1. Introduction
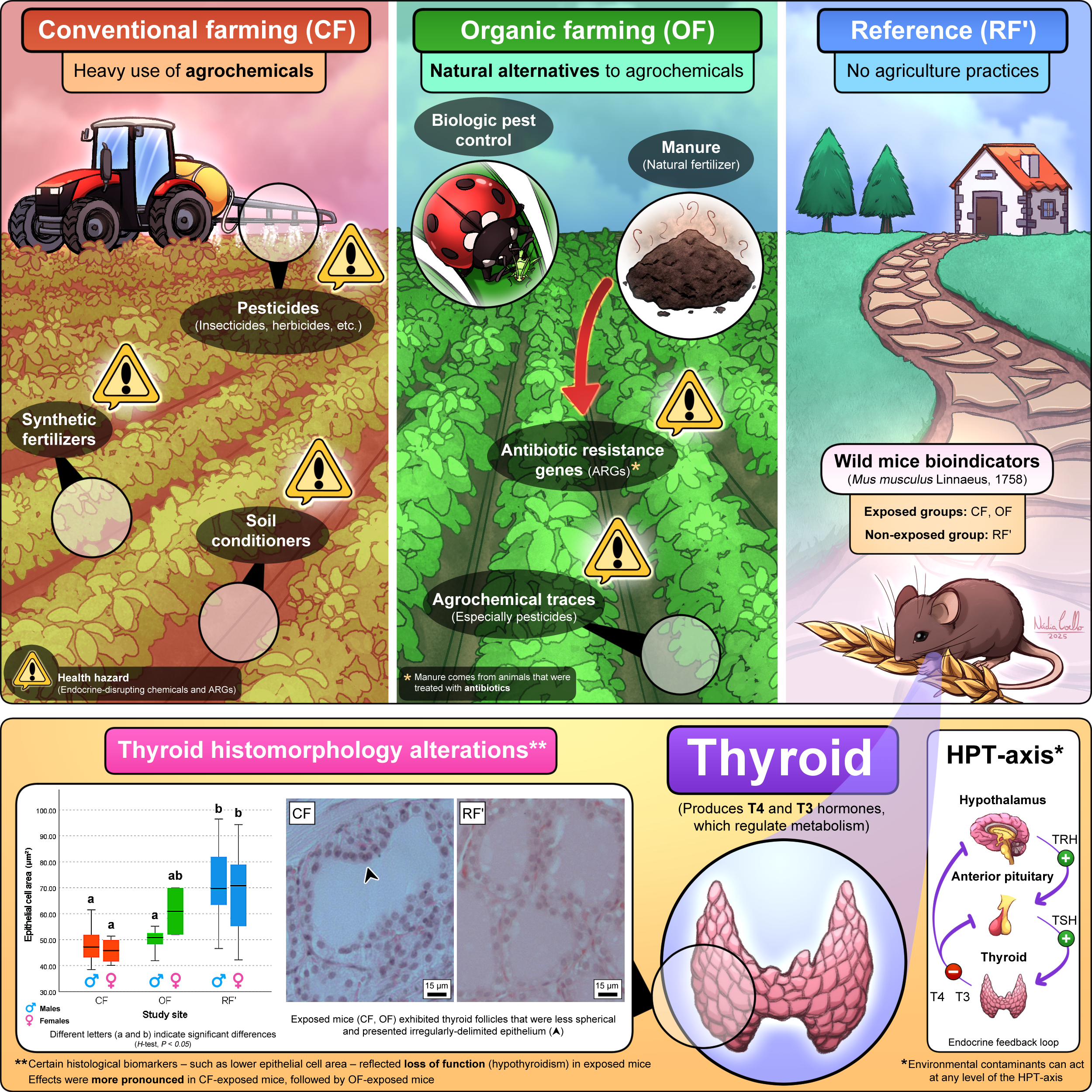
Agriculture is among the most important anthropogenic activities, providing human populations with a steady supply of food, fuel, and fiber (Fanzo, 2018; Viana et al., 2022). However, agriculture is one of the major causes of environmental pollution, adversely affecting ecosystems condition and human health – especially when involving the use of synthetic agrochemicals (Kaur et al., 2024). Based on the nature of approaches towards food and livestock production, agriculture is divided into two major systems: conventional farming (CF), and organic farming (OF). In essence, while the first heavily relies on synthetic inputs (i.e., agrochemicals) to maximize yields, the latter excludes synthetic inputs to promote environmental sustainability (Azarbad, 2022). Worldwide, CF is the dominant farming system (Gaitán-Cremaschi et al., 2019). By contrast, although growing, OF represents only about 1% of global cropland (Crowder et al., 2015). There is debate about whether OF is an overall better alternative to CF, namely when it comes to the balance between production costs, yields, and even the presence of hazardous substances in produce (Seufert et al., 2012).
Conventional farming (also known as industrialized agriculture) refers to agricultural methods heavily reliant on synthetic inputs, such as the use of agrochemicals and genetically-modified organisms (GMOs), to achieve the highest possible yields. Most modern agriculture systems rely on CF practices for production, due to the rise in human population numbers and consequent ever-increasing demand for food products (Milner et al., 2023). The presence of several cancer-promoting pesticide residues has been documented in CF food products, highlighting the serious health hazard associated with CF contaminants (Sadighara et al., 2023). On the other hand, organic farming (also referred to as biological agriculture) is characterized by the exclusion of synthetic fertilizers, pesticides, and GMOs in its practices. Organic farming prioritizes quality over quantity, with the aim of producing safer and healthier food products despite relatively lower yields (Wilbois et al., 2019). Focusing on environmental sustainability, OF employs techniques like the use of composting and manure as natural fertilizers, crop rotation, cover cropping, and biological pest control – i.e., the implementation of natural predators, parasites, and pathogens of pest populations – in its practices (Strenner et al., 2023; Panday et al., 2024). Data suggests that OF positively impacts the environment by promoting a decrease in nitrate leaching and in greenhouse gas emissions, along with increasing farmland biodiversity, and even promoting carbon sequestration via increasing soil organic matter (Meng et al., 2017). According to Meng et al. (2017), all these benefits compensate for the economic costs of a lower crop production.
The trend is for current CF systems to transition to OF ones, due to the perception that the latter are more beneficial for both the environment and humans. However, agrochemical residues, especially synthetic pesticide traces, are commonly detected in OF produce (Giampieri et al., 2022; Lazarević-Pašti et al., 2025). The review of Lazarević-Pašti et al. (2025) points to the presence of pesticide residues in 28% of organic food in Southern Germany, 9% in the Swiss market, and 6% in the overall European market. Another problem is the fact that OF practices increase the amount and distribution of antibiotic resistance genes (ARGs) in the environment. This is because the manure used as natural fertilizer often comes from livestock that was treated with antibiotics, growth factors, and other substances (Parelho et al., 2018; Silva et al., 2018; Bassitta et al., 2022). Bacterial populations in the soil are exposed to these compounds, gain resistance towards them, and pass on the relevant genes both intra- (vertical gene transfer) and interspecies (horizontal gene transfer) (Zhuang et al., 2024).
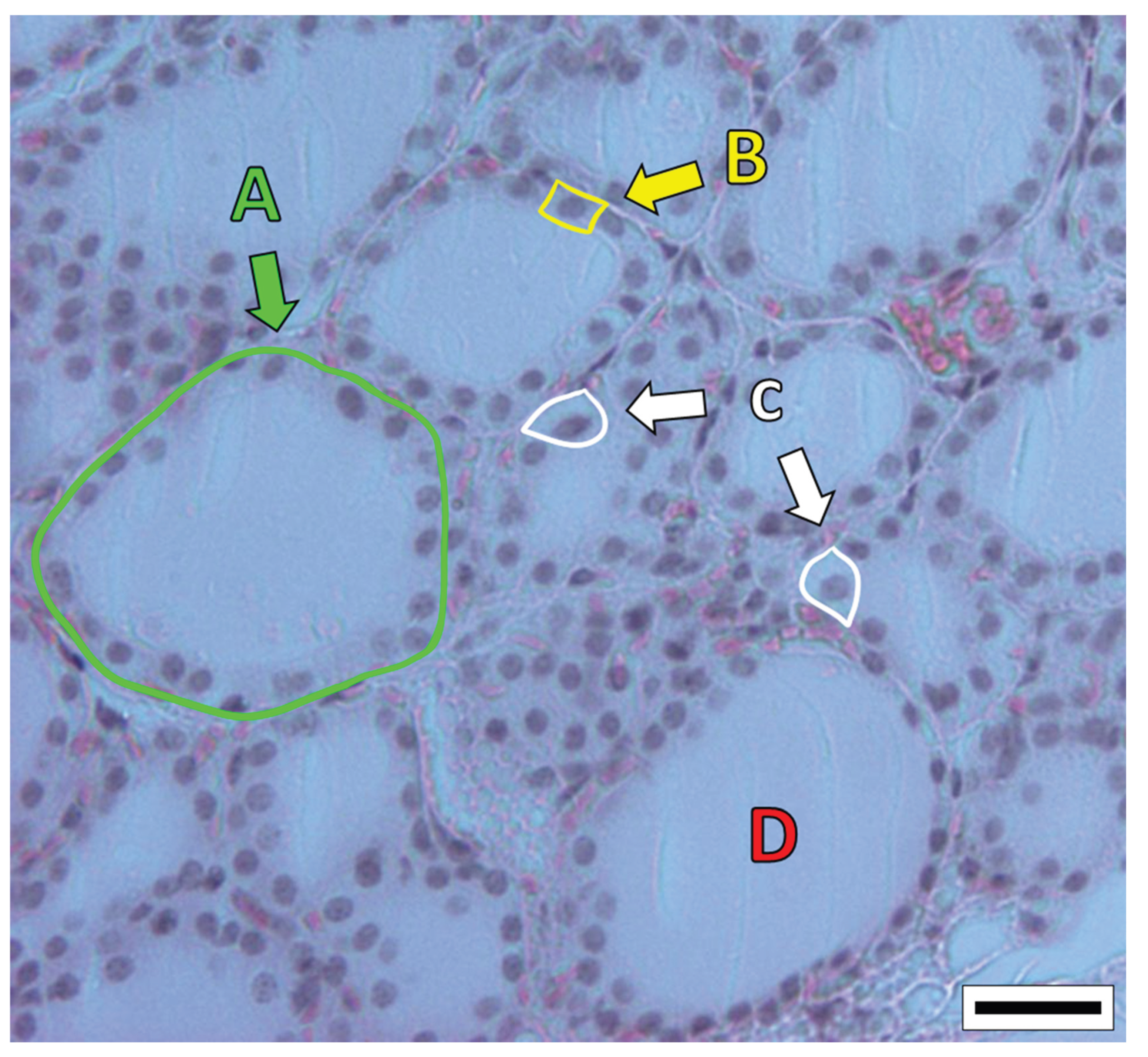
The thyroid is a butterfly-shaped gland of the endocrine system, located in front of the neck, which exerts a fundamental role in metabolism. Its purpose is linked to the production of thyroid hormones (TH) – tetraiodothyronine (thyroxine or T4), and triiodothyronine (T3). In essence, TH boost metabolism by influencing the speed at which cells make use of oxygen and nutrients, which are needed for cellular activity (Yavuz
et al., 2019). Thyroid hormones regulate a plethora of processes, ranging from nervous system development, linear growth, and thermogenesis, to even the hepatic metabolism of nutrients, fluid balance, and cardiovascular system function. Thyrocytes (also known as follicular cells) are the cells that produce TH, which are organized in spherical structures (known as follicles) composed of a simple cuboidal epithelium, inside of which the colloid is stored (Ortiga-Carvalho
et al., 2016). C cells (also referred to as parafollicular cells) are present in the spaces between thyroid follicles, producing the calcitonin hormone to decrease blood calcium levels when appropriate (Cote
et al., 2015) (
Figure 1). Thyroid function is kept in check via the endocrine feedback loop spanning the hypothalamus-pituitary-thyroid axis (HPT-axis), via which TH production is controlled by negative feedback, according to TH levels in the bloodstream. The hypothalamus secretes thyrotropin-releasing hormone (TRH) to induce the synthesis and secretion of thyrotropin (or thyroid-stimulating hormone, TSH) by the anterior pituitary. In turn, TSH acts on the thyroid to promote the biosynthesis and secretion of TH (Boelen
et al., 2012; Ortiga-Carvalho
et al., 2016).
