1. Introduction
1.1. Dual Metabolic Syndrome Burden and the Critical Need for Alternative Therapies for Menopausal Symptom Management
The modern public health landscape is increasingly shaped by the concurrent increase in the prevalence of metabolic disorders such as obesity and metabolic syndrome (MS), necessitating innovative and sustainable strategies for metabolic regulation [
1]. Simultaneously, quality of life is compromised in a growing demographic of postmenopausal women due to a constellation of adverse symptoms, including vasomotor instability, sleep disruption, and mood changes [
2].
The conventional management of debilitating menopausal symptoms typically involves hormone replacement therapy (HRT). However, patients manifest widespread reluctance toward HRT due to significant safety concerns, such as increased risk of cardiovascular events, venous thromboembolism, and specific hormone-dependent cancers [
3].
This pervasive reluctance in patients has created an urgent clinical need for effective, safe, and natural dietary alternatives that can alleviate menopausal distress. This critical need has directed research toward functional foods rich in phytoestrogens, which can serve as viable therapeutic options.
Traditional fermented foods are globally recognized for their unique and diverse functional health benefits, which are significantly enhanced through microbial biotransformation [
4].
Gochujang, a traditional Korean fermented red pepper paste, is a highly relevant functional food matrix for addressing this dual health challenge. Its unique composition, resulting from a combination of capsaicin-rich red pepper powder and fermented soybeans (Meju), yields a potent blend of bioactive compounds, including capsaicinoids and highly bioavailable isoflavone aglycones [
5,
6].
1.2. Established Metabolic and Anti-Inflammatory Efficacy of Gochujang
Extensive scientific research on Gochujang, primarily focusing on its metabolic health- and systemic protection-promoting effects, has robustly demonstrated its functional properties.
Anti-Obesity and Lipid Regulatory Effects: Preclinical models have shown that Gochujang supplementation suppresses weight gain, decreases lipid accumulation in adipose tissue and the liver, and enhances energy expenditure [
7]. Of note, human randomized controlled trials have provided clinical validation of its efficacy, revealing that it significantly decreases the visceral fat area and favorably modulates blood lipid profiles (Total Cholesterol, LDL-C) in overweight subjects [
7]. These anti-obesity effects are mechanistically linked to capsaicin-mediated activation of the TRPV1/SIRT1 pathway, which drives white adipose tissue browning and the anti-adipogenic actions of isoflavone aglycones that modulate key fat synthesis transcription factors (SREBP−1c, PPARγ) via AMPK activation [
8,
9].
Anti-inflammatory and Gut Health-promoting Effects: Gochujang exerts potent anti-inflammatory effects, effectively suppressing pro-inflammatory cytokine (TNF−α, IL−6) expression and inhibiting the NF−κB signaling pathway in colitis models [
10]. Its protective effects extend to the intestinal barrier, where capsaicin has been shown to reduce chronic low-grade inflammation (CLGI) by stimulating tight junction protein (ZO−1 and Occludin) expression, thereby restoring intestinal integrity [
11]. Furthermore, its consumption promotes a positive shift in the gut microbiota by increasing the population of beneficial bacteria [
12].
Protective Effects on the Cardiovascular System: Studies have shown that Gochujang possesses anti-hypertensive potential and that it can suppress high-salt-diet-induced increase in blood pressure through the downregulation of the Renin-Angiotensin-Aldosterone System (RAAS) [
13]. Gochujang incorporates fermented soybean blocks (meju), in which the fermentation process converts isoflavone glycosides, the native forms in soybeans, to their highly bioavailable aglycone forms (daidzein, genistein, and glycitein). These isoflavone aglycones exert anti-hypertensive effects by potentiating the release of vasorelaxant mediators; more specifically, they exert their effects primarily by increasing nitric oxide (NO) production in vascular endothelial cells [
14].
1.3. Knowledge Gap and Research Objectives
The Meju component of Gochujang, which yields bioavailable isoflavone aglycones at high concentrations, is derived from fermented soybeans. Given the high phytoestrogenic content of Gochujang, its clinical usefulness as an alternative therapy for the management of menopausal symptoms is highly plausible. Despite the pervasive reluctance of patients toward HRT, no rigorous randomized, controlled clinical trial has been conducted to specifically evaluate the efficacy of Gochujang supplementation in relieving symptoms of menopausal syndrome.
