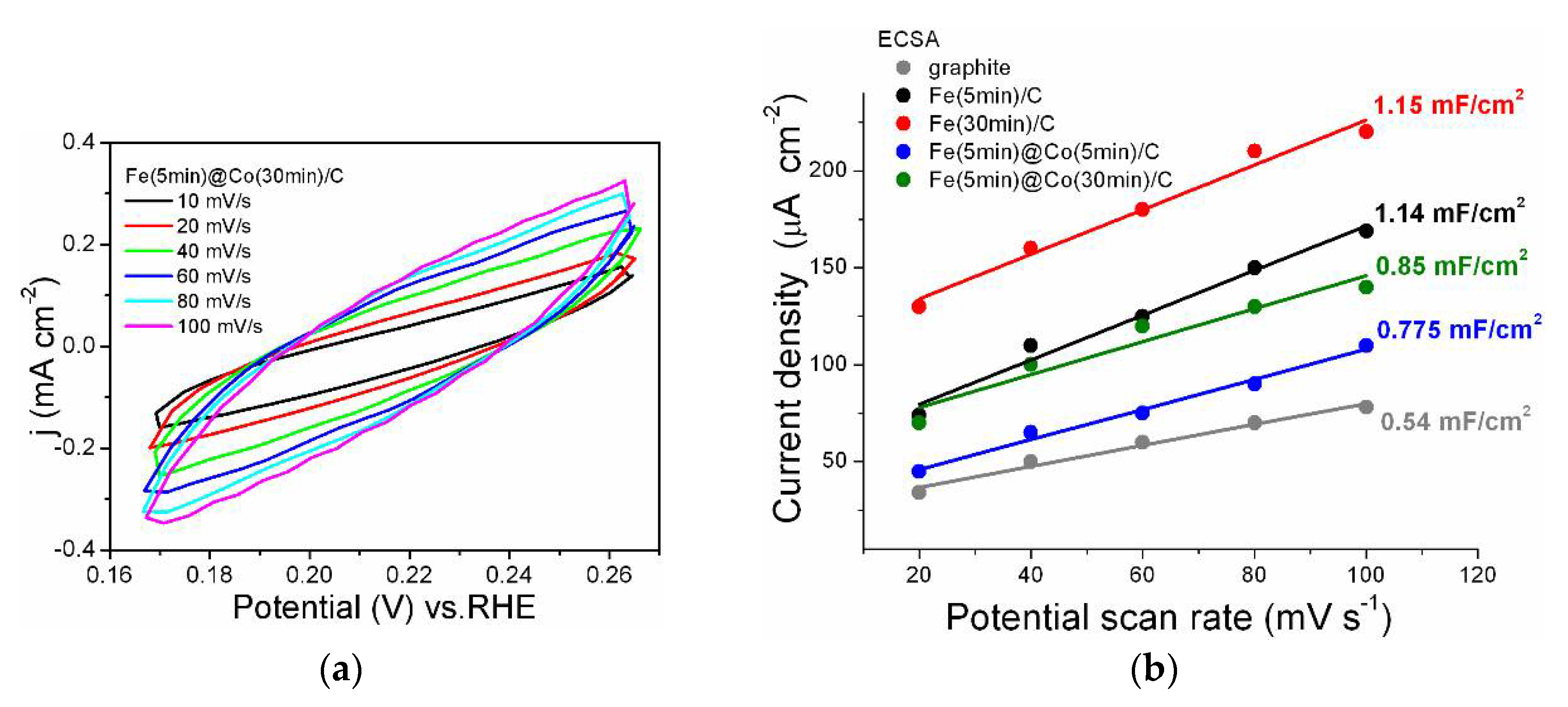
2.5. Determination of Electrochemically Active Surface Area (ECSA)
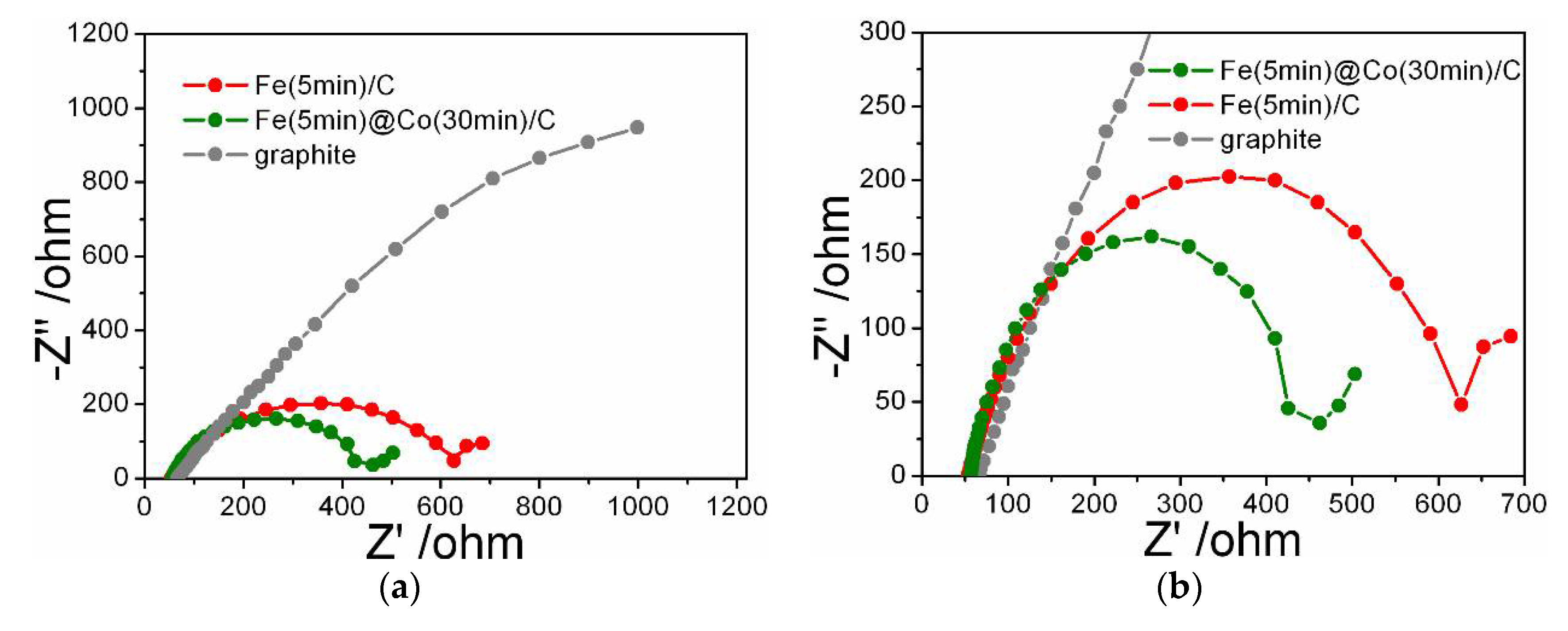
2.5. Electrochemical Impedance Spectroscopy (EIS)
2.6. A brief Summary of the Elucidation for Proposed Mechanism of Electrocatalysis
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Electrochemical Measurements
3.4. Detection of Ammonia
3.5. ECSA Evaluation
3.6. Impedance Response Testing
3.7. Material Characterization
4. Conclusions
- The surface morphology and NPs size, defining the further efficiency of electrocatalysts in NO3RR, were determined by scanning electron microscopy. XPS and XRD revealed the state and composition of catalytic nanoparticles.
- According to the results of linear voltammetric studies, five potential values were selected at which NO3RR was performed for 1 hour for each sample of the electrocatalyst.
- A clearly expressed volcano-like FE-E relationship (Figure 7a) and the highest FE result of 58.2% for E = -0.785 V (RHE) and ammonia yield rate of 14.6 μmol h-1 cm-2 highlight the Fe(5min)@Co (30min)/C bimetallic catalyst in NO3RR compared to other investigated catalysts.