The thyroid retains high plasticity even in adults, reflecting its ability to adapt to stimuli by changing both its structure and function according to physiological needs. Because thyroid structure is intimately associated with glandular activity levels, its morphology is a good indicator of its functional state (Perez-Montiel & Suster, 2008; Lee et al., 2016; Candanedo-Gonzalez et al., 2021). Thyroid follicle size and epithelium height are examples of where histological alterations of note can be observed (Hallgren & Darnerud, 2002). Thyroid disorders are the most common endocrine disorders worldwide, estimated at around 200 million people having been diagnosed (Song et al., 2024). Estimates suggest that 1 in 10 men, and 1 in 8 women have some form of thyroid condition. It is further believed that 50% of thyroid disorders remain undiagnosed, underlining the seriousness of the topic (Bano et al., 2019). Hypothyroidism – a thyroid endocrine disorder in which TH are produced in insufficient amounts – is thought to affect approximately 5% of world population (≈ 380 million), with another 5% remaining undiagnosed (Chiovato et al., 2019). This data emphasizes the need for the development of studies that can accurately identify the causes of endocrine disruption of the thyroid and countermeasures against it.
Endocrine disruption is the process by which natural or man-made chemicals interfere with hormones, frequently by mimicking their structure or blocking their receptors (NIEHS, 2024). These exogenous chemicals are called endocrine disruptors (or endocrine-disrupting chemicals, EDCs), which lead to changes in the physiological function of endogenous hormones (Ahn & Jeung, 2023). Agrochemicals, with particular emphasis on pesticides, are widespread environmental EDCs (McKinlay et al., 2008; Campos & Freire, 2016; Kongtip et al., 2019). The term “thyroid disruption” encompasses any adverse thyroid outcomes derived of exposures to EDCs, such as hypothyroidism, hyperthyroidism, and cancer, via their endocrine-disrupting potential (He et al., 2024; Coelho et al., 2025). The majority of research linking thyroid disruption with exposure to CF and OF contaminants are epidemiological studies. In general, hypothyroidism was often reported in occupationally exposed farm workers (Goldner et al., 2010; Piccoli et al., 2016). However, a recent study showed that mice chronically exposed to an environment where CF is practiced had thyroid histomorphology alterations (Coelho et al., 2024). Such alterations, namely when it comes to lower epithelial cell area and volume, revealed indications of hypothyroidism in the exposed mice. These results highlighted the usefulness of thyroid histomorphology evaluation as an early and sensitive biomarker of glandular activity, therefore providing indications of thyroid disruption.
The purpose of this study is assessing the potential negative impact of OF on the thyroid gland and comparing it with the negative impact of CF on this organ, using histological techniques for the analysis of the thyroid of wild mice chronically exposed to these agricultural practices. The topic is especially relevant given the growing demand for agricultural products, and the tendency of regarding OF as a better alternative to CF. Therefore, this study aims to answer the following questions: (i) Does chronic exposure to an environment in which OF is practiced have a negative impact on the thyroid? (ii) If so, is such impact less severe than that caused by CF? The formulated hypothesis was: Chronic exposure to OF has a negative but less severe impact on the thyroid than that caused by CF.
2. Methodology
2.1. Study Sites
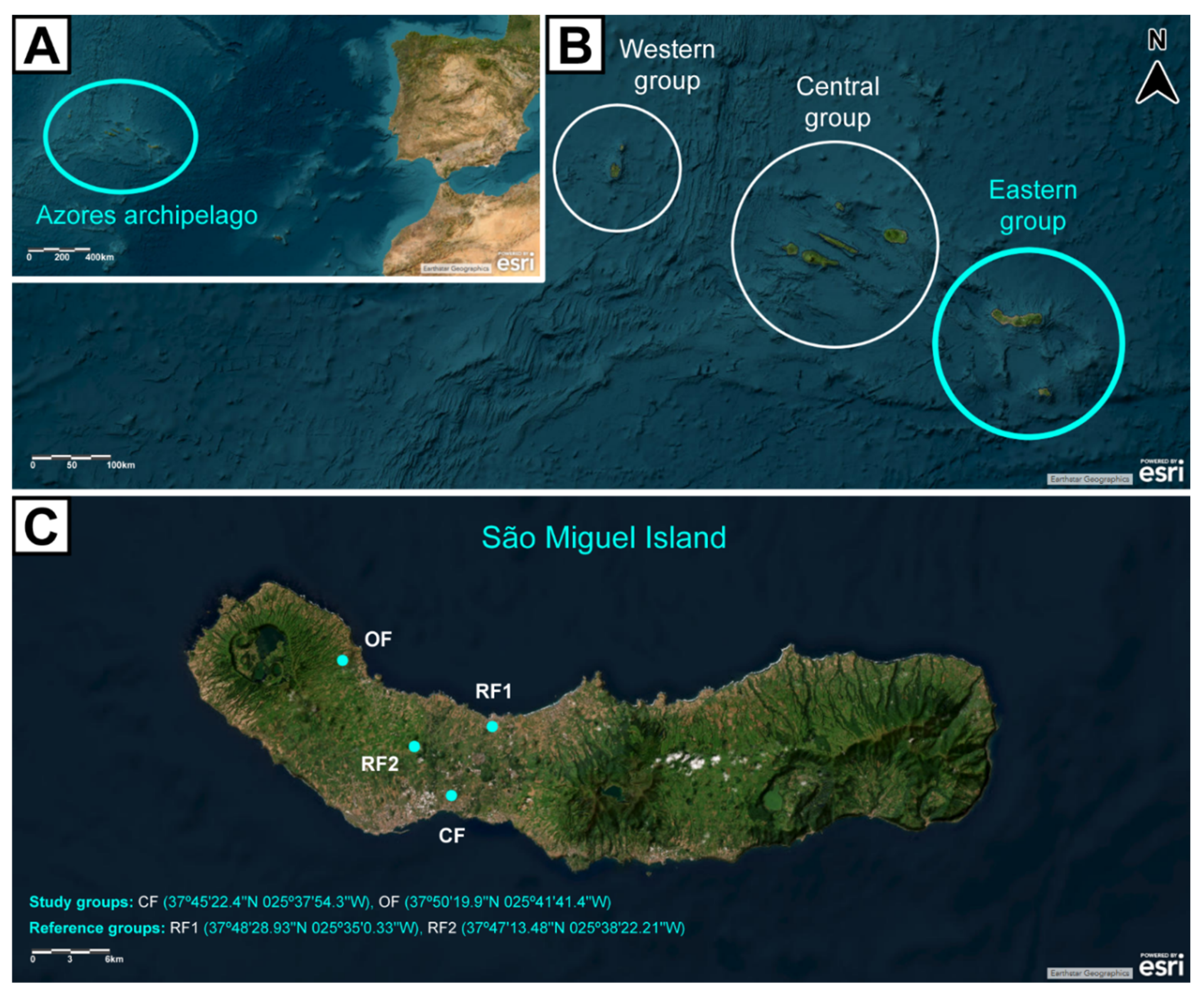
This work was carried out in São Miguel Island, Azores, Portugal. Study sites were selected based on the presence or absence of agricultural practices – including the nature of those practices. A total of 4 sites was selected: 2 agricultural areas (study groups), and 2 areas without agriculture (reference groups) (
Figure 2). The 2 study groups corresponded to a CF site and an OF site, explored under the respective agricultural system for at least over a decade. Concerning the 2 reference groups, one was a small village (Rabo de Peixe, RF1), while the other corresponded to a pristine area (Pinhal da Paz, RF2); in neither of which agricultural practices were made for at least over a decade. All sites are located in the geological complex of Picos Fissural Volcanic System, granting them the same bedrock and pedological conditions. This ensures that each site is distinguished mostly on the occurrence and nature of agricultural practices (Parelho
et al., 2014; Parelho
et al., 2016).
