Submitted:
31 December 2025
Posted:
31 December 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Evidence for Parental Autoimmune Disease-Autism Association
2.1. Epidemiological Evidence
2.2. The TNF-α Common Denominator
- Psoriasis: TNF-α drives keratinocyte proliferation and inflammatory cascade; anti-TNF biologics are first-line therapy
- Type 1 Diabetes: TNF-α directly induces β-cell apoptosis and promotes autoimmune destruction of pancreatic islets
- Rheumatoid Arthritis: TNF-α orchestrates synovial inflammation and joint destruction; anti-TNF therapy revolutionized treatment
- Normal-Tension Glaucoma: TNF-α mediates retinal ganglion cell death independent of intraocular pressure elevation
3. TNF-α and Mitochondrial Dysfunction: The Mechanistic Link
3.1. Direct Effects of TNF-α on Mitochondrial Function
3.2. Rapid Neurotoxicity of TNF-α
- Reduction in mitochondrial basal respiration within 1.5 hours of TNF-α exposure
- Decreased ATP production preceding neuronal cell death
- Effects mediated specifically through TNF-R1 receptor signaling
- Cascade involving caspase-8 activation, membrane potential collapse, and cytochrome c release
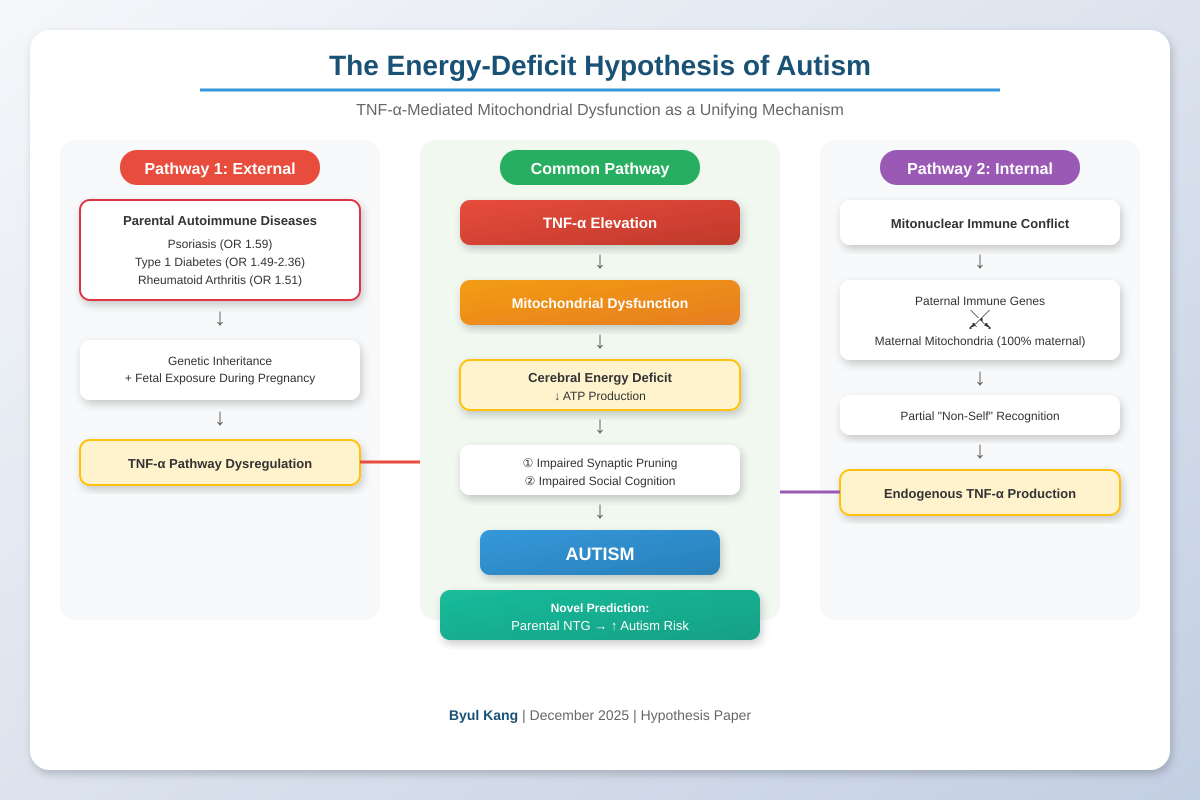
4. The Energy-Deficit Hypothesis of Autism
4.1. Impaired Synaptic Pruning
- Microglia actively phagocytose synapses, requiring substantial ATP
- The infant brain consumes 40% of total body energy—far exceeding adult proportions
- Complement cascade activation and autophagy pathways require ATP
| Parameter | Neurotypical | Autism |
|---|---|---|
| Synaptic density reduction (childhood→adolescence) | ~50% | ~16% |
| Dendritic spine density | Normal | Elevated |
| mTOR pathway activity | Normal | Hyperactive |
| Autophagy function | Normal | Impaired |
- Local over-connectivity: Excess short-range connections creating "neural noise"
- Long-distance under-connectivity: Insufficient resources for developing major "highway" connections between brain regions
- Reduced signal-to-noise ratio: Difficulty filtering relevant from irrelevant information
- Sensory overload: Heightened sensitivity due to failure to attenuate sensory inputs
4.2. Impaired Social Cognition and Gaze Avoidance
- Fusiform Face Area (FFA): Face identity processing
- Superior Temporal Sulcus (STS): Gaze direction and biological motion
- Amygdala: Emotional salience and threat detection
- Prefrontal Cortex: Social context integration and decision-making
| Experience Category | Representative Quote |
|---|---|
| Energy Exertion | "Eye contact feels like I'm using up a lot of energy. Maximum 2-6 seconds." |
| Audiovisual Integration Failure | "I cannot listen to someone while making eye contact at the same time." |
| Cognitive Trade-off | "When I focus on eye contact, I can't process what's being said." |
| Recovery Requirement | "The longer I maintain eye contact, the more recovery time I need afterward." |
5. Integrated Pathophysiological Model
6. The Mitonuclear Immune Conflict Hypothesis: An Endogenous Source of TNF-α
6.1. The Gap in the TNF-α Hypothesis
6.2. The Unique Inheritance Pattern of Mitochondria
