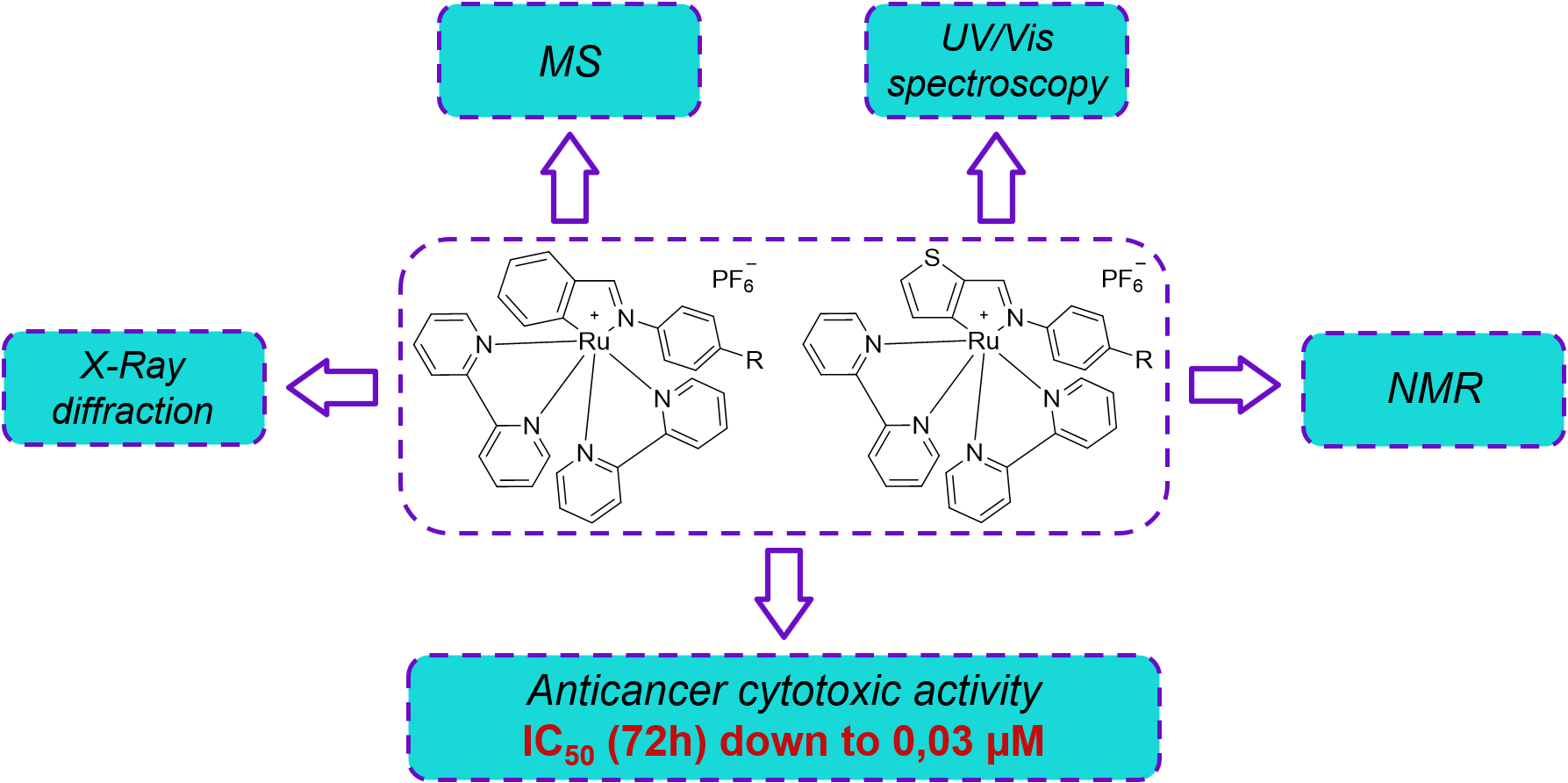
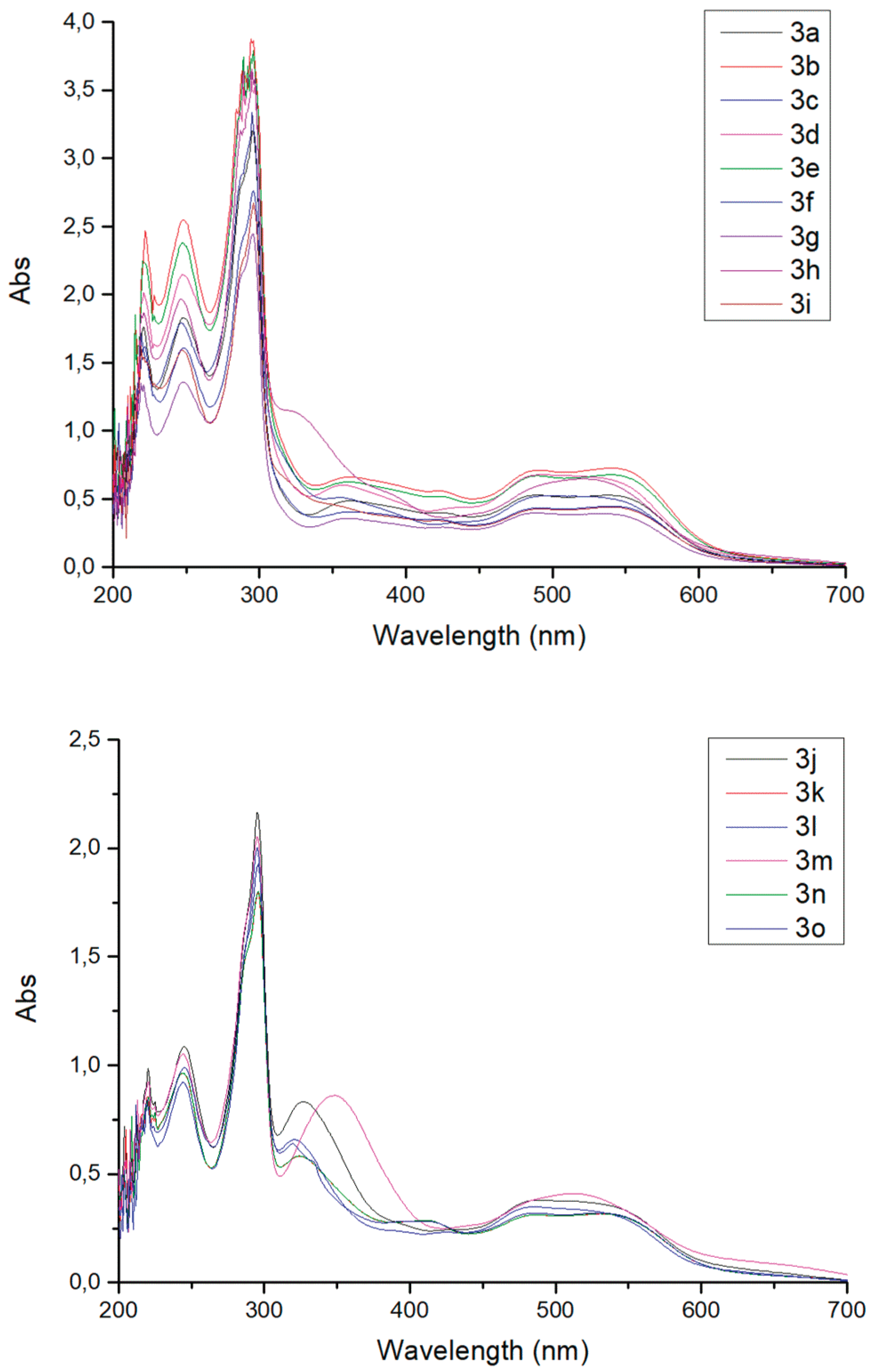
3.1. General Procedure for Compounds 2a-2o.
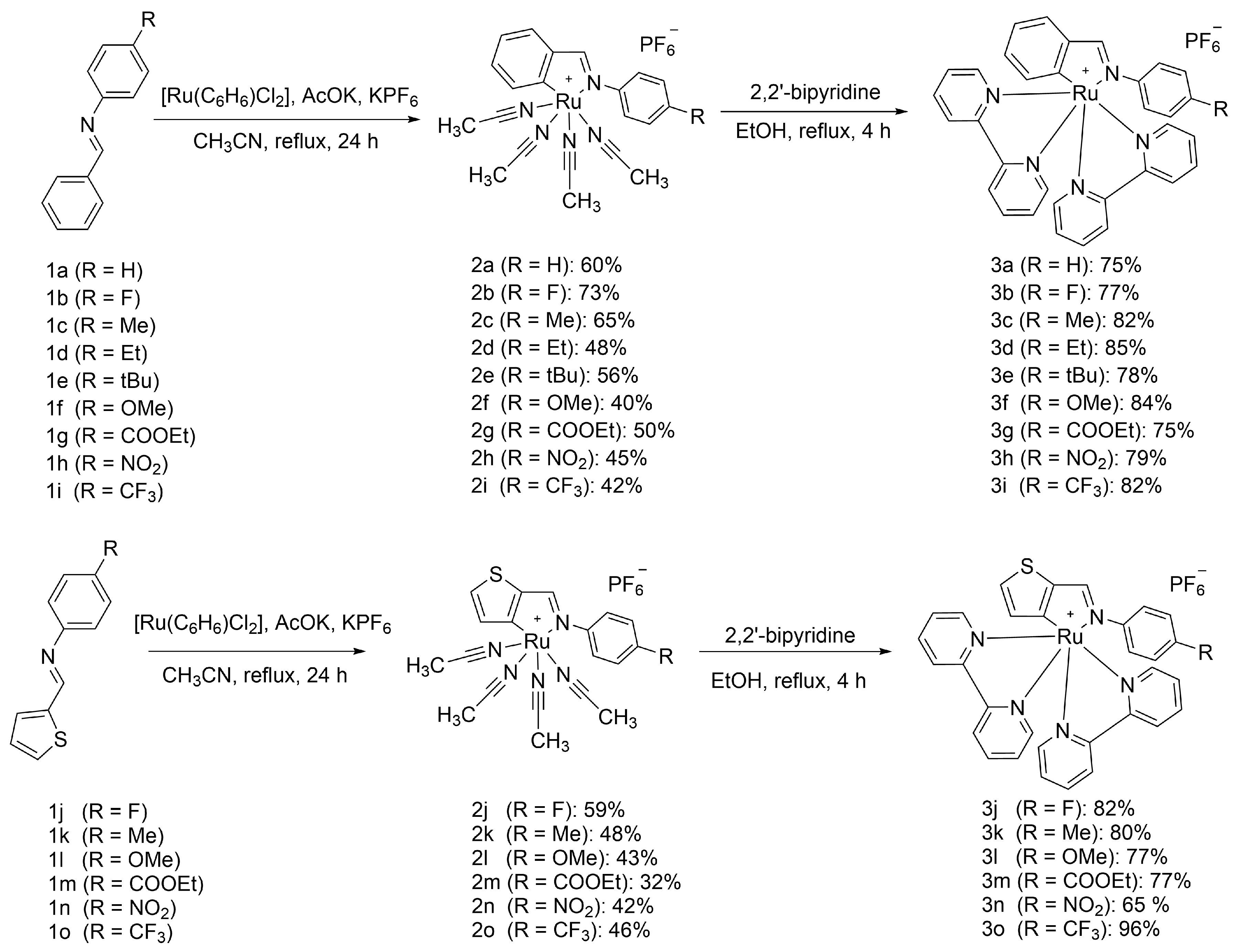
Imine 1 (0.40 mmol, 2 eq) dissolved in acetonitrile (15 ml) was added to a three-necked flask equipped with a reflux condenser with [Ru(C6H6)Cl2]2 (0.20 mmol, 1 eq), potassium hexafluorophosphate (0.80 mmol, 4 eq), potassium acetate (0.60 mmol, 3 eq) and refluxed in an argon atmosphere for 24 hours. The reaction mixture was evaporated, residue was dissolved in DCM (15 ml) and of water (10 ml), organic layer was separated, aqueous layer was washed with DCM (5 ml), organic phases were combined, dried over sodium sulfate and evaporated to dryness. Crude product was purified with column chromatography on silica gel (CHCl3:CH3CN 10:1). Crystals suitable for X-ray analysis were grown by diethyl ether vapor diffusion on acetonitrile solution of complexes.