Conventional farming systems involve agricultural practices in which the use of agrochemicals is frequent and legally framed by European and national guidelines. Agrochemicals used in the CF study site include organic fertilizers, inorganic fertilizers, and pesticides like insecticides, herbicides, nematicides, fungicides, molluscicides, and acaricides. Parelho et al. (2014) mention that agriculture is a diffuse source of metal pollution in farming soils of São Miguel Island. According to the authors, CF soils in São Miguel Island are particularly contaminated with lithium (Li), potassium (K), and molybdenum (Mo), due to intensive and repeated use of inorganic fertilizers and pesticides. On the other hand, OF systems are certified by the European Commission, which prohibit the use of synthetic agrochemicals. Soil amendments are confined to organic fertilizers, namely compost and manure. Both farms have been explored under the same system for at least a decade (Parelho et al., 2016). RF1 is known for being the biggest fishing village in the Azores. There are no major agricultural practices within the village, with the exception of some home gardening for a small part of its population. Meanwhile, RF2 is a forest reserve of centennial Japanese cedar [Cryptomeria japonica (Thunb. ex L.f.) D.Don], in a region with no historical records or evidence of farming activity. According to Parelho et al. (2014), the soils from OF and RF2 have trace metal background values significantly lower than those found in the CF site. An exception was found for vanadium (V) and barium (Ba), which are present in higher concentrations in RF2 than in CF and OF soils. Further details on the characteristics of the study sites can be found in Parelho et al. (2016).
2.2. Mice Sampling and Histological Slides Preparation
Data from animals living in – or otherwise exposed to – contaminated areas is important in research, given it provides early warnings for environmental hazards and potential human health risks. The assessment of toxic effects on bioindicator species at environmentally relevant concentrations helps in understanding long-term effects of environmental stressors, monitoring contaminant flow through ecosystems and food chains, developing conservation strategies, and protecting public health. Additionally, field studies with animal models provide invaluable ecotoxicological data under the complexity of the assessed environment, which would be impossible to recreate in laboratory (Chen et al., 2024). The main criteria for selecting bioindicators are (i) high abundance, (ii) a life expectancy wide enough for the estimation of possible long-term effects (Marcheselli et al., 2010), and (iii) a fairly small home range of, on average, 145 m2 (Lidicker, 1966) (which is much smaller than the surface area of the study sites – i.e., farming areas of around 5000 m2). Wild mice Mus musculus Linnaeus, 1758, fulfill these criteria, hence they were selected as bioindicator species for this work.
In accordance with the methodology described by Coelho et al. (2024), wild M. musculus mice were captured in each of the selected sites (CF, OF, RF1, and RF2). Following euthanasia with isoflurane, the thyroids of the mice were removed en-bloc, fixed in 4% buffered formaldehyde, then subjected to standard histological processing. Both males and females were included in this work. Histological slides with sections of the thyroid of 64 individuals were made (each slide corresponding to a single individual, containing several 5 µm thick sections): 12 from CF, 12 from OF, 27 from RF1, and 13 from RF2. The slides were then stained with hematoxylin and eosin (Martoja et al., 1970). A series of histomorphometric and histomorphologic data was collected from the slides with the aim of evaluating the thyroid status of each individual.
Because males and females were not equally distributed between groups (χ2-test, P < 0.05), their data was first treated separately (Supplementary material). For both males and females, groups RF1 and RF2 did not differ in the majority of the evaluated parameters (t-test, P > 0.05 for normal data; U-test, P > 0.05 for non-normal data), hence they were merged into a new group, designated RF’ (Supplementary material). After assessing differences within groups between males and females, it was found that they did not differ in the majority of the evaluated parameters (t-test, P > 0.05 for normal data; U-test, P > 0.05 for non-normal data), hence statistical analysis was finally performed between groups CF, OF, and RF’ including their respective males and females population (Supplementary material). The inclusion of both males and females within groups ensured balanced samples across the CF and OF groups. On the other hand, a higher number of individuals in the RF’ group in comparison to the CF and OF groups ensured a stronger statistical power, increasing the likelihood of detecting real differences between groups (Andrade, 2020). There were no significant differences (F-test, P > 0.05) in the weight of mice between groups [weight (g): 14.0792 ± 0.47019 (CF); 13.4467 ± 0.40168 (OF); 14.5988 ± 0.42182 (RF’)]. Mice from CF and OF were, on average, significantly (F-test, P > 0.05) younger than those from RF’ [age (days): 106.7733 ± 5.32363 (CF); 116.1783 ± 10.47914 (OF); 211.9335 ± 11.05431 (RF’)].
The experimental procedures involved in this work were approved by the Ethics Committee of the University of Azores (REF: 10/2020). The procedures were performed under the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123) recommendations, directive 2010/63EU and Portuguese legislation (DL 113/2013).
2.3. Histomorphometric Analysis
Following the methodology from Coelho et al. (2024), 5 fields of thyroid were selected per slide to take photomicrographs using the 20X and 40X objectives, separated by a minimum of 20 µm. Photomicrographs were taken of the 4 largest follicles within each field, making up a total of 20 follicles studied per individual.
From the images obtained with the 20X objective, data regarding the number of follicles in an area of 30,000 µm2 of thyroid, per individual, was collected. From the images obtained with the 40X objective, data was collected regarding the following parameters: (i) the area and (ii) perimeter of the colloid, (iii) the thickness of the epithelium, and (iv) the number of epithelial cells nuclei on a 50 µm transect (Supplementary material). All the data obtained per each analyzed parameter was registered as the average per individual. The average width of epithelial cells, the average area of epithelial cells, and the average volume of epithelial cells were calculated per individual as described in Coelho et al. (2024).
The thickness of the epithelium varies with thyroid activity, with cuboidal cells when this gland functions normally, and smaller, squamous cells when it is underactive. In light of this, the average epithelium thickness, along with the average area and volume of epithelial cells, were selected as the main biomarkers of thyroid function (De Felice & Di Lauro, 2010).
2.4. Histomorphological Analysis
Following the criteria from Coelho et al. (2024), based on the photomicrographs taken with the 20X objective, a set of 5 different parameters were analyzed semi-quantitatively through visual qualitative scales, per field, per individual. The greater the assigned value in the scales, the greater the degree of deviation from normality – i.e., the greater the degree of thyroid disruption. The evaluated aspects were: (i) follicular sphericity, (ii) epithelium irregularity, (iii) degree of exfoliation, (iv) degree of inflammation, and (v) degree of colloid vacuolization (based on the presence of vacuoles in the colloid). The analyses were performed by two observers independently. The final values were registered by consensus, from a total of observations equivalent to: 60 for CF, 60 for OF, 135 for RF1, and 65 for RF2. Both the independent analyses and large number of observations served as a way to ensure that the recorded abnormalities were not influenced by histological processing artifacts. Examples of photomicrographs to which extreme values were assigned can be found in Supplementary material.
2.5. Statistical Analysis
Statistical analysis was performed in the IBM SPSS Statistics ® v. 28.0.1 (142) software. Statistical significance was considered when P < 0.05.
The normality of the morphometric analysis was verified through Q-Q plots. Differences of the means between groups CF, OF, and RF’ were assessed via One-way analysis of variance (ANOVA) tests [followed by post-hoc Tukey’s honest significance (HSD) tests, to assess which groups differed from one another], for normal data, and non-parametric independent samples Kruskal-Wallis tests (followed by pairwise comparisons, to assess which groups differed from one another), for non-normal data.
Box plots portraying data regarding colloid area and perimeter, epithelium thickness, and epithelial cell area and volume were made, with the aim of improving the perception of differences between their distributions per group. Much like a squamous epithelium, follicles with larger colloid area and perimeter are also indicative of an underactive thyroid. In light of this, the mentioned parameters were chosen for graphical representation due to better representing indications of thyroid disruption (De Felice & Di Lauro, 2010; Lee et al., 2016).
Regarding the morphologic analysis, for each of the evaluated aspects, non-parametric Kruskal-Wallis tests were used to compare the distributions between the values registered in observations between groups CF, OF, and RF’. The relative frequency of each category was calculated, per group, to improve the perception of possible differences between their distributions per group.
Associations between data were determined via Spearman correlations (in all correlations assessed, at least one parameter had either non-normal data or qualitative data).
3. Results
3.1. Histomorphometric Analysis
The table below displays the descriptive statistics and differences between groups regarding morphometric (quantitative) data (
Table 1).