6.3. The Conflict Hypothesis: Paternal Immune Genes vs. Maternal Mitochondria
- Immune misrecognition: The paternal contribution to immune recognition machinery (HLA genes, innate immune pathways) may be calibrated to recognize mitochondrial signatures that differ from those inherited from the mother.
- Chronic immune attack: The immune system may mount persistent inflammatory responses against the individual's own mitochondria, treating them as partially foreign.
- Endogenous TNF-α production: This chronic immune activation would result in sustained TNF-α release—activating the same pathogenic cascade described in previous sections, even without external TNF-α exposure from parental autoimmune disease.
6.4. Two Pathways to the Same Outcome
| Pathway 1: External | Pathway 2: Internal | |
|---|---|---|
| Source of TNF-α | Parental autoimmune disease | Mitonuclear immune conflict |
| Mechanism | Genetic inheritance + fetal exposure during pregnancy | Paternal immune attack on maternal mitochondria |
| Parental disease required? | Yes | No |
| Final common pathway | TNF-α elevation → Mitochondrial dysfunction → Energy deficit → Autism | |
- Parental autoimmune disease increases autism risk (Pathway 1)
- Autism also occurs without parental autoimmune disease (Pathway 2)
- Only a subset of children with autoimmune parents develop autism (variable mitonuclear compatibility may be protective or additive)
6.5. Testable Predictions
- Anti-mitochondrial antibodies or mitochondria-targeted immune markers may be elevated in autistic individuals without parental autoimmune history
- Inflammatory cytokines including TNF-α may be elevated even in autism cases without parental autoimmune disease
- Specific HLA haplotype combinations from parents may show associations with autism risk
7. Novel Prediction: Normal-Tension Glaucoma and Autism
7.1. NTG as a TNF-α-Mediated Condition
- Elevated TNF-α levels in aqueous humor and serum of NTG patients
- TNF-α directly induces RGC apoptosis via TNF-R1 signaling
- Anti-TNF therapy shows protective effects in animal models
- NTG frequently co-occurs with systemic inflammatory conditions (e.g., psoriasis)
- Disease progression occurs despite normal intraocular pressure, implicating IOP-independent mechanisms
7.2. The Untested Hypothesis
| Parental Condition | TNF-α Role | Autism Association Studied? |
|---|---|---|
| Psoriasis | Central | Yes (OR 1.59) |
| Type 1 Diabetes | Central | Yes (OR 1.49-2.36) |
| Rheumatoid Arthritis | Central | Yes (OR 1.51) |
| Normal-Tension Glaucoma | Central | NO STUDIES EXIST |
8. Therapeutic Implications
8.1. Anti-TNF-α Interventions
8.2. Mitochondrial Support
- Coenzyme Q10: Essential electron carrier in ETC
- L-Carnitine: Facilitates fatty acid transport into mitochondria
- NAD+ Precursors (NR, NMN): Support ETC function and cellular energy production
- B Vitamins: Cofactors for mitochondrial enzymes
8.3. Early Identification
9. Limitations and Future Directions
10. Conclusion
References