To address this critical knowledge gap, we conducted an 8-week, randomized, double-blind, placebo-controlled clinical trial using formulated Gochujang pills. The primary objective of the study was to rigorously evaluate the efficacy of Gochujang supplementation in relieving symptoms of menopausal syndrome. The secondary objectives were to assess the effects of different Gochujang formulations on key metabolic parameters, including body composition and blood lipid profiles, as well as to evaluate their impact on gut microbial composition.
Gochujang induced a statistically significant decrease in the total Kupperman Index (KMI) score in both the high-Gochujang (HGC) and low-Gochujang (LGC) groups, indicating significant overall improvement of menopausal symptoms, and was well-tolerated as it was not associated with any safety issues during the 8-week intervention period.
2. Materials and Methods
2.1. Clinical Trial Design
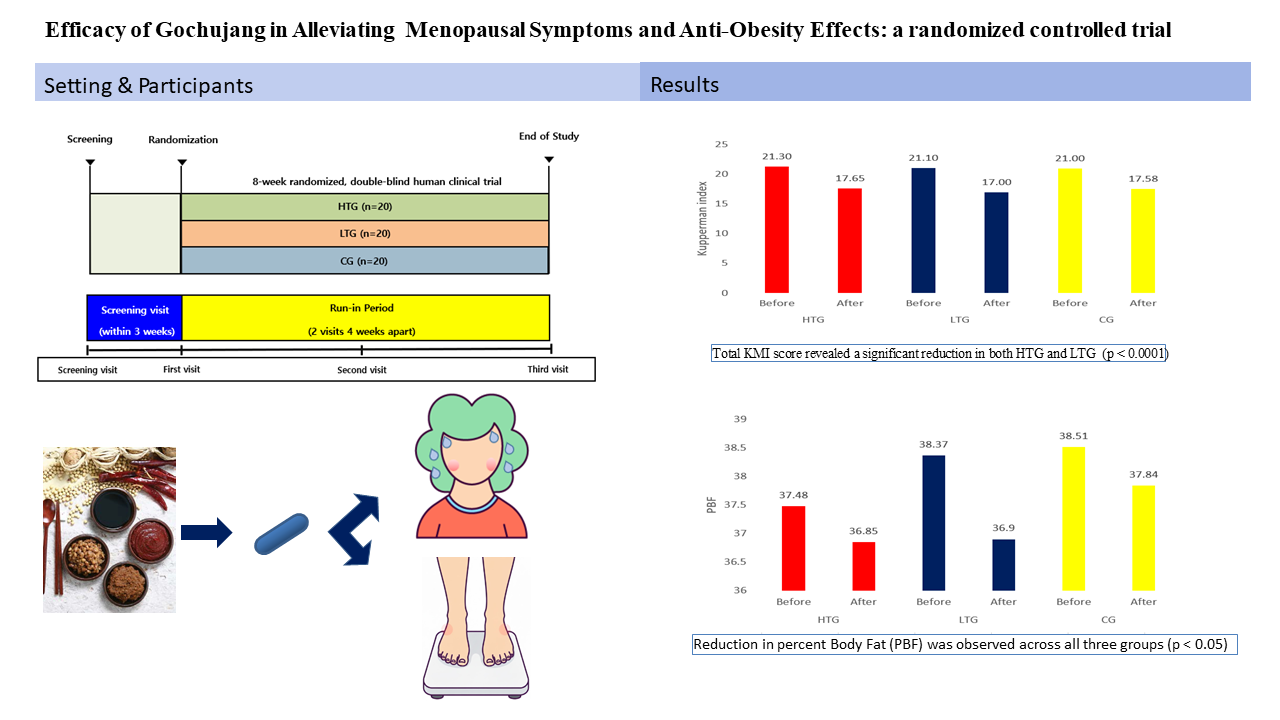
This was an 8-week, randomized, double-blind, controlled clinical trial registered with the Korean Clinical Research Information Service (CRiS) under the identification number, KCT0011085. Participants were required to visit the clinical center on three occasions, i.e., at screening/baseline (week 0), at an interim visit (week 4), and at the final assessment (week 8). The interim visit was implemented to monitor vital signs, assess adherence to the dosing regimen, and document potential adverse events.
The primary efficacy endpoint was a change in the Kupperman Index (KMI) score from baseline to week 8. Secondary endpoints included changes in body composition, including body weight, body mass index (BMI), percent body fat (PBF), and fat-free mass (FFM), measured via a bioelectrical impedance analysis (BIA). Standard biochemical assays, including lipid profile assays, were also conducted at baseline and week 8.