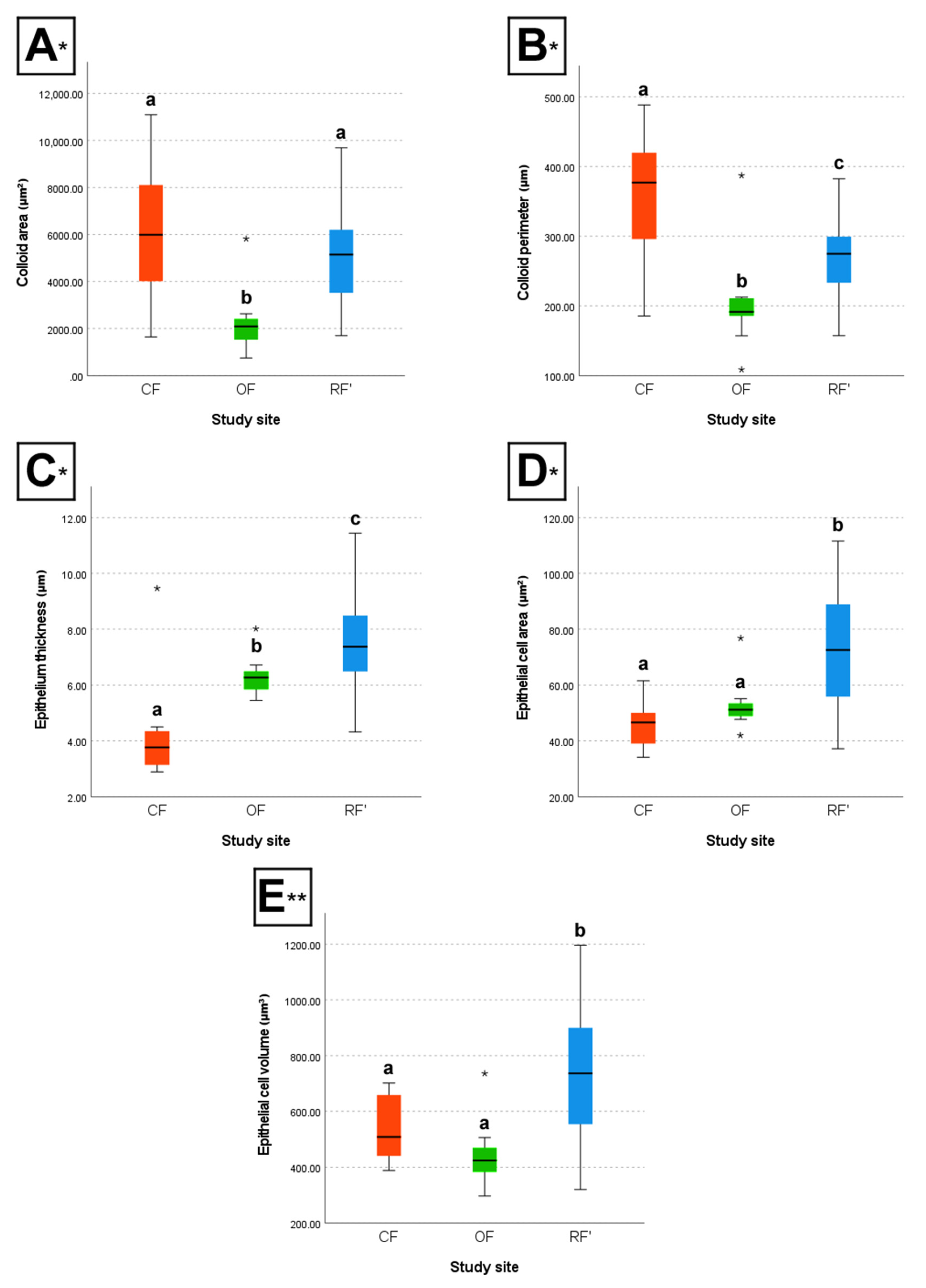
The box plots below illustrate the differences between groups concerning colloid area and perimeter, epithelium thickness, and epithelial cell area and volume (
Figure 3).
Between CF and OF, results show that individuals from CF had, on average, significantly larger colloid area (H-test, P < 0.05), larger colloid perimeter (H-test, P < 0.05), lower epithelium thickness (H-test, P < 0.05), a lower number of epithelial cell nuclei per 50 µm (H-test, P < 0.05), larger epithelial cell width (F-test, P < 0.05), and a lower number of follicles per 30,000 µm2 of thyroid (H-test, P < 0.05), when compared to individuals from OF. However, CF and OF individuals did not differ significantly regarding epithelial cell area (H-test, P > 0.05), nor epithelial cell volume (F-test, P > 0.05).
Between CF and RF’, results show that individuals from CF had, on average, significantly larger colloid perimeter (H-test, P < 0.05), lower epithelium thickness (H-test, P < 0.05), a lower number of epithelial cell nuclei per 50 µm (H-test, P < 0.05), larger epithelial cell width (F-test, P < 0.05), lower epithelial cell area (H-test, P < 0.05), lower epithelial cell volume (F-test, P < 0.05), and a lower number of follicles per 30,000 µm2 of thyroid (H-test, P < 0.05), when compared to individuals from RF’. However, CF and RF’ individuals did not differ significantly regarding colloid area (H-test, P > 0.05).
Finally, between OF and RF’, results show that individuals from OF had, on average, significantly lower colloid area (H-test, P < 0.05), lower colloid perimeter (H-test, P < 0.05), lower epithelium thickness (H-test, P < 0.05), a higher number of epithelial cell nuclei per 50 µm (H-test, P < 0.05), lower epithelial cell width (F-test, P < 0.05), lower epithelial cell area (H-test, P < 0.05), lower epithelial cell volume (F-test, P < 0.05), and a higher number of follicles per 30,000 µm2 of thyroid (H-test, P < 0.05), when compared to individuals from RF’.
3.2. Histomorphological Analysis
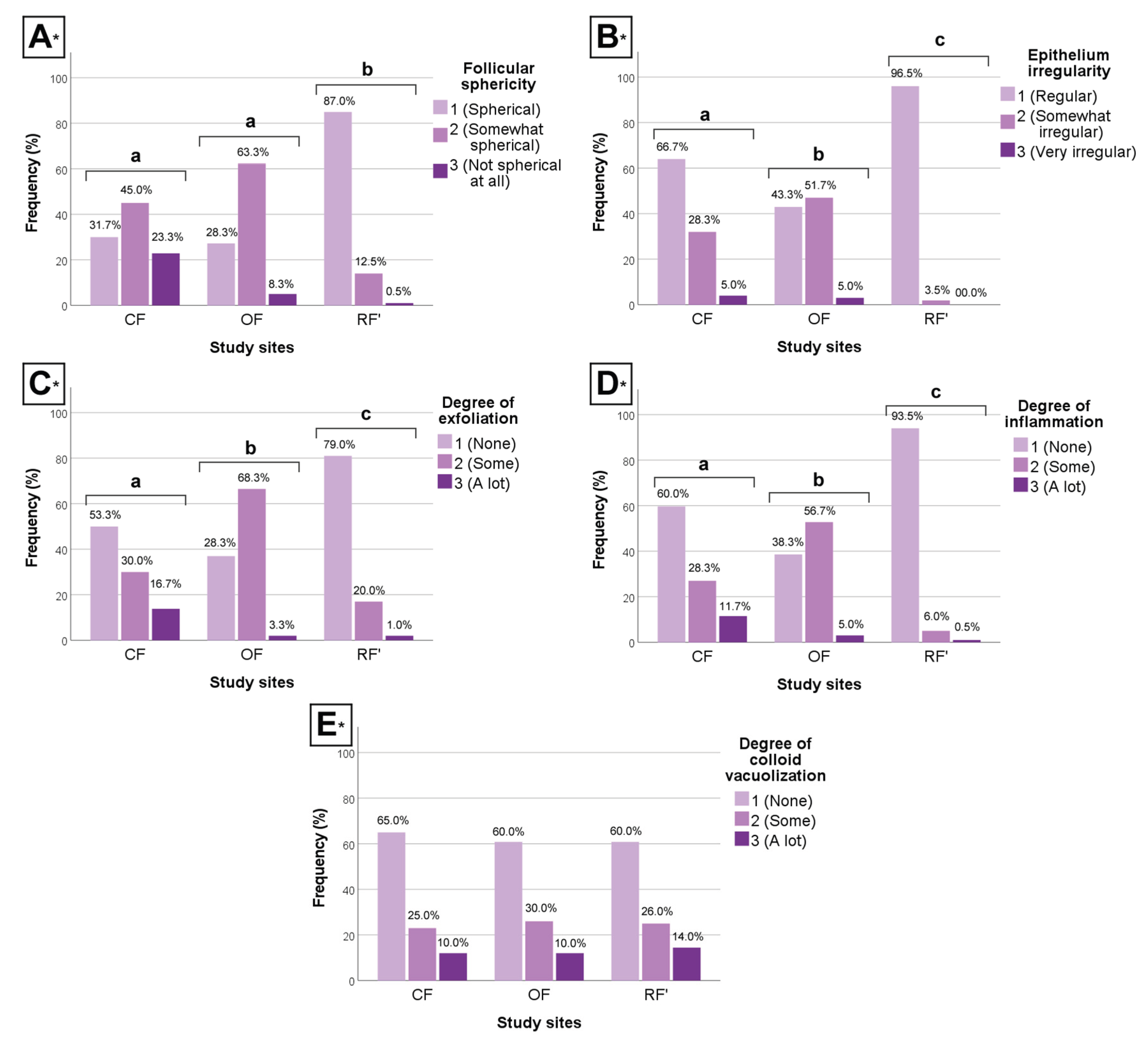
The clustered bar charts below illustrate the differences between groups regarding morphologic (qualitative) data (
Figure 4).
Between CF and OF, results show that individuals from CF had significantly more regular epithelium, less exfoliation, and less inflammation (H-test, P < 0.05, all parameters), when compared to individuals from OF. However, CF and OF individuals did not differ significantly regarding follicular sphericity (H-test, P > 0.05).
Between CF and RF’, results show that individuals from CF had significantly less spherical follicles, more irregular epithelium, more exfoliation, and more inflammation (H-test, P < 0.05, all parameters), when compared to individuals from RF’.
Finally, between OF and RF’, results show that individuals from OF had significantly less spherical follicles, more irregular epithelium, more exfoliation, and more inflammation (H-test, P < 0.05, all parameters), when compared to individuals from RF’.
3.3. Data Correlations
Regarding morphometric data, results showed a significant (P < 0.05) positive association between (i) weight and colloid area [rs(64) = 0.274, P = 0.028], (ii) weight and colloid perimeter [rs(64) = 0.257, P = 0.040], (iii) age and epithelium thickness [rs(64) = 0.429, P < 0.001], (iv) age and epithelial cell area [rs(64) = 0.480, P < 0.001], and (v) age and epithelial cell volume [rs(64) = 0.389, P = 0.001]. On the other hand, there was a significant (P < 0.05) negative association between weight and number of follicles per 30,000 µm2 of thyroid [rs(64) = -0.312, P = 0.012].