- Wu, S; Ding, Y; Wu, F; et al. Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2015, 55, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Xiang, AH; Wang, X; Martinez, MP; et al. Maternal Type 1 Diabetes and Risk of Autism in Offspring. JAMA 2018, 320, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Persson, M; et al. Maternal type 1 diabetes, pre-term birth and risk of autism spectrum disorder. Int J Epidemiol. 2023, 52, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Keil, A; Daniels, JL; Forssen, U; et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology 2010, 21, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Atladóttir, HO; Pedersen, MG; Thorsen, P; et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 2009, 124, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Tang, G; Gudsnuk, K; Kuo, SH; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, DA; Roberts, N; Lin, C; Birmingham, E. How do adults and teens with self-declared Autism Spectrum Disorder experience eye contact? PLOS ONE 2017, 12, e0188446. [Google Scholar] [CrossRef] [PubMed]
- Dalton, KM; Nacewicz, BM; Johnstone, T; et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005, 8, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Morris, G; Berk, M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Quattrin, T; Haller, MJ; Steck, AK; et al. Golimumab and Beta-Cell Function in Youth with New-Onset Type 1 Diabetes. N Engl J Med. 2020, 383, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y; et al. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar]
- Rossignol, DA; Frye, RE. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, T; et al. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J Neurosci. 2006, 26, 12633–12641. [Google Scholar] [CrossRef] [PubMed]
- Deng, W; et al. Rapid mitochondrial dysfunction mediates TNF-alpha-induced neurotoxicity. J Neurochem. 2015, 132, 443–451. [Google Scholar]
- Ashwood, P; et al. Immunological cytokine profiling identifies TNF-α as a key molecule dysregulated in autistic children. Mol Psychiatry 2017, 22, 809–816. [Google Scholar]

| Parental Disease | Odds Ratio | 95% CI | Key Reference |
|---|---|---|---|
| Psoriasis | 1.59 | 1.21-2.10 | Wu et al. 2015 |
| Type 1 Diabetes (T1D) | 1.49-2.36 | 1.21-4.12 | JAMA 2018, IJE 2023 |
| Rheumatoid Arthritis | 1.51 | 1.14-2.00 | Keil et al. 2010 |
| Hypothyroidism | 1.64 | 1.16-2.32 | Atladóttir et al. 2009 |
| Any Autoimmune Disease | 1.28-1.50 | 1.11-1.75 | Wu et al. 2015 Meta |
| Mechanism | Effect on Energy Metabolism |
|---|---|
| ETC Complex I Inhibition | Blocks electron transfer at the first step of oxidative phosphorylation |
| ETC Complex III Inhibition | Disrupts cytochrome bc1 complex function |
| Cytochrome c Oxidase (COX) | Reduces terminal electron transfer and oxygen consumption |
| Membrane Depolarization | Collapses mitochondrial membrane potential (ΔΨm), halting ATP synthesis |
| PDH Suppression | Inhibits pyruvate dehydrogenase, blocking glucose entry into TCA cycle |
| ROS Overproduction | Increases reactive oxygen species, causing oxidative damage to mitochondrial components |
| Warburg Effect Induction | Shifts metabolism to inefficient aerobic glycolysis (2 vs 36 ATP per glucose) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