The investigational products consisted of three distinct Gochujang formulations in pill form: (1) Traditional Gochujang with high beneficial microorganism content (HGC), (2) Traditional Gochujang with low beneficial microorganism content (LGC), and (3) Commercial Gochujang (CG). Sixty volunteers were recruited and randomly allocated to one of the three treatment arms (n=20 per group). During the 8-week study, two participants withdrew, with 58 participants successfully completing the protocol.
2.2. Study Population and Dosing Protocol
Sixty female volunteers aged 45–70 years were enrolled in the trial. All participants were confirmed to be postmenopausal, defined either as 12 consecutive months of amenorrhea or based on formal medical certification from an obstetrician-gynecologist. Furthermore, all participants were required to manifest menopausal syndrome of at least moderate severity, defined as a baseline KMI score ≥15.
Exclusion criteria: >10% change in body weight within the previous three months; existing cardiovascular morbidities; known allergy to any component of the test material; chronic gastrointestinal disease; prior history of major gastrointestinal surgery; participation in another clinical trial within the two preceding months; impaired hepatic or renal function; consumption of antipsychotic agents within the two previous months; clinically significant abnormal laboratory results; psychological instability; history of substance abuse; or pregnancy/lactation. The study protocol was approved by the Institutional Review Board (IRB) of Wonkwang University Hospital (Approval no.: WKUH IRB 2025-03-030-002) and all subjects provided written informed consent.
Participants were instructed to swallow three capsules twice daily i.e., after their morning and evening meals. This regimen resulted in a total daily supplement consumption of 25.2 g, corresponding to 19 g/day of dehydrated Gochujang powder. This daily target dosage of Gochujang powder (19 g) was selected based on the 2014 Korean National Health and Nutrition Survey, which identified the average daily red pepper paste intake and extreme consumption level for the Korean population to be 10.75 and 3,686 g, respectively [
15].
Table 1.
Composition of Gochujang pills.
Table 1.
Composition of Gochujang pills.
| Ingredient |
HTG |
LTG |
CG |
| Content (g) |
Ratio (%) |
Content (g) |
Ratio (%) |
Content (g) |
Ratio (%) |
| Freeze-dried Gochujang powder |
19.0 |
75 |
19.0 |
75 |
19.0 |
75 |
| Microcrystalline cellulose |
5.1 |
20 |
5.1 |
20 |
5.1 |
20 |
| Magnesium stearate |
1.1 |
5 |
1.1 |
5 |
1.1 |
5 |
| Total |
25.2 |
100 |
25.2 |
100 |
25.2 |
100 |
2.3. Safety Assessment
Safety was assessed via physical examination and laboratory testing at each visit. Laboratory safety evaluations included a complete blood count and measurement of biochemical indicators of hepatic (ALT, AST, total protein, and albumin) and renal (BUN and creatinine) function. Vital signs (pulse rate, systolic blood pressure (SBP) and diastolic blood pressure (DBP)) were recorded at every visit after a ten-minute rest period.
2.4. Efficacy Assessment
The primary efficacy endpoint was a change in the KMI score, which reflects the severity of 11 climacteric symptoms [
16]. Secondary endpoints included metabolic, hepatic, and inflammatory biomarker levels, including the levels of complete lipid profile parameters (TC, LDL-C, HDL-C, triglyceride, non-HDL-C, and TG/HDL-C), hepatic enzyme (ALT, AST, and GGT) levels, renal indicator (BUN and creatinine) levels, glucose/insulin metabolism marker (fasting glucose and insulin) levels, and high-sensitivity C-reactive protein (hs-CRP) levels. Surrogate indices for insulin sensitivity (HOMA-IR, QUICKI) and β-cell function (HOMA-β) were also calculated.
2.5. Statistical Analysis
All statistical analyses were performed using PASW Statistics version 23.0 (IBM Corporation, Armonk, NY, USA). Continuous variables were reported as mean ± standard deviation (SD). Statistical significance was set at p < 0.05. Within-group changes from baseline (week 0) to week 8 were analyzed using a paired sample t-test for normally distributed data or the Wilcoxon signed-rank test for non-normally distributed data.
3. Results
3.1. Changes in Menopausal Symptoms as Assessed Using the Kupperman Index
The effects of the intervention on menopausal symptoms were evaluated using the KMI score, and detailed results are presented in
Table 2. Primary analysis of the total KMI score revealed a statistically significant decrease in both the HGC (p < 0.0001) and LGC (p < 0.0001) groups, indicating significant overall improvement of menopausal symptoms. In contrast, the change in total KMI score in the CG group was not statistically significant (p = 0.429). Analysis of the individual KMI items revealed a significant improvement in the scores for hot flashes and paresthesia in all three groups, as well as a decrease in other group-specific symptoms (
Table 2).
