Submitted:
16 December 2025
Posted:
17 December 2025
You are already at the latest version
Abstract
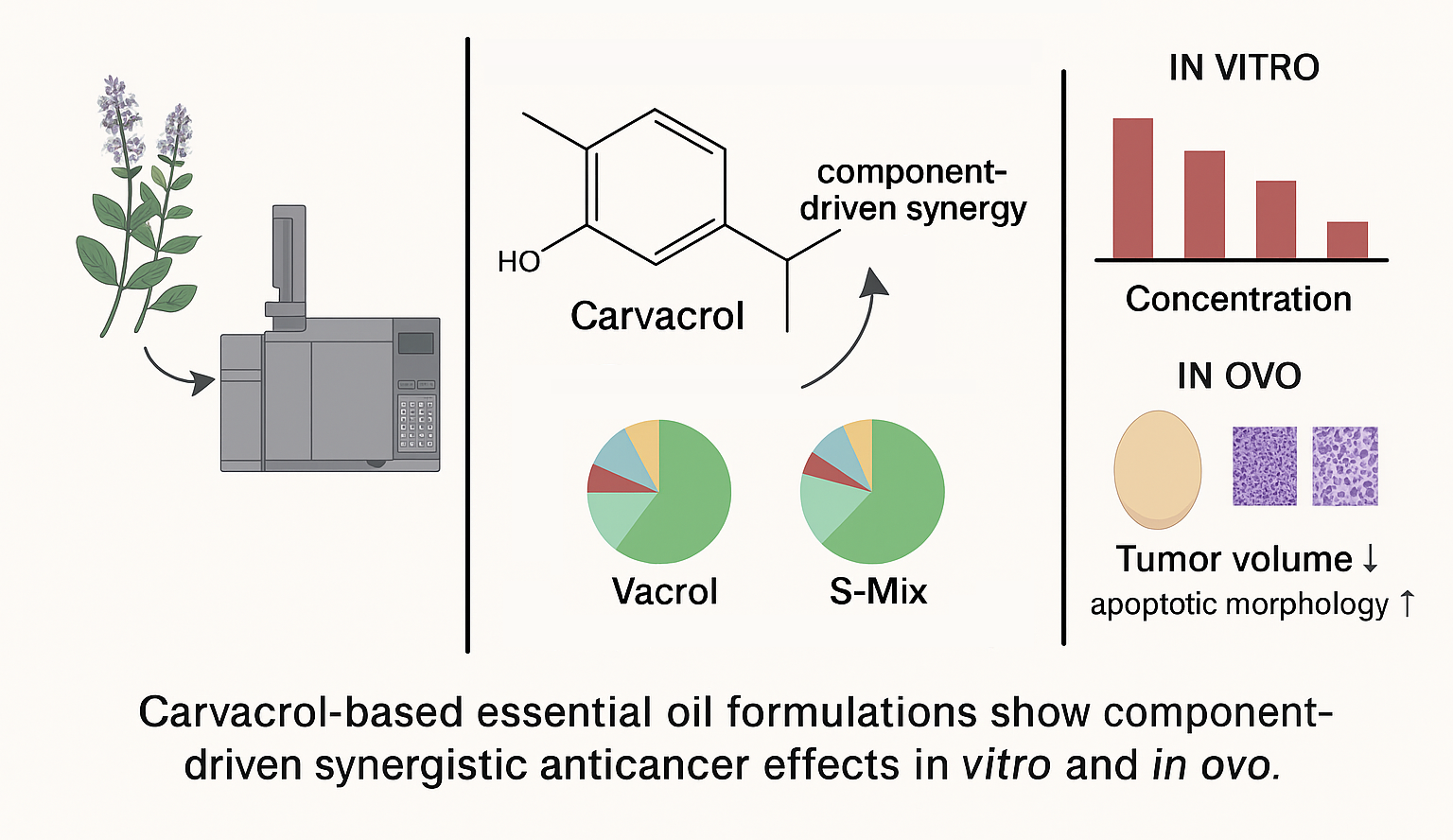
Keywords:
1. Introduction
2. Results
2.1. GC-MS Analysis Results
2.2. In Vitro Analysis Results
2.3. In Ovo Analysis Results
3. Discussion
4. Materials and Methods
4.1. GC-MS Analysis
4.2. In Vitro Analyses
4.2.1. Cell Culture
4.2.2. Cell Viability Assay
4.3. In Ovo Analyses
4.3.1. Incubation Process
4.3.2. Histopathological Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SRB | Sulforhodamine B |
| CAM | Chorioallantoic Membrane |
| TNBC | Triple-Negative Breast Cancer |
| EDD | Egg Development Day |
References
- Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. - Wiley Online Libr. 2021, 71(3), 209–249. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; et al. Breast Cancer. Nat. Rev. Dis. Primer 2019, 5(66), 1–31. [Google Scholar] [CrossRef]
- Waks, A. G.; Winer, E. P. Breast Cancer Treatment: A Review. JAMA 2019, 321(3), 288–300. [Google Scholar] [CrossRef]
- Mardale, G.; Caruntu, F.; Mioc, A.; Mioc, M.; Lukinich-Gruia, A. T.; Pricop, M.-A.; Jianu, C.; Gogulescu, A.; Maksimovic, T.; Șoica, C.; Mardale, G.; Caruntu, F.; Mioc, A.; Mioc, M.; Lukinich-Gruia, A. T.; Pricop, M.-A.; Jianu, C.; Gogulescu, A.; Maksimovic, T.; Șoica, C. Integrated In Silico and In Vitro Assessment of the Anticancer Potential of Origanum Vulgare L. Essential Oil. Processes 2025, 13(6), 1695. [Google Scholar] [CrossRef]
- Savini, I.; Arnone, R.; Catani, M. V.; Avigliano, L. Origanum Vulgare Induces Apoptosis in Human Colon Cancer Caco2 Cells. Nutr. Cancer 2009, 61(3), 381–389. [Google Scholar] [CrossRef]
- Baser, K. H. C.; Özek, T.; Tümen, G.; Sezik, E. Composition of the Essential Oils of Turkish Origanum Species with Commercial Importance. J. Essent. Oil Res. 1993, 5(6), 619–623. [Google Scholar] [CrossRef]
- Musmula, M.; Akkoc, S. Apoptotic Effect of Carvacrol on Different Cancer Cells and Its Potential as an Active Medicine Ingredient. J. Asian Nat. Prod. Res. 2025, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The Antibacterial Properties of Phenolic Isomers, Carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2020, 60(18), 3042–3053. [Google Scholar] [CrossRef]
- Suntres, Z.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55(3), 304–318. [Google Scholar] [CrossRef] [PubMed]
- Moradipour, A.; Dariushnejad, H.; Ahmadizadeh, C.; Lashgarian, H. E. Dietary Flavonoid Carvacrol Triggers the Apoptosis of Human Breast Cancer MCF-7 Cells via the P53/Bax/Bcl-2 Axis. Med. Oncol. 2022, 40(1), 46. [Google Scholar] [CrossRef]