- The ECSA method showed that despite the lower value of Cdl = 0.85 mF/cm2 for the Fe(5min)@Co(30min)/C catalyst, its selectivity for the investigated reaction is significantly higher, as confirmed by the EF data discussed above. It was found by the EIS method that the addition of Co-nanolayer promotes charge transfer at the cathode and increases the reaction rate of conversion of nitrate to ammonia.
- The nature of Fe- and Co- nanoparticles suggests a joint catalysis to accelerate the early and intermediate stages of NO3RR.
Author Contributions
Funding
Conflicts of Interest
References
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Каlenchuk, A.; Кustov, L. Advances and Prospects in Electrocatalytic Hydrogenation of Aromatic Hydrocarbons for Synthesis of “Loaded” Liquid Organic Hydrogen Carriers. Curr. Opin. Electrochem. 2023, 38, 101207. [Google Scholar] [CrossRef]
- Shen, H.; Choi, C.; Masa, J.; Li, X.; Qiu, J.; Jung, Y.; Sun, Z. Electrochemical Ammonia Synthesis: Mechanistic Understanding and Catalyst Design. Chem 2021, 7, 1708–1754. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Yahya, Z.; Ali khan, W.; Faraz, A. Sustainable Pathways to Ammonia: A Comprehensive Review of Green Production Approaches. Clean Energy 2024, 8, 60–72. [Google Scholar] [CrossRef]
- Maximov, A.L.; Beletskaya, I.P. Carbon Dioxide and “Methanol” Economy: Advances in the Catalytic Synthesis of Methanol from CO2. Russ. Chem. Rev. 2024, 93, RCR5101. [Google Scholar] [CrossRef]
- Garagounis; Vourros; Stoukides; Dasopoulos; Stoukides Electrochemical Synthesis of Ammonia: Recent Efforts and Future Outlook. Membranes 2019, 9, 112. [CrossRef] [PubMed]
- Anastasiadou, D.; van Beek, Y.; Hensen, E.J.M.; Costa Figueiredo, M. Ammonia Electrocatalytic Synthesis from Nitrate. Electrochemical Science Adv. 2023, 3, e2100220. [Google Scholar] [CrossRef]
- Zhu, X.; Mou, S.; Peng, Q.; Liu, Q.; Luo, Y.; Chen, G.; Gao, S.; Sun, X. Aqueous Electrocatalytic N 2 Reduction for Ambient NH 3 Synthesis: Recent Advances in Catalyst Development and Performance Improvement. J. Mater. Chem. A 2020, 8, 1545–1556. [Google Scholar] [CrossRef]
- Utomo, W.P.; Wu, H.; Ng, Y.H. Quantification Methodology of Ammonia Produced from Electrocatalytic and Photocatalytic Nitrogen/Nitrate Reduction. Energies 2022, 16, 27. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Nørskov, J.K.; Chorkendorff, I. The Difficulty of Proving Electrochemical Ammonia Synthesis. ACS Energy Lett. 2019, 4, 2986–2988. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, K.; Yang, J.; Chen, H.; Ning, J.; Wang, H.; Hu, Y. Strategies and Applications of Electrocatalytic Nitrate Reduction towards Ammonia. Coord. Chem. Rev. 2024, 506, 215723. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, P.; Liang, J.; Li, H.; Wang, F. Opportunities and Challenges in Aqueous Nitrate and Nitrite Reduction beyond Electrocatalysis. Inorg. Chem. Front. 2023, 10, 4610–4631. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.-W.; Gu, K.; Chen, C.; Liu, Y.; Wei, X.; Singh, C.V.; Wang, S. Hexagonal Cobalt Nanosheets for High-Performance Electrocatalytic NO Reduction to NH 3. J. Am. Chem. Soc. 2023, 145, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Jing, H.; Wei, P.; Fu, X.; Pang, L.; Song, Y.; Ye, K.; Li, M.; Jiang, L.; Ma, J.; et al. Electrochemical Synthesis of Ammonia from Nitric Oxide Using a Copper–Tin Alloy Catalyst. Nat. Energy 2023, 8, 1273–1283. [Google Scholar] [CrossRef]