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]aniline-κN]ruthenium(II) hexafluorophosphate (2a).
Orange powder. Yield 60%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.46 (s, 1H), 8.03 (d, J = 7.5 Hz, 1H), 7.64 (dd, J = 7.5, 1.1 Hz, 1H), 7.49–7.42 (m, 2H), 7.39–7.34 (m, 1H), 7.34–7.28 (m, 2H), 7.13 (td, J = 7.4, 1.5 Hz, 1H), 6.96 (td, J = 7.3, 1.2 Hz, 1H), 2.51 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H).
13C NМR (76 MHz, Acetonitrile-d3): 191.61, 176.62, 152.09, 149.52, 138.15, 129.68, 128.76, 126.63, 124.03, 122.69, 121.81, 120.57, 117.35, 65.30, 14.66, 3.41, 2.93.
HRMS-ESI: calc for [C19H19N4Ru-CH3CN]+ 405.0647, found 405.0643
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-fluoroaniline-κN]ruthenium(II) hexafluorophosphate (2b).
Orange powder. Yield 73%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.45 (s, 1H), 8.02 (d, J = 7.5 Hz, 1H), 7.63 (d, J = 7.4 Hz, 1H), 7.35–7.28 (m, 2H), 7.22–7.10 (m, 3H), 6.96 (t, J = 7.3 Hz, 1H), 2.51 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 191.83, 176.98, 162.05, 160.44, 149.45, 148.55, 138.21, 129.83, 128.73, 124.55, 121.91, 120.63, 117.40, 115.22, 3.41, 2.94.
HRMS-ESI: calc for [C19H18FN14Ru-CH3CN]+ 423.0534, found 423.0568.
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-methylaniline-κN]ruthenium(II) hexafluorophosphate (2c).
Orange powder. Yield 65%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.43 (s, 1H), 8.01 (d, J = 7.5 Hz, 1H), 7.61 (dd, J = 7.5, 1.1 Hz, 1H), 7.26 (d, J = 8.3 Hz, 2H), 7.19 (d, J = 8.4 Hz, 2H), 7.12 (td, J = 7.4, 1.5 Hz, 1H), 6.96 (td, J = 7.3, 1.1 Hz, 1H), 2.51 (s, 3H), 2.39 (s, 3H), 2.11 (s, 6H), 1.96 (s, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 191.40, 176.19, 149.80, 138.14, 136.59, 129.52, 129.20, 128.53, 123.97, 122.54, 121.76, 120.57, 117.36, 20.11, 3.45, 2.96.
HRMS-ESI: calc for [C20H21N4Ru-CH3CN]+ 419.0822, found 419.0814.
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-ethylaniline-κN]ruthenium(II) hexafluorophosphate (2d)
Orange powder. Yield 48%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.44 (s, 1H), 8.01 (d, J = 7.5 Hz, 1H), 7.63–7.58 (m, 1H), 7.29 (d, J = 8.2 Hz, 2H), 7.21 (d, J = 8.3 Hz, 2H), 7.12 (t, J = 7.3 Hz, 1H), 6.96 (t, J = 7.3 Hz, 1H), 2.70 (q, J = 7.6 Hz, 2H), 2.51 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H), 1.26 (t, J = 7.6 Hz, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 191.39, 176.22, 149.97, 149.58, 143.02, 138.14, 129.54, 128.55, 128.08, 123.98, 122.63, 121.77, 120.57, 117.36, 28.10, 15.25, 3.44, 2.97.
HRMS-ESI: calc for [C21H23N4Ru-CH3CN]+ 433.1210, found 433.1215
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-(tert-butyl)aniline-κN]ruthenium(II) hexafluorophosphate (2e).
Orange powder. Yield 56%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.45 (s, 1H), 8.01 (d, J = 7.4 Hz, 1H), 7.62 (d, J = 7.4 Hz, 1H), 7.48 (d, J = 8.5 Hz, 2H), 7.24 (d, J = 8.4 Hz, 2H), 7.12 (t, J = 7.3 Hz, 1H), 6.96 (t, J = 7.3 Hz, 1H), 2.51 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H), 1.37 (s, 9H).
13C NМR (151 MHz, Acetonitrile-d3): 191.43, 176.25, 149.73, 138.15, 129.57, 128.57, 125.58, 124.00, 122.31, 121.79, 120.59, 117.38, 34.30, 30.68, 3.43, 2.96.
HRMS-ESI: calc for [C23H27N4Ru-CH3CN]+ 315.6547, found 315.6548.
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-methoxyaniline-κN]ruthenium(II) hexafluorophosphate (2f).
Orange powder. Yield 40%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.42 (s, 1H), 8.01 (d, J = 7.5 Hz, 1H), 7.61 (d, J = 7.4 Hz, 1H), 7.26 (d, J = 8.8 Hz, 2H), 7.11 (t, J = 7.3 Hz, 1H), 7.00–6.92 (m, 3H), 3.84 (s, 3H), 2.51 (s, 3H), 2.11 (s, 6H), 1.96 (s, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 191.19, 175.85, 158.52, 149.61, 145.56, 138.10, 129.41, 128.45, 123.79, 121.74, 120.57, 117.36, 113.75, 55.28, 2.99.
HRMS-ESI: calc for [C19H18N4ORu]+ 419.0812, found 419.0814.
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-ethoxycarbonylaniline-κN]ruthenium(II) hexafluorophosphate (2g).