- Pandey, P.; Ramniwas, S.; Verma, M.; Sharma, N.; Upadhye, V. J.; Khan, F.; Shah, M. A. A Study to Investigate the Anticancer Potential of Carvacrol via Targeting Notch Signaling in Breast Cancer. Open Chem. 2024, 22(1), 1–9. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol Affects Breast Cancer Cells through TRPM7 Mediated Cell Cycle Regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef] [PubMed]
- Abuaisha, A.; Nekay, E.; Yilmaz, O.; Yildiz, B.; Mecit, T.; Yavas, C.; Papila, B.; Arslan, H. I.; Gumus, A. H.; Nazligul, E.; Akbas, S.; Emiroglu, S.; Abuaisha, A.; Nekay, E.; Yilmaz, O.; Yildiz, B.; Mecit, T.; Yavas, C.; Papila, B.; Arslan, H. I.; Gumus, A. H.; Nazligul, E.; Akbas, S.; Emiroglu, S. Carvacrol as a Therapeutic Candidate in Breast Cancer: Insights into Subtype-Specific Cellular Modulation. Biology 2025, 14(10), 1443. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Ou-Yahia, D.; Bellaouchi, R.; Mothana, R. A.; Al-Yousef, H. M.; Asehraou, A.; Addi, M.; Guerrouj, B. E.; Chaabane, K.; Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Ou-Yahia, D.; Bellaouchi, R.; Mothana, R. A.; Al-Yousef, H. M.; Asehraou, A.; Addi, M.; Guerrouj, B. E.; Chaabane, K. Evaluation of the Interaction between Carvacrol and Thymol, Major Compounds of Ptychotis Verticillata Essential Oil: Antioxidant, Anti-Inflammatory and Anticancer Activities against Breast Cancer Lines. Life 2024, 14(8). [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; Patel, M.; Ahmad, A. Improved Efficacy of Antifungal Drugs in Combination with Monoterpene Phenols against Candida Auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Ranjan, R. A.; Muenzner, J. K.; Kunze, P.; Geppert, C. I.; Ruebner, M.; Huebner, H.; Fasching, P. A.; Beckmann, M. W.; Bäuerle, T.; Hartmann, A.; Walther, W.; Eckstein, M.; Erber, R.; Schneider-Stock, R.; Ranjan, R. A.; Muenzner, J. K.; Kunze, P.; Geppert, C. I.; Ruebner, M.; Huebner, H.; Fasching, P. A.; Beckmann, M. W.; Bäuerle, T.; Hartmann, A.; Walther, W.; Eckstein, M.; Erber, R.; Schneider-Stock, R. The Chorioallantoic Membrane Xenograft Assay as a Reliable Model for Investigating the Biology of Breast Cancer. Cancers 2023, 15(6), 1704. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, L. A.; Pina, L. T. S.; Serafini, M. R.; Tavares, D. dos S.; Guimarães, A. G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Gautam, N.; Mantha, A. K.; Mittal, S. Essential Oils and Their Constituents as Anticancer Agents: A Mechanistic View. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef]
- Jaafari, A.; Tilaoui, M.; Mouse, H. A.; M’bark, L. A.; Aboufatima, R.; Chait, A.; Lepoivre, M.; Zyad, A. Comparative Study of the Antitumor Effect of Natural Monoterpenes: Relationship to Cell Cycle Analysis. Rev. Bras. Farmacogn. 2012, 22(3), 534–540. [Google Scholar] [CrossRef]