4. Discussion
Several studies have addressed the effects of exposure to environmental EDCs on the thyroid (Ferrari et al., 2017; EFSA et al., 2019; Coelho et al., 2025). Within the scope, some works also involved the use of morphological parameters to evaluate thyroid function, under exposure to chemicals known to cause thyroid endocrine disruption (Schnitzler et al., 2008; El-Mehi & Amin, 2012; Andrade et al., 2018). In Coelho et al. (2024), connections were found between chronic exposure to an environment in which CF is practiced and thyroid disruption, particularly the appearance of hypothyroidism-like histological signs in exposed wild mice. The methodology followed in this work corroborated those results, while also revealing the same – albeit less strikingly, in certain parameters – for chronic exposure to an environment in which OF is practiced. The obtained results are in accordance with those of in vivo studies linking hypothyroidism with exposure to CF contaminants, with emphasis on pesticides (Liu et al., 2006; Mallem et al., 2006; Stoker et al., 2007; Bhanu, 2016). Results also support the findings of other studies indicating an association between hypothyroidism in humans and exposure to CF contaminants (e.g., Goldner et al., 2013; Shrestha et al., 2018; Sirikul & Sapbamrer, 2023) and OF contaminants (Kongtip et al., 2019).
In research, it is important that study populations are equally distributed, namely when it comes to parameters such as number of subjects per group, age, and sex. This ensures representative samples which support the validity and generalization of results towards larger, more diverse populations, while minimizing the risk of bias (Haas et al., 2012). Confounding factors potentially influencing the results of this work were the weight and age of the mice, which should be taken into account when interpreting results (Kim et al., 2025). As seen by data correlations, weight could have influenced colloid area, colloid perimeter (heavier mice had larger colloid area and perimeter), and number of follicles per 30,000 µm2 of thyroid (heavier mice had a lower number of follicles per 30,000 µm2 of thyroid). Even so, the influence of weight on results was negligible, due to the lack of statistically significant differences in weight between groups. On the other hand, age may have influenced epithelium thickness, epithelial cell area, and epithelial cell volume (younger mice presented lower epithelium thickness, and lower epithelial cell area and volume). This is an important aspect, given that both CF and OF mice were significantly younger than RF’ mice.
Collectively, indications of a higher degree of thyroid disruption in CF, followed by OF, in comparison to RF’ were found for both males and females – especially when it comes to lower epithelium thickness, lower epithelial cell area and volume, and a higher degree of thyroid disruption registered in the majority of the evaluated histomorphologic parameters. A noteworthy observation was that indications of thyroid disruption in OF mice were of an intermediate level between CF and RF’ mice.
A healthy thyroid is crucial for young individuals, given that TH contribute to the growth, development, and proper functioning of every organ system in the body (Tarım, 2011). These hormones play a critical role in brain development, to the point where even minor TH deficiencies in youth can cause irreversible brain damage (Segni, 2017). However, thyroid function is known to decline with age, marked by a reduction in TH secretion, and a decline in T3 levels due to decreased peripheral conversion of T4. The HPT-axis also loses response plasticity to shifts in TH production, which can be seen, for instance, in a less pronounced TSH response to TH deficiency (Bégin et al., 2008; Taylor et al., 2023). In light of this, the fact that the thyroids of exposed mice presented more indications of disruption, even while CF and OF mice were significantly younger than non-exposed RF’ mice (practically half their age), is very alarming. While it would have been expected for the thyroid of younger mice to show signs of much more activity (i.e., smaller follicles, a thicker epithelium, and epithelial cells of larger area and volume), they instead presented lower epithelium thickness, lower epithelial cell area, and lower epithelial cell volume, all of which indicate loss of function (hypothyroidism) (Lee et al., 2016). The presence of significant differences between groups regardless of age highlights the severity of the impact of chronic exposure to CF and OF contaminants on thyroid function. Moreover, it is plausible that the discrepancy of the measured values between groups could have been even greater if the age of the mice had not differed significantly, accounting for longer exposure to the farming environments. Increased colloid area and perimeter are also often associated with hypothyroidism (Lee et al., 2016); nevertheless, in this work, differences between groups were mostly not significant for these parameters. Therefore, epithelium thickness, along with epithelial cell area and volume, might be a better alternative to assess thyroid disruption than colloid area and perimeter alone. The usefulness of biomarkers of effect, like the thickness of epithelia, and the area and volume of cells, for studies to assess chronic exposures to contaminants is also highlighted. These parameters can be especially relevant for organs that play a fundamental role in metabolism for an extended life period.
In addition to decreasing the ability of the thyroid to produce hormones – as seen through a thinner epithelium, and epithelial cells of smaller area and volume –, chronic exposure to CF and OF seemed to alter the histomorphology of the gland. Lower follicular sphericity, irregularly-delimited epithelium, a higher degree of exfoliation, and a higher degree of inflammation were observed in the thyroids of OF-exposed mice, though less pronouncedly than in CF mice. No differences were found between groups when it comes to degree of vacuolization [when in abundance, colloid vacuoles tend to be a sign of thyroid epithelial cell hyperactivity, which is in line with other studies (Nilsson et al., 1988; Andrade et al., 2018; Pirahanchi et al., 2023)].
A lower follicular sphericity, an irregularly-delimited epithelium, and a higher degree of exfoliation are alterations that reflect thyroid function status. Because of this, these aspects are the ones most likely tied with exposure to CF and OF environments. On one hand, the appearance of less spherical follicles and an epithelium with irregular delimitation are commonly associated with injuries on the thyroid (Mense & Boorman, 2018; Al-Maathidy et al., 2019). In turn, exfoliation, while being a part of normal thyroid function, is tied to the degeneration of epithelial cells, which remains are shed into the colloid – where apoptosis (cell death) processes take place (Al-Maathidy et al., 2019; Zhang et al., 2021). Considering this, results suggest that thyroid disruption arising in CF and OF-exposed mice not only involves indications of loss of function, but also observable alterations to tissue histomorphology. On the other hand, evidence of a higher abundance of lymphocytes in the thyroid of CF and OF-exposed mice suggests inflammation that may be related to agrochemical residues and their endocrine-disrupting potential. Agrochemicals have been reported to trigger abnormal inflammatory responses, given they can interfere with the natural physiology and metabolism of immune system cells (Lopes-Ferreira et al., 2023; Ruíz-Arias et al., 2023). In Hashimoto’s thyroiditis, the immune system actively attacks the thyroid, destroying thyroid cells via cell- and antibody-mediated processes, which can lead to hypothyroidism or underactive thyroid (Erge et al., 2023). The causes for Hashimoto’s thyroiditis are still unclear; however, it is plausible that environmental factors, such as exposures to contaminants, may contribute to its onset (Babić Leko et al., 2021; Cyna et al., 2024).
It has been shown that EDCs can act at any level of the HPT-axis communication, including by interfering with tissue-specific factors such as iodothyronine deiodinase (DIO) enzymes. Some mechanisms of EDCs interference with thyroid metabolism and signaling pathways are (i) mimicking TH structure, (ii) binding to TH receptors, (iii) binding to TH transport proteins, (iv) inhibiting or stimulating DIO activity, (v) inhibiting or stimulating thyroperoxidase (TPO) activity, and (vi) inhibiting iodide (I–) transport into thyrocytes (Van den Berg et al., 1991; Kackar et al., 1997; Thambirajah et al., 2022). The heavy metals (also designated potentially toxic elements, PTEs) present in the composition of agrochemicals – with special emphasis on pesticides – often mimic the chemical structure of TH (Fiore et al., 2019). Because inorganic fertilizers and several pesticides were used in the CF study site, the observed histological effects on CF-exposed mice were expected and can likely be attributed to the intensive application of these contaminants. In contrast, relatively fewer studies have addressed the impact of OF contaminants on health (Ramakrishnan et al., 2021; Zhuang et al., 2024; Kongtip et al., 2019; Nankongnab et al., 2020a, 2020b). In the study of Zhuang et al. (2024), 8 types of major ARGs and 10 mobile genetic elements (MGEs) were detected in the gut microbiome of mice exposed to a diet consisting of organic foods, including tetracycline, multidrug, sulfonamide, aminoglycoside, beta-lactamase, chloramphenicol, MLSB, and vancomycin resistance genes. It was also found that the abundance and diversity of ARGs, MGEs, and potential antibiotic-resistant bacteria (ARB) in the gut increased with time after organic food consumption. ARGs are not known to directly disrupt the thyroid; however, environmental antibiotic-related contaminants can cause thyroid disruption via TH synthesis inhibition or by causing chemical thyroiditis. It has been documented that long-term exposures to certain antibiotics – like tetracyclines – present in contaminated food and water can cause thyroid disruption (Yu et al., 2020). The immune system, which is dysregulated in thyroid autoimmune disorders, can also be influenced by the microbiome (Pollock et al., 2016). The works of Kongtip et al. (2019) and Nankongnab et al. (2020a) were the only ones to directly study thyroid effects on OF farmers. Their works involved the same population of CF and OF farmers in Thailand. In both studies, the authors found that the TSH, free T3 (FT3), T3, and T4 levels were significantly higher in CF farmers than OF farmers. In both groups, several individuals suffered from hypothyroidism, though there was a higher proportion of hypothyroid individuals in the OF group. The authors suggested the higher number of females in the OF group – some of which were nearing or in postmenopausal age – as a likely explanation for a higher proportion of hypothyroid individuals. Another relevant factor was that many individuals in the OF group had handled agrochemicals, particularly pesticides, in the past. The studies lacked a population non-exposed to farming environments to serve as a reference group, which makes it difficult to establish the true extent of the impact of OF on the thyroid. Still, their results align with those of the present work in that thyroid effects were overall more pronounced on the CF-exposed group than the OF-exposed group.