The KMI is a validated rating scale for quantifying the severity of 11 common climacteric symptoms. Each symptom is rated on a 4-point severity scale (0=none, 1=mild, 2=moderate, and 3=severe), and the total score is calculated as a weighted sum. A higher score indicates greater symptom severity, and a decrease from baseline represents clinical improvement. In this study, a baseline KMI score ≥15 was adopted as an inclusion criterion for defining moderate-to-severe menopausal syndrome.
Figure 1.
Efficacy evaluation of the Kupperman index between the three groups. HTG, traditional Gochujang containing a high dose of beneficial microbes; LTG, traditional Gochujang, containing a low dose of effective microbes; CG, commercially prepared Gochujang.
Figure 1.
Efficacy evaluation of the Kupperman index between the three groups. HTG, traditional Gochujang containing a high dose of beneficial microbes; LTG, traditional Gochujang, containing a low dose of effective microbes; CG, commercially prepared Gochujang.
3.2. Changes in Anthropometric and Body Composition Parameters
Changes in the anthropometric data and body composition of subjects after the 8-week intervention period are presented in
Table 3. A statistically significant decrease in percent body fat (PBF) was observed in all three groups (p < 0.05). In addition, there were significant changes in other parameters in certain groups. There was also a significant decrease in body fat mass (BFM) in the HGC group (p = 0.002). A significant increase in fat-free mass (FFM) and basal metabolic rate (BMR) was observed in the LGC group (p = 0.025 and 0.024, respectively). For all other measured parameters, including weight, BMI, waist circumference (WC), and the waist-to-hip ratio (WHR), no statistically significant changes were observed in any group (p > 0.05).
Table 2.
Efficacy evaluation of the Kupperman index between the three groups.
Table 2.
Efficacy evaluation of the Kupperman index between the three groups.
| Value |
Group |
| HTG (n=20) |
LTG (n=20) |
CG (n=20) |
| Before |
After |
p-value |
Before |
After |
p-value |
Before |
After |
p-value |
| Vasomotor symptoms |
9±2.55 |
7±2.2 |
<0.0001 |
8.8±2.78 |
6.8±2.28 |
0.002 |
8.84±2.85 |
7.16±2.85 |
0.002 |
| Paresthesia |
1.5±1.43 |
1±1.03 |
0.021 |
2±1.3 |
1.2±1.2 |
0.008 |
1.79±1.75 |
1.05±1.22 |
0.015 |
| Insomnia |
3.7±0.98 |
3.6±1.39 |
0.789 |
3.8±1.94 |
3.7±1.17 |
0.853 |
3.37±1.5 |
3.47±1.47 |
0.716 |
| Nervousness |
1.1±1.37 |
0.5±0.89 |
0.030 |
0.9±1.37 |
0.3±0.73 |
0.055 |
0.84±1.38 |
0.32±0.75 |
0.021 |
| Melancholia |
0±0 |
0±0 |
- |
0.05±0.22 |
0±0 |
0.330 |
0.16±0.5 |
0±0 |
0.187 |
| Vertigo |
0.95±0.69 |
0.55±0.83 |
0.042 |
0.75±0.64 |
0.5±0.61 |
0.056 |
1±0.82 |
0.53±0.61 |
0.001 |
| Fatigue |
2.4±0.75 |
2.45±0.76 |
0.834 |
2.3±0.66 |
2.5±0.61 |
0.330 |
2.21±0.71 |
2.58±0.61 |
0.049 |
| Headache |
0.6±0.75 |
0.4±0.6 |
0.258 |
0.65±0.59 |
0.35±0.49 |
0.030 |
0.89±0.74 |
0.53±0.51 |
0.031 |
| Arthralgia and myalgia |
1.8±0.95 |
2.1±0.85 |
0.267 |
1.6±0.94 |
1.6±0.82 |
1.000 |
1.84±0.69 |
1.95±0.62 |
0.650 |
| Palpitation |
0.35±0.88 |
0.05±0.22 |
0.110 |
0.15±0.67 |
0.05±0.22 |
0.541 |
#VALUE! |
#VALUE! |
0.331 |
| Formication |
0.05±0.22 |
0.0±0.0 |
0.330 |
0.1±0.45 |
0.0±0.0 |
0.330 |
0.05±0.23 |
0.0±0.0 |
<0.0001 |
| Total |
21.3±2.39 |
17.65±2.25 |
<0.0001 |
21.1±3.67 |
17±3.29 |
<0.0001 |
21±2.87 |
17.58±2.69 |
0.429 |
| Vaginal dryness |
1.3±0.57 |
1±0.46 |
0.010 |
1.45±0.83 |
1.15±0.59 |
0.030 |
1.21±0.71 |
1.11±0.46 |
0.187 |
3.3. Effects of Gochujang on Markers of Lipid and Glucose Metabolism
The effects of the intervention on key inflammation-, lipid metabolism-, and glucose metabolism-related markers were assessed, and the results are summarized in
Table 4. Overall, the 8-week intervention did not result in any statistically significant changes in the levels of inflammation- or glucose metabolism-related markers (including fasting blood glucose, insulin, HOMA-IR, and HOMA-β) in any of the three groups. As concerns lipid metabolism, most parameters, including total cholesterol and LDL cholesterol, remained stable, with no significant alterations observed in their levels with respect to those at baseline. The only statistically significant change observed was a slight decrease in HDL cholesterol levels following the intervention. However, the magnitude of this decrease was small, and mean values remained well within the normal clinical range. Therefore, this change was not considered clinically significant.