Orange powder. Yield 50%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.50 (s, 1H), 8.10–8.02 (m, 3H), 7.67 (d, J = 7.3 Hz, 1H), 7.38 (d, J = 8.4 Hz, 2H), 7.14 (t, J = 7.4 Hz, 1H), 6.98 (t, J = 7.3 Hz, 1H), 4.37 (q, J = 7.1 Hz, 2H), 2.52 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H), 1.38 (t, J = 7.1 Hz, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 192.72, 177.59, 165.84, 155.89, 149.44, 138.28, 130.31, 130.03, 128.98, 124.23, 123.06, 122.02, 120.71, 117.38, 61.00, 13.68, 3.45, 2.96.
HRMS-ESI: calc for [C22H23N4O2Ru-CH3CN]+ 477.0853, found 477.0859.
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-nitroaniline-κN]ruthenium(II) hexafluorophosphate (2h).
Orange powder. Yield 45%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.54 (s, 1H), 8.33–8.27 (m, 2H),
8.06 (d, J = 7.5 Hz, 1H), 7.71 (dd, J = 7.5, 1.1 Hz, 1H), 7.51–7.46 (m, 2H), 7.16 (td, J = 7.3, 1.4 Hz, 1H), 6.99 (td, J = 7.4, 1.1 Hz, 1H), 2.52 (s, 3H), 2.13 (s, 6H), 1.96 (s, 3H).13C NМR (151 MHz, Acetonitrile-d3): 193.64, 178.56, 157.44, 149.33, 146.21, 138.35, 130. 82, 129.27, 124.44, 123.99, 122.18, 120.82, 117.37, 3.46, 2.99.
HRMS-ESI: calc for [C19H18N5O2Ru-CH3CN]+ 435.0765, found 435.0766.
Tetrakis(acetonitrile)[N-((phenyl-κC2)methyliden]-4-(trifluoromethyl)aniline-κN]ruthenium(II) hexafluorophosphate (2i).
Orange powder. Yield 42%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.51 (s, 1H), 8.04 (d, J = 7.5 Hz, 1H), 7.77 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 7.5 Hz, 1H), 7.46 (d, J = 8.3 Hz, 2H), 7.15 (t, J = 7.4 Hz, 1H), 6.98 (t, J = 7.4 Hz, 1H), 2.52 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 192.73, 177.99, 155.22, 149.38, 138.29, 130.39, 129.05, 126.01, 124.27, 123.64, 122.06, 120.74, 117.37, 3.46, 2.99.
HRMS-ESI: calc for [C20H18F3N4Ru-CH3CN]+ 473.0528, 473.0524.
Tetrakis(acetonitrile)[N-((thiophene-2-yl-κC2)methyliden]-4-fluoroaniline-κN]ruthenium(II) hexafluorophosphate (2j).
Orange powder. Yield 59%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.38 (s, 1H), 7.82 (d, J = 4.6 Hz, 1H), 7.63 (d, J = 4.1 Hz, 1H), 7.34–7.27 (m, 2H), 7.20–7.13 (m, 2H), 2.51 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 202.67, 168.15, 163.47, 160.26, 149.81, 149.78, 138.48, 137.04, 134.08, 125.66, 125.55, 125.23, 123.44, 116.33, 116.04, 31.61, 30.35, 4.32, 3.89, 1.77.
HRMS-ESI: calc for [C19H19BrN5RuS-CH3CN]+ 470.0387, found 470.0397
Tetrakis(acetonitrile)[N-((thiophene-2-yl-κC2)methyliden]-4-methylaniline-κN]ruthenium(II) hexafluorophosphate (2k).
Orange powder. Yield 48%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.38 (s, 1H), 7.80 (d, J = 4.6 Hz, 1H), 7.62 (d, J = 4.6 Hz, 1H), 7.25–7.16 (m, 4H), 2.51 (s, 3H), 2.38 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H).
13C NMR (75 MHz, Acetonitrile-d3) δ 201.70, 167.47, 151.03, 138.48, 136.98, 136.91, 133.58, 130.16, 125.11, 123.73, 123.29, 21.05, 4.34, 3.91, 1.77.
HRMS-ESI: calc for [C19H19BrN5RuS-CH3CN]+ 425.0372, found 425.0362.
Tetrakis(acetonitrile)[N-((thiophene-2-yl-κC2)methyliden]-4-methoxyaniline-κN]ruthenium(II) hexafluorophosphate (2l).
Orange powder. Yield 43%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.39 (s, 1H), 7.82 (d, J = 4.6 Hz, 1H), 7.64 (d, J = 4.6 Hz, 1H), 7.30–7.23 (m, 2H), 7.02–6.96 (m, 2H), 3.86 (s, 3H), 2.54 (s, 3H), 2.14 (s, 6H), 1.99 (s, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 201.18, 167.17, 159.05, 146.78, 138.41, 136.93, 133.36, 132.27, 129.73, 125.06, 124.87, 123.27, 114.69, 56.19, 4.34, 3.92.
HRMS-ESI: calc for [C19H19BrN5RuS-CH3CN]+ 482.0587, found 482.0595.
Tetrakis(acetonitrile)[N-((thiophene-2-yl-κC2)methyliden]-4-ethoxycarbonylaniline-κN]ruthenium(II) hexafluorophosphate (2m).
Orange powder. Yield 32%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.45 (s, 1H), 8.05 (d, J = 8.5 Hz, 2H), 7.88 (d, J = 4.7 Hz, 1H), 7.66 (d, J = 4.7 Hz, 1H), 7.39 (d, J = 8.4 Hz, 2H), 4.36 (q, J = 7.1 Hz, 2H), 2.52 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H), 1.38 (t, J = 7.1 Hz, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 204.97, 168.66, 166.87, 157.31, 138.94, 137.13, 135.02, 130.96, 129.02, 125.33, 124.17, 123.56, 61.85, 14.63, 4.37, 3.92.
HRMS-ESI: calc for [C19H19BrN5RuS-CH3CN]+ 524.0694, found 524.0695.
Tetrakis(acetonitrile)[N-((thiophene-2-yl-κC2)methyliden]-4-nitroaniline-κN]ruthenium(II) hexafluorophosphate (2n).