The results obtained in this work underline the risks of chronic exposure to both CF and OF environments, which supports the first hypothesis. The thyroids of exposed mice showed several histological signs of disruption (particularly indicating hypothyroidism), when compared to the RF’ group, though less pronouncedly on OF group. While OF might be a better alternative to CF, it is not completely free of health hazards, mainly because OF products can be indirectly contaminated by the agrochemicals used in CF practices. Considering this, organic certification must guarantee total exemption of agrochemical contamination in OF products. Overall, seeing as exposure to an OF environment seemed to have a less severe impact on the thyroid, the benefit of transitioning from CF systems towards OF systems is supported. Petit et al. (2024) alert to the fact that thyroid disorders among farmers “likely arise from the combined effects of various causal agents and triggering factors (agricultural exposome)”. In light of this, it is important to keep researching about what exactly causes thyroid disruption in both CF and, especially, OF farming practices, so that countermeasures can be taken against so. Filling in these knowledge gaps is of utmost importance for occupationally exposed farmers as well as for the general population through food consumption. Efforts made towards monitoring the presence of pesticide residues in food products have been substantial in the European Union (EU) (EFSA, 2023, 2024, 2025). Unfortunately, the same still cannot be said about other regions of the world.
5. Conclusion
The findings of this study indicate that chronic exposure to an OF environment – although less pronouncedly than in a CF environment – still leads to thyroid disruption. This is evidenced by clear histological alterations observed in the thyroid tissue of exposed wild mice, namely (i) lower epithelium thickness, (ii) lower epithelial cell area and (iii) volume, (iv) lower follicular sphericity, (v) irregularly-delimited epithelium, (vi) increased exfoliation into the colloid, and (vii) increased inflammation of thyroid tissue. Lower epithelium thickness, along with lower epithelial cell area and volume, are indications commonly associated with hypothyroidism.
Because the OF environment seemed to have a less severe impact on the thyroid than the CF environment, the transition from the globally predominant CF systems to OF alternatives is supported. Still, knowledge gaps remain in regards of the general impact of OF on human health. More research is needed about what exactly causes thyroid disruption in both CF and, especially, OF farms, so that preventive countermeasures can be taken against so. The topic is of utmost importance, given that not only are farmers occupationally exposed to such hazards, but also the general population through food consumption.
Declaration of generative AI and AI-associated technologies in the writing process
The authors declare that there were no generative AI and AI-associated technologies in the writing process.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
N. M. P. Coelho: Conceptualization, Methodology, Investigation, Writing - original draft, Illustrating graphical abstract; R. Camarinho: Conceptualization, Methodology, Investigation, Writing - Review and editing; P. Garcia: Conceptualization, Funding acquisition, Writing - Review and editing; F. Bernardo: Conceptualization, Writing - Review and editing; A. S. Rodrigues: Conceptualization, Funding acquisition, Writing - Review and editing.
Funding
This research was funded by cE3c (DOI 10.54499/UID/00329/2023) and IVAR (DOI 10.54499/UID/PRR/00643/2025) projects from FCT national funds.
Institutional Review Board Statement
The experimental procedures involved in this work were approved by the Ethics Committee of the University of Azores (REF: 10/2020). The procedures were performed under the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123) recommendations, directive 2010/63EU and Portuguese legislation (DL 113/2013).
Acknowledgments
The authors would like to thank Paulo Melo for the support in fieldwork.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Abbreviations
ARB, Antibiotic-resistant bacteria; ARGs, Antibiotic resistance genes; CF, Conventional farming; DIO, Iodothyronine deiodinases; EDCs, Endocrine-disrupting chemicals; EU, European Union; FT3, Free triiodothyronine; GMOs, Genetically-modified organisms; HPT-axis, Hypothalamus-pituitary-thyroid axis; I, Iodine; I–, Iodide; MGEs, Mobile genetic elements; OF, Organic farming; PTEs, Potentially toxic elements; RF1, Reference group 1; RF2, Reference group 2; RF’, New reference group (merging of RF1 and RF2 groups); T3, Triiodothyronine; T4, Thyroxine, Tetraiodothyronine; TH, Thyroid hormones; TPO, Thyroperoxidase; TRH, Thyrotropin-releasing hormone; TSH, Thyroid-stimulating hormone.
References
- Ahn, C.; Jeung, E. B. Endocrine-disrupting chemicals and disease endpoints. Int. J. Mol. Sci. 2023, 24(6), 5342. [Google Scholar] [CrossRef] [PubMed]
- Al-Maathidy, A.; Alzyoud, J. A. M.; Al-Dalaen, S.; Al-Qtaitat, A. Histological alterations in the thyroid follicular cells induced by lead acetate toxicity in adult male albino rats. Int. J. Pharm. Phytopharm. Res. 2019, 9, 19–26. [Google Scholar] [CrossRef]
- Andrade, C. Sample size and its importance in research. Indian J. Psychol. Med. 2020, 42(1), 102–103. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M. N.; Santos-Silva, A. P.; Rodrigues-Pereira, P.; Paiva-Melo, F. D.; de Lima Junior, N. C.; Teixeira, M. P.; Soares, P.; Dias, G. R. M.; Graceli, J. B.; de Carvalho, D. P.; Ferreira, A. C. F.; Miranda-Alves, L. The environmental contaminant tributyltin leads to abnormalities in different levels of the hypothalamus-pituitary-thyroid axis in female rats. Environ. Pollut. 2018, 241, 636–645. [Google Scholar] [CrossRef]
- Azarbad, H. Conventional vs. organic agriculture–Which one promotes better yields and microbial resilience in rapidly changing climates? Front. Microbiol. 2022, 13, 903500. [Google Scholar] [CrossRef]
- Babić Leko, M.; Gunjača, I.; Pleić, N.; Zemunik, T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int. J. Mol. Sci. 2021, 22(12), 6521. [Google Scholar] [CrossRef]
- Bano, A.; Chaker, L.; Mattace-Raso, F. U. S.; Terzikhan, N.; Kavousi, M.; Ikram, M. A.; Peeters, R. P.; Franco, O. H. Thyroid function and life expectancy with and without noncommunicable diseases: A population-based study. PLoS Med. 2019, 16(10), e1002957. [Google Scholar] [CrossRef]
- Bassitta, R.; Nottensteiner, A.; Bauer, J.; Straubinger, R. K.; Hölzel, C. S. Spread of antimicrobial resistance genes via pig manure from organic and conventional farms in the presence or absence of antibiotic use. J. Appl. Microbiol. 2022, 133(4), 2457–2465. [Google Scholar] [CrossRef]
- Bégin, M. E.; Langlois, M. F.; Lorrain, D.; Cunnane, S. C. Thyroid function and cognition during aging. Curr. Gerontol. Geriatr. Res. 2008, 2008, 474868. [Google Scholar] [CrossRef]
- Bhanu, A. P. Disrupting action of cypermethrin on thyroid and cortisol hormones in the serum of Cyprinus carpio. J. Entomol. Zool. Stud. 2016, 4, 340–341. [Google Scholar]
- Boelen, A.; Kwakkel, J.; Fliers, E. Thyroid hormone receptors in health and disease. Minerva Endocrinol. 2012, 37(4), 291–304. [Google Scholar] [PubMed]
- Campos, É.; Freire, C. Exposure to non-persistent pesticides and thyroid function: A systematic review of epidemiological evidence. Int. J. Hyg. Environ. Health 2016, 219(6), 481–497. [Google Scholar] [CrossRef] [PubMed]
- Candanedo-Gonzalez, F.; Rios-Valencia, J.; Noemi Pacheco-Garcilazo, D.; Valenzuela-Gonzalez, W.; Gamboa-Dominguez, A. Morphology aspects of hypothyroidism. IntechOpen 2021. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, F.; Ma, J.; Alghamdi, M. A.; Zhu, Y.; Yong, J. W. H. Intersecting planetary health: Exploring the impacts of environmental stressors on wildlife and human health. Ecotoxicol. Environ. Saf. 2024, 283, 116848. [Google Scholar] [CrossRef]
- Chiovato, L.; Magri, F.; Carlé, A. Hypothyroidism in context: Where we’ve been and where we’re going. Adv. Ther. 2019, 36(4), 47–58. [Google Scholar] [CrossRef]
- Coelho, N. M. P.; Bernardo, F.; Rodrigues, A. S.; Garcia, P. Volcanic environments and thyroid disruption – A review focused on As, Hg, and Co. Sci. Total Environ. 2025, 993, 180018. [Google Scholar] [CrossRef]
- Coelho, N.; Camarinho, R.; Garcia, P.; Rodrigues, A. S. Histological evidence of hypothyroidism in mice chronically exposed to conventional farming. Environ. Toxicol. Pharmacol. 2024, 106, 104387. [Google Scholar] [CrossRef]
- Cote, G. J.; Grubbs, E. G.; Hofmann, M. C. Thyroid c-cell biology and oncogenic transformation. Recent Results Cancer Res. 2015, 204, 1–39. [Google Scholar] [CrossRef]
- Crowder, D. W.; Reganold, J. P. Financial competitiveness of organic agriculture on a global scale. Proc. Natl. Acad. Sci. USA 2015, 112(24), 7611–7616. [Google Scholar] [CrossRef]
- Cyna, W.; Wojciechowska, A.; Szybiak-Skora, W.; Lacka, K. The Impact of environmental factors on the development of autoimmune thyroiditis—Review. Biomedicines 2024, 12(8), 1788. [Google Scholar] [CrossRef]
- De Felice, M.; Di Lauro, R. Chapter 72 – Anatomy and development of the thyroid. In Endocrinology, 6th Ed.; Jameson, J. L., De Groot, L. J., Eds.; W.B. Saunders, 2010; pp. 1342–1361. [Google Scholar] [CrossRef]