Figure 2.
Efficacy evaluation of antiobesity between the three groups. HTG, traditional Gochujang containing a high dose of beneficial microbes; LTG, traditional Gochujang, containing a low dose of effective microbes; CG, commercially prepared Gochujang; PBF, Percent Body Fat.
Figure 2.
Efficacy evaluation of antiobesity between the three groups. HTG, traditional Gochujang containing a high dose of beneficial microbes; LTG, traditional Gochujang, containing a low dose of effective microbes; CG, commercially prepared Gochujang; PBF, Percent Body Fat.
3.4. Safety and Tolerability Assessment
Gochujang safety and tolerability were assessed by monitoring vital signs and conducting hematological and biochemical analyses before and after the 8-week intervention period; the results are summarized in
Table 5. Throughout the study period, no statistically significant changes were observed in vital signs, including systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse, in any of the groups. Key indicators of liver function (GGT, AST, and ALT) also remained stable, with no significant changes observed with respect to their levels at baseline. Several other biochemical and hematological parameters, including RBC counts, hemoglobin levels, hematocrit, creatinine levels, uric acid levels, total protein levels, and albumin levels, showed statistically significant changes from their levels at baseline in one or more groups. However, all of these changes were minor, and the mean values for all parameters remained well within their normal clinical ranges throughout the study period. No adverse events related to these changes were reported by the participants. Overall, these findings suggest that the Gochujang-based supplement was well-tolerated and was not associated with any safety issues during the 8-week intervention period.
4. Discussion
Menopause, an important physiological transition associated with a decline in ovarian function, results in a complex array of climacteric symptoms, including disruptive vasomotor events, disturbed sleep, and affective mood alterations [
17,
18]. These manifestations significantly compromise quality of life in a large proportion of postmenopausal women, highlighting the need for the development of effective therapeutic strategies [
19,
20]. Although HRT is the most effective pharmaceutical intervention for managing these symptoms, its long-term application remains controversial owing to documented safety concerns, particularly those related to cardiovascular events and hormone-sensitive malignancies [
21,
22]. Consequently, there is a persistent patient preference for scientifically substantiated, non-hormonal, natural alternatives [
22,
23].
Our rationale for selecting an alternative functional food for use in the postmenopausal context was driven by the imperative to address the dual pathological burden common in this population. Estrogen deficiency following menopause increases the risk for conditions such as metabolic syndrome and central adiposity, which occur concurrently with severe climacteric symptoms. Therefore, an optimal nutraceutical should possess a pleiotropic therapeutic profile, being capable of concurrently mitigating climacteric symptoms and attenuating metabolic dysregulation. Gochujang, a complex Korean traditional fermented food (KTFF), was specifically selected owing to its potential to provide this dual synergistic benefit. It integrates fermentation-enhanced isoflavone aglycones with red pepper-derived capsaicinoids and provides a chemical matrix distinct from that of single-component phytoestrogen supplements [
24].
Previous studies have established a robust functional profile for Gochujang, particularly in relation to the mitigation of chronic metabolic and inflammatory pathologies [
12]. Human clinical trials carried out in overweight adults have demonstrated that Gochujang supplementation induces a verifiable decrease in visceral adipose tissue mass [
25] and favorable modulation of blood lipid profiles, primarily through TRPV1 channel activation [
26]. This activation promotes WAT browning and enhanced thermogenesis [
27]. Preclinical data have also confirmed the potent anti-inflammatory action of Gochujang, mediated via a decrease in pro-inflammatory cytokine levels and NF-κB signaling pathway inhibition [
28,
29]. Furthermore, evidence suggests that Gochujang positively modulates the RAAS, thereby exerting anti-hypertensive effects regardless of its characteristic high sodium content [
13,
30].