Orange powder. Yield 42%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.50 (s, 1H), 8.27 (d, J = 9.0 Hz, 2H), 7.92 (d, J = 4.7 Hz, 1H), 7.68 (d, J = 4.7 Hz, 1H), 7.49 (d, J = 9.0 Hz, 2H), 2.52 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 207.50, 169.44, 159.04, 146.53, 139.30, 137.28, 136.07, 125.48, 125.39, 124.97, 123.77, 31.59, 30.34, 4.37, 3.94, 1.77.
HRMS-ESI: calc for [C19H19BrN5RuS-CH3CN]+ 497.0332, found 497.0344.
Tetrakis(acetonitrile)[N-((thiophene-2-yl-κC2)methyliden]-4-(trifluoromethyl)aniline-κN]ruthenium(II) hexafluorophosphate (2o).
Orange powder. Yield 46%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.46 (s, 1H), 7.88 (d, J = 4.6 Hz, 1H), 7.74 (d, J = 8.3 Hz, 2H), 7.66 (d, J = 4.6 Hz, 1H), 7.46 (d, J = 8.2 Hz, 2H), 2.52 (s, 3H), 2.12 (s, 6H), 1.96 (s, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 205.10, 169.01, 156.59, 138.89, 137.13, 135.13, 128.29, 127.86, 126.88 (q, J = 3.8 Hz), 125.36, 124.75, 123.60, 4.38, 3.94.
HRMS-ESI: calc for [C19H19BrN5RuS-CH3CN]+ 520.0356, found 520.0367.
3.1. General Procedure for Compounds 3a-3o.
Complex 2 (0.30 mmol, 1 eq) and 2,2’-bipyridine (0.60 mmol, 2 eq) were introduced into a flask equipped with a reflux condenser, ethanol (18 ml) was added, and the mixture was refluxed in an argon atmosphere for 4 hours. The reaction mixture was evaporated and purified with column chromatography on silica gel (CHCl3:CH3CN 10:1). The product was dissolved in minimal volume of CH3CN and added dropwise to large excess of diethyl ether under vigorous stirring. The precipitate was filtered, washed with diethyl ether and dried in vacuo for 3 h. Crystals suitable for X-ray analysis were grown by diethyl ether vapor diffusion on acetonitrile solution of complexes.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]aniline-κN]ruthenium(II) hexafluorophosphate (3a).
Dark purple powder. Yield 75%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.73 (s, 1H), 8.63 (d, J = 5.7 Hz, 1H), 8.33 (d, J = 8.2 Hz, 2H), 8.13 (d, J = 8.2 Hz, 1H), 8.00 (dd, J = 12.8, 6.9 Hz, 2H), 7.84 (p, J = 7.7 Hz, 3H), 7.78–7.64 (m, 4H), 7.40 (t, J = 6.6 Hz, 1H), 7.31 (t, J = 6.6 Hz, 1H), 7.19 (t, J = 6.5 Hz, 2H), 7.02–6.81 (m, 5H), 6.53 (dd, J = 12.5, 7.3 Hz, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 201.76, 176.18, 158.78, 158.01, 157.64, 155.56, 155.43, 153.29, 151.82, 151.24, 150.07, 149.36, 136.77, 136.36, 136.09, 135.10, 134.81, 131.82, 130.09, 129.47, 127.43, 127.39, 127.35, 127.16, 127.05, 124.02, 123.67, 123.21, 122.64, 121.63.
CHN calc. for С33H26N5RuPF6*0.2C4H10O: C: 53.29; H: 3.53; N: 9.31. Obs. C: 53.29; H: 3.52; N: 9.39.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-fluoroaniline-κN]ruthenium(II) hexafluorophosphate (3b).
Dark purple powder. Yield 77%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.72 (s, 1H), 8.59 (d, J = 5.7 Hz, 1H), 8.34 (d, J = 8.1 Hz, 2H), 8.15 (d, J = 8.1 Hz, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.96 (d, J = 5.6 Hz, 1H), 7.84 (p, J = 7.2 Hz, 3H), 7.78–7.70 (m, 3H), 7.66 (d, J = 5.3 Hz, 1H), 7.39 (t, J = 6.6 Hz, 1H), 7.31 (t, J = 6.7 Hz, 1H), 7.20 (q, J = 6.2 Hz, 2H), 6.87 (p, J = 7.4 Hz, 2H), 6.66 (t, J = 8.7 Hz, 2H), 6.58–6.47 (m, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 200.85, 175.57, 161.43, 159.82, 157.77, 157.04, 156.67, 154.49, 152.32, 150.34, 149.15, 148.33, 147.22, 135.94, 135.21, 134.22, 133.92, 130.96, 129.22, 126.58, 123.49, 122.37, 120.73, 117.38, 115.10.
CHN calc. for C33H25N5RuPF7: C: 52.39; H: 3.33; N: 9.26. Obs. C: 52.23; H: 3.21; N: 9.09.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-methylaniline-κN]ruthenium(II) hexafluorophosphate (3c).
Dark purple powder. Yield 82%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.70 (s, 1H), 8.61 (d, J = 5.7 Hz, 1H), 8.33 (d, J = 8.2 Hz, 2H), 8.14 (d, J = 8.1 Hz, 1H), 8.04 (d, J = 8.1 Hz, 1H), 7.96 (d, J = 5.6 Hz, 1H), 7.88–7.78 (m, 3H), 7.76–7.68 (m, 3H), 7.66 (d, J = 5.3 Hz, 1H), 7.39 (t, J = 6.7 Hz, 1H), 7.30 (t, J = 6.7 Hz, 1H), 7.22–7.16 (m, 2H), 6.91–6.80 (m, 2H), 6.73 (d, J = 8.0 Hz, 2H), 6.52 (d, J = 7.1 Hz, 1H), 6.41 (d, J = 8.0 Hz, 2H), 2.12 (s, 3H).