- Huang, T.-C.; Fu, H.-Y.; Ho, C.-T.; Tan, D.; Huang, Y.-T.; Pan, M.-H. Induction of Apoptosis by Cinnamaldehyde from Indigenous Cinnamon Cinnamomum Osmophloeum Kaneh through Reactive Oxygen Species Production, Glutathione Depletion, and Caspase Activation in Human Leukemia K562 Cells. Food Chem. 2007, 103(2), 434–443. [Google Scholar] [CrossRef]
- Aggarwal, S.; Bhadana, K.; Singh, B.; Rawat, M.; Mohammad, T.; Al-Keridis, L. A.; Alshammari, N.; Hassan, M. I.; Das, S. N. Cinnamomum Zeylanicum Extract and Its Bioactive Component Cinnamaldehyde Show Anti-Tumor Effects via Inhibition of Multiple Cellular Pathways. Front. Pharmacol. 2022, 13, 918479. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, A.; Putri, H.; Utomo, R. Y. Exploration of Targets and Molecular Mechanisms of Cinnamaldehyde in Overcoming Fulvestrant-Resistant Breast Cancer: A Bioinformatics Study. Netw. Model. Anal. Health Inform. Bioinforma 2021, 10, 30. [Google Scholar] [CrossRef]
- Peng, J.; Song, X.; Yu, W.; Pan, Y.; Zhang, Y.; Jian, H.; He, B. The Role and Mechanism of Cinnamaldehyde in Cancer. J. Food Drug Anal. 2024, 32(2), 140–154. [Google Scholar] [CrossRef]
- Liu, Y.; An, T.; Wan, D.; Yu, B.; Fan, Y.; Pei, X. Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer. Front. Pharmacol. 2020, 11, 582719. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, H.; Liu, W.; Liu, E.; Pang, Y.; Gao, H.; He, Q.; Liao, W.; Yao, Y.; Zeng, J.; Guo, J. Menthol: An Underestimated Anticancer Agent. Front. Pharmacol. 2023, 14, 1148790. [Google Scholar] [CrossRef] [PubMed]
- Naksawat, M.; Norkaew, C.; Charoensedtasin, K.; Roytrakul, S.; Tanyong, D. Anti-Leukemic Effect of Menthol, a Peppermint Compound, on Induction of Apoptosis and Autophagy. PeerJ 2023, 11, e15049. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical Composition and Anti-Inflammatory, Cytotoxic and Antioxidant Activities of Essential Oil from Leaves of Mentha Piperita Grown in China. PLOS ONE 2014, 9(12), e114767. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; Zubor, P.; Smejkal, K.; Svajdlenka, E.; Solar, P.; Samuel, S. M.; Zulli, A.; Kassayova, M.; Lasabova, Z.; Kwon, T. K.; Pec, M.; Danko, J.; Büsselberg, D. Anticancer Activities of Thymus Vulgaris L. in Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20(7), 1749. [Google Scholar] [CrossRef]
- Uchôa, A. F. C.; Formiga, A. L. D.; Cardoso, A. L. M. R.; Pereira, G. M. A.; Carvalho, L. M. M.; Souza, P. H. O.; Silva, A. L.; Souza, R. R. M.; Sobral, M. V.; Silva, M. S.; Barbosa-Filho, J. M.; Xavier-Júnior, F. H.; Uchôa, A. F. C.; Formiga, A. L. D.; Cardoso, A. L. M. R.; Pereira, G. M. A.; Carvalho, L. M. M.; Souza, P. H. O.; Silva, A. L.; Souza, R. R. M.; Sobral, M. V.; Silva, M. S.; Barbosa-Filho, J. M.; Xavier-Júnior, F. H. Optimized and Functionalized Carvacrol-Loaded Nanostructured Lipid Carriers for Enhanced Cytotoxicity in Breast Cancer Cells. Pharmaceutics 2025, 17(3), 363. [Google Scholar] [CrossRef]