Despite this significant evidence of the systemic health benefits of Gochujang, data supporting its clinical efficacy against menopausal syndrome is lacking. Research on climacteric symptom relief has mostly focused on isolated phytoestrogens or other structurally related KTFFs such as Cheonggukjang, which has been clinically proven to alleviate menopausal symptoms through the use of the KMI as primary endpoint [
31,
32].
This study represents a pioneering effort to bridge this gap. To the best of our knowledge, this is the first randomized, double-blind, controlled clinical trial designed to assess the therapeutic effects of various Gochujang preparations on climacteric symptom severity in postmenopausal women. We selected the KMI as the primary measure for efficacy as it is globally established and recognized as an authoritative and validated quantitative rating scale for assessing climacteric symptoms in controlled clinical trials [
33].
Our findings, for the first time, provide clinical evidence to substantiate this therapeutic potential. Regarding the primary outcome, we demonstrated a statistically significant decrease in the total KMI score in both the HGC and LGC groups (P < 0.0001), with no significant overall improvement observed in the CG group (P = 0.429). This indicates that Gochujang supplementation provides significant and comprehensive climacteric symptom relief. Of note, improvements in vasomotor symptoms, specifically, hot flashes and paresthesia, were observed across all three groups, suggesting a more general effect of the intervention on these symptoms.
Furthermore, our results on the anti-obesity effects of Gochujang align with those of previous studies, although we present some novel insights. We observed a statistically significant decrease in PBF in all three groups. There was also a significant decrease in total BFM in the HGC group. Intriguingly, subjects in the LGC group exhibited a unique and favorable change in body composition, with a significant increase in FFM and a corresponding increase in BMR. These improvements in body composition were not accompanied by significant changes in overall weight or BMI. However, unlike in some previous studies which focused on assessing metabolic profiles, with the exception of a minor, clinically insignificant decrease in HDL-C, we did not observe significant changes in broader lipid profiles and glucose metabolism markers in this study. The functional rationale for selecting Gochujang is bolstered by its documented anti-obesogenic effects in human intervention trials [
34]. Systematic reviews have confirmed that primarily via TRPV1 receptor activation, capsaicinoid increases thermogenesis, as well as daily energy expenditure by approximately 50 kcal/day [
35]. Mechanistically, capsinoid supplementation for 8 weeks at a dose of 9 mg/day has been shown to increase brown adipose tissue activity in humans, thereby promoting non-shivering thermogenesis [
36]. These physiological changes result in improvements in body composition, with randomized controlled trials on the effects of Gochujang in overweight adults demonstrating a verifiable decrease in visceral fat content over 12 weeks [
24]. Specifically, Gochujang consumption for 12 weeks significantly improved cardiovascular risk profiles by decreasing serum triglyceride levels (-14.1%) and the TG/HDL ratio. This evidence confirms that Gochujang is an effective functional food for concurrently managing the high metabolic risk associated with the postmenopausal state.
As concerns safety and tolerability, the intervention was well-tolerated by all participants. In line with previous studies which suggested that Gochujang exerts a benign or even favorable effect on the RAAS [
37], we observed no adverse changes in blood pressure or other vital signs. Although minor, statistically significant fluctuations were observed in the levels of some hematological and biochemical markers, all mean values remained well within normal clinical ranges, confirming the safety of Gochujang during the 8-week intervention. These safety findings are crucial for establishing Gochujang as a viable long-term nutritional supplement for postmenopausal women.
5. Conclusions
This randomized, controlled trial demonstrated the efficacy of Gochujang in mitigating the dual pathological burden of climacteric symptoms and metabolic risk in postmenopausal women. The primary endpoint showed that Gochujang supplementation induced a statistically significant decrease in the total KMI score (p < 0.0001) across the intervention groups. Favorable secondary outcomes included a significant decrease in PBF across all groups and an increase in FFM in the LGC group, which indicated beneficial modulation of body composition. Safety assessments confirmed excellent Gochujang tolerability, with no adverse changes observed in hematological profiles, vital signs, or self-reported symptoms. These data suggest that Gochujang is a safe, non-hormonal nutraceutical for the concurrent clinical management of climacteric symptoms and metabolic health in postmenopausal women.