13C NМR (76 MHz, Acetonitrile-d3): 200.41, 174.77, 157.82, 157.03, 156.67, 154.60, 154.43, 152.31, 150.23, 149.08, 148.62, 148.44, 136.06, 135.78, 135.38, 135.05, 134.08, 133.79, 130.62, 128.91, 126.40, 126.17, 123.02, 122.70, 122.29, 121.47, 120.68, 117.36, 19.80.
CHN calc. for C34H28N5RuPF6: C: 54.26; H: 3.75; N: 9.30. Obs. C: 54.07; H: 3.70; N: 9.34.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-ethylaniline-κN]ruthenium(II) hexafluorophosphate (3d).
Dark purple powder. Yield 85%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.71 (s, 1H), 8.62 (d, J = 5.5 Hz, 1H), 8.33 (d, J = 8.3 Hz, 2H), 8.12 (d, J = 8.1 Hz, 1H), 8.02–7.95 (m, 2H), 7.89–7.78 (m, 3H), 7.76–7.63 (m, 4H), 7.39 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.30 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.19 (ddd, J = 7.5, 6.0, 1.1 Hz, 2H), 6.89 (td, J = 7.3, 1.5 Hz, 1H), 6.83 (td, J = 7.2, 1.7 Hz, 1H), 6.76–6.71 (m, 2H), 6.56–6.51 (m, 1H), 6.41–6.36 (m, 2H), 2.42 (q, J = 7.6 Hz, 2H), 1.05 (t, J = 7.6 Hz, 3H).
13C NМR (76 MHz, Acetonitrile-d3): 200.43, 174.66, 157.85, 157.04, 156.66, 154.40, 152.33, 150.29, 149.07, 148.48, 142.53, 135.77, 135.33, 135.05, 134.04, 133.76, 130.61, 129.01, 127.76, 126.40, 126.15, 123.03, 122.63, 122.22, 121.52, 120.64, 117.35, 27.80, 15.28.
CHN calc. for C35H30N5RuPF6: C: 54.66; H: 3.94; N: 9.31. Obs. C: 54.87; H: 3.93; N :9.13.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-(tert-butyl)aniline-κN]ruthenium(II) hexafluorophosphate (3e).
Dark purple powder. Yield 78%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.71 (s, 1H), 8.64 (d, J = 5.8 Hz, 1H), 8.34 (d, J = 8.1 Hz, 2H), 8.09 (d, J = 8.1 Hz, 1H), 7.98 (d, J = 5.7 Hz, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.89–7.77 (m, 4H), 7.73 (d, J = 7.3 Hz, 1H), 7.67 (t, J = 7.8 Hz, 1H), 7.62 (d, J = 5.3 Hz, 1H), 7.39 (t, J = 6.6 Hz, 1H), 7.30 (t, J = 6.6 Hz, 1H), 7.19 (q, J = 6.0 Hz, 2H), 6.92–6.81 (m, 4H), 6.55 (d, J = 7.2 Hz, 1H), 6.33 (d, J = 8.0 Hz, 2H), 1.15 (s, 9H).
13C NМR (76 MHz, Acetonitrile-d3): 205.61, 179.79, 163.22, 162.35, 161.97, 159.96, 159.68, 157.66, 155.67, 154.44, 154.39, 153.81, 153.10, 141.05, 140.62, 140.38, 139.31, 139.03, 135.87, 134.34, 131.42, 130.49, 128.36, 127.86, 127.48, 126.43, 125.94, 122.66, 39.21, 35.77.
CHN calc. for C37H34N5PF6Ru: C: 55.92; H: 3.84; N: 8.73. Obs. C: 55.90; H: 4.24; N :9.10.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-methoxyaniline-κN]ruthenium(II) hexafluorophosphate (3f).
Dark purple powder. Yield 84%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.69 (s, 1H), 8.60 (d, J = 5.0 Hz, 1H), 8.33 (d, J = 8.2 Hz, 2H), 8.14 (d, J = 8.1 Hz, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.98–7.94 (m, 1H), 7.88–7.78 (m, 3H), 7.76–7.70 (m, 3H), 7.68–7.65 (m, 1H), 7.39 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.30 (ddd, J = 7.4, 5.7, 1.4 Hz, 1H), 7.23–7.16 (m, 2H), 6.88 (td, J = 7.2, 1.5 Hz, 1H), 6.82 (td, J = 7.2, 1.7 Hz, 1H), 6.55–6.50 (m, 1H), 6.45 (s, 4H), 3.62 (s, 3H).
13C NМR (151 MHz, Acetonitrile-d3): 200.28, 174.42, 157.92, 157.83, 157.07, 156.71, 154.68, 154.42, 152.33, 150.31, 149.09, 148.46, 144.23, 135.82, 135.32, 135.04, 134.08, 133.78, 130.53, 128.94, 126.45, 126.19, 123.06, 122.71, 120.66, 117.37, 113.52, 55.12.
CHN calc. for C34H28N5ORuPF6: C: 53.13; H: 3.67; N: 9.11. Obs. C: 52.87; H: 3.76; N: 9.17.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-ethoxycarbonylaniline-κN]ruthenium(II) hexafluorophosphate (3g).
Dark purple powder. Yield 75%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.78 (s, 1H), 8.60 (dd, J = 5.7, 0.7 Hz, 1H), 8.34 (dq, J = 8.2, 1.2 Hz, 2H), 8.14 (d, J = 8.0 Hz, 1H), 8.02 (d, J = 8.2 Hz, 1H), 7.97–7.94 (m, 1H), 7.90–7.78 (m, 4H), 7.73–7.65 (m, 3H), 7.57–7.52 (m, 2H), 7.40 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.32 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.24–7.17 (m, 2H), 6.91 (td, J = 7.3, 1.5 Hz, 1H), 6.86 (td, J = 7.3, 1.7 Hz, 1H), 6.63–6.56 (m, 3H), 4.25 (q, J = 7.1 Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H).