- EFSA. National summary reports on pesticide residue analysis performed in 2021. EFSA Support. Publ. 2023, 20(4). [Google Scholar] [CrossRef]
- EFSA. National summary reports on pesticide residue analyses performed in 2022. EFSA Support. Publ. 2024, 21(4), EN-8751. [Google Scholar] [CrossRef]
- EFSA. National summary reports on pesticide residue analyses performed in 2023. EFSA Support. Publ. 2025, 22(5), EN-9320. [Google Scholar] [CrossRef]
- Crivellente, F.; Hart, A.; Hernandez-Jerez, A.F.; Hougaard Bennekou, S.; Pedersen, R.; Terron, A.; Wolterink, G.; Mohimont, L. Establishment of cumulative assessment groups of pesticides for their effects on the thyroid. EFSA J. 2019, 17(9), e05801. [Google Scholar] [CrossRef] [PubMed]
- El-Mehi, A. E.; Amin, S. A. Effect of lead acetate on the thyroid gland of adult male albino rats and the possible protective role of zinc supplementation: A biochemical, histological and morphometric study. Am. J. Sci. 2012, 8, 61–71. [Google Scholar] [CrossRef]
- Erge, E.; Kiziltunc, C.; Balci, S. B.; Atak Tel, B. M.; Bilgin, S.; Duman, T. T.; Aktas, G. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: Platelet-count-to-lymphocyte-count ratio. Diseases 2023, 11(1), 15. [Google Scholar] [CrossRef]
- Fanzo, J. The role of farming and rural development as central to our diets. Physiol. Behav. 2018, 193 Pt B, 291–297. [Google Scholar] [CrossRef]
- Ferrari, S. M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental issues in thyroid diseases. Front. Endocrinol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Fiore, M.; Oliveri Conti, G.; Caltabiano, R.; Buffone, A.; Zuccarello, P.; Cormaci, L.; Cannizzaro, M. A.; Ferrante, M. Role of emerging environmental risk factors in thyroid cancer: A brief review. Int. J. Environ. Res. Public Health 2019, 16(7), 1185. [Google Scholar] [CrossRef]
- Gaitán-Cremaschi, D.; Klerkx, L.; Duncan, J.; Trienekens, J. H.; Huenchuleo, C.; Dogliotti, S.; Contesse, M. E.; Rossing, W. A. H. Characterizing diversity of food systems in view of sustainability transitions. A review. Agron. Sustain. Dev. 2019, 39(1), 1. [Google Scholar] [CrossRef]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J. M.; Regolo, L.; Sánchez-González, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, M. Organic vs conventional plant-based foods: A review. Food Chem. 2022, 383, 132352. [Google Scholar] [CrossRef] [PubMed]
- Goldner, W. S.; Sandler, D. P.; Yu, F.; Hoppin, J. A.; Kamel, F.; Levan, T. D. Pesticide use and thyroid disease among women in the Agricultural Health Study. Am. J. Epidemiol. 2010, 171(4), 455–464. [Google Scholar] [CrossRef] [PubMed]
- Goldner, W. S.; Sandler, D. P.; Yu, F.; Shostrom, V.; Hoppin, J. A.; Kamel, F.; LeVan, T. D. Hypothyroidism and pesticide use among male private pesticide applicators in the Agricultural Health Study. J. Occup. Environ. Med. 2013, 55(10), 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M. L.; Chernock, R. D.; Mansour, M. Environmental factors and anatomic pathology of the thyroid gland: Review of literature. Diagn. Histopathol. 2020, 26(5), 207–215. [Google Scholar] [CrossRef]
- Gudeta, K.; Kumar, V.; Bhagat, A.; Julka, J. M.; Bhat, S. A.; Ameen, F.; Qadri, H.; Singh, S.; Amarowicz, R. Ecological adaptation of earthworms for coping with plant polyphenols, heavy metals, and microplastics in the soil: A review. Heliyon 2023, 9(3), e14572. [Google Scholar] [CrossRef]
- Haas, J.; Manro, J.; Shannon, H.; et al. In vivo assay guidelines (last updated on October 2012). In Assay guidance manual [Internet]; Markossian, S., Grossman, A., Baskir, H., et al., Eds.; Eli Lilly & Company and the National Center For Advancing Translational Sciences: Bethesda (MD), 2012). In vivo assay guidelines (last updated on October 2012; p. 2004. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK92013/.
- Hallgren, S.; Darnerud, P. O. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats–Testing interactions and mechanisms for thyroid hormone effects. Toxicology 2002, 177(2-3), 227–243. [Google Scholar] [CrossRef]
- He, J.; Xu, J.; Zheng, M.; Pan, K.; Yang, L.; Ma, L.; Wang, C.; Yu, J. Thyroid dysfunction caused by exposure to environmental endocrine disruptors and the underlying mechanism: A review. Chem. Biol. Interact. 2024, 391, 110909. [Google Scholar] [CrossRef]
- Imholt, C.; Abdulla, T.; Stevens, A.; Edwards, P.; Jacob, J.; Woods, D.; Rogers, E.; Aarons, L.; Segelcke, D. Establishment and validation of microsampling techniques in wild rodents for ecotoxicological research. J. Appl. Toxicol. 2018, 38(9), 1244–1250. [Google Scholar] [CrossRef]
- Kackar, R.; Srivastava, M. K.; Raizada, R. B. Studies on rat thyroid after oral administration of mancozeb: morphological and biochemical evaluations. J. Appl. Toxicol. 1997, 17(6), 369–375. [Google Scholar] [CrossRef]
- Kaur, R.; Choudhary, D.; Bali, S.; Bandral, S. S.; Singh, V.; Ahmad, M. A.; Rani, N.; Singh, T. G.; Chandrasekaran, B. Pesticides: An alarming detrimental to health and environment. Sci. Total Environ. 2024, 915, 170113. [Google Scholar] [CrossRef]
- Kim, J. H.; Ko, Y.; Kim, H. J.; Park, S. J. Age and sex differences in the relationship of body weight changes with colon cancer risks: A nationwide cohort study. Sci. Rep. 2025, 15(1), 678. [Google Scholar] [CrossRef]
- Kongtip, P.; Nankongnab, N.; Kallayanatham, N.; Pundee, R.; Choochouy, N.; Yimsabai, J.; Woskie, S. Thyroid hormones in conventional and organic farmers in Thailand. Int. J. Environ. Res. Public Health 2019, 16(15), 2704. [Google Scholar] [CrossRef] [PubMed]
- Lazarević-Pašti, T.; Milanković, V.; Tasić, T.; Petrović, S.; Leskovac, A. With or without you?—A critical review on pesticides in food. Foods 2025, 14(7), 1128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yi, S.; Kang, Y. E.; Kim, H. W.; Joung, K. H.; Sul, H. J.; Kim, K. S.; Shong, M. Morphological and functional changes in the thyroid follicles of the aged murine and humans. J. Pathol. Transl. Med. 2016, 50(6), 426–435. [Google Scholar] [CrossRef] [PubMed]
- Leemans, M.; Couderq, S.; Demeneix, B.; Fini, J. B. Pesticides with potential thyroid hormone-disrupting effects: A review of recent data. Front. Endocrinol. 2019, 10, 743. [Google Scholar] [CrossRef]
- Lidicker, W. Z. Ecological observations on a feral house mouse population declining to extinction. Ecol. Monogr. 1966, 36(1), 27–50. [Google Scholar] [CrossRef]
- Liu, P.; Song, X.; Yuan, W.; Wen, W.; Wu, X.; Li, J.; Chen, X. Effects of cypermethrin and methyl parathion mixtures on hormone levels and immune functions in Wistar rats. Arch. Toxicol. 2006, 80(7), 449–457. [Google Scholar] [CrossRef]
- Lombardo, L.; Zelasco, S. Biotech approaches to overcome the limitations of using transgenic plants in organic farming. Sustainability 2016, 8(5), 497. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Farinha, L. R. L.; Costa, Y. S. O.; Pinto, F. J.; Disner, G. R.; da Rosa, J. G. D. S.; Lima, C. Pesticide-induced inflammation at a glance. Toxics 2023, 11(11), 896. [Google Scholar] [CrossRef]
- Mallem, L.; Boulakoud, M. S.; Franck, M. Hypothyroidism after medium exposure to the fungicide maneb in the rabbit Cuniculus lepus. Commun. Agric. Appl. Biol. Sci. 2006, 71 2 Pt A, 91–99. [Google Scholar]
- Marcheselli, M.; Sala, L.; Mauri, M. Bioaccumulation of PGEs and other traffic-related metals in populations of the small mammal Apodemus sylvaticus. Chemosphere 2010, 80(11), 1247–1254. [Google Scholar] [CrossRef]
- Martoja, R.; Martoja-Pierson, M.; Grumbles, L. C.; Moncanut, M. E.; Coll, M. D. Técnicas de histología animal [Animal histology techniques], 1st ed.; Toray-Masson: Barcelona, 1970. [Google Scholar]
- McClure, M. T.; Stupans, I. Hormonal perturbation as a possible mechanism for the alteration of cytochrome P450 by cyclophosphamide. Biochem. Pharmacol. 1995, 49(12), 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, R.; Plant, J. A.; Bell, J. N.; Voulvoulis, N. Endocrine disrupting pesticides: Implications for risk assessment. Environ. Int. 2008, 34(2), 168–183. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Qiao, Y.; Wu, W.; Smith, P.; Scott, S. Environmental impacts and production performances of organic agriculture in China: A monetary valuation. J. Environ. Manage. 2017, 188, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mense, M. G.; Boorman, G. A. Chapter 33 – Thyroid gland. In Boorman’s pathology of the rat, 2nd ed.; Suttie, A. W., Ed.; Academic Press, 2018; pp. 669–686. [Google Scholar] [CrossRef]
- Milner, G. R.; Boldsen, J. L. Population trends and the transition to agriculture: Global processes as seen from North America. Proc. Natl. Acad. Sci. USA 2023, 120(4), e2209478119. [Google Scholar] [CrossRef]
- Nankongnab, N.; Kongtip, P.; Kallayanatham, N.; Pundee, R.; Yimsabai, J.; Woskie, S. Longitudinal study of thyroid hormones between conventional and organic farmers in Thailand. Toxics 2020a, 8(4), 82. [Google Scholar] [CrossRef]
- Nankongnab, N.; Kongtip, P.; Tipayamongkholgul, M.; Bunngamchairat, A.; Sitthisak, S.; Woskie, S. Difference in accidents, health symptoms, and ergonomic problems between conventional farmers using pesticides and organic farmers. J. Agromed. 2020b, 25(2), 158–165. [Google Scholar] [CrossRef]
- NIEHS (National Institute of Environmental Health Sciences). Endocrine disruptors. 2024. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine.