Author Contributions
Author Contributions: Conceptualization, Project administration, D.Y.J.; Methodology, M.S.R.; Resources, Funding acquisition, M.S.R. and H.J.Y.; Visualization, S.J.J. and H.J.Y.; Writing—review, A.L.H.; Editing, K.H.C and S.R.S. All authors have read and agreed to the submitted version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture, Food and Rural Affairs and the Korea Agro-Fisheries and Food Trade Corporation.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Wonkwang University Hospital (IRB Approval no.: WKUH IRB 2025-03-030-002).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study
Data Availability Statement
Raw data can be provided upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI |
Body mass index |
| CG |
Control group |
| FFM |
Fat-free mass |
| HGC |
High-Gochujang |
| HRT |
Hormone replacement therapy |
| LGC |
Low-Gochujang |
| KMI |
Kupperman Menopausal Index |
| PBF |
Percent body fat |
| RAAS |
Renin-Angiotensin-Aldosterone System |
References
- Martínez-Vázquez, S.; Hernández-Martínez, A.; Peinado-Molina, R.A.; Martínez-Galiano, J.M. Impact of overweight and obesity in postmenopausal women. Climacteric 2023, 26, 577–582. [Google Scholar] [CrossRef]
- Bachmann, G.A. Menopausal vasomotor symptoms: a review of causes, effects and evidence-based treatment options. J Reprod Med 2005, 50(3), 155–65. [Google Scholar] [PubMed]
- Panay, N.; Rees, M. Alternatives to Hormone Replacement Therapy for Management of Menopause Symptoms. Curr Obstet Gynaecol 2005, 15, 259–266. [Google Scholar] [CrossRef]
- Sampsell, K.; Schultz Marcolla, C.; Tapping, S.; Fan, Y.; Sánchez-Lafuente, C.L.; Willing, B.P.; Reimer, R.A.; Burton, J.P. Current Research in Fermented Foods: Bridging Tradition and Science. Adv Nutr 2025, 16, 100554. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Jang, D.J.; Yang, H.J.; Chung, K.R. History of Korean Gochu, Gochujang, and Kimchi. J Ethn Foods 2014, 1, 3–7. [Google Scholar] [CrossRef]
- Jung, S.J.; Chae, S.W.; Shin, D.H. Fermented Foods of Korea and Their Functionalities. Fermentation 2022, 8, 645. [Google Scholar] [CrossRef]
- Shin, H.W.; Jang, E.S.; Moon, B.S.; Lee, J.J.; Lee, D.E.; Lee, C.H.; Shin, C.S. Anti-obesity Effects of Gochujang Products Prepared Using Rice Koji and Soybean Meju in Rats. J Food Sci Technol 2016, 53, 1004–1013. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol 2016, 173(15), 2369–2389. [Google Scholar] [CrossRef]
- Lee, Y.K.; Cha, Y.S.; Park, Y.S.; Lee, M.S. PPARγ2 C1431T Polymorphism Interacts with the Antiobesogenic Effects of Kochujang, a Korean Fermented, Soybean-Based Red Pepper Paste, in Overweight/Obese Subjects: A 12-Week, Double-Blind Randomized Clinical Trial. J Med Food 2016, 20((6).). [Google Scholar] [CrossRef]
- Mahoro, P.; Moon, H.J.; Yang, H.J.; Kim, K.A.; Cha, Y.S. Protective Effect of Gochujang on Inflammation in a DSS-Induced Colitis Rat Model. Foods 2021, 10(5), 1072. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Wang, Y. Capsaicin Reduces Obesity by Reducing Chronic Low-Grade Inflammation. Int J Mol Sci 2024, 25(16), 8979. [Google Scholar] [CrossRef]
- Lee, E.J.; Edward, O.C.; Seo, E.B.; Mun, E.G.; Jeong, S.J.; Ha, G.; Han, A.; Cha, Y.S. Gochujang Ameliorates Hepatic Inflammation by Improving Dysbiosis of Gut Microbiota in High-Fat Diet-Induced Obese Mice. Microorganisms 2023, 11(4), 911. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Han, A.; Mun, E.G.; Cha, Y.S. A traditional Korean fermented food, Gochujang exerts anti-hypertensive effects, regardless of its high salt content by regulating renin-angiotensin-aldosterone system in SD rats. Heliyon 2024, 10(9), e30451. [Google Scholar] [CrossRef] [PubMed]
- Silva, H. The Vascular Effects of Isolated Isoflavones—A Focus on the Determinants of Blood Pressure Regulation. Biology (Basel) 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. The Korea National Health and Nutrition Examination Survey (KNHANES): Current Status and Challenges. Epidemiol Health 2014, 36, e2014002. [Google Scholar] [CrossRef]