13C NМR (76 MHz, Acetonitrile-d3): 201.83, 176.20, 165.42, 157.69, 157.00, 156.65, 154.68, 154.52, 152.36, 150.30, 149.12, 148.29, 135.94, 135.50, 135.29, 134.32, 134.01, 131.44, 129.69, 129.41, 128.07, 126.62, 126.25, 123.10, 122.82, 122.38, 122.02, 120.77, 117.35, 60.92, 13.54.
CHN calc. for C36H30N5O2RuPF6*0.16CHCl3: C: 52.13; H:3.88; N: 8.71. Obs. C: 52.40; H:3.84; N: 8.73.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-nitroaniline-κN]ruthenium(II) hexafluorophosphate (3h).
Dark purple powder. Yield 79%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.85–8.78 (m, 1H), 8.60–8.53 (m, 1H), 8.38–8.31 (m, 2H), 8.15–8.09 (m, 1H), 8.04–7.98 (m, 1H), 7.93 (t, J = 5.6 Hz, 1H), 7.90–7.79 (m, 4H), 7.77–7.64 (m, 5H), 7.43–7.36 (m, 1H), 7.33 (t, J = 6.6 Hz, 1H), 7.27–7.17 (m, 2H), 6.96–6.83 (m, 2H), 6.72–6.64 (m, 2H), 6.63–6.57 (m, 1H).
13C NМR (151 MHz, Acetonitrile-d3): 202.80, 177.17, 157.64, 157.02, 156.67, 156.18, 154.60, 154.42, 152.42, 150.40, 149.12, 148.25, 145.46, 136.15, 135.60, 135.46, 134.51, 134.19, 131.98, 129.73, 126.87, 126.72, 126.50, 126.31, 124.05, 123.20, 123.16, 122.95, 122.60, 120.87, 117.35.
CHN calc. for C33H25N6O2PF6Ru*0.44CHCl3*0.34C4H10O: C: 48.51; H: 3.38; N: 9.75. Obs. C: 48.51; H: 3.21; N :9.75.
Bis(2,2՛-bipiridine)[N-((phenyl-κC2)methyliden]-4-(trifluoromethyl)aniline-κN]ruthenium(II) hexafluorophosphate (3i).
Dark purple powder. Yield 82%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.78 (s, 1H), 8.59 (d, J = 5.7 Hz, 1H), 8.34 (d, J = 8.1 Hz, 2H), 8.13 (d, J = 8.1 Hz, 1H), 8.02–7.95 (m, 2H), 7.91–7.78 (m, 4H), 7.74–7.63 (m, 3H), 7.40 (t, J = 6.7 Hz, 1H), 7.33 (t, J = 6.7 Hz, 1H), 7.25–7.17 (m, 4H), 6.89 (q, J = 6.6 Hz, 2H), 6.64 (d, J = 8.0 Hz, 2H), 6.59 (d, J = 6.9 Hz, 1H).
13C NМR (76 MHz, Acetonitrile-d3): 201.70, 176.46, 157.69, 157.00, 156.63, 154.57, 152.34, 150.34, 149.16, 148.27, 135.92, 135.52, 135.34, 135.27, 134.34, 134.04, 131.46, 129.48, 126.64, 126.42, 126.24, 125.65, 123.11, 122.82, 122.53, 122.33, 120.80, 117.34.
CHN calc. for C34H25N5RuPF9*0.16CH3CN: C: 50.47; H: 3.15; N: 8.85. Obs. C: 50.47; H: 3.15; N: 8.84.
Bis(2,2՛-bipiridine)[N-((thiophene-2-yl-κC2)methyliden]-4-fluoroaniline-κN]ruthenium(II) hexafluorophosphate (3j).
Dark purple powder. Yield 82%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.64 (s, 1H), 8.51 (d, J = 4.9 Hz, 1H), 8.32 (d, J = 8.1 Hz, 2H), 8.14 (d, J = 8.0 Hz, 1H), 8.07 (d, J = 8.2 Hz, 1H), 7.91–7.71 (m, 6H), 7.68 (d, J = 4.7 Hz, 1H), 7.61 (d, J = 4.7 Hz, 1H), 7.41 (ddd, 1H), 7.31 (ddd, 1H), 7.26–7.16 (m, 2H), 6.68–6.58 (m, 2H), 6.51–6.43 (m, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 212.13, 166.43, 162.82, 159.60, 158.52, 158.39, 157.85, 155.74, 155.42, 153.95, 151.77, 150.03, 148.60, 148.56, 138.59, 136.93, 136.22, 135.82, 135.46, 135.18, 134.74, 127.58, 127.50, 127.31, 127.16, 124.61, 124.50, 124.03, 123.85, 123.69, 123.38, 116.06, 115.76.
CHN calc. for C31H23F7N5OPRuS: C: 48.82; H: 3.04; N: 9.18 Obs. C: 48.83; H: 3.04; N: 9.18.
Bis(2,2՛-bipiridine)[N-((thiophene-2-yl-κC2)methyliden]-4-methylaniline-κN]ruthenium(II) hexafluorophosphate (3k).
Dark purple powder. Yield 80%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.63 (s, 1H), 8.53 (d, J = 5.7 Hz, 1H), 8.31 (d, J = 8.1 Hz, 2H), 8.14 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 8.1 Hz, 1H), 7.90–7.69 (m, 6H), 7.67–7.63 (m, 1H), 7.61 (d, J = 5.4 Hz, 1H), 7.44–7.36 (m, 1H), 7.33–7.26 (m, 1H), 7.19 (q, 2H), 6.71 (d, J = 8.1 Hz, 2H), 6.45 (d, J = 4.6 Hz, 1H), 6.39 (d, J = 8.3 Hz, 2H), 2.11 (s, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 211.31, 165.81, 158.60, 158.40, 157.88, 155.77, 155.43, 153.94, 151.67, 149.99, 138.53, 136.81, 136.32, 136.08, 135.36, 135.33, 135.08, 134.70, 129.90, 127.44, 127.37, 127.28, 127.16, 123.99, 123.80, 123.65, 123.34, 122.66, 20.75.