- He, W.; Zhang, J.; Dieckhöfer, S.; Varhade, S.; Brix, A.C.; Lielpetere, A.; Seisel, S.; Junqueira, J.R.C.; Schuhmann, W. Splicing the Active Phases of Copper/Cobalt-Based Catalysts Achieves High-Rate Tandem Electroreduction of Nitrate to Ammonia. Nat. Commun. 2022, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Ling, Y.; Yang, M.; Zhao, X.; Osman, A.I.; Al-Muhtaseb, A.H.; Rooney, D.W.; Yap, P.-S. Recent Research Progress of Electrocatalytic Reduction Technology for Nitrate Wastewater: A Review. J. Environ. Chem. Eng. 2023, 11, 109418. [Google Scholar] [CrossRef]
- Choueiri, R.M.; Tatarchuk, S.W.; Klinkova, A.; Chen, L.D. Mechanism of Ammonia Oxidation to Dinitrogen, Nitrite, and Nitrate on β-Ni(OH) 2 from First-principles Simulations. Electrochemical Science Adv. 2022, 2, e2100142. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Pan, Z.; Xia, Q.; Huo, X.; Esan, O.C.; Zhang, X.; An, L. Cu-Based Catalysts for Electrocatalytic Nitrate Reduction to Ammonia: Fundamentals and Recent Advances. EES. Catal. 2024, 2, 727–752. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Lin, H.; Lu, X.; Zhou, C.; Li, Y. Copper-Based Electro-Catalytic Nitrate Reduction to Ammonia from Water: Mechanism, Preparation, and Research Directions. Environ. Sci. Ecotechnology 2024, 20, 100383. [Google Scholar] [CrossRef]
- Niu, S.; Yang, J.; Qian, L.; Zhou, D.; Du, P.; Si, N.; Gu, X.; Jiang, D.; Feng, Y. Electrochemical Nitrate Reduction to Ammonia – Recent Progress. ChemElectroChem 2023, 10, e202300419. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Zhang, Y.; Li, H.; Huang, W.; Yang, Y.; Ye, M.; Liu, Y. The Interface-Mediated Electron Structure Tuning of RuOx–Co3O4 Nano-Particles for Efficient Electrocatalytic Nitrate Reduction. Dalton Trans. 2024, 53, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Jiang, X.; Zhang, Y.; Liu, Y.; Liu, Y.; Zhao, L. Enhanced Electrocatalytic Nitrate Reduction to Ammonia Using Functionalized Multi-Walled Carbon Nanotube-Supported Cobalt Catalyst. Nanomaterials 2024, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhu, W.; Ma, D.; Liang, C.; Wang, Z.; Liang, H. Screening of Transition Metal Oxides for Electrocatalytic Nitrate Reduction to Ammonia at Large Currents. Nano Res. 2024, 17, 3902–3910. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Y.; Chu, J.; Liu, S.; Bai, F.; Luo, W. Electrocatalytic Nitrate Reduction to Ammonia: A Perspective on Fe/Cu-Containing Catalysts. Chin. J. Catal. 2024, 58, 25–36. [Google Scholar] [CrossRef]
- Yuan, S.; Xue, Y.; Ma, R.; Ma, Q.; Chen, Y.; Fan, J. Advances in Iron-Based Electrocatalysts for Nitrate Reduction. Sci. Total Environ. 2023, 866, 161444. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ji, X.; Kou, J. Rational Design of Iron Single-Atom Catalysts for Electrochemical Nitrate Reduction to Produce Ammonia. Discov. Chem. Eng. 2023, 3, 21. [Google Scholar] [CrossRef]
- Yu, Z.; Gu, M.; Wang, Y.; Li, H.; Chen, Y.; Wei, L. Recent Progress of Electrochemical Nitrate Reduction to Ammonia on Copper-Based Catalysts: From Nanoparticles to Single Atoms. Adv. Energy and Sustain. Res. 2024, 5, 2300284. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Wang, X.; Si, R.; Xu, J.; Han, Y.-F. Promotional Effects of Multiwalled Carbon Nanotubes on Iron Catalysts for Fischer-Tropsch to Olefins. J. Catal. 2018, 365, 71–85. [Google Scholar] [CrossRef]