- Tao, M.F.; Shao, H.; Li, C.; Teng, Y. Correlation Between the Modified Kupperman Index and the Menopause Rating Scale in Chinese Women. Patient Prefer Adherence 2013, 7, 223–229. [Google Scholar] [CrossRef]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The Menopause Transition: Signs, Symptoms, and Management Options. J Clin Endocrinol Metab 2021, 106, 1–15. [Google Scholar] [CrossRef]
- Gatenby, C.; Simpson, P. Menopause: Physiology, definitions, and symptoms. Best Pract Res Clin Endocrinol Metab 2024, 38, 101855. [Google Scholar] [CrossRef]
- Crandall, C.J.; Mehta, J.M.; Manson, J.E. Management of menopausal symptoms: A review. JAMA 2023, 329, 405–20. [Google Scholar] [CrossRef]
- Madsen, T.E.; Sobel, T.; Negash, S.; Shrout Allen, T.; Stefanick, M.L.; Manson, J.E.; et al. A review of hormone and non-hormonal therapy options for the treatment of menopause. Int J Women’s Health 2023, 15, 825–36. [Google Scholar] [CrossRef]
- The 2023 nonhormone therapy position statement of The North American Menopause Society. Menopause 2023, 30(6), 573–90. [CrossRef] [PubMed]
- The 2022 Hormone Therapy Position Statement of The North American Menopause Society. Menopause 2022, 29(7), 767–94. [CrossRef] [PubMed]
- Panay, N.; Ang, S.B.; Cheshire, R.; Goldstein, S.R.; Maki, P.; Nappi, R.E. Menopause and MHT in 2024: addressing the key controversies – an International Menopause Society White Paper. Climacteric 2024, 27(5), 441–457. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.S.; Kim, S.R.; Yang, J.A.; Back, H.I.; Kim, M.G.; Jung, S.J.; et al. Kochujang, fermented soybean-based red pepper paste, decreases visceral fat and improves blood lipid profiles in overweight adults. Nutr Metab (Lond) 2013, 10(1), 24. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Wang, L.; Wang, J.; Guo, W.; Tang, T.; Zhang, Y.; Dong, Y. The effects of capsaicin intake on weight loss among overweight and obese subjects: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr 2023, 130, 1645–1656. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol Epub. 2016, 173(15), 2369–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; Accili, D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012, 150(3), 620–32. [Google Scholar] [CrossRef]
- Park, Y.K.; Kim, J.; Ryu, M.S.; Yang, H.-J.; Jeong, D.-Y.; Shin, D.-H. The Health Benefits and Functional Properties of Gochujang: A Comprehensive Review of Fermentation and Bioactive Compounds. Fermentation 2025, 11, 67. [Google Scholar] [CrossRef]
- Shen, J.; Li, N.; Zhang, X. Daidzein ameliorates dextran sulfate sodium-induced experimental colitis in mice by regulating NF-κB signaling. J Environ Pathol Toxicol Oncol 2019, 38(1). [Google Scholar] [CrossRef]
- Edward, O.C. Gochujang consumption prevents metabolic syndrome in a high-fat diet induced obese mouse model. J Med Food 2023, 26, 244–254. [Google Scholar] [CrossRef]
- Rasheed, S.; Rehman, K.; Shahid, M.; Suhail, S.; Akash, M.S.H. Therapeutic potentials of genistein: New insights and perspectives. J Food Biochem 2022, 46(9), e14228. [Google Scholar] [CrossRef]
- Han, A-.L.; Ryu, M.-S.; Yang, H.-J.; Jeong, D.-Y.; Choi, K.-H. The Efficacy of Cheonggukjang in Alleviating Menopausal Syndrome and Its Effects on the Gut Microbiome: A Randomized, Double-Blind Trial. Nutrients 2025, 17(3), 505. [Google Scholar] [CrossRef]
- Davis, S.R. The kupperman index undressed. Maturitas 2019, 126, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Urbina, S.L.; Taylor, L.W.; Wilborn, C.D.; Purpura, M.; Jäger, R.; Juturu, V. Capsaicinoids supplementation decreases percent body fat and fat mass: adjustment using covariates in a post hoc analysis. BMC Obes 2018, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite 2012, 59, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zheng, S.; Feng, Q.; Zhang, Q.; Xiao, X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci Rep 2017, 37, e00286. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).