CHN calc. for C32H26F6N5PRuS: C: 50.66; H: 3.45; N: 9.23 Obs. C: 50.48; H: 3.48; N: 9.20.
Bis(2,2՛-bipiridine)[N-((thiophene-2-yl-κC2)methyliden]-4-methoxyaniline-κN]ruthenium(II) hexafluorophosphate (3l).
Dark purple powder. Yield 77%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.61 (s, 1H), 8.53 (d, J = 4.9 Hz, 1H), 8.31 (d, J = 8.1 Hz, 2H), 8.14 (d, J = 8.1 Hz, 1H), 8.06 (d, J = 8.2 Hz, 1H), 7.90–7.70 (m, 6H), 7.64 (d, J = 4.6 Hz, 1H), 7.61 (d, J = 4.6 Hz, 1H), 7.40 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.30 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.25–7.15 (m, 2H), 6.45 (d, J = 4.6 Hz, 1H), 6.42 (br.s, 3H), 3.61 (s, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 210.71, 165.46, 158.59, 158.44, 158.41, 157.88, 155.82, 155.39, 153.93, 151.72, 149.99, 145.56, 138.48, 136.82, 136.06, 135.32, 135.10, 135.04, 134.66, 127.45, 127.37, 127.27, 127.13, 123.99, 123.81, 123.61, 123.32, 114.48, 56.03, 15.64.
CHN calc. for C32H26F6N5OPRuS: C: 49.61; H: 3.38; N: 9.04 Obs. C: 49.60; H: 3.39; N: 9.08.
Bis(2,2՛-bipiridine)[N-((thiophene-2-yl-κC2)methyliden]-4-ethoxycarbonylaniline-κN]ruthenium(II) hexafluorophosphate (3m).
Dark purple powder. Yield 77%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.72 (s, 1H), 8.50 (d, J = 4.9 Hz, 1H), 8.33 (d, J = 8.4 Hz, 2H), 8.13 (d, J = 8.1 Hz, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.92–7.70 (m, 7H), 7.63 (d, J = 4.7 Hz, 1H), 7.55–7.49 (m, 2H), 7.42 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.32 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.25 (ddd, J = 7.5, 5.4, 1.2 Hz, 1H), 7.19 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 6.63–6.56 (m, 2H), 6.49 (d, J = 4.6 Hz, 1H), 4.24 (q, J = 7.1 Hz, 2H), 1.29 (t, J = 7.1 Hz, 3H).
13C NMR (76 MHz, Acetonitrile-d3) δ 166.89, 166.51, 158.46, 158.41, 157.89, 156.34, 155.61, 155.45, 154.08, 151.80, 150.05, 137.02, 136.80, 136.35, 135.63, 135.37, 134.88, 130.66, 128.29, 127.70, 127.53, 127.36, 127.25, 124.07, 123.90, 123.76, 123.46, 123.19, 61.80, 14.52.
CHN calc. for C34H28F6N5O2PRuS: C: 50.00; H: 3.46; N: 8.57 Obs. C: 50.03; H: 3.48; N: 8.56.
Bis(2,2՛-bipiridine)[N-((thiophene-2-yl-κC2)methyliden]-4-nitroaniline-κN]ruthenium(II) hexafluorophosphate (3n).
Dark purple powder. Yield 65%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.78 (s, 1H), 8.48 (d, J = 5.6 Hz, 1H), 8.34 (d, J = 7.6 Hz, 2H), 8.12 (d, J = 8.0 Hz, 1H), 8.05 (d, J = 8.2 Hz, 1H), 7.95–7.71 (m, 9H), 7.64 (d, J = 5.3 Hz, 1H), 7.43 (t, J = 6.6 Hz, 1H), 7.35–7.26 (m, 2H), 7.20 (t, J = 6.7 Hz, 1H), 6.68 (d, J = 9.0 Hz, 2H), 6.52 (d, J = 4.7 Hz, 1H).
13C NMR (76 MHz, Acetonitrile-d3) δ 217.12, 167.65, 158.41, 158.36, 158.11, 157.88, 155.48, 155.42, 154.14, 151.90, 149.98, 145.73, 139.52, 137.86, 137.24, 136.52, 135.82, 135.56, 135.02, 127.96, 127.64, 127.43, 127.30, 125.02, 124.13, 124.00, 123.95, 123.86, 123.67.
CHN calc. for C31H23F6N6O2PRuS: C: 47.15; H: 2.94; N: 10.64 Obs. C: 46.99; H: 2.96; N: 10.61.
Bis(2,2՛-bipiridine)[N-((thiophene-2-yl-κC2)methyliden]-4-(trifluoromethyl)aniline-κN]ruthenium(II) hexafluorophosphate (3o).
Dark purple powder. Yield 96%.
1H NMR (300 MHz, Acetonitrile-d3) δ 8.73 (s, 1H), 8.51 (d, J = 4.9 Hz, 1H), 8.33 (d, J = 7.2 Hz, 2H), 8.13 (d, J = 8.1 Hz, 1H), 8.04 (d, J = 8.2 Hz, 1H), 7.93–7.70 (m, 7H), 7.62 (d, J = 4.8 Hz, 1H), 7.42 (ddd, J = 7.4, 5.7, 1.4 Hz, 1H), 7.32 (ddd, J = 7.3, 5.7, 1.4 Hz, 1H), 7.27–7.17 (m, 4H), 6.63 (d, J = 8.0 Hz, 2H), 6.51 (d, J = 4.7 Hz, 1H).
13C NMR (76 MHz, Acetonitrile-d3) δ 214.53, 167.20, 158.45, 158.41, 157.87, 155.63, 155.49, 155.38, 154.06, 151.82, 150.06, 139.05, 137.01, 136.87, 136.40, 135.65, 135.38, 134.87, 127.73, 127.59, 127.37, 127.23, 126.64, 126.59, 126.54, 126.48, 124.09, 123.92, 123.76, 123.71, 123.41.
CHN calc. for C32H23F9N5PRuS: C: 47.30; H: 2.85; N: 8.62 Obs. C: 47.28; H: 2.79; N: 8.63.