- Mu, J.; Chen, B.; Guo, Z.; Zhang, M.; Zhang, Z.; Zhang, P.; Shao, C.; Liu, Y. Highly Dispersed Fe3O4 Nanosheets on One-Dimensional Carbon Nanofibers: Synthesis, Formation Mechanism, and Electrochemical Performance as Supercapacitor Electrode Materials. Nanoscale 2011, 3, 5034. [Google Scholar] [CrossRef]
- Kempler, P.A.; Nielander, A.C. Reliable Reporting of Faradaic Efficiencies for Electrocatalysis Research. Nat. Commun. 2023, 14, 1158. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Z.; Zhao, H.; Liu, X.; Zheng, M.; Zhang, L.; Zhou, Y.; Sun, L.; Liu, J.; Zhang, H. High Faraday Efficiency of Cu 1 Co 1 –BCN Based on a Dodecahydro- Closo -Dodecaborate Hybrid for Electrocatalytic Reduction of Nitrate to Ammonia. J. Mater. Chem. A 2023, 11, 20234–20241. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Smith, R.L.; Liu, L.; Qi, X. Synthesis of Self-Renewing Fe(0)-Dispersed Ordered Mesoporous Carbon for Electrocatalytic Reduction of Nitrates to Nitrogen. Sci. Total Environ. 2022, 836, 155640. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, K.; Yan, C.; Li, S.; Zhang, H.; Cheng, L.; Huang, F. Interfacial Engineering of Cu–Fe 2 O 3 Nanotube Arrays with Built-in Electric Field and Oxygen Vacancies for Boosting the Electrocatalytic Reduction of Nitrates. Mater. Adv. 2022, 3, 7107–7115. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, G.; Zhang, G.; Chen, K.; Chu, K. Electrochemical Nitrate-to-Ammonia Reduction over Atomic Fe-Dopants Incorporated in CoS2. Chem. Eng. J. 2023, 474, 145861. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Cai, C.; Liu, Y.; Wu, D.; Wang, M.; Li, M.; Wei, X.; Shao, M.; Gu, M. Cu-Doped Iron Oxide for the Efficient Electrocatalytic Nitrate Reduction Reaction. Nano Lett. 2023, 23, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, D.; Zhao, Q.; Feng, S.; Peng, X.; Chu, P.K. Electrochemical Reduction of Nitrate to Ammonia Using Non-Precious Metal-Based Catalysts. Coord. Chem. Rev. 2024, 502, 215609. [Google Scholar] [CrossRef]
- Wei, P.; Liang, J.; Liu, Q.; Xie, L.; Tong, X.; Ren, Y.; Li, T.; Luo, Y.; Li, N.; Tang, B.; et al. Iron-Doped Cobalt Oxide Nanoarray for Efficient Electrocatalytic Nitrate-to-Ammonia Conversion. J. Colloid Interface Sci. 2022, 615, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, P.G.; Altimari, P.; Pagnanelli, F.; Moscardini, E.; Toro, L. Synthesis of Cobalt Nanoparticles by Electrodeposition onto Aluminium Foils. Chem. Eng. Trans. 2015, 43, 673–678. [Google Scholar] [CrossRef]
- Niu, Z.; Fan, S.; Li, X.; Duan, J.; Chen, A. Interfacial Engineering of CoMn2O4/NC Induced Electronic Delocalization Boosts Electrocatalytic Nitrogen Oxyanions Reduction to Ammonia. Appl. Catal., B 2023, 322, 122090. [Google Scholar] [CrossRef]
| Designation | General brief description |
|---|---|
| C | Graphite is the initial substrate |
| Fe(30min)/C | Fe-NPs (deposited at 30 min) on the substrate С |
| Fe(5min)/C | Fe-NPs (deposited at 5 min) on the substrate С |
| Fe(5min)@Co(5min)/C | Fe-NPs (deposited at 5 min) on the Сo-NPs layer (deposited at 5 min) on the substrate C |
| Fe(5min)@Co(30min)/C | Fe-NPs (deposited at 5 min) on the Сo-NPs layer (deposited at 30 min) on the substrate C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