- Nilsson, M.; Mölne, J.; Jörtsö, E.; Smeds, S.; Ericson, L. E. Plasma membrane shedding and colloid vacuoles in hyperactive human thyroid tissue. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1988, 56(2), 85–94. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T. M.; Chiamolera, M. I.; Pazos-Moura, C. C.; Wondisford, F. E. Hypothalamus-pituitary-thyroid axis. Compr. Physiol. 2016, 6(3), 1387–1428. [Google Scholar] [CrossRef]
- Panday, D.; Bhusal, N.; Das, S.; Ghalehgolabbehbahani, A. Rooted in nature: The rise, challenges, and potential of organic farming and fertilizers in agroecosystems. Sustainability 2024, 16(4), 1530. [Google Scholar] [CrossRef]
- Parelho, C.; Bernardo, F.; Camarinho, R.; Rodrigues, A. S.; Garcia, P. Testicular damage and farming environments – An integrative ecotoxicological link. Chemosphere 2016, 155, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Parelho, C.; Rodrigues, A.; Bernardo, F.; Barreto, M. C.; Cunha, L.; Poeta, P.; Garcia, P. Biological endpoints in earthworms (Amynthas gracilis) as tools for the ecotoxicity assessment of soils from livestock production systems. Ecol. Indic. 2018, 95, 984–990. [Google Scholar] [CrossRef]
- Parelho, C.; Rodrigues, A. S.; Cruz, J. V.; Garcia, P. Linking trace metals and agricultural land use in volcanic soils--A multivariate approach. Sci. Total Environ. 2014, 496, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Perez-Montiel, M. D.; Suster, S. The spectrum of histologic changes in thyroid hyperplasia: A clinicopathologic study of 300 cases. Human Pathol. 2008, 39(7), 1080–1087. [Google Scholar] [CrossRef]
- Petit, P.; Chamot, S.; Al-Salameh, A.; Cancé, C.; Desailloud, R.; Bonneterre, V. Farming activity and risk of treated thyroid disorders: Insights from the TRACTOR project, a nationwide cohort study. Environ. Res. 2024, 249, 118458. [Google Scholar] [CrossRef]
- Piccoli, C.; Cremonese, C.; Koifman, R. J.; Koifman, S.; Freire, C. Pesticide exposure and thyroid function in an agricultural population in Brazil. Environ. Res. 2016, 151, 389–398. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Tariq, M. A.; Jialal, I. Physiology, thyroid (last updated on February 2023), in: StatPearls [Internet]; StatPearls Publishing: Treasure Island (FL), 2023) Physiology, thyroid (last updated on February 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK519566/.
- Pollock, A. J.; Seibert, T.; Allen, D. B. Severe and persistent thyroid dysfunction associated with tetracycline-antibiotic treatment in youth. J. Pediatr. 2016, 173, 232–234. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Maddela, N. R.; Venkateswarlu, K.; Megharaj, M. Organic farming: Does it contribute to contaminant-free produce and ensure food safety? Sci. Total Environ. 2021, 769, 145079. [Google Scholar] [CrossRef]
- Ruíz-Arias, M. A.; Medina-Díaz, I. M.; Bernal-Hernández, Y. Y.; Agraz-Cibrián, J. M.; González-Arias, C. A.; Barrón-Vivanco, B. S.; Herrera-Moreno, J. F.; Verdín-Betancourt, F. A.; Zambrano-Zaragoza, J. F.; Rojas-García, A. E. Hematological indices as indicators of inflammation induced by exposure to pesticides. Environ. Sci. Pollut. Res. Int. 2023, 30(7), 19466–19476. [Google Scholar] [CrossRef]
- Sadighara, P.; Mahmudiono, T.; Marufi, N.; Yazdanfar, N.; Fakhri, Y.; Rikabadi, A. K.; Khaneghah, A. M. Residues of carcinogenic pesticides in food: A systematic review. Rev. Environ. Health 2023, 39(4), 659–666. [Google Scholar] [CrossRef]
- Schnitzler, J. G.; Koutrakis, E.; Siebert, U.; Thomé, J. P.; Das, K. Effects of persistent organic pollutants on the thyroid function of the European sea bass (Dicentrarchus labrax) from the Aegean sea, is it an endocrine disruption? Mar. Pollut. Bull. 2008, 56(10), 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Segni, M. Disorders of the thyroid gland in infancy, childhood and adolescence (last updated on March 2017). In Endotext [Internet]; Feingold, K. R., Ahmed, S. F., Anawalt, B., et al., Eds.; MDText.com, Inc.: South Dartmouth (MA), 2017; p. 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279032/.
- Seufert, V.; Ramankutty, N.; Foley, J. A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485(7397), 229–232. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liu, J.; Zhang, M.; Ma, M.; Kang, L.; Liu, F.; Du, L. Organic farming is an important way to achieve low accumulation of heavy metals in soil. Sustainability 2025, 17(3), 899. [Google Scholar] [CrossRef]
- Shore, R. F.; Douben, P. E. Predicting ecotoxicological impacts of environmental contaminants on terrestrial small mammals. Rev. Environ. Contam. Toxicol. 1994, 134, 49–89. [Google Scholar] [CrossRef]
- Shrestha, S.; Parks, C. G.; Goldner, W. S.; Kamel, F.; Umbach, D. M.; Ward, M. H.; Lerro, C. C.; Koutros, S.; Hofmann, J. N.; Beane Freeman, L. E.; Sandler, D. P. Pesticide use and incident hypothyroidism in pesticide applicators in the Agricultural Health Study. Environ. Health Perspect. 2018, 126(9), 97008. [Google Scholar] [CrossRef]
- Silva, V.; Peixoto, F.; Igrejas, G.; Parelho, C.; Garcia, P.; Carvalho, I.; Pereira, J.; Rodrigues, A.; Poeta, P. First report on vanA-Enterococcus faecalis recovered from soils subjected to long-term livestock agricultural practices in Azores archipelago. Int. J. Environ. Res. 2018, 12(1), 39–44. [Google Scholar] [CrossRef]
- Singh, A.; Schurman, S. H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D. M., 3rd; Sen, R.; Ferrucci, L. Aging and Inflammation. Cold Spring Harb. Perspect. Med. 2024, 14(6), a041197. [Google Scholar] [CrossRef]
- Sirikul, W.; Sapbamrer, R. Exposure to pesticides and the risk of hypothyroidism: A systematic review and meta-analysis. BMC Public Health 2023, 23(1), 1867. [Google Scholar] [CrossRef]
- Song, M.; Sun, W.; Liu, Q.; Wang, Z.; Zhang, H. Global scientific trends on thyroid disease in early 21st century: A bibliometric and visualized analysis. Front. Endocrinol. 2024, 14, 1306232. [Google Scholar] [CrossRef]
- Stoker, T.; Kaydos, E.; Jeffay, S.; Cooper, R. Effect of 2,4-D exposure on pubertal development and thyroid function in the male Wistar rat. Biol. Reprod. 2007, 77, 75. [Google Scholar] [CrossRef]
- Strenner, M.; Chmelíková, L.; Hülsbergen, K.-J. Compost fertilization in organic agriculture—A comparison of the impact on corn plants using field spectroscopy. Applied Sci. 2023, 13(6), 3676. [Google Scholar] [CrossRef]
- Tarım, Ö. Thyroid hormones and growth in health and disease. J. Clin. Res. Pediatr. Endocrinol. 2011, 3(2), 51–55. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P. N.; Lansdown, A.; Witczak, J.; Khan, R.; Rees, A.; Dayan, C. M.; Okosieme, O. Age-related variation in thyroid function – A narrative review highlighting important implications for research and clinical practice. Thyroid Res. 2023, 16(1), 7. [Google Scholar] [CrossRef] [PubMed]
- Thambirajah, A. A.; Wade, M. G.; Verreault, J.; Buisine, N.; Alves, V. A.; Langlois, V. S.; Helbing, C. C. Disruption by stealth – Interference of endocrine disrupting chemicals on hormonal crosstalk with thyroid axis function in humans and other animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef]
- Van den Berg, K. J.; van Raaij, J. A.; Bragt, P. C.; Notten, W. R. Interactions of halogenated industrial chemicals with transthyretin and effects on thyroid hormone levels in vivo. Arch. Toxicol. 1991, 65(1), 15–19. [Google Scholar] [CrossRef]
- Viana, C. M.; Freire, D.; Abrantes, P.; Rocha, J.; Pereira, P. Agricultural land systems importance for supporting food security and sustainable development goals: A systematic review. Sci. Total Environ. 2022, 806 Pt 3, 150718. [Google Scholar] [CrossRef]
- Wilbois, K.-P.; Schmidt, J. E. Reframing the debate surrounding the yield gap between organic and conventional farming. Agronomy 2019, 9(2), 82. [Google Scholar] [CrossRef]
- Yavuz, S.; Salgado Nunez Del Prado, S.; Celi, F. S. Thyroid hormone action and energy expenditure. J. Endocr. Soc. 2019, 3(7), 1345–1356. [Google Scholar] [CrossRef]
- Yu, K.; Li, X.; Qiu, Y.; Zeng, X.; Yu, X.; Wang, W.; Yi, X.; Huang, L. Low-dose effects on thyroid disruption in zebrafish by long-term exposure to oxytetracycline. Aquat. Toxicol. 2020, 227, 105608. [Google Scholar] [CrossRef]
- Zhang, X.; Kellogg, A. P.; Citterio, C. E.; Zhang, H.; Larkin, D.; Morishita, Y.; Targovnik, H. M.; Balbi, V. A.; Arvan, P. Thyroid hormone synthesis continues despite biallelic thyroglobulin mutation with cell death. J. Clin. Invest. 2021, 6(11), e148496. [Google Scholar] [CrossRef]
- Zhuang, X.; Fan, H.; Li, X.; Dong, Y.; Wang, S.; Zhao, B.; Wu, S. Transfer and accumulation of antibiotic resistance genes and bacterial pathogens in the mice gut due to consumption of organic foods. Sci. Total Environ. 2024, 915, 169842. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).