- Arunasree, K. M. Anti-Proliferative Effects of Carvacrol on a Human Metastatic Breast Cancer Cell Line, MDA-MB 231. Phytomedicine 2010, No. 17(8-9), 581–588. [Google Scholar] [CrossRef]
- Hassan, S. B.; Gali-Muhtasib, H.; Göransson, H.; Larsson, R. Alpha Terpineol: A Potential Anticancer Agent Which Acts through Suppressing NF-kappaB Signalling. Anticancer Res. 2010, 30(6), 1911–1919. [Google Scholar]
- Meijer, M. M. Y.; van den Brand, H.; Niknafs, S.; Stark, T.; Navarro, M.; Khaskheli, A. A.; Roura, E. Carvacrol in Ovo Delivery Optimization and Flow Dynamics in Broiler Chicken Eggs. Poult. Sci. 2024, 103(3), 103443. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Sabatino, M.; Valentini, E.; D’Aguanno, S.; Ragno, R.; Del Bufalo, D. Antitumor Effect of Melaleuca Alternifolia Essential Oil and Its Main Component Terpinen-4-Ol in Combination with Target Therapy in Melanoma Models. Cell Death Discov. 2021, 7(1), 127. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E. A.; Zahra, H. A.; Ammar, R. B.; Mohamed, M. E.; Ibrahim, H.-I. M.; Ahmed, E. A.; Zahra, H. A.; Ammar, R. B.; Mohamed, M. E.; Ibrahim, H.-I. M. Beta-Caryophyllene Enhances the Anti-Tumor Activity of Cisplatin in Lung Cancer Cell Lines through Regulating Cell Cycle and Apoptosis Signaling Molecules. Molecules 2022, 27(23), 8354. [Google Scholar] [CrossRef]
- Azimi, S.; Esmaeil Lashgarian, H.; Ghorbanzadeh, V.; Moradipour, A.; Pirzeh, L.; Dariushnejad, H. 5-FU and the Dietary Flavonoid Carvacrol: A Synergistic Combination That Induces Apoptosis in MCF-7 Breast Cancer Cells. Med. Oncol. 2022, 39(12), 253. [Google Scholar] [CrossRef]
- Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Ou-Yahia, D.; Bellaouchi, R.; Mothana, R. A.; Al-Yousef, H. M.; Asehraou, A.; Addi, M.; Guerrouj, B. E.; Chaabane, K.; Taibi, M.; Elbouzidi, A.; Haddou, M.; Baraich, A.; Ou-Yahia, D.; Bellaouchi, R.; Mothana, R. A.; Al-Yousef, H. M.; Asehraou, A.; Addi, M.; Guerrouj, B. E.; Chaabane, K. Evaluation of the Interaction between Carvacrol and Thymol, Major Compounds of Ptychotis Verticillata Essential Oil: Antioxidant, Anti-Inflammatory and Anticancer Activities against Breast Cancer Lines. Life 2024, 14(8), 1037. [Google Scholar] [CrossRef]
- Jamali, T.; Kavoosi, G.; Safavi, M.; Ardestani, S. K. In-Vitro Evaluation of Apoptotic Effect of OEO and Thymol in 2D and 3D Cell Cultures and the Study of Their Interaction Mode with DNA. Sci. Rep. 2018, 8(1), 15787. [Google Scholar] [CrossRef]
- Chung, K.-S.; Hong, J. Y.; Lee, J.-H.; Lee, H.-J.; Park, J. Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T.; Chung, K.-S.; Hong, J. Y.; Lee, J.-H.; Lee, H.-J.; Park, J. Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24(20), 3754. [Google Scholar] [CrossRef]
- Chen, W.-L.; Barszczyk, A.; Turlova, E.; Deurloo, M.; Liu, B.; Yang, B. B.; Rutka, J. T.; Feng, Z.-P.; Sun, H.-S. Inhibition of TRPM7 by Carvacrol Suppresses Glioblastoma Cell Proliferation, Migration and Invasion. Oncotarget 2015, 6(18), 16321–16340. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, X.; Cao, Y.; Qi, H.; Li, L.; Zhang, Q.; Sun, H. Carvacrol Inhibits Proliferation and Induces Apoptosis in Human Colon Cancer Cells. Anticancer. Drugs 2015, 26(8), 813. [Google Scholar] [CrossRef] [PubMed]
- Rrustemi, T.; Geyik, Ö. G.; Özkaya, A. B.; Öztürk, T. K.; Yüce, Z.; Kılınç, A. Acrylamide-Encapsulated Glucose Oxidase Inhibits Breast Cancer Cell Viability. Turk. J. Biochem. 2020, 45(6), 811–816. [Google Scholar] [CrossRef]
- Sys, G. M. L.; Lapeire, L.; Stevens, N.; Favoreel, H.; Forsyth, R.; Bracke, M.; De Wever, O. The in Ovo CAM-Assay as a Xenograft Model for Sarcoma. J. Vis. Exp. JoVE 2013, 77, e50522. [Google Scholar] [CrossRef]







| Component | Vacrol* | RTVacrol (min) | S-Mix* | RTS-Mix (min) |
|---|---|---|---|---|
| carvacrol | 50.1 | 33.16 | 24 | 33.13 |
| limonene | 0.8 | 9.17 | 13 | 9.24 |
| α-pinene | 3.5 | 4.30 | 12.7 | 4.33 |
| 1,8-cineole | 9.7 | 9.42 | 11.7 | 9.44 |
| eugenol | 4.7 | 32.17 | 7.6 | 32.17 |
| cinnamaldehyde | 4.3 | 29.71 | 7.1 | 29.72 |
| p-cymene | 3.2 | 11.38 | 3.4 | 11.37 |
| linalool | 6.3 | 19.06 | 2.3 | 19.04 |
| menthol | 1.9 | 21.22 | 2.2 | 21.22 |
| α-thujone | 1.3 | 15.59 | 1.5 | 15.59 |
| δ-3-carene | 0.4 | 7.65 | 1.5 | 7.66 |
| camphor | 1.2 | 18.03 | 1.4 | 18.03 |
| β-pinene | 0.7 | 6.41 | 1.3 | 6.41 |
| terpinenyl acetate | 0.8 | 22.44 | 1.1 | 22.44 |
| caryophyllene | 0.9 | 20.07 | 0.9 | 20.07 |
| menthone | 0.8 | 16.68 | 0.9 | 16.70 |
| 4-terpineol | 1.3 | 20.31 | 0.8 | 20.31 |
| sabinene | - | - | 0.7 | 6.83 |
| δ-terpinene | 1.1 | 10.64 | 0.7 | 10.66 |
| bisabolene | 1.1 | 23.14 | 0.7 | 23.13 |
| hexadecadienoic acid. methyl ester | 0.6 | 51.10 | 0.6 | 51.10 |
| 9-octadecenoic acid | - | - | 0.5 | 49.49 |
| thymol | 2.1 | 32.60 | 0.5 | 32.60 |
| camphene | 0.4 | 5.30 | 0.5 | 5.29 |
| myrcene | 0.3 | 8.21 | 0.5 | 8.21 |
| borneol | 0.9 | 22.65 | 0.4 | 22.65 |
| fencyl alcohol | 0.6 | 22.55 | 0.4 | 22.55 |
| β-thujone | 0.4 | 16.11 | 0.4 | 16.11 |
| humulene | 0.3 | 21.76 | 0.4 | 21.76 |
| eugenol acetate | - | - | 0.3 | 33.72 |
| α-terpinene | 0.3 | 8.59